Abstract
Malaria, caused by the protozoan Plasmodium is a devastating mosquito-borne disease, that puts nearly half the world’s population at risk1. Despite mounting substantial T and B cell responses, humans fail to efficiently control blood-stage malaria or develop sterilizing immunity to reinfections2. Though Foxp3+ regulatory T cells (Tregs) form a part of these responses3–5, their influence remains disputed, and mode of action unknown. Here we show that Tregs, which expand in both humans and rodents during blood-stage malaria, interfere with conventional T helper (Th) cell responses and the Follicular T helper (Tfh) cell:B cell partnership in germinal centers, in a critical temporal window to impede protective immunity, through the Cytotoxic T-lymphocyte-Associated protein (CTLA)-4. Targeting Tregs or CTLA-4 with precisely timed depletion or blocking enhanced immune responses, accelerated clearance, and generated species-transcending immunity to blood-stage malaria in mice. Our study uncovers a critical mechanism of immunosuppression associated with blood-stage malaria that delays parasite clearance and prevents development of potent adaptive immunity to reinfection. These data also reveal a temporally discrete and therapeutically amenable functional role for Tregs in limiting anti-malarial immunity.
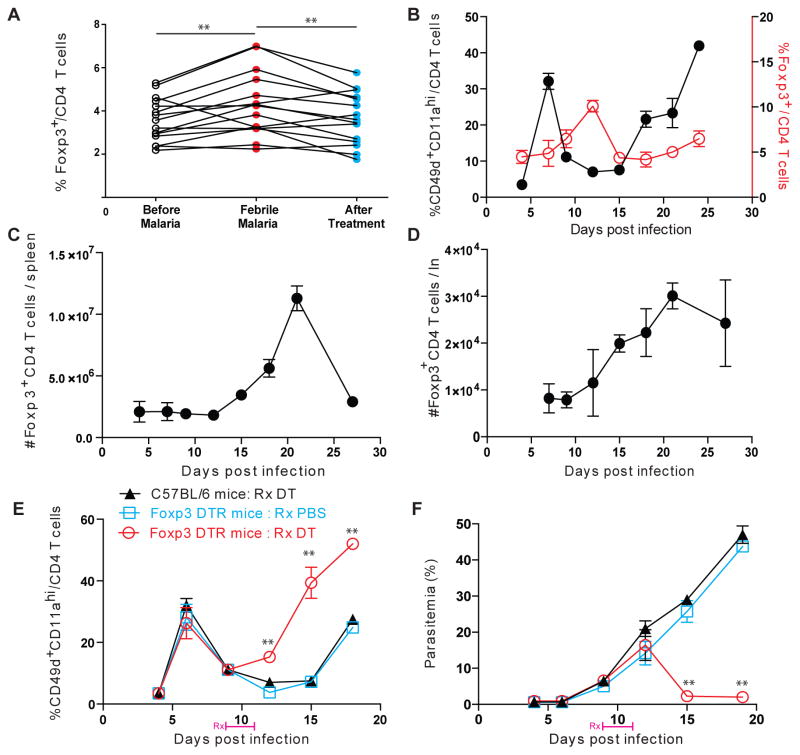
CD4 T helper (Th) cells are vital to the control of malaria in humans and the rodent models of disease6. Frequencies of activated Th cells increased during the course of malaria in mice and humans (Supplementary figures 1C, 2) and their depletion after established infection resulted in uncontrolled parasitemia and death in mice infected with the normally non-lethal rodent parasite, Plasmodium yoelii 17XNL (Py) (Supplementary figure 1A,B). However, unlike in most other infections (eg. L. monocytogenes), the expansion of pathogen-specific Th cells (defined as CD49d+CD11ahiCD4+)7 in Py infection is distinctly and inexplicably biphasic. Specifically, the frequency and total numbers of pathogen-specific Th cells temporarily fall or plateau before rising again prior to clearance of Py infection (Supplementary figure 1D–F). Though the mechanism behind this hiatus in Th cell expansion has remained unknown, we perceived it as a tangible sign of the general immunosuppression associated with blood-stage malaria. This assumption was underscored by the consistently observed increase in the key immunosuppressive cell population: Foxp3+ Tregs, during blood-stage malaria in humans (Supplementary table 1) and mice (Figure 1A–D), as well as the numerical and functional correlation between Tregs and disease susceptibility5,8,9. Higher parasite densities in blood were associated with higher Treg frequencies in humans; and chloroquine treatment to decrease parasitemia reduced Treg frequencies in mice (Supplementary figure 3A–B), also tempering the drop in Th frequencies (Supplementary figure 3C). The impact and function of Tregs in malaria has remained controversial5,10,11, with independent studies suggesting that Tregs suppress8,12,13, or enhance14,15 protection in this infection. While some concluded that depleting Tregs helped control parasitemia, disease severity or morality8,12,16, others saw no impact14,17,18 or even increased parasitemia and disease severity in some cases15. Importantly, these studies invariably manipulated the Treg response prior to or shortly after Plasmodium infection, mostly using anti-CD25 depleting antibodies8,12,14,15,18 with some studies using the more precise Foxp3-DTR system16,17. Although variations in model systems may have contributed to some of these inconsistencies, the timing of interventions that target Tregs may also be a critical consideration in malaria. Here, we observed that the expansion of Tregs in Py infected mice preceded or matched in time the hiatus in Th cell responses, suggesting a causal relationship that manifests ~10 days post infection. To test this, we depleted circulating and lymphoid19–21 Tregs in Py infected Foxp3-DTR (Supplementary figure 4A–B) or C57BL/6 (Supplementary figure 5A) mice with diphtheria toxin22 or anti-CD25 antibody8 respectively, beginning at day 9 just prior to the Th cell hiatus. Treg depletion at this time with both treatments interrupted the hiatus, restored Plasmodium-specific Th cell expansion and substantially accelerated control of Py infection (Figures1E–F, Supplementary figure 5B–C). In contrast, Treg depletion in Foxp3-DTR mice at the onset of Py infection resulted in death of infected mice (Supplementary Figure 4C).
