Abstract
Over the past decade, vectors derived from adeno-associated virus (AAV) have established themselves as a powerful tool for in vivo gene transfer, allowing long-lasting and safe transgene expression in a variety of human tissues. Nevertheless, clinical trials demonstrated how B and T cell immune responses directed against the AAV capsid, likely arising after natural infection with wild-type AAV, might potentially impact gene transfer safety and efficacy in patients. Seroprevalence studies have evidenced that most individuals carry anti-AAV neutralizing antibodies that can inhibit recombinant AAV transduction of target cells following in vivo administration of vector particles. Likewise, liver- and muscle-directed clinical trials have shown that capsid-reactive memory CD8+ T cells could be reactivated and expanded upon presentation of capsid-derived antigens on transduced cells, potentially leading to loss of transgene expression and immune-mediated toxicities. In celebration of the 25th anniversary of the European Society of Gene and Cell Therapy, this review article summarizes progress made during the past decade in understanding and modulating AAV vector immunogenicity. While the knowledge generated has contributed to yield impressive clinical results, several important questions remain unanswered, making the study of immune responses to AAV a priority for the field of in vivo transfer.
Keywords: : AAV vectors, immune responses, T cells, antibody responses, gene therapy
Introduction
Recombinant adeno-associated viruses (rAAV) are derived from small, non-enveloped, 4.7 kb DNA dependo-viruses belonging to the Parvoviridae family. Over the past decade, they have emerged as a promising vector platform for in vivo gene delivery. Used in >100 gene therapy clinical trials worldwide, sustained therapeutic effect has been achieved in the frame of a variety of inherited diseases, including Leber's congenital amaurosis type 2,1,2 hemophilia B,3 M-type α-1 antitrypsin deficiency,4,5 and lipoprotein lipase deficiency.6,7 Additional ongoing trials for indications such as hemophilia A (NCT 02576795), hemophilia B (NCT 00979238, NCT01687608, NCT02484092, NCT02396342, NCT02618915, NCT02971969), or spinal muscular atrophy (NCT 02122952) are yielding extremely promising results. Nevertheless, these successes have been tempered by rising concerns over the immunogenicity of the AAV capsid in patients, especially when the vector is systemically administered.
Though widely disseminated among the human population,8 wild-type (WT) AAV human infection has not been clearly associated with any clinical pathology or disease.9 After primary infection, WT AAV genomes can persist years in host cells, either episomally or integrated within the host DNA, and be reactivated by a helper virus or a genotoxic reagent. Seroprevalence studies have indicated that initial exposure to WT AAV often occurs early during childhood,10,11 when humoral and cellular immune responses directed against the AAV capsid might be mounted.12,13 As such, memory AAV-specific T and B cells might persist lifelong and be recalled upon rAAV-mediated gene transfer.
This review summarizes what is currently known on the prevalence of AAV capsid-specific B and T cell responses in the general population, as well as their impact on rAAV-mediated gene transfer in clinical trials, and discusses open controversies on AAV-mediated immunogenicity.
Generalities on Immune Responses
Immunity can be broadly defined as all the processes that enable an organism to defend itself against antigens perceived as causing a rupture of homeostatic welfare. Since rAAV vectors do not contain any viral gene, the only sources of foreign antigens brought in during gene transfer are derived from the viral capsid and the transgene product. The nucleic acid contained in the virion may also concur to activate immunity via engagement of Toll-like receptors.
Immune responses can be divided into two closely interwoven and collaborative subsystems: innate and adaptive immune responses. Innate immunity mounts rapidly, is non-specific, and does not result in immunological memory. Innate immune responses are initiated through the recognition of pathogen-associated molecular patterns (PAMPs), exhibited on pathogens, by pattern recognition receptors (PRRs) expressed at the surface or within immune cells. These PRRs recognize viral nucleic acids, as well as membrane glycoproteins, or even chemical messengers. Through a variety of signaling pathways, the engagement of PRRs mainly leads to the activation of nuclear factor κB (NF-κB) and interferon regulatory factor transcription factors, both of which play a central role in inducing the expression of pro-inflammatory cytokines or type I interferons (IFN), respectively.14
Adaptive immunity occurs after innate immunity and allows the recognition and elimination of pathogens that would have escaped the innate immune system, or persisted despite its action. The key feature of adaptive immune responses lies in the establishment of immunological memory after the first contact with a definite pathogen: in case of ulterior encounters with the same pathogen, this memory response is both faster and more efficient. Adaptive immunity can be decomposed into four main stages15: (1) antigen presentation by antigen-presenting cells and antigen recognition by T and B lymphocytes; (2) lymphocytes activation, with clonal expansion and differentiation into effector cells; (3) antigen elimination through humoral responses (secretion of antigen-specific antibodies by B lymphocytes) and/or cellular responses (destruction of antigen-containing cells by CD8+ cytotoxic T lymphocytes); (4) homeostatic contraction of immune responses, with apoptosis of activated lymphocytes, and installation of immunological memory with long-term persisting antigen-specific memory T and B lymphocytes.
Considering that rAAV vectors have a similar or even identical capsid to their wild-type counterpart, vector-directed adaptive immune responses triggered after gene transfer can potentially be greatly influenced by prior exposure to the WT virus during natural infections (Fig. 1).
Figure 1.
Initiation and reactivation of adaptive immune responses to adeno-associated virus (AAV). During natural infection with wild-type (WT) AAV, capsid-specific adaptive immune responses can be triggered, with the development of anti-AAV antibodies and the establishment of a pool of long-lasting capsid-reactive memory B and T lymphocytes. Upon in vivo administration of recombinant AAV (rAAV) vectors, pre-existing anti-AAV antibodies can neutralize vector particles, while memory lymphocytes can be reactivated and expanded, leading to the de novo production of anti-AAV antibodies or, potentially, to the destruction of transduced cells presenting capsid-derived antigens.
Pre-Existing Immunity to WT AAV Capsid in Humans
Prevalence of anti-AAV humoral immunity
Since the 1960s-1970s, numerous studies have investigated the seroprevalence of neutralizing antibodies directed against various AAV serotypes among the general population.10,16–31 Some detection methods are based on the direct fixation of antibodies onto AAV capsids, as is the case for enzyme-linked immunosorbent assay, while others detect the neutralization of rAAV-mediated transduction by neutralizing antibodies present in serum samples. Importantly, all these assays are difficult to standardize across laboratories, particularly in terms of thresholds of positivity, leading to variations in prevalence and cutoff values among the different reports.10,16–31 While seroprevalence varies geographically, anti-AAV2 neutralizing antibodies display the highest prevalence, ranging from 30% to 60% of the population. In comparison, anti-AAV7, -AAV8, and -AAV9 neutralizing antibodies have a prevalence ranging from 15% to 30% of the population. Although the prevalence of anti-AAV1 neutralizing antibodies is lower than that of AAV2 NAbs, it is still higher than anti-AAV7, -AAV8, and -AAV9 antibodies in most regions.
Generally speaking, neutralizing antibodies recognizing virtually all serotypes can be found in almost all subjects.32 This can be explained either by multiple infections with various WT AAV serotypes, or by broad cross-reactivity between neutralizing antibodies.16,17,33 This cross-reaction is likely the result of high amino acid sequence homologies between the capsids of different AAV serotypes.19
It is worth noting that not all anti-AAV antibodies have a neutralizing activity. The role of non-neutralizing antibodies is ill-defined, and can enhance the clearance of rAAV vector particles through their opsonization,34 or else have been documented to have an opposite effect to that of neutralizing antibodies.35 The prevalence of total anti-AAV antibodies is close to 70% of the population for AAV1 and AAV2, 45% for AAV6 and AAV9, and 38% for AAV8.16 Importantly, titers of anti-AAV immunoglobulin G (IgG) antibodies correlate significantly, though not completely, with titers of anti-AAV neutralizing antibodies.16,22,25,28,34,36–38
In terms of immunoglobulin subclasses, IgG1 levels are often the highest in AAV seropositive individuals,16,28 though for some subjects IgG2 and IgG3 prevail. Titers of IgG1, IgG2, and IgM are well-correlated with neutralizing factor titers, which is not the case for IgG3 and IgG4.28 Similarly, in subjects undergoing AAV gene transfer, development of high-titer IgG1 antibodies has been documented,28 with IgG3 subclasses identified as the predominant isotype in subjects developing T cell reactivity to AAV.39
Given that in some hereditary diseases characterized by early lethality it is desirable to administer gene therapy as early as possible, one important question is at what age individuals seroconvert to AAV. Antibodies specific for different AAV serotypes can already be detected at birth, which suggest vertical transmission of maternal antibodies.18 Antibody titers then decrease during the first year of life, when most humans are seronegative for most AAV serotypes,31,35 and thereafter IgG levels raise to reach a plateau at teenage years.18,36 Consequently, the time window during which humans are devoid of any anti-AAV antibodies is quite narrow.