Fig. 1. Regulatory T cells expand; modulate helper T cell responses and immunity to malaria.
(A) Longitudinal frequencies of Foxp3+ regulatory T cells in PBMCs in a cohort of children in Mali (Supplementary table 1) before, during and after acute febrile malaria. Each connected line indicates a separate subject. (B) Kinetics of activated CD4 helper or Foxp3+ regulatory T cell frequencies in circulation in Py infected C57BL/6 mice. (C–D) Absolute numbers of Foxp3+ Treg cells in spleen (C) or lymph nodes (D) at the indicated time points in Py infected C57BL/6 mice. (E–F) Frequencies of activated CD4 helper T cells in circulation (E) or parasitemia (F) at various time points post infection with Py in C57BL/6 or Foxp3 DTR mice treated with DT (Rx DT) or PBS (Rx PBS) on 9 and 11dpi. All experimental data represent 1 of at least 3 separate experiments with 5 mice/group and are presented as mean ± s.e.m. **indicates P≤ 0.01 comparing the indicated groups using one-way ANOVA with Bonferroni’s correction (A) or Foxp3 DTR:Rx DT or Foxp3 DTR:Rx PBS groups at the indicated time points using two-way ANOVA with Tukey’s correction (B)
To further address the opposing contributions of Tregs and Th cells in the control of parasitemia during the Th hiatus in Py infected mice, we selectively expanded Tregs (with IL-2/JES6 Ab complexes) or pathogen-specific Th cells (with IL-2/S4B6 Ab complexes)23 starting at day 9 post infection. Increasing the frequencies of Tregs further dampened the pathogen-specific Th cell response and resulted in higher parasitemia and death, while increasing the frequencies of pathogen-specific Th cells resulted in better control of the infection (Supplementary figure 5D–F). Together, these data suggested that Tregs suppress Th cell responses during a critical window of time during blood-stage malaria, compromising control of acute infection.
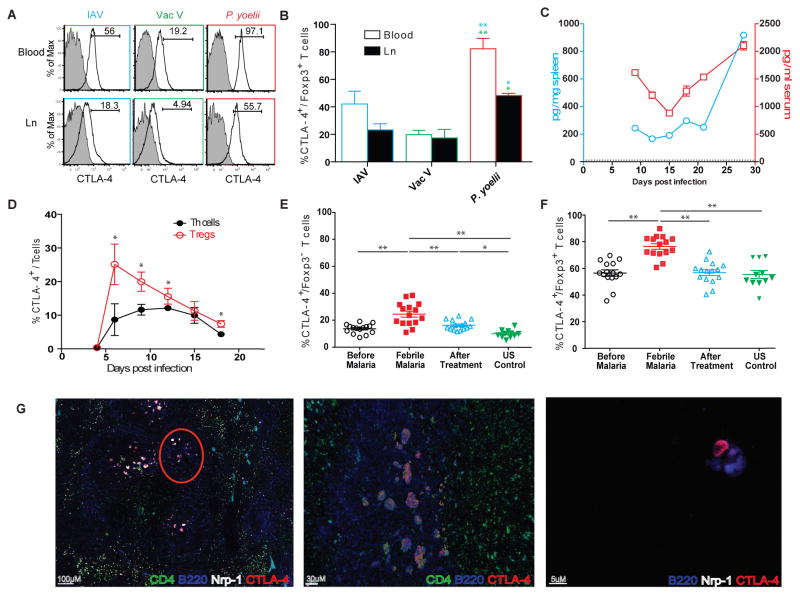
There are two prominent mechanisms by which Tregs counter Th cell responses in the context of infection: through contact-independent, IL-10 mediated inhibition, or through contact-dependent, CTLA-4 mediated repression of co-stimulation by antigen presenting cells (APCs)24. Tregs in mouse malaria transcriptionally upregulate both IL-10 and CTLA-416. Consistent with another study16, blocking IL-10 after established infection altered neither the kinetics of Th cell response nor the course of parasitemia in Py infected mice (Supplementary figure 6). However, Tregs in Py infected mice exhibited enhanced upregulation of CTLA-4, compared to Tregs in acute infections with influenza or vaccinia viruses (Figure 2A,B). Additionally, CTLA-4 was detectable in serum and spleen lysates in Py infected mice (Figure 2C), where the fraction of Tregs expressing CTLA-4 remained higher than the small percentages of Th cells with detectable CTLA-4 expression (Figure 2D)16. Of note, longitudinal analyses in humans showed that blood-stage (febrile) malaria increased the frequencies of circulating Th cells and Tregs expressing CTLA-4 (Figure 2E–F, Supplementary figure 7A). Febrile malaria in humans was also associated with higher frequencies of circulating, Helios (a marker of superior suppressive function25,26) expressing Tregs, Helios+ Tregs expressing CTLA-4, as well as CTLA-4 expressing Tregs of lymphoid follicular origin (Tfr)27,28 (Supplementary figure 7B–D). Taken together, these data suggested the hypothesis that Tregs may modulate Th and possibly humoral immunity to blood-stage malaria through CTLA-4.
Fig. 2. CTLA-4 expression enhanced in malaria, integral to Tfh:B:Tfr cell interactions in the germinal centers.
(A–B) Representative histograms showing CTLA-4 expression in Foxp3+ Tregs in Influenza A virus (IAV), vaccinia virus (VacV) or Py infected C57BL/6 mice, 9dpi (A). Gates and numbers inset indicate the proportions of CTLA4+ Foxp3+ Tregs, summarized in (B). (C–D) Kinetics of CTLA-4 expressed in spleen or serum (C) or in splenic Foxp3+ Tregs or Th cells (D) after Py infection in C57BL/6 mice. Dotted line in (C) is threshold of detection. (E–F) Proportions of CTLA-4 expressing Th cells (E) or Tregs (F) in a cohort of children in Mali before, during and after acute febrile malaria compared to healthy controls. (G) Representative pseudocolored images of fluorescently labeled sections from a C57BL/6 mouse spleen (left, middle panels) or lymph node (right panel) with a resolving (21–27dpi) Py infection, indicating Tfh:B:Tfr clusters in the GCs. Middle panel represents the encircled portion in the left panel. The right panel shows a single B cell and CTLA-4 expressing Tfr cell in close apposition. All experimental data represent 1 of at least 3 experiments with 5 mice per group, presented as mean ± s.e.m. *or **indicate P≤ 0.05 or 0.01 respectively, comparing the indicated groups using two-way ANOVA with Bonferroni correction (B), two-tailed student t-test (D) or one-way ANOVA with Tukey’s correction (E–F).
Humoral immunity, built on efficient follicular T helper (Tfh) cell: B cell cooperation in the secondary lymphoid organs, is perhaps the most important component of acquired immunity that controls blood-stage malaria29,30. To examine if either Tregs or CTLA-4 interfered with humoral immunity against malaria, we examined their roles in the Tfh:B cell partnership in the germinal centers (GCs). Within the GCs in secondary lymphoid organs, (CD4+) Th cells and (GL-7+B220+) B cells appeared to form discrete clusters of interaction (Video 1) after Py infection. These clusters were composed of CTLA-4-expressing follicular Treg (Tfr) and Th (Tfh) cells31, in close apposition with the GC plasmablasts or B cells (Figure 2G, Video 2). Expression of Neuropilin (Nrp)-1 or Foxp3 distinguished Tfr from Tfh cells32.
In the context of infection, CTLA-4 expressed on T cells bind to B7 ligands on APCs and limits immune responses by 1) competitive inhibition of B7:CD28 co-stimulatory interactions, 2) inducing inhibitory indoleamine 2,3-dioxygenase (IDO) in the APCs or 3) transendocytosing the B7 molecules from the surface of APCs 33. Since B cells (and not follicular dendritic cells) are the primary APCs that sustain Tfh cell responses34 and dictate protective antibody responses in malaria, we investigated if B cells might broker the CTLA-4-mediated immunomodulation. After Py infection, the CTLA-4+ Tfr cells appeared to directly associate with GC B cells (Video 3, Figure 2G), with CTLA-4 detectable on the B cells at their interface with Tfr cells (Video 4). Further, individual Tfr cells appeared to transiently interact with multiple B cells in GCs (Video 5), indicating how the relatively few Tfr cells could effectively modulate the GC reaction. We failed to observe any induced IDO (in vitro) in, or discernable transendocytosis of B7 molecules (in vitro or in vivo) from, Th or B cells after Py infection (data not shown). These observations suggested that CTLA-4 expressed or secreted by the Tfr cells might be directly binding to B7 ligands on the B cell surface, restricting productive co-stimulation of Tfh cells in the B cell follicles. Additionally, it also implied that blocking the CTLA-4: B7 interactions (checkpoint blockade) might augment immunity and clearance of blood-stage malaria.