Prevalence of T cell reactivity to the AAV capsid in healthy donors
Anti-capsid humoral responses were initially thought to be the only component of anti-AAV immunity that could explain the inefficiency of rAAV-mediated gene transfer in a number of preclinical and clinical studies. However, in 2006, the first liver-directed clinical trial for hemophilia B40 revealed that CD8+ T cell–mediated cytotoxic responses directed against the vector could completely annihilate the benefits of rAAV-mediated gene therapy41 (Fig. 2). This discovery prompted the scientific community to take more interest in pre-existing anti-AAV cellular immunity and its impact on rAAV-based gene transfer.12,32
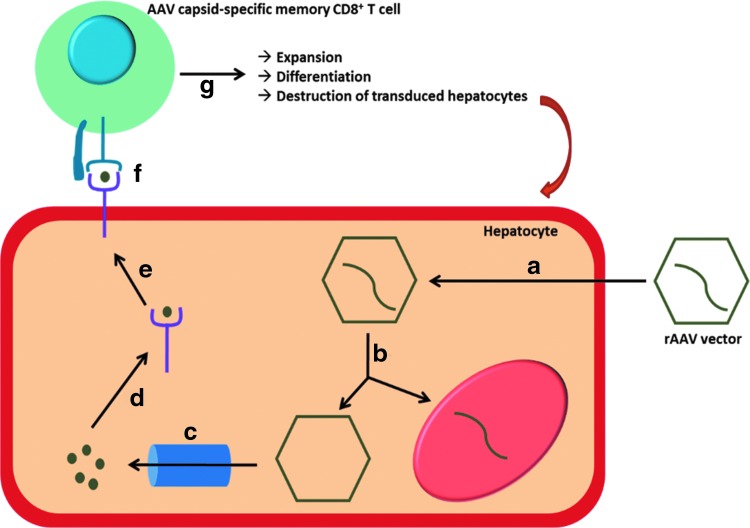
Figure 2.
Working model of capsid processing in hepatocyte and presentation to AAV-specific memory CD8+ T cells. (a) After administration, rAAV vectors enter hepatocytes via receptor-mediated endocytosis. (b) Following escape from the endosome and uncoating, vector DNA traffics to the nucleus where it drives the expression of the transgene. (c) Capsids are cleaved by the proteasome (or immune-proteasome) into short peptides. (d) Capsid-derived peptides are transported to the endoplasmic reticulum and loaded onto MHC class I molecules. (e) AAV-peptide/MHC complexes are transported to the plasmalemma, where they flag transduced hepatocytes as targets for AAV capsid-specific memory CD8+ T cells. (f) AAV-derived epitopes are presented to AAV capsid-specific memory CD8+ T cells through interaction between the TCR and the AAV-peptide/MHC complex. (g) Upon antigen recognition, AAV capsid-specific memory CD8+ T cells undergo expansion and differentiation into cytotoxic effector cells which can clear transduced hepatocytes through secretion of cytolytic factors or expression of death-inducing ligands.
The prevalence of T cells directed against AAV1 and AAV2 in the general population has been investigated through a variety of functional assays and is summarized in Table 1.22,25,41–43 Although prevalence can vary across studies, depending on the sensitivity of the assay used or on how the positive threshold was defined, data collected so far suggest that, overall, anti-capsid cellular responses are less preponderant than humoral responses. Interestingly, capsid-reactive T cells can be detected in a larger number of individuals in splenocytes compared to peripheral blood mononuclear cells (PBMCs), suggesting that AAV-specific T cells might fail to recirculate in peripheral blood, and preferentially home to lymphoid organs.41,43 In addition, a higher prevalence of T cell responses in PBMCs or splenocytes is observed after several rounds of in vitro expansion, suggesting that the frequency of AAV-specific T cells might be too low to be systematically detected ex vivo.41,43 Correlation studies between anti-AAV humoral and cellular responses suggest that there is no link between both parameters, at least for the AAV1 and AAV2 serotypes,22,41 and show that both seronegative and seropositive individuals could harbor T cells reactive to AAV.
Table 1.
Prevalence of AAV capsid-specific T cell responses in healthy donors
| Serotypes | Samples | Assays | Phenotype | Functionality | Positive/total |
|---|---|---|---|---|---|
| AAV225 | PBMCs | Lymphocyte proliferation; IFN-γ secretion in response to AAV capsid (ELISA) | N.A | N.A | 3/57 (6%) |
| AAV241 | PBMCs | IFN-γ ELISpot on unexpanded cells | CD45RA+ CD27+ CCR7- Resting central memory cells |
IFN-γ | 2/46 (4%) |
| Splenocytes | IFN-γ ELISpot on unexpanded cells | 2/28 (7%) | |||
| PBMCs | IFN-γ ELISpot on cells expanded with AAV peptides or whole capsid | 2/7 (28%) | |||
| Splenocytes | IFN-γ ELISpot on cells expanded with AAV peptides or whole capsid | 9/15 (60%) | |||
| AAV242 | PBMCs | Intracellular cytokine staining on cells stimulated with AAV peptides in the presence of anti-CD28 and anti-CD49d | CD45ROhi CD27hi Central memory Cells |
IFN-γ, IL-2, TNF-α | 8/17 (47%) |
| AAV122 | PBMCs | IFN-γ ELISpot on LV/VP1-stimulated cells | CD45RA- CD62L- Effector memory cells |
IFN-γ | 16/55 (29%) |
| AAV2/AAV143 | Splenocytes | IFN-γ ELIspot on unexpanded cells | CD45RO+ memory cells | IFN-γ, IL-2, TNF-α, CD107a, cytotoxicity | 2/44 (4.55%) |
| IFN-γ ELISpot on cells expanded with AAV peptides or whole capsid | 20/32 (62.5%) |
AAV, adeno-associated virus; PBMCs, peripheral blood mononuclear cells; IFN-γ, interferon gamma; ELISA, enzyme-linked immunosorbent assay; ELISpot, enzyme-linked immunospot; IL-2, interleukin-2; TNF-α, tumor necrosis factor alpha.
Interestingly, capsid-reactive T cells were also found in splenocytes isolated from children41,43 (5% of samples assessed ex vivo; 62.5% of samples assessed after in vitro expansion). As flow cytometry–based assessment of differentiation markers evidenced that the majority of AAV-specific T cells exhibits a memory phenotype,22,41,42 it is likely that they arise during infancy after naturally occurring WT AAV infections and persist throughout lifetime as a pool of memory T cells in secondary lymphoid organs, such as the spleen. Concerning their functionality, AAV-specific T cells have been shown to be able to produce IFN-γ,22,41–43 interleukin-2, and tumor necrosis factor alpha,42,43 as well as to express the CD107a degranulation marker and to be able to mediate cytotoxicity.43
Importantly, in a manner similar to AAV-specific antibodies, capsid-reactive CD8+ T cells are broadly cross-reactive through recognition of conserved epitopes across various AAV serotypes.41,43
Immune Responses to rAAV Vectors in Clinical Trials
While rAAV vectors do not encode viral proteins, the viral particles have an identical composition to WT AAV. Therefore, high doses of rAAV vectors can potentially activate recall responses generated against WT AAV capsid following cross-presentation of capsid antigens on target cells (Figs. 1 and 2).
Impact of neutralizing anti-AAV antibodies
Pre-existing anti-AAV humoral immunity represents one of the most efficient barrier to prevent successful gene transfer through systemic administration of rAAV vectors.32 The first hemophilia B clinical trial where rAAV vectors were injected into the bloodstream revealed that relatively low titers of neutralizing antibodies were sufficient to neutralize high doses of vectors completely.40 Indeed, among the two subjects enrolled in the high-dose cohort (2 × 1012 vg/kg), the one exhibiting a titer of neutralizing antibodies of 1:17 never experienced detectable levels of factor IX (FIX) transgene expression, while the other subject, with a titer of 1:2, developed circulating FIX levels at around 10% of normal range. Subsequent studies in mice44,45 and nonhuman primates (NHP)46 revealed that antibody titers as low as 1:5 were sufficient to block liver transduction by rAAV vectors completely, and that vectors remained susceptible to neutralization even hours after intravascular administration.
Likewise, rAAV vector transduction may be inhibited by anti-AAV antibodies when the vector is administered: in the synovial fluid of the articular space to target synoviocytes,21,47 in the vasculature of muscle limb to target muscles,48,49 and in the coronary artery to target cardiac muscle.50
Conversely, the presence of anti-AAV antibodies do not seem to impede transduction when the vector is administered through the intra-parenchymal route or in the subretinal space in the eye2,51,52 or in the cerebral ventricle to target the central nervous system.53
Administration of rAAV vectors triggers anti-AAV humoral responses in seronegative murine models,54 large animal models,33,46,55,56 and humans.57,58 Seroconversion is independent of species, vector, or administration route, and prevents successful re-administration of the same rAAV vectors38,59 (other than in immuno-privileged sites such as the subretinal space). Data emerging from human trials in adult subjects seem to indicate that long-term multi-year transgene expression can be obtained following a single AAV vector infusion.3,5,60 However, loss of expression may be observed in pediatric subjects61–63 due to cell proliferation and dilution of vector genomes, thus highlighting the need for strategies that allow for rAAV vector re-administration.