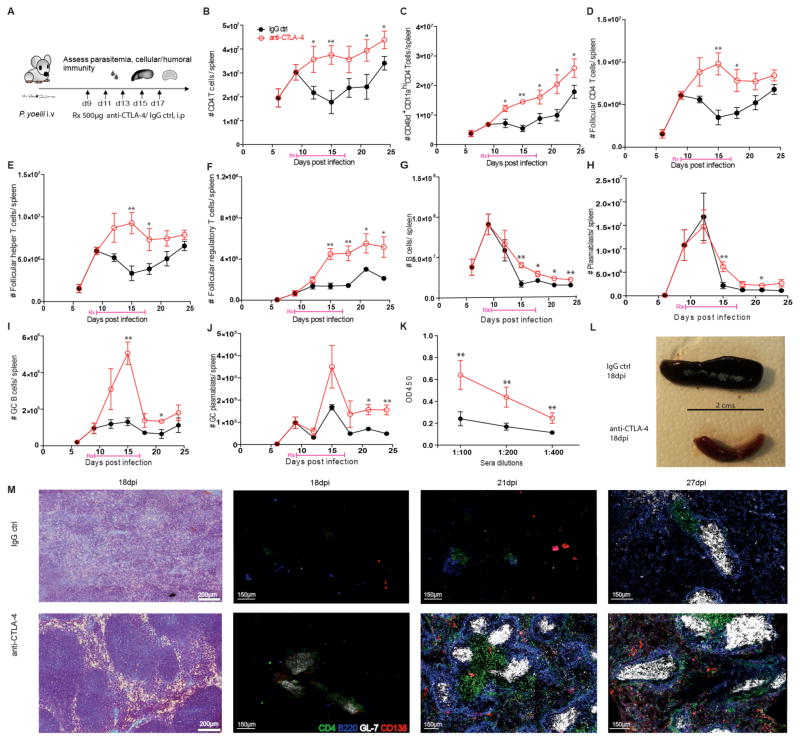
Although checkpoint blockade regimens applied without an understanding of the underlying immunology or disease progression could be ineffective or even detrimental to the host, they can be intuitively tailored to generate safe, clinically approved therapeutic choices35,36. For example, therapeutic blockade of CTLA-4 at the onset of blood-stage malaria or the absence of PD-L1 in a non-lethal model of LCMV infection, resulted in severe immunopathology and death in mice37–39. The precise definition of Treg kinetics, CTLA-4 expression dynamics, and timing of GC reactions uncovered here suggested that CTLA-4: B7 interactions may be meaningfully targeted post-establishment of Py infection. Hence, Py infected C57BL/6 mice were treated with CTLA-4 blocking (anti-CTLA-4) or IgG control (IgG ctrl) antibodies at the onset of the hiatus in the expansion of pathogen-specific Th cells (Figure 3a). Similar to Treg depletion, therapeutic blockade of CTLA-4 truncated the hiatus and enhanced the total numbers of CD4 T cells, pathogen-specific Th, follicular CD4 T cells, and Tfh cells in the spleen (and peripheral lymph nodes, data not shown) compared to IgG ctrl treated mice (Figure 3B–E). Hypothetically, CTLA-4 blockade could target Tregs, Th cells or both (Figure 2D). However, CTLA-4 blockade failed to further improve the Th cell response in Treg-depleted mice, suggesting a minor role for CTLA-4 expressed on Th cells in shaping their kinetics during this interval (Supplementary figure 8). Also, unlike in tumor models40, anti-CTLA-4 treatment during malaria did not deplete Tregs or Tfr cells in spleen (Supplementary figure 9, Figure 3F). We also observed a corresponding increase in the total numbers of splenic B cells, plasmablasts, GC B cells, GC plasmablasts, and Py-specific, protective serum-antibodies41 in anti-CTLA-4 treated mice (Figure 3G–K). Depleting GC B cells with anti-CD40L treatment34 prevented the revival of Th cell responses after CTLA-4 blockade, indicating that Treg interaction with GC B cells likely mediated the repression of Th cell responses in Py infection (Supplementary figure 10), although effects on other APC cannot be ruled out. Plasmodium infection notoriously causes severe splenomegaly and obliterates the splenic architecture in its hosts. In Py infected mice, CTLA-4 blockade resulted in considerably improved resolution of the spleen (Figure 3L) and its architecture, with distinct T cell zones, B cell follicles and GC reactions visible shortly after treatment (Figure 3M). Strikingly, therapeutic blockade of CTLA-4 also dramatically accelerated control of Py infection in C57BL/6 mice and the relatively more susceptible BALB/c mice (Figure 4A–B). Additionally, CTLA-4 blockade controlled parasitemia better and partially rescued (40% survival) BALB/c mice from lethal P. berghei ANKA infection (Figure 4C). However, CTLA-4 blockade before or after the critical window of expansion of Tregs was unable to productively alter the Th cell responses or accelerate control of Py infection (Supplementary figure 11), consistent with some previous results17,37. We previously showed that blockade of PD-1 and LAG-3 signaling during the post-hiatus revival of Th cell responses resulted in accelerated clearance of Py infection in mice7. In contrast, blocking PD-1 and LAG-3 signaling during the Treg-mediated hiatus in Th cell responses after Py infection provided no tangible improvement in immunity or parasite clearance, indicating a minimal contribution of these pathways to dampening immune responses during this critical interval (Supplementary figure 12). Of note, a suitably timed stimulation of OX40 signaling can also improve immunity against blood-stage malaria42. Together, these results reinforce the notion that immunomodulation during blood-stage malaria is complex and based on multiple molecular pathways that may be dominant during discrete time-windows during infection.
Fig. 3. CTLA-4 blockade enhances CD4 T and B cell responses, GC reaction and antibody titers after Py infection.
(A) A schematic of therapeutic blockade in Py infected C57BL/6 mice. (B–J) Total numbers of CD4 Th (B), Plasmodium-specific CD49d+CD11ahiCD4+Th (C), CXCR5+ICOS+PD-1+CD4+T follicular cells (D), CXCR5+ICOS+PD-1+Foxp3−CD4+Tfh (E) CXCR5+ICOS+PD-1+Foxp3+CD4+Tfr (F) cells, CD19+B220+B cells (G), CD138+IgD−CD19+B220+plasmablasts (H), CD95+GL7+CD19+B220+GC B cells (I) and CD95+GL7+CD138+IgD−CD19+B220+GC plasmablasts (J) in spleen at various time points and Py MSP1–19 specific relative serum antibody titers at 18 days (K), post Py infection in C57BL/6 mice with or without CTLA-4 blockade as in Figure 3(A). Data presented as mean ± s.e.m at each time point or sera dilution and represent 1 of 3 separate experiments, each with at least 5 mice per group. * or ** indicate P≤ 0.05 or 0.01 respectively, comparing the treatment and control groups at the indicated time points or sera dilutions with two-tailed student t-tests. Representative image of the gross appearance (18dpi, L), H&E stained or pseudocolored fluorescently labeled sections (M) of spleen from Py infected C57BL/6 mice at the indicated time points with or without CTLA-4 blockade. Markers and colors are indicated in the figure.
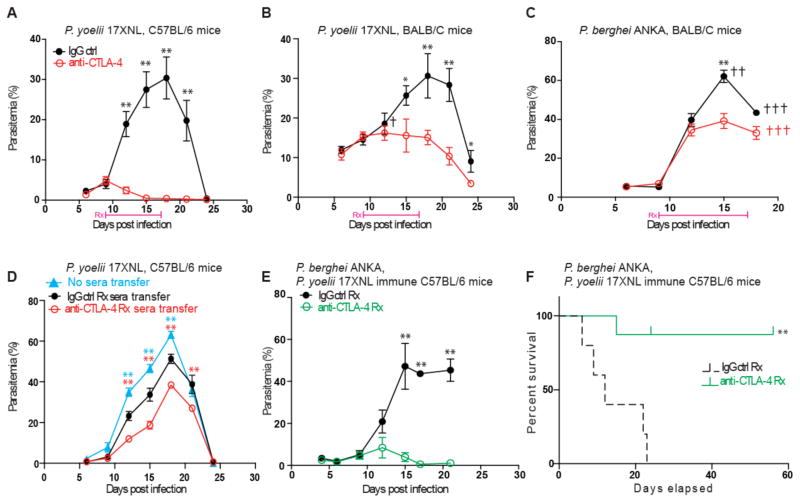
Fig. 4. Therapeutic blockade of CTLA-4 enhances immunity to malaria.
(A–C) Percentages of parasitemia at the indicated time points in C57BL/6 (A) or BALB/C (B–C) mice infected with Py (A–B) or P. berghei (C) with or without CTLA-4 blockade as in Figure 3(A). (D) Parasitemia at the indicated time points in Py infected C57BL/6 mice, that received sera (100μl) from 56 dpi donor mice as in panel (A), at 0 dpi. (E–F) Parasitemia at the indicated time points (E) or survival (F) in mice from panel (A) heterologously challenged with P. berghei at 56 dpi. All data represent 1 of at least 3 separate experiments, each started with 5 mice per group. Error bars represent s.e.m and color coded *or **indicate P≤ 0.05 or 0.01 respectively, comparing the corresponding treatment and control groups at the indicated time points with two-tailed student t-tests (A–E) or chi-square test (F). †indicates death of one mouse in the corresponding group.
Growing resistance to anti-malarial drugs is a major therapeutic concern that would not arise with approaches such as CTLA-4 blockade that target host molecules43. Yet, CTLA-4 blockade might be a less realistic independent treatment option for malaria in endemic areas, with its frequent dosages, parenteral administration, precise timing requirements, and currently prohibitive costs. Nevertheless, a major unresolved issue in malaria is why humans fail to generate potent adaptive immunity to subsequent Plasmodium infections, which may also involve multiple species of the parasite in endemic areas4,44. To address a potential role of Tregs/CTLA-4 in limiting generation of long-term immunity, we transferred (56 days post infection (dpi)) sera from C57BL/6 mice that cleared Py infection with or without CTLA-4 blockade, into C57BL/6 recipients with fresh (0 dpi) or established (10 dpi) Py infections. Recipients of sera from anti-CTLA-4 treated mice (that contain no detectable residual anti-CTLA-4 antibodies, Supplementary figure 13A) exhibited significantly lower parasitemia (Figure 4D, Supplementary figure 14). To test if anti-CTLA-4 therapy aided long-term and perhaps species-transcending immunity, we re-challenged C57BL/6 mice originally infected with Py, and cured with or without CTLA-4 blockade, using the lethal, cerebral malaria-causing P. berghei ANKA strain at 56 or 100 dpi after initial Py infection. CTLA-4 blockade during Py infection resulted in durable, CD4 T cell driven immunity against P. berghei ANKA, and dramatically improved long-term survival of the mice (Figure 4E–F, Supplementary Figure 13B–D) with no incidence of cerebral malaria. Taken together, these findings revealed that Treg expression of CTLA-4 is a major mechanism that limits acquired, cross-species immunity to malaria. Direct evidence of a role for Tregs and CTLA-4 in limiting protection from Plasmodium reinfection in humans can only be obtained through clinical trials and, like the translation of checkpoint blockade from animal models to human cancer immunotherapy45–47, the precise pathway forward for malaria must be carefully defined. However, in the face of rapidly developing drug resistance by Plasmodium, our findings provide important mechanistic insights to consider while designing and evaluating evidence-based interventions to target host-immunity for improved control of malaria.
Malaria is a global health threat, with close to 200 million clinical cases and many deaths reported annually. It is critically important to understand how Plasmodia circumvent effective immune responses in humans2. Here, we build on high-resolution studies of immune cell dynamics during blood-stage malaria of humans and mice, to show how Tregs can act in a discrete temporal window through CTLA-4 to suppress the Th and humoral immune responses. Thus, Tregs may function as an essential component of the immunoregulation observed in blood-stage malaria to inhibit clearance of acute infection and development of long-term sterilizing immunity to future infections.