Impact of anti-AAV T cell responses
The initial report of a deleterious effect of anti-AAV cellular immune responses was the first clinical trial of liver-directed gene transfer for hemophilia B40 (Fig. 2). The FIX transgene, placed under the control of a liver-specific promoter, was packaged into a rAAV2 vector that was infused through the hepatic artery into seven subjects suffering from severe hemophilia B. In agreement with the preclinical studies in hemophilic dogs,64 the first subject from the high-dose cohort (Subject E; 2 × 1012 vg/kg) initially expressed FIX levels at ∼10% of normal range. Nonetheless, 4–6 weeks after rAAV2-based gene transfer, FIX expression decreased down to pretreatment baseline levels, concomitantly with a self-limited transient and asymptomatic rise in liver transaminase levels. A similar series of events was observed in the next patient enrolled in the mid-dose cohort (Subject G; 4 × 1011 vg/kg), from whom PBMCs were collected in order to perform a posteriori immune analyses.41 IFN-γ enzyme-linked immunospot (ELISpot) assays showed a response to AAV2 capsid but not FIX, and allowed the identification of a HLA-B*0702-restricted epitope derived from the AAV2 capsid (AAV2-p74). Kinetics of PBMCs staining with AAV2-p74/B7 MHC class I pentamer complexes finally revealed that the time course of AAV2 capsid-specific CD8+ T cells' frequency closely mirrored the rise in serum transaminases.
In much the same way, activation of AAV capsid-specific cellular response was also reported by Nathwani et al. during a subsequent liver-directed gene transfer clinical trial for hemophilia B.58 In this case, an rAAV8 vector encoding the self-complementary, codon-optimized FIX transgene (still under the control of a liver-specific promoter) was infused in a peripheral vein in patients suffering from severe hemophilia B with no detectable levels of anti-AAV neutralizing antibodies. While the first two dose cohorts proceeded uneventfully, subjects from the high-dose cohort (2 × 1012 vg/kg) once more displayed FIX expression at levels of 8–10% of normal over a period of 8 weeks, at which point FIX levels began to drop while serum transaminase levels rapidly increased, along with a marked rise in circulating capsid-specific T cells that were assessed by IFN-γ ELISpot assay. As soon as the rise in transaminase levels began, subjects were placed on a tapered regimen of high-dose steroids, which lead to a resolution in transaminitis and partial rescue FIX.58 Though effective at the currently used vector doses, ongoing studies will address if this corticosteroid regimen will be effective at higher vector doses and with different AAV serotypes. Of note, subjects from the intermediate dose cohort (6 × 1011 vg/kg) also exhibited detectable numbers of circulating AAV8 capsid-specific T cells when assessed through IFN-γ ELISpot assay, though this did not translate into either decline of FIX levels or rise in transaminase levels.
A number of additional clinical trials of hemophilia gene transfer confirmed the initial findings about occurrence of enzyme elevation together with loss of transgene expression (Table 2). Association of an increase in liver enzymes with T cell reactivity to the AAV capsid and loss of transgene expression in some cases has not been straightforward, underlying the complexity of the variables shaping the immunogenicity of rAAV vectors (vide infra).
Table 2.
Overview of transgene expression and enzyme elevation in hemophilia clinical trials
| Sponsor(s) | Capsid | Indication (transgene) | Results |
|---|---|---|---|
| Avigen40 | AAV2 | Hemophilia B (wild-type FIX) | 7 subjects treated |
| Transient expression of 10–12% of normal, at a dose of 2 × 1012 vg/kg | |||
| Liver enzyme elevation in two subjects | |||
| University College London and St. Jude Children's Research Hospital (NCT00979238)3 | AAV8 | Hemophilia B (wild-type FIX) | 6 subject treated |
| Long-term expression of 2.9–7.2% of normal (average 5.1%), at dose of 2 × 1012 vg/kg | |||
| 4/6 subjects dosed at 2 × 1012 vg/kg required a short course of steroids following a raise in liver enzymes | |||
| Baxalta/Shire (NCT01687608)125 | AAV8 | Hemophilia B (FIX Padua126) | Long-term expression at levels of ∼20% of normal in one subject |
| Loss of expression in most of the remaining subjects, despite a course of steroids (at doses from 2 × 1011 to 3 × 1012 vg/kg) | |||
| Spark Therapeutics and Pfizer (NCT02484092)127 | Engineered capsid | Hemophilia B (FIX Padua126) | 10 subjects treated |
| Long-term expression in all subjects at average plateau levels of >28% of normal at a dose of 5 × 1011 vg/kg | |||
| Two subjects required a short course of steroids | |||
| UniQure (NCT02396342)128 | AAV5 | Hemophilia B (wild-type FIX) | 10 subjects treated |
| Long-term expression at ∼5% of normal in 4/5 subjects in the low-dose cohort (5 × 1012 vg/kg) | |||
| Average levels at 7% of normal in 5 subjects from the second dose cohort (2 × 1013 vg/kg) | |||
| 3 subjects treated with course of steroids | |||
| Dimension Therapeutics (NCT02618915, NCT02971969) | AAVrh10 | Hemophilia B (wild-type FIX) | 6 subjects treated, all had evidence for transgene expression |
| 5/6 patients experienced transaminitis (ALT at 914 IU/L in one subject treated at 3.5 × 1012 gc/kg) | |||
| BioMarin (NCT02576795)129 | AAV5 | Hemophilia A (BDD FVIII) | 15 subjects treated |
| 7/7 subjects of the high-dose cohort (6 × 1013 vg/kg) expressed FVIII at levels ranging from 10% to >20% | |||
| Steroids administered to all high-dose subjects |
A plethora of data is available from numerous reports of intramuscular gene transfer clinical trials.65 Overall, the results from these trials indicate that the magnitude of AAV-specific T cells responses roughly correlates with the administered vector dose, as seen with liver-directed gene transfer. Though T cell reactivity in PBMCs and T cell infiltrates in the injected muscle have been detected in some cases, their presence is not always associated with a loss of transgene expression.4,5,39,57,66–68 A potential explanation for this is the presence of CD4+ CD25+ FoxP3+ regulatory T cells in muscle cell infiltrates, concomitant with PD-1/PD-1L expressing T cells.4,5,67 The ability of regulatory T cells and exhausted T cells to initiate tolerance to the AAV capsid after muscle-directed gene transfer has recently been extensively reviewed by Gernoux et al.69
Finally, during rAAV-mediated gene transfer to immune-privileged body compartments (such as the eye or the central nervous system), little to no capsid-specific cellular response has been detected so far in PBMCs from subjects infused directly into the brain or in the eye.32 One important feature to consider in this particular clinical setting is that the doses of rAAV vector administered are relatively small compared to muscle- or liver-directed gene transfer. Whether this immunologic unresponsiveness will endure upon administration of higher vector doses to allow the global transduction of the central nervous system remains to be seen. Data emerging from a gene transfer trial for spinal muscular atrophy (NCT 02122952), and other systemic diseases treated at high vector doses, suggest that careful management of vector immunogenicity is a requirement to limit or avoid toxicities. Similarly, for indications such as Duchenne muscular dystrophy,70 myotubularin myopathy,71 and other neuromuscular diseases treatable with high rAAV vector doses, as recently shown in large-animal models, careful monitoring and immunomodulatory plans in humans need to be devised.
Clinical management of anti-AAV immune responses
The easiest way to bypass the impact of pre-existing immune responses to AAV would be simply to exclude from clinical trials the subjects exhibiting high amounts of anti-AAV antibodies/neutralizing factors or capsid-reactive T cells. Considering that AAV-seropositive individuals represent up to 70% of the population, exclusion is difficult. Similarly, pre-screening patients to exclude those with pre-existing anti-AAV cellular immunity is not a sound approach, as the frequency of pre-existing circulating AAV-specific T cells in PBMCs is too low to permit their systematic detection through ELISpot or flow cytometry assays. Furthermore, positive anti-capsid cellular responses in clinical trials are not systematically translated into deleterious clinical consequences, and there is currently no means of predicting which parameters will trigger the onset of harmful responses. Importantly, though anti-AAV immune responses can result in loss of transgene expression, they do not inflict other harmful sequelae to the patient and seem to be so far more an “efficiency” than a “safety” issue. Nevertheless, for new indications needing high vector doses or targeting inflammatory tissues such as Duchenne muscular dystrophy,70 careful clinical and immune monitoring will be required.
The approaches most commonly investigated to circumvent AAV-capsid-specific humoral and cellular responses are summarized in Table 3. They can be divided into two categories—those impacting the vector itself and those impacting the patient or clinical setting–and might be combined in order to yield the best outcome.
Table 3.