Supplementary Material
Acknowledgments
We thank L. Epping, S. Hartwig, S. Perlman and V. Badovinac for reagents and comments, the UI Central Microscopy Research facility and the NYU Insectary Core. Support for these studies was provided by grants from NIAID/NIH (AI85515, AI95178, AI100527 to JTH). Support for the NSB lab was provided by grants from NIAID/NIH (AI125446, AI127481) and GMS/NIH (GM103447). The Mali study and the analysis of human samples were funded by the Division of Intramural Research, NIAID/NIH.
Footnotes
Author contributions
S.P.K. designed, performed, analyzed and interpreted experiments and wrote the paper. N.O-A, S.M.A designed, performed, analyzed and interpreted experiments. N.S.B designed, performed, analyzed and interpreted experiments and wrote the paper. B.T. and O.K.D. supervised the human studies, designed, analyzed and interpreted experiments. P.D.C supervised the human studies, designed, analyzed and interpreted experiments and wrote the paper. J.T.H supervised the project, designed and interpreted experiments and wrote the paper.
References
- 1.World Malaria Report 2014. World Health Organization; 2014. [Google Scholar]
- 2.Tran TM, Li S, Doumbo S, Doumtabe D, Huang CY, Dia S, Bathily A, Sangala J, Kone Y, Traore A, Niangaly M, Dara C, Kayentao K, Ongoiba A, Doumbo OK, Traore B, Crompton PD. An intensive longitudinal cohort study of Malian children and adults reveals no evidence of acquired immunity to Plasmodium falciparum infection. Clinical infectious diseases: an official publication of the Infectious Diseases Society of America. 2013;57:40–47. doi: 10.1093/cid/cit174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Ho M, Webster HK, Looareesuwan S, Supanaranond W, Phillips RE, Chanthavanich P, Warrell DA. Antigen-specific immunosuppression in human malaria due to Plasmodium falciparum. The Journal of infectious diseases. 1986;153:763–771. doi: 10.1093/infdis/153.4.763. [DOI] [PubMed] [Google Scholar]
- 4.Crompton PD, Moebius J, Portugal S, Waisberg M, Hart G, Garver LS, Miller LH, Barillas-Mury C, Pierce SK. Malaria immunity in man and mosquito: insights into unsolved mysteries of a deadly infectious disease. Annu Rev Immunol. 2014;32:157–187. doi: 10.1146/annurev-immunol-032713-120220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Van Braeckel-Budimir N, Kurup SP, Harty JT. Regulatory issues in immunity to liver and blood-stage malaria. Current opinion in immunology. 2016;42:91–97. doi: 10.1016/j.coi.2016.06.008. [DOI] [PubMed] [Google Scholar]
- 6.Perez-Mazliah D, Langhorne J. CD4 T-cell subsets in malaria: TH1/TH2 revisited. Frontiers in immunology. 2014;5:671. doi: 10.3389/fimmu.2014.00671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Butler NS, Moebius J, Pewe LL, Traore B, Doumbo OK, Tygrett LT, Waldschmidt TJ, Crompton PD, Harty JT. Therapeutic blockade of PD-L1 and LAG-3 rapidly clears established blood-stage Plasmodium infection. Nature immunology. 2012;13:188–195. doi: 10.1038/ni.2180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Hisaeda H, Maekawa Y, Iwakawa D, Okada H, Himeno K, Kishihara K, Tsukumo S, Yasutomo K. Escape of malaria parasites from host immunity requires CD4+ CD25+ regulatory T cells. Nature medicine. 2004;10:29–30. doi: 10.1038/nm975. [DOI] [PubMed] [Google Scholar]
- 9.Torcia MG, Santarlasci V, Cosmi L, Clemente A, Maggi L, Mangano VD, Verra F, Bancone G, Nebie I, Sirima BS, Liotta F, Frosali F, Angeli R, Severini C, Sannella AR, Bonini P, Lucibello M, Maggi E, Garaci E, Coluzzi M, Cozzolino F, Annunziato F, Romagnani S, Modiano D. Functional deficit of T regulatory cells in Fulani, an ethnic group with low susceptibility to Plasmodium falciparum malaria. Proceedings of the National Academy of Sciences of the United States of America. 2008;105:646–651. doi: 10.1073/pnas.0709969105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Finney OC, Riley EM, Walther M. Regulatory T cells in malaria--friend or foe? Trends in immunology. 2010;31:63–70. doi: 10.1016/j.it.2009.12.002. [DOI] [PubMed] [Google Scholar]
- 11.Hansen DS, Schofield L. Natural regulatory T cells in malaria: host or parasite allies? PLoS pathogens. 2010;6:e1000771. doi: 10.1371/journal.ppat.1000771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Amante FH, Stanley AC, Randall LM, Zhou Y, Haque A, McSweeney K, Waters AP, Janse CJ, Good MF, Hill GR, Engwerda CR. A role for natural regulatory T cells in the pathogenesis of experimental cerebral malaria. The American journal of pathology. 2007;171:548–559. doi: 10.2353/ajpath.2007.061033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Randall LM, Amante FH, McSweeney KA, Zhou Y, Stanley AC, Haque A, Jones MK, Hill GR, Boyle GM, Engwerda CR. Common strategies to prevent and modulate experimental cerebral malaria in mouse strains with different susceptibilities. Infection and immunity. 2008;76:3312–3320. doi: 10.1128/IAI.01475-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Nie CQ, Bernard NJ, Schofield L, Hansen DS. CD4+ CD25+ regulatory T cells suppress CD4+ T-cell function and inhibit the development of Plasmodium berghei-specific TH1 responses involved in cerebral malaria pathogenesis. Infection and immunity. 2007;75:2275–2282. doi: 10.1128/IAI.01783-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Cambos M, Belanger B, Jacques A, Roulet A, Scorza T. Natural regulatory (CD4+CD25+FOXP+) T cells control the production of pro-inflammatory cytokines during Plasmodium chabaudi adami infection and do not contribute to immune evasion. International journal for parasitology. 2008;38:229–238. doi: 10.1016/j.ijpara.2007.07.006. [DOI] [PubMed] [Google Scholar]