Main approaches currently under investigation to modulate AAV-specific B and T cell responses
| Effective on | |||
|---|---|---|---|
| Strategies | B cells | T cells | Main drawbacks |
| Vector-oriented actions | |||
| Administer higher doses of vectors to titrate out NAb | Yes | No | - High doses can be neutralized by low titers of NAb (1:5/1:17) - Increase the antigenic charge susceptible to trigger capsid-reactive CD8+ T cells |
| Use empty capsid as “decoys” to titrate NAb124 | Yes | No | - Increase the antigenic charge susceptible to trigger capsid-reactive CD8+ T cells |
| Modify rAAV serotypes to prevent immune recognition: - Isolate new natural variants130 - Modify existing AAV capsids to shield them from neutralization131 - Construct new capsids by molecular engineering94,132 (disruption of known epitopes; tyrosine mutation to limit ubiquitination and proteasomal processing) |
Yes | Maybe | - Technically difficult and time-consuming - Potential alteration of vector tropism, production, and purification processes - Possibly inefficient due to cross-reactive responses |
| Improve manufacturing and characterization of rAAV batches to reduce immune recognition: - Reduce the presence of contaminants and/or adjuvants - Increase the ratio full/empty capsids |
Yes No |
Yes Yes |
- Technically difficult and time-consuming - Serotype-specific |
| Decrease the therapeutic dose needed to reduce antigen load127: - Improve transduction specificity and efficiency - Design hyperactive variants of the therapeutic transgene |
No | Yes | - Not feasible for all transgenes - Potential alteration of transgene packaging - Transgene-specific |
| Patient-oriented actions | |||
| Reduce exposition of vectors to neutralizing blood components: - Perform plasmapheresis to reduce circulating NAb titers133,134 - Use balloon catheters with saline flushes to deliver vectors135 |
Yes | No | - Several rounds of plasmapheresis needed to significantly decrease NAb titers - Transient immune-suppression induced by plasmapheresis - Balloon catheters cannot be used for all administration routes/target tissues; invasive procedure |
| Administer proteasome inhibitors to limit capsid-derived MHC class I antigen presentation77 | No | Yes | - Prolonged pharmacotherapy for a limited effect likely to be required |
| Administer immune-suppressive drugs to prevent or eradicate immune responses21,136 | Yes | Yes | - Risks associated to systemic immunosuppression - Interference with Treg induction - Difficult pre-clinical evaluation (drugs often not efficient in animals) - No eradication of memory lymphocytes |
| Induce peripheral tolerance to capsid-derived antigens to prevent activation of capsid-specific immune responses79 | Yes | Yes | - No effect on pre-existing NAb - Additional clinical intervention needed - Technically difficult and time-consuming |
It is worth noting that despite promising advances, it is currently still not possible to wholly circumvent pre-existing anti-AAV humoral immunity. On the other hand, management of anti-AAV cellular responses seems to be efficiently achieved, in the clinical settings tested so far, through broadly immuno-suppressive drugs administered either pre-emptively or as soon as a rise in liver or muscle transaminases is observed in blood samples.3,32,72 The caveats of immune responses triggered by capsid-derived antigens bear an uncanny resemblance to the immunological issues encountered during solid organ transplantation, which is why the immunosuppressive drugs currently used in rAAV-mediated gene transfer stem from immunosuppression regimen initially designed to allow long-term graft survival.73 In this fashion, some adverse events associated to immunosuppression are common between both clinical settings, such as the issue of specificity (i.e., the ability to limit only immune responses directed against a given antigen) or the preclusion of tolerance induction (since immunosuppressive drugs also prevent the development of Treg sometimes necessary to establish robust antigen-specific long-term tolerance to the transgene product for instance74). Other potential issues, however, are specifically related to the context of rAAV-mediated gene transfer, notably how drugs might influence tissue biodistribution of vector particles or transduction efficiency. Of interest, immunosuppressive regimens in rAAV-mediated gene therapy clinical trials are only used transiently, therefore limiting the risk of complications classically associated with long-term immunosuppression in organ transplantation (mainly cardiovascular disease and cancer). It is noteworthy that one drawback of transient immunosuppression is that the timeframe of the in vivo persistence of intact rAAV particles is not known, though some microscopy data from retinal- and muscle-directed gene transfer indicate that whole particles might persist years in large-animal and human tissues.4,75 The implications of intact capsids persistence for years after gene transfer are not fully understood. Intact capsids gaining access to MHC class I antigen presentation pathway76,77 might trigger capsid-directed immune responses at a moment when patients are not closely monitored anymore. On the other hand, it has been suggested that persisting intact capsid may also mimic a chronic viral infection and promote tolerance maintenance via induction or Tregs and expression of PD1/PDL1.4,69
Ideally, the development of new immunosuppressive or immunomodulatory strategies for future clinical applications should comply with the following key requirements: capsid-derived antigen specificity, limited spatial range of action (e.g., in situ immunosuppression where antigens are locally presented), induction of robust long-term peripheral tolerance (rather than transient immunosuppression), and with no heavy additional medical procedures for the patient. With this aim in mind, recent work with novel immunomodulatory strategies based on biodegradable nanoparticles yielded promising results in the context of anti-drug antibodies78 and rAAV vector–mediated gene transfer.79
Open Controversies
Which preclinical animal models would be best to study anti-AAV cellular immune responses?
While some preclinical small- and/or large-animal models have been useful in predicting the impact of anti-AAV humoral responses, the onset of anti-capsid cellular responses observed in liver-directed clinical trials had never been observed before in any of the preclinical animal models employed, even in those susceptible to natural AAV infections such as NHP.46,80 The lack of relevant animal models to study anti-capsid cellular responses upon administration of rAAV vectors is an undeniable hindrance to understanding the machinery of this phenomenon and to developing efficient and safe strategies to circumvent them.
Comparing capsid-specific T cell responses to natural AAV infections in humans and in NHP, Li et al. highlighted differences in the frequencies and subset distributions of capsid-specific CD8+ and CD4+ T cells between both species.42 These disparities might stem from differences between AAV2 and AAV8 life cycles in humans and NHP, respectively. Furthermore, the loss of inhibitory sialic acid–recognizing Ig superfamily lectins on human T cells and a more efficient recruitment of primed human T cells to the liver have also been proposed as potential reasons why humans more readily mount capsid-specific T cell responses in liver-directed gene transfer than NHP.81–83
It must be noted that preclinical studies on NHP models were mainly carried out in rhesus or cynomolgus monkeys, and that other species might provide better insights. For instance, baboons (Papio anubis), which are more widely used as preclinical models in transplantation studies, have been shown to reflect more closely the reactivation process of human memory CD4+ T cells than macaques, consequently providing better predictions of human immune responses in transplantation protocols.84–86 Few studies have used baboons to assess rAAV vectors, and none of them dealt with capsid-directed cellular immune responses,87–89 so that this field of research is yet to be filled.
Accounting for the ethical and economic considerations related to the use of NHP, multiple efforts were independently made to set up a small-animal model recapitulating what is observed in humans. First attempts at generating a mouse model consisted in immunizing mice against the AAV capsid through intramuscular injection of an adenovirus expressing the AAV capsid, or through repeated injections of capsid-pulsed dendritic cells.90–92 While these mice did develop capsid-specific CD8+ T cells, no clearance of transduced hepatocytes was seen upon rAAV vector administration. More recently, two groups have made noteworthy progress in setting up mouse models that, in part, managed to reproduce some of the features of anti-capsid cellular immunity observed in human liver-directed clinical trials. Ertl's group developed an adoptive transfer mouse model where animals received rAAV2 vectors, whose VP2 capsid protein contained multiple copies of the immunodominant, ovalbumin-derived, CD8+ T cell SIINFEKL epitope, and splenocytes derived from OT-1 mice, which bear transgenic SIINFEKL-specific CD8+ TCRs.93 This model allowed proliferation of epitope-specific CD8+ T cells and permitted assessment of the rate of AAV2 capsid degradation in vivo. Herzog's group developed an adoptive transfer mouse model where immune-deficient animals received rAAV vectors and capsid-specific CD8+ T cells (expanded ex vivo from splenocytes isolated from AAV-immunized immune-competent mice) in addition to adjuvants.94 In this model, transaminitis, loss of transgene expression, and infiltration of capsid-specific CD8+ T cells in rAAV2- or rAAV8- transduced livers were observed, therefore reproducing human observations. Though promising, the pertinence of these new models in assessing immuno-modulating strategies for clinical application remains untested. Of note, high expectations have been placed lately in chimeric mouse models where livers are partly reconstituted with human hepatocytes.95,96
How do peripheral anti-AAV cellular immune responses compare to in situ responses?
Attempts at correlating transgene expression and AAV capsid-specific cellular immune responses during clinical trials have essentially relied on the assessment of capsid-reactive T cells in PBMC samples. However, observations made in the periphery might not accurately reflect the local immunological events taking place in the target tissue, where capsid antigens are present. Only in muscle-directed gene transfer have the authors sought to correlate anti-capsid cellular immune responses to transgene expression in muscle biopsies, which permitted the presence of in situ immune infiltrates in the target tissue to be assessed.3–5,65 In particular, analysis of in situ immune responses might help refine our understanding of the mechanisms by which loss or maintenance of transgene expression may occur. Of note, up to now, published records of muscle-directed gene transfer clinical trials have exclusively been operated through intramuscular injections. Biopsies can therefore be biased, as transduction of muscle cells might not be homogeneous within the whole tissue. Additionally, the overall comparison of results from all muscle-directed clinical trials can be somewhat hampered by several caveats: (1) safety and efficacy endpoints are not always straightforward; (2) muscle physiopathology, in particular underlying inflammation, is not always well defined; (3) some transgene products possess immune-modulating properties or in contract immunogenic features; (4) immune-suppression regimen are often administered prior to or concomitantly with rAAV vectors.
Elucidating whether anti-AAV immune responses assessed in the periphery faithfully depict in situ events will be of particular interest when administration of high doses of vectors to target a pathologically inflamed tissue is attempted, as is the case for muscle-directed gene therapy in patients suffering from Duchenne muscular dystrophy, for instance.
Does innate immunity play a role in shaping adaptive immune responses to rAAV vectors in humans?
It is known that rAAV vectors trigger innate immune responses. However, despite the growing body of evidence, the role of early activation of innate immunity following rAAV-mediated gene transfer remains elusive. In vitro studies indicate that rAAV vectors carrying a single-stranded DNA genome interact with the innate immune system through the Toll-like receptor (TLR)9/MyD88 and type I interferon cascades.97 Additionally, the capsid of AAV2 may also interact with the innate immune system via TLR2 in liver non-parenchymal cells (Kupffer and liver sinusoidal endothelial cells).98 In vivo, it has been demonstrated that rAAV vectors also trigger the NF-κB-dependent release of cytokines and chemokines in the mouse liver.99 Furthermore, MyD88 signaling in B cells seems to control the production of capsid-specific Th1 antibody responses,100 while TLR9-dependent release of inflammatory cytokines may also result in enhanced transgene immunogenicity, as shown by Martino et al. for self-complementary AAV vectors in mice.101 AAV vectors were also shown to interact with the complement pathway through iC3b factor.102,103 This interaction could be a mechanism used by AAV to limit the innate response as shown for other pathogens. While these studies provide strong evidence that innate immune recognition of AAV occurs in animals, little is known about the consequences of these interactions in the clinical setting, and particularly about how innate immunity to AAV impacts adaptive immune responses to the recombinant vector. To this end, recent work from the Herzog lab104 provides compelling evidence on the role of innate immunity in the cross-priming of CD8+ T cell responses directed against rAAV vectors. While these findings may seem controversial, as no evidence of systemic activation of innate immunity has been observed in human trials to date, they suggest that early control of innate immunity could lead to decreased vector immunogenicity.
Should we worry about transgene-specific immune responses and how they might impact the onset of anti-AAV immune responses?
Despite the fact that a wealth of preclinical data is available on immune responses to the transgene product in rAAV gene transfer, relatively little information is available on whether this knowledge would faithfully translate to the clinic. Several groups showed that delivery of rAAV vectors to the liver induces transgene-specific tolerance.105–109 Accordingly, rAAV-mediated liver gene transfer has also been used in inhibitor-prone hemophilia A dogs to eradicate low-titer anti-FVIII neutralizing antibodies,110 and in mice to eradicate antibodies to FIX111 or alpha-acid glucosidase.112 Although these preclinical data on liver tolerance are highly convincing, the open question is whether this concept will reliably translate to humans. Thus far, with only one exception,113 clinical studies of liver gene transfer have been conducted in the context of hemophilia trials in which patients were enrolled only if at low risk of developing immune responses to the transgene product.3,40 Therefore, both the transgene immunogenicity profile in the setting of liver gene transfer in at-risk patients and the outcome of gene therapy in humans that are cross-reactive immunological material (CRIM)-negative or pre-immunized to the transgene product (e.g., hemophilia patients with inhibitor) are crucial questions that remain open.
What seems to be clear is that the development of transgene-specific immune responses is highly dependent on the route of rAAV vector administration. For example, preclinical studies of rAAV gene transfer to the muscle suggest that immunosuppression may be needed to maintain transgene expression following vector intramuscular delivery to non-tolerant animals.55,114–116 Accordingly, transgene immunogenicity was observed in children with Duchenne muscular dystrophy following the intramuscular administration of an rAAV vector encoding the micro-dystrophin (a functional truncated version of the dystrophin protein).117 Importantly in this study, an immune response to the vector was also triggered, likely the result of both the intramuscular delivery route and the overall proinflammatory environment of the dystrophic muscle.118 As skeletal muscle is an important target tissue for the treatment of a broad range of diseases, promising strategies are currently under investigation to reduce the risk of onset of anti-transgene immunity, such as less immunogenic administration routes where the vector is perfused locoregionally in a limb,55,70,71,119 immunosuppression,55 and liver expression of the same antigen expressed in the muscle.120 Finally, based on recent data, non-secreted transgenes are predicted to be more immunogenic than secreted ones,121 a phenomenon that is likely due to antigen availability systemically, which can to some extent promote induction of Tregs both in the periphery and in the thymus.122
Conclusions
All things considered, little is known about natural AAV infection, which undoubtedly adds a level of difficulty in predicting AAV capsid-directed responses in rAAV-mediated gene therapies. The various clinical trials conducted so far have shown that a wide variety of parameters can influence these responses, including the configuration of the therapeutic DNA, the transgene properties, the AAV serotype, the vector production and purification process, the clinical settings, and the patient's natural history of WT AAV infection. Henceforth, AAV immunogenicity remains very much a puzzle, and the field of rAAV gene therapy research requires further efforts to resolve the complexity of capsid-related immune issues. The harmonization of patient monitoring using standard guidelines and external quality controls to check immune assay performance over time and across clinical trials would greatly facilitate the comparison of data and subsequently the understanding of the complexity of anti-AAV immune responses.
The best trade-off one can currently imagine is to engineer rAAV vectors with better transduction efficiency, carrying optimized therapeutic transgenes and with reduced immunogenic profiles (CpG-depleted genome,123 inert capsids, contaminant-free batches, minimum amounts of empty capsids,124 etc.). Such vectors would provide a higher therapeutic index, as they would permit therapeutic efficiency at doses sufficient to bypass pre-existing humoral immunity, but not high enough to trigger deleterious cellular immunity. Importantly, the relationship between therapeutic efficiency and rAAV vector dose is all about finding the right balance to remain above the therapeutic threshold. In this manner, pharmacological interventions improving the general state of the patient might help decrease this threshold and therefore contribute to the efficiency of rAAV-mediated gene therapy.
As the European Society of Gene and Cell Therapy reaches its 25th anniversary, the gene therapy field is experiencing one of its most exciting periods. Long-term efficacy has finally been achieved in several clinical trials, and gene therapy drugs are reaching late-stage clinical development and market approval. The years to come will bring forward a wealth of data that should give precious insights on several crucial questions. Does long-term therapeutic efficiency mean lifelong? Does the recombinant AAV genome persist indefinitely in transduced cells? Is it subjected to gene silencing after some time? Is genotoxicity a concern at all with AAV vectors? How long can intact rAAV particles be detected in vivo? While our understanding of the rAAV vector technology becomes more and more refined, answering some of these questions will provide a path forward to extend further the success of this novel therapeutic paradigm.
Acknowledgments
F.M.'s work was supported by Genethon. It was also supported by the European Union's research and innovation program under Grant Agreement No. 667751 (MYOCURE to F.M.), the European Research Council Consolidator Grant under Grant Agreement No. 617432 (MoMAAV to F.M.), and the R-Rare2 consortium grant SMART-HaemoCare. O.A. and C.V. were supported by the Inserm, the French Ministry of Research, the F.R.M. (Fondation pour la Recherche Médicale), the University Hospital of Nantes, the Fondation pour la Thérapie Génique en Pays de Loire, the AFM-Téléthon (Association Française contre les Myopathies), the Région Pays de la Loire (IMBIO-DC consortium), and the IHU-CESTI, which is supported by the French National Research Agency (ANR) via the “Investment Into The Future” program ANR-10-IBHU-005.
Author Disclosure
F.M. has consulted for companies developing AAV vector-based therapies. F.M. received sponsored research support from gene therapy companies. C.V. and O.A. have no conflict of interests to disclose.