- 16.Abel S, Luckheide N, Westendorf AM, Geffers R, Roers A, Muller W, Sparwasser T, Matuschewski K, Buer J, Hansen W. Strong impact of CD4+ Foxp3+ regulatory T cells and limited effect of T cell-derived IL-10 on pathogen clearance during Plasmodium yoelii infection. Journal of immunology. 2012;188:5467–5477. doi: 10.4049/jimmunol.1102223. [DOI] [PubMed] [Google Scholar]
- 17.Haque A, Best SE, Amante FH, Mustafah S, Desbarrieres L, de Labastida F, Sparwasser T, Hill GR, Engwerda CR. CD4+ natural regulatory T cells prevent experimental cerebral malaria via CTLA-4 when expanded in vivo. PLoS pathogens. 2010;6:e1001221. doi: 10.1371/journal.ppat.1001221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Couper KN, Blount DG, Wilson MS, Hafalla JC, Belkaid Y, Kamanaka M, Flavell RA, de Souza JB, Riley EM. IL-10 from CD4CD25Foxp3CD127 adaptive regulatory T cells modulates parasite clearance and pathology during malaria infection. PLoS pathogens. 2008;4:e1000004. doi: 10.1371/journal.ppat.1000004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Linterman MA, Pierson W, Lee SK, Kallies A, Kawamoto S, Rayner TF, Srivastava M, Divekar DP, Beaton L, Hogan JJ, Fagarasan S, Liston A, Smith KG, Vinuesa CG. Foxp3+ follicular regulatory T cells control the germinal center response. Nature medicine. 2011;17:975–982. doi: 10.1038/nm.2425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Aloulou M, Carr EJ, Gador M, Bignon A, Liblau RS, Fazilleau N, Linterman MA. Follicular regulatory T cells can be specific for the immunizing antigen and derive from naive T cells. Nature communications. 2016;7:10579. doi: 10.1038/ncomms10579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Setiady YY, Coccia JA, Park PU. In vivo depletion of CD4+FOXP3+ Treg cells by the PC61 anti-CD25 monoclonal antibody is mediated by FcgammaRIII+ phagocytes. European journal of immunology. 2010;40:780–786. doi: 10.1002/eji.200939613. [DOI] [PubMed] [Google Scholar]
- 22.Penaloza-MacMaster P, Kamphorst AO, Wieland A, Araki K, Iyer SS, West EE, O’Mara L, Yang S, Konieczny BT, Sharpe AH, Freeman GJ, Rudensky AY, Ahmed R. Interplay between regulatory T cells and PD-1 in modulating T cell exhaustion and viral control during chronic LCMV infection. The Journal of experimental medicine. 2014;211:1905–1918. doi: 10.1084/jem.20132577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Boyman O, Sprent J. The role of interleukin-2 during homeostasis and activation of the immune system. Nature reviews. Immunology. 2012;12:180–190. doi: 10.1038/nri3156. [DOI] [PubMed] [Google Scholar]
- 24.Schmidt A, Oberle N, Krammer PH. Molecular mechanisms of treg-mediated T cell suppression. Frontiers in immunology. 2012;3:51. doi: 10.3389/fimmu.2012.00051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Sebastian M, Lopez-Ocasio M, Metidji A, Rieder SA, Shevach EM, Thornton AM. Helios Controls a Limited Subset of Regulatory T Cell Functions. Journal of immunology. 2016;196:144–155. doi: 10.4049/jimmunol.1501704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kim HJ, Barnitz RA, Kreslavsky T, Brown FD, Moffett H, Lemieux ME, Kaygusuz Y, Meissner T, Holderried TA, Chan S, Kastner P, Haining WN, Cantor H. Stable inhibitory activity of regulatory T cells requires the transcription factor Helios. Science. 2015;350:334–339. doi: 10.1126/science.aad0616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Wei Y, Feng J, Hou Z, Wang XM, Yu D. Flow cytometric analysis of circulating follicular helper T (Tfh) and follicular regulatory T (Tfr) populations in human blood. Methods Mol Biol. 2015;1291:199–207. doi: 10.1007/978-1-4939-2498-1_17. [DOI] [PubMed] [Google Scholar]
- 28.Maceiras AR, Graca L. Identification of Foxp3(+) T follicular regulatory (Tfr) cells by flow cytometry. Methods Mol Biol. 2015;1291:143–150. doi: 10.1007/978-1-4939-2498-1_12. [DOI] [PubMed] [Google Scholar]
- 29.Boyle MJ, Reiling L, Feng G, Langer C, Osier FH, Aspeling-Jones H, Cheng YS, Stubbs J, Tetteh KK, Conway DJ, McCarthy JS, Muller I, Marsh K, Anders RF, Beeson JG. Human antibodies fix complement to inhibit Plasmodium falciparum invasion of erythrocytes and are associated with protection against malaria. Immunity. 2015;42:580–590. doi: 10.1016/j.immuni.2015.02.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Cohen S, Mc GI, Carrington S. Gamma-globulin and acquired immunity to human malaria. Nature. 1961;192:733–737. doi: 10.1038/192733a0. [DOI] [PubMed] [Google Scholar]