References
- 1.Bainbridge JWB, Mehat MS, Sundaram V, et al. Long-term effect of gene therapy on Leber's congenital amaurosis. N Engl J Med 2015;372:1887–1897 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Maguire AM, High KA, Auricchio A, et al. Age-dependent effects of RPE65 gene therapy for Leber's congenital amaurosis: a Phase 1 dose-escalation trial. Lancet 2009;374:1597–1605 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Nathwani AC, Reiss UM, Tuddenham EGD, et al. Long-term safety and efficacy of factor IX gene therapy in hemophilia B. N Engl J Med 2014;371:1994–2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Mueller C, Chulay JD, Trapnell BC, et al. Human Treg responses allow sustained recombinant adeno-associated virus–mediated transgene expression. J Clin Invest 2013;123:5310–5318 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Mueller C, Gernoux G, Gruntman AM, et al. 5 Year expression and neutrophil defect repair after gene therapy in alpha-1 antitrypsin deficiency. Mol Ther 2017;25:1387–1394 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Carpentier AC, Frisch F, Labbé SM, et al. Effect of alipogene tiparvovec (AAV1-LPLS447X) on postprandial chylomicron metabolism in lipoprotein lipase-deficient patients. J Clin Endocrinol Metab 2012;97:1635–1644 [DOI] [PubMed] [Google Scholar]
- 7.Burnett JR, Hooper AJ. Alipogene tiparvovec, an adeno-associated virus encoding the Ser(447)X variant of the human lipoprotein lipase gene for the treatment of patients with lipoprotein lipase deficiency. Curr Opin Mol Ther 2009;11:681–691 [PubMed] [Google Scholar]
- 8.Gao G, Vandenberghe LH, Alvira MR, et al. Clades of adeno-associated viruses are widely disseminated in human tissues. J Virol 2004;78:6381–6388 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Berns KI, Muzyczka N. AAV: an overview of unanswered questions. Hum Gene Ther 2017;28:308–313 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Blacklow NR, Hoggan MD, Kapikian AZ, et al. Epidemiology of adenovirus-associated virus infection in a nursery population. Am J Epidemiol 1968;88:368–378 [DOI] [PubMed] [Google Scholar]
- 11.Blacklow NR, Hoggan MD, Rowe WP. Serologic evidence for human infection with adenovirus-associated viruses. J Natl Cancer Inst 1968;40:319–327 [PubMed] [Google Scholar]
- 12.Basner-Tschakarjan E, Mingozzi F. Cell-mediated immunity to AAV vectors, evolving concepts and potential solutions. Front Immunol 2014;5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Tse LV, Moller-Tank S, Asokan A. Strategies to circumvent humoral immunity to adeno-associated viral vectors. Expert Opin Biol Ther 2015;15:845–855 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Trinchieri G, Sher A. Cooperation of Toll-like receptor signals in innate immune defence. Nat Rev Immunol 2007;7:179–190 [DOI] [PubMed] [Google Scholar]
- 15.Abbas AK, Lichtman AH. Basic Immunology: Functions and Disorders of the Immune System. 3rd ed. Philadelphia, PA: Saunders/Elsevier, 2010 [Google Scholar]
- 16.Boutin S, Monteilhet V, Veron P, et al. Prevalence of serum IgG and neutralizing factors against adeno-associated virus (AAV) types 1, 2, 5, 6, 8, and 9 in the healthy population: implications for gene therapy using AAV vectors. Hum Gene Ther 2010;21:704–712 [DOI] [PubMed] [Google Scholar]
- 17.Calcedo R, Vandenberghe LH, Gao G, et al. Worldwide epidemiology of neutralizing antibodies to adeno-associated viruses. J Infect Dis 2009;199:381–390 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Calcedo R, Morizono H, Wang L, et al. Adeno-associated virus antibody profiles in newborns, children, and adolescents. Clin Vaccine Immunol 2011;18:1586–1588 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Calcedo R, Wilson JM. Humoral immune response to AAV. Front Immunol 2013;4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Halbert CL, Miller AD, McNamara S, et al. Prevalence of neutralizing antibodies against adeno-associated virus (AAV) types 2, 5, and 6 in cystic fibrosis and normal populations: implications for gene therapy using AAV vectors. Hum Gene Ther 2006;17:440–447 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Mingozzi F, Chen Y, Edmonson SC, et al. Prevalence and pharmacological modulation of humoral immunity to AAV vectors in gene transfer to synovial tissue. Gene Ther 2013;20:417–424 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Veron P, Leborgne C, Monteilhet V, et al. Humoral and cellular capsid-specific immune responses to adeno-associated virus type 1 in randomized healthy donors. J Immunol 2012;188:6418–6424 [DOI] [PubMed] [Google Scholar]
- 23.Thwaite R, Pagès G, Chillón M, et al. AAVrh.10 immunogenicity in mice and humans. Relevance of antibody cross-reactivity in human gene therapy. Gene Ther 2015;22:196–201 [DOI] [PubMed] [Google Scholar]
- 24.Parks WP, Boucher DW, Melnick JL, et al. Seroepidemiological and ecological studies of the adenovirus-associated satellite viruses. Infect Immun 1970;2:716–722 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Chirmule N, Propert K, Magosin S, et al. Immune responses to adenovirus and adeno-associated virus in humans. Gene Ther 1999;6:1574–1583 [DOI] [PubMed] [Google Scholar]
- 26.Wagner JA, Nepomuceno IB, Messner AH, et al. A Phase II, double-blind, randomized, placebo-controlled clinical trial of tgAAVCF using maxillary sinus delivery in patients with cystic fibrosis with antrostomies. Hum Gene Ther 2002;13:1349–1359 [DOI] [PubMed] [Google Scholar]
- 27.Moss RB, Rodman D, Spencer LT, et al. Repeated adeno-associated virus serotype 2 aerosol-mediated cystic fibrosis transmembrane regulator gene transfer to the lungs of patients with cystic fibrosis: a multicenter, double-blind, placebo-controlled trial. Chest 2004;125:509–521 [DOI] [PubMed] [Google Scholar]
- 28.Murphy SL, Li H, Mingozzi F, et al. Diverse IgG subclass responses to adeno-associated virus infection and vector administration. J Med Virol 2009;81:65–74 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Corden A, Handelman B, Yin H, et al. Neutralizing antibodies against adeno-associated viruses in Sjögren's patients: implications for gene therapy. Gene Ther 2017;24:241–244 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Ferla R, Claudiani P, Savarese M, et al. Prevalence of anti–adeno-associated virus serotype 8 neutralizing antibodies and arylsulfatase B cross-reactive immunologic material in mucopolysaccharidosis VI patient candidates for a gene therapy trial. Hum Gene Ther 2015;26:145–152 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Li C, Narkbunnam N, Samulski RJ, et al. Neutralizing antibodies against adeno-associated virus examined prospectively in pediatric patients with hemophilia. Gene Ther 2012;19:288–294 [DOI] [PubMed] [Google Scholar]
- 32.Mingozzi F, High KA. Immune responses to AAV vectors: overcoming barriers to successful gene therapy. Blood 2013;122:23–36 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Calcedo R, Wilson JM. AAV natural infection induces broad cross-neutralizing antibody responses to multiple AAV serotypes in chimpanzees. Hum Gene Ther Clin Dev 2016;27:79–82 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Wang L, Calcedo R, Bell P, et al. Impact of pre-existing immunity on gene transfer to nonhuman primate liver with adeno-associated virus 8 vectors. Hum Gene Ther 2011;22:1389–1401 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Fitzpatrick Z, Leborgne C, Barbon E, et al. Influence of pre-existing anti-capsid neutralizing and binding antibodies on AAV-mediated liver transduction. ASGCT abstract. 2017 [DOI] [PMC free article] [PubMed]
- 36.Erles K, Sebökovà P, Schlehofer JR. Update on the prevalence of serum antibodies (IgG and IgM) to adeno-associated virus (AAV). J Med Virol 1999;59:406–411 [DOI] [PubMed] [Google Scholar]
- 37.Hurlbut GD, Ziegler RJ, Nietupski JB, et al. Preexisting immunity and low expression in primates highlight translational challenges for liver-directed AAV8-mediated gene therapy. Mol Ther J Am Soc Gene Ther 2010;18:1983–1994 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Jeune VL, Joergensen JA, Hajjar RJ, et al. Pre-existing anti–adeno-associated virus antibodies as a challenge in AAV gene therapy. Hum Gene Ther Methods 2013;24:59–67 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Mingozzi F, Meulenberg JJ, Hui DJ, et al. AAV-1–mediated gene transfer to skeletal muscle in humans results in dose-dependent activation of capsid-specific T cells. Blood 2009;114:2077–2086 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Manno CS, Glenn F. Pierce, Arruda VR, et al. Successful transduction of liver in hemophilia by AAV-Factor IX and limitations imposed by the host immune response. Nat Med 2006;12:342–347 [DOI] [PubMed] [Google Scholar]
- 41.Mingozzi F, Maus MV, Hui DJ, et al. CD8+ T-cell responses to adeno-associated virus capsid in humans. Nat Med 2007;13:419–422 [DOI] [PubMed] [Google Scholar]
- 42.Li H, Lasaro MO, Jia B, et al. Capsid-specific T-cell responses to natural infections with adeno-associated viruses in humans differ from those of nonhuman primates. Mol Ther 2011;19:2021–2030 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Hui DJ, Edmonson SC, Podsakoff GM, et al. AAV capsid CD8+ T-cell epitopes are highly conserved across AAV serotypes. Mol Ther Methods Clin Dev 2015;2:15029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Murphy SL, Li H, Zhou S, et al. Prolonged susceptibility to antibody-mediated neutralization for adeno-associated vectors targeted to the liver. Mol Ther 2008;16:138–145 [DOI] [PubMed] [Google Scholar]
- 45.Scallan CD, Jiang H, Liu T, et al. Human immunoglobulin inhibits liver transduction by AAV vectors at low AAV2 neutralizing titers in SCID mice. Blood 2006;107:1810–1817 [DOI] [PubMed] [Google Scholar]
- 46.Jiang H, Couto LB, Patarroyo-White S, et al. Effects of transient immunosuppression on adenoassociated, virus-mediated, liver-directed gene transfer in rhesus macaques and implications for human gene therapy. Blood 2006;108:3321–3328 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Cottard V, Valvason C, Falgarone G, et al. Immune response against gene therapy vectors: influence of synovial fluid on adeno-associated virus mediated gene transfer to chondrocytes. J Clin Immunol 2004;24:162–169 [DOI] [PubMed] [Google Scholar]
- 48.Arruda VR, Stedman HH, Haurigot V, et al. Peripheral transvenular delivery of adeno-associated viral vectors to skeletal muscle as a novel therapy for hemophilia B. Blood 2010;115:4678–4688 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Rodino-Klapac LR, Montgomery CL, Mendell JR, et al. AAV-mediated gene therapy to the isolated limb in rhesus macaques. Methods Mol Biol 2011;709:287–298 [DOI] [PubMed] [Google Scholar]
- 50.Hajjar RJ, Zsebo K, Deckelbaum L, et al. Design of a Phase 1/2 trial of intracoronary administration of AAV1/SERCA2a in patients with heart failure. J Card Fail 2008;14:355–367 [DOI] [PubMed] [Google Scholar]
- 51.Bennett J, Ashtari M, Wellman J, et al. AAV2 gene therapy readministration in three adults with congenital blindness. Sci Transl Med 2012;4:120ra15–120ra15 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Kaplitt MG, Feigin A, Tang C, et al. Safety and tolerability of gene therapy with an adeno-associated virus (AAV) borne GAD gene for Parkinson's disease: an open label, Phase I trial. Lancet 2007;369:2097–2105 [DOI] [PubMed] [Google Scholar]
- 53.Samaranch L, Salegio EA, San Sebastian W, et al. Adeno-associated virus serotype 9 transduction in the central nervous system of nonhuman primates. Hum Gene Ther 2012;23:382–389 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Chirmule N, Xiao W, Truneh A, et al. Humoral immunity to adeno-associated virus type 2 vectors following administration to murine and nonhuman primate muscle. J Virol 2000;74:2420–2425 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Haurigot V, Mingozzi F, Buchlis G, et al. Safety of AAV Factor IX peripheral transvenular gene delivery to muscle in hemophilia B dogs. Mol Ther 2010;18:1318–1329 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Nathwani AC, Gray JT, McIntosh J, et al. Safe and efficient transduction of the liver after peripheral vein infusion of self-complementary AAV vector results in stable therapeutic expression of human FIX in nonhuman primates. Blood 2007;109:1414–1421 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Flotte TR, Trapnell BC, Humphries M, et al. Phase 2 clinical trial of a recombinant adeno-associated viral vector expressing α1-antitrypsin: interim results. Hum Gene Ther 2011;22:1239–1247 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Nathwani AC, Tuddenham EGD, Rangarajan S, et al. Adenovirus-associated virus vector–mediated gene transfer in hemophilia B. N Engl J Med 2011;365:2357–2365 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Amado D, Mingozzi F, Hui D, et al. Safety and efficacy of subretinal readministration of a viral vector in large animals to treat congenital blindness. Sci Transl Med 2010;2:21ra16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Buchlis G, Podsakoff GM, Radu A, et al. Factor IX expression in skeletal muscle of a severe hemophilia B patient 10 years after AAV-mediated gene transfer. Blood 2012;119:3038–3041 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Bortolussi G, Zentillin L, Vaníkova J, et al. Life-long correction of hyperbilirubinemia with a neonatal liver-specific AAV-mediated gene transfer in a lethal mouse model of Crigler–Najjar syndrome. Hum Gene Ther 2014;25:844–855 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Ronzitti G, Bortolussi G, van Dijk R, et al. A translationally optimized AAV-UGT1A1 vector drives safe and long-lasting correction of Crigler–Najjar syndrome. Mol Ther Methods Clin Dev 2016;3:16049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Wang L, Bell P, Lin J, et al. AAV8-mediated hepatic gene transfer in infant rhesus monkeys (Macaca mulatta). Mol Ther 2011;19:2012–2020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Mount JD, Herzog RW, Tillson DM, et al. Sustained phenotypic correction of hemophilia B dogs with a factor IX null mutation by liver-directed gene therapy. Blood 2002;99:2670–2676 [DOI] [PubMed] [Google Scholar]
- 65.Boisgérault F, Mingozzi F. The skeletal muscle environment and its role in immunity and tolerance to AAV vector-mediated gene transfer. Curr Gene Ther 2015;15:381–394 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Brantly ML, Chulay JD, Wang L, et al. Sustained transgene expression despite T lymphocyte responses in a clinical trial of rAAV1-AAT gene therapy. Proc Natl Acad Sci U S A 2009;106:16363–16368 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Ferreira V, Petry H, Salmon F. Immune responses to AAV-vectors, the Glybera example from bench to bedside. Front Immunol 2014;5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Mendell JR, Rodino-Klapac LR, Rosales XQ, et al. Sustained alpha-sarcoglycan gene expression following gene transfer in LGMD2D. Ann Neurol 2010;68:629–638 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Gernoux G, Wilson JM, Mueller C. Regulatory and exhausted t cell responses to AAV capsid. Hum Gene Ther 2017;28:338–349 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Le Guiner C, Servais L, Montus M, et al. Long-term microdystrophin gene therapy is effective in a canine model of Duchenne muscular dystrophy. Nat Commun 2017;8:16105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Mak KY, Rajapaksha IG, Angus PW, et al. The adeno-associated virus—a safe and effective vehicle for liver-specific gene therapy of inherited and non-inherited diseases. Curr Gene Ther 2017;17:4–16 [DOI] [PubMed] [Google Scholar]
- 72.Flotte TR, Mueller C. What is suppression of anti–adeno-associated virus capsid T-cells achieving? Hum Gene Ther 2014;25:178–179 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Arruda VR, Favaro P, Finn JD. Strategies to modulate immune responses: a new frontier for gene therapy. Mol Ther J Am Soc Gene Ther 2009;17:1492–1503 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Mingozzi F, Hasbrouck NC, Basner-Tschakarjan E, et al. Modulation of tolerance to the transgene product in a nonhuman primate model of AAV-mediated gene transfer to liver. Blood 2007;110:2334–2341 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Stieger K, Schroeder J, Provost N, et al. Detection of intact rAAV particles up to 6 years after successful gene transfer in the retina of dogs and primates. Mol Ther 2009;17:516–523 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Pien GC, Basner-Tschakarjan E, Hui DJ, et al. Capsid antigen presentation flags human hepatocytes for destruction after transduction by adeno-associated viral vectors. J Clin Invest 2009;119:1688–1695 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Finn JD, Hui DJ, Downey H, et al. Proteasome inhibitors decrease AAV2 capsid derived peptide epitope presentation on MHC class I following transduction. Mol Ther 2010;18:135–142 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Kishimoto TK, Ferrari JD, LaMothe RA, et al. Improving the efficacy and safety of biologic drugs with tolerogenic nanoparticles. Nat Nanotechnol 2016;11:890–899 [DOI] [PubMed] [Google Scholar]
- 79.Meliani A, Boisgerault F, Marmier S, et al. Modulation of AAV vector dosing and avoidance of capsid immune responses via repeated co-administration of vector with tolerogenic rapamycin nanoparticles. Res Pract Thromb Haemost 2017;1:1–1451 [Google Scholar]
- 80.Gao G, Wang Q, Calcedo R, et al. Adeno-associated virus-mediated gene transfer to nonhuman primate liver can elicit destructive transgene-specific T cell responses. Hum Gene Ther 2009;20:930–942 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Guidotti LG, Iannacone M. Effector CD8 T cell trafficking within the liver. Mol Immunol 2013;55:94–99 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Lang KS, Georgiev P, Recher M, et al. Immunoprivileged status of the liver is controlled by Toll-like receptor 3 signaling. J Clin Invest 2006;116:2456–2463 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Nguyen DH, Hurtado-Ziola N, Gagneux P, et al. Loss of Siglec expression on T lymphocytes during human evolution. Proc Natl Acad Sci U S A 2006;103:7765–7770 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Eastwood D, Findlay L, Poole S, et al. Monoclonal antibody TGN1412 trial failure explained by species differences in CD28 expression on CD4+ effector memory T-cells. Br J Pharmacol 2010;161:512–526 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Poirier N, Mary C, Le Bas-Bernardet S, et al. Advantages of Papio anubis for preclinical testing of immunotoxicity of candidate therapeutic antagonist antibodies targeting CD28. MAbs 2014;6:697–706 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Porter CM, Horvath-Arcidiacono JA, Singh AK, et al. Characterization and expansion of baboon CD4+CD25+ Treg cells for potential use in a non-human primate xenotransplantation model. Xenotransplantation 2007;14:298–308 [DOI] [PubMed] [Google Scholar]
- 87.McTiernan CF, Mathier MA, Zhu X, et al. Myocarditis following adeno-associated viral gene expression of human soluble TNF receptor (TNFRII-Fc) in baboon hearts. Gene Ther 2007;14:1613–1622 [DOI] [PubMed] [Google Scholar]
- 88.Song S, Scott-Jorgensen M, Wang J, et al. Intramuscular administration of recombinant adeno-associated virus 2 α-1 antitrypsin (rAAV-SERPINA1) vectors in a nonhuman primate model: safety and immunologic aspects. Mol Ther 2002;6:329–335 [DOI] [PubMed] [Google Scholar]
- 89.Zhou S, Murphy J, Escobedo J, et al. Adeno-associated virus-mediated delivery of erythropoietin leads to sustained elevation of hematocrit in nonhuman primates. Gene Ther 1998;5:665–670 [DOI] [PubMed] [Google Scholar]
- 90.Li C, Hirsch M, Asokan A, et al. Adeno-associated virus type 2 (AAV2) capsid-specific cytotoxic T lymphocytes eliminate only vector-transduced cells coexpressing the AAV2 capsid in vivo. J Virol 2007;81:7540–7547 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Li H, Murphy SL, Giles-Davis W, et al. Pre-existing AAV capsid-specific CD8+ T cells are unable to eliminate AAV-transduced hepatocytes. Mol Ther 2007;15:792–800 [DOI] [PubMed] [Google Scholar]