- 31.Sage PT, Paterson AM, Lovitch SB, Sharpe AH. The coinhibitory receptor CTLA-4 controls B cell responses by modulating T follicular helper, T follicular regulatory, and T regulatory cells. Immunity. 2014;41:1026–1039. doi: 10.1016/j.immuni.2014.12.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Yadav M, Stephan S, Bluestone JA. Peripherally induced tregs - role in immune homeostasis and autoimmunity. Frontiers in immunology. 2013;4:232. doi: 10.3389/fimmu.2013.00232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Walker LS, Sansom DM. The emerging role of CTLA4 as a cell-extrinsic regulator of T cell responses. Nature reviews. Immunology. 2011;11:852–863. doi: 10.1038/nri3108. [DOI] [PubMed] [Google Scholar]
- 34.Yusuf I, Stern J, McCaughtry TM, Gallagher S, Sun H, Gao C, Tedder T, Carlesso G, Carter L, Herbst R, Wang Y. Germinal center B cell depletion diminishes CD4+ follicular T helper cells in autoimmune mice. PloS one. 2014;9:e102791. doi: 10.1371/journal.pone.0102791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Zou W, Wolchok JD, Chen L. PD-L1 (B7-H1) and PD-1 pathway blockade for cancer therapy: Mechanisms, response biomarkers, and combinations. Science translational medicine. 2016;8:328rv324. doi: 10.1126/scitranslmed.aad7118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Porichis F, Kaufmann DE. Role of PD-1 in HIV pathogenesis and as target for therapy. Curr HIV/AIDS Rep. 2012;9:81–90. doi: 10.1007/s11904-011-0106-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Hafalla JC, Claser C, Couper KN, Grau GE, Renia L, de Souza JB, Riley EM. The CTLA-4 and PD-1/PD-L1 inhibitory pathways independently regulate host resistance to Plasmodium-induced acute immune pathology. PLoS pathogens. 2012;8:e1002504. doi: 10.1371/journal.ppat.1002504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Jacobs T, Graefe SE, Niknafs S, Gaworski I, Fleischer B. Murine malaria is exacerbated by CTLA-4 blockade. Journal of immunology. 2002;169:2323–2329. doi: 10.4049/jimmunol.169.5.2323. [DOI] [PubMed] [Google Scholar]
- 39.Barber DL, Wherry EJ, Masopust D, Zhu B, Allison JP, Sharpe AH, Freeman GJ, Ahmed R. Restoring function in exhausted CD8 T cells during chronic viral infection. Nature. 2006;439:682–687. doi: 10.1038/nature04444. [DOI] [PubMed] [Google Scholar]
- 40.Hannani D, Vetizou M, Enot D, Rusakiewicz S, Chaput N, Klatzmann D, Desbois M, Jacquelot N, Vimond N, Chouaib S, Mateus C, Allison JP, Ribas A, Wolchok JD, Yuan J, Wong P, Postow M, Mackiewicz A, Mackiewicz J, Schadendorff D, Jaeger D, Zornig I, Hassel J, Korman AJ, Bahjat K, Maio M, Calabro L, Teng MW, Smyth MJ, Eggermont A, Robert C, Kroemer G, Zitvogel L. Anticancer immunotherapy by CTLA-4 blockade: obligatory contribution of IL-2 receptors and negative prognostic impact of soluble CD25. Cell Res. 2015;25:208–224. doi: 10.1038/cr.2015.3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Ahlborg N, Ling IT, Howard W, Holder AA, Riley EM. Protective immune responses to the 42-kilodalton (kDa) region of Plasmodium yoelii merozoite surface protein 1 are induced by the C-terminal 19-kDa region but not by the adjacent 33-kDa region. Infection and immunity. 2002;70:820–825. doi: 10.1128/IAI.70.2.820-825.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Zander RA, Obeng-Adjei N, Guthmiller JJ, Kulu DI, Li J, Ongoiba A, Traore B, Crompton PD, Butler NS. PD-1 Co-inhibitory and OX40 Co-stimulatory Crosstalk Regulates Helper T Cell Differentiation and Anti-Plasmodium Humoral Immunity. Cell host & microbe. 2015;17:628–641. doi: 10.1016/j.chom.2015.03.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Cui L, Mharakurwa S, Ndiaye D, Rathod PK, Rosenthal PJ. Antimalarial Drug Resistance: Literature Review and Activities and Findings of the ICEMR Network. The American journal of tropical medicine and hygiene. 2015;93:57–68. doi: 10.4269/ajtmh.15-0007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.McKenzie FE, Bossert WH. Multispecies Plasmodium infections of humans. J Parasitol. 1999;85:12–18. [PMC free article] [PubMed] [Google Scholar]
- 45.Korman AJ, Peggs KS, Allison JP. Checkpoint blockade in cancer immunotherapy. Adv Immunol. 2006;90:297–339. doi: 10.1016/S0065-2776(06)90008-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Postow MA, Callahan MK, Wolchok JD. Immune Checkpoint Blockade in Cancer Therapy. J Clin Oncol. 2015;33:1974–1982. doi: 10.1200/JCO.2014.59.4358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Topalian SL, Drake CG, Pardoll DM. Immune checkpoint blockade: a common denominator approach to cancer therapy. Cancer Cell. 2015;27:450–461. doi: 10.1016/j.ccell.2015.03.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.