- 92.Wang L, Figueredo J, Calcedo R, et al. Cross-presentation of adeno-associated virus serotype 2 capsids activates cytotoxic T cells but does not render hepatocytes effective cytolytic targets. Hum Gene Ther 2007;18:185–194 [DOI] [PubMed] [Google Scholar]
- 93.Li H, Tuyishime S, Wu T-L, et al. Adeno-associated virus vectors serotype 2 induce prolonged proliferation of capsid-specific CD8+ T cells in mice. Mol Ther 2011;19:536–546 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Martino AT, Basner-Tschakarjan E, Markusic DM, et al. Engineered AAV vector minimizes in vivo targeting of transduced hepatocytes by capsid-specific CD8+ T cells. Blood 2013;121:2224–2233 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Vercauteren K, Hoffman BE, Zolotukhin I, et al. Superior in vivo transduction of human hepatocytes using engineered AAV3 capsid. Mol Ther 2016;24:1042–1049 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Lisowski L, Dane AP, Chu K, et al. Selection and evaluation of clinically relevant AAV variants in a xenograft liver model. Nature 2014;506:382–386 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Zhu J, Huang X, Yang Y. The TLR9-MyD88 pathway is critical for adaptive immune responses to adeno-associated virus gene therapy vectors in mice. J Clin Invest 2009;119:2388–2398 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Hösel M, Broxtermann M, Janicki H, et al. Toll-like receptor 2–mediated innate immune response in human nonparenchymal liver cells toward adeno-associated viral vectors. Hepatology 2012;55:287–297 [DOI] [PubMed] [Google Scholar]
- 99.Jayandharan GR, Aslanidi G, Martino AT, et al. Activation of the NF-κB pathway by adeno-associated virus (AAV) vectors and its implications in immune response and gene therapy. Proc Natl Acad Sci 2011;108:3743–3748 [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 100.Sudres M, Ciré S, Vasseur V, et al. MyD88 signaling in B cells regulates the production of Th1-dependent antibodies to AAV. Mol Ther 2012;20:1571–1581 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Martino AT, Suzuki M, Markusic DM, et al. The genome of self-complementary adeno-associated viral vectors increases Toll-like receptor 9–dependent innate immune responses in the liver. Blood 2011;117:6459–6468 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Zaiss AK, Cotter MJ, White LR, et al. Complement is an essential component of the immune response to adeno-associated virus vectors. J Virol 2008;82:2727–2740 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Zaiss A-K, Liu Q, Bowen GP, et al. Differential activation of innate immune responses by adenovirus and adeno-associated virus vectors. J Virol 2002;76:4580–4590 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Rogers GL, Shirley JL, Zolotukhin I, et al. Plasmacytoid and conventional dendritic cells cooperate in crosspriming AAV capsid-specific CD8+ T cells. Blood 2017;129:3184–3195 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Mingozzi F, Liu Y-L, Dobrzynski E, et al. Induction of immune tolerance to coagulation factor IX antigen by in vivo hepatic gene transfer. J Clin Invest 2003;111:1347–1356 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Dobrzynski E, Mingozzi F, Liu Y-L, et al. Induction of antigen-specific CD4+ T-cell anergy and deletion by in vivo viral gene transfer. Blood 2004;104:969–977 [DOI] [PubMed] [Google Scholar]
- 107.Franco LM, Sun B, Yang X, et al. Evasion of immune responses to introduced human acid α-glucosidase by liver-restricted expression in glycogen storage disease type II. Mol Ther 2005;12:876–884 [DOI] [PubMed] [Google Scholar]
- 108.Ziegler RJ, Lonning SM, Armentano D, et al. AAV2 vector harboring a liver-restricted promoter facilitates sustained expression of therapeutic levels of α-galactosidase A and the induction of immune tolerance in Fabry mice. Mol Ther 2004;9:231–240 [DOI] [PubMed] [Google Scholar]
- 109.Martino AT, Nayak S, Hoffman BE, et al. Tolerance induction to cytoplasmic β-galactosidase by hepatic AAV gene transfer—implications for antigen presentation and immunotoxicity. PLoS One 2009;4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Finn JD, Ozelo MC, Sabatino DE, et al. Eradication of neutralizing antibodies to factor VIII in canine hemophilia A after liver gene therapy. Blood 2010;116:5842–5848 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Markusic DM, Hoffman BE, Perrin GQ, et al. Effective gene therapy for haemophilic mice with pathogenic factor IX antibodies. EMBO Mol Med 2013;5:1698–1709 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Han S, Ronzitti G, Arnson B, et al. Low-dose liver-targeted gene therapy for Pompe disease enhances therapeutic efficacy of ERT via immune tolerance induction. Mol Ther Methods Clin Dev 2017;4:126–136 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.D'Avola D, López-Franco E, Sangro B, et al. Phase I open label liver-directed gene therapy clinical trial for acute intermittent porphyria. J Hepatol 2016;65:776–783 [DOI] [PubMed] [Google Scholar]
- 114.Gao G, Lebherz C, Weiner DJ, et al. Erythropoietin gene therapy leads to autoimmune anemia in macaques. Blood 2004;103:3300–3302 [DOI] [PubMed] [Google Scholar]
- 115.Herzog RW, Mount JD, Arruda VR, et al. Muscle-directed gene transfer and transient immune suppression result in sustained partial correction of canine hemophilia B caused by a null mutation. Mol Ther 2001;4:192–200 [DOI] [PubMed] [Google Scholar]
- 116.Wang Z, Kuhr CS, Allen JM, et al. Sustained AAV-mediated dystrophin expression in a canine model of Duchenne muscular dystrophy with a brief course of immunosuppression. Mol Ther J Am Soc Gene Ther 2007;15:1160–1166 [DOI] [PubMed] [Google Scholar]
- 117.Mendell JR, Campbell K, Rodino-Klapac L, et al. Dystrophin immunity in Duchenne's muscular dystrophy. N Engl J Med 2010;363:1429–1437 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Flanigan KM, Campbell K, Viollet L, et al. Anti-dystrophin T cell responses in Duchenne muscular dystrophy: prevalence and a glucocorticoid treatment effect. Hum Gene Ther 2013;24:797–806 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Toromanoff A, Adjali O, Larcher T, et al. Lack of immunotoxicity after regional intravenous (RI) delivery of rAAV to nonhuman primate skeletal muscle. Mol Ther 2010;18:151–160 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Doerfler PA, Nayak S, Corti M, et al. Targeted approaches to induce immune tolerance for Pompe disease therapy. Mol Ther Methods Clin Dev 2016;3:15053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Puzzo F, Colella P, Biferi MG, et al. Whole-body rescue of Pompe disease with AAV liver delivery of engineered secretable GAA transgenes. Sci Transl Med (in press) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Perrin GQ, Zolotukhin I, Sherman A, et al. Dynamics of antigen presentation to transgene product-specific CD4+ T cells and of Treg induction upon hepatic AAV gene transfer. Mol Ther Methods Clin Dev 2016;3:16083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Faust SM, Bell P, Cutler BJ, et al. CpG-depleted adeno-associated virus vectors evade immune detection. J Clin Invest 2013;123:2994–3001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Mingozzi F, Anguela XM, Pavani G, et al. Overcoming preexisting humoral immunity to AAV using capsid decoys. Sci Transl Med 2013;5:194ra92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Chapin J, Rottensteiner H, Scheiflinger F, et al. An analysis of bleeding rates and Factor IX consumption in the Phase I/II BAX 335 gene therapy trial in subjects with hemophilia B. Res Pract Thromb Haemost 2017;1:1–1451 [Google Scholar]
- 126.Simioni P, Tormene D, Tognin G, et al. X-linked thrombophilia with a mutant factor IX (factor IX Padua). N Engl J Med 2009;361:1671–1675 [DOI] [PubMed] [Google Scholar]
- 127.George L, Giermasz A, Sullivan S, et al. SPK-9001: adeno-associated virus mediated gene transfer for haemophilia B achieved durable endogenous prophylaxis at levels of activity sufficient to achieve significant mean reduction in annual bleeding and infusions rates in preliminary data from an on-going Phase 1/2a trial. Res Pract Thromb Haemost 2017;1:1–1451 [Google Scholar]
- 128.Miesbach W, Tangelder M, Meijer K, et al. Updated results from a dose escalation study in adults with severe or moderate-severe hemophilia B treated with AMT-060 (AAV5-hFIX) gene therapy: up to 1.5 years follow-up. Res Pract Thromb Haemost 2017;1:1–1451 [Google Scholar]
- 129.Pasi J, Rangarajan S, Walsh L, et al. Interim results from a Phase 1/2 AAV5-FVIII gene transfer in patients with severe hemophilia A. Res Pract Thromb Haemost 2017;1:1–1451 [Google Scholar]
- 130.Zinn E, Pacouret S, Khaychuk V, et al. In silico reconstruction of the viral evolutionary lineage yields a potent gene therapy vector. Cell Rep 2015;12:1056–1068 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.György B, Fitzpatrick Z, Crommentuijn MHW, et al. Naturally enveloped AAV vectors for shielding neutralizing antibodies and robust gene delivery in vivo. Biomaterials 2014;35:7598–7609 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Tse LV, Klinc KA, Madigan VJ, et al. Structure-guided evolution of antigenically distinct adeno-associated virus variants for immune evasion. Proc Natl Acad Sci U S A 2017;114:E4812–E4821 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Monteilhet V, Saheb S, Boutin S, et al. A 10 patient case report on the impact of plasmapheresis upon neutralizing factors against adeno-associated virus (AAV) types 1, 2, 6, and 8. Mol Ther J Am Soc Gene Ther 2011;19:2084–2091 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Chicoine LG, Montgomery CL, Bremer WG, et al. Plasmapheresis eliminates the negative impact of AAV antibodies on microdystrophin gene expression following vascular delivery. Mol Ther J Am Soc Gene Ther 2014;22:338–347 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Mimuro J, Mizukami H, Hishikawa S, et al. Minimizing the inhibitory effect of neutralizing antibody for efficient gene expression in the liver with adeno-associated virus 8 vectors. Mol Ther J Am Soc Gene Ther 2013;21:318–323 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Unzu C, Hervás-Stubbs S, Sampedro A, et al. Transient and intensive pharmacological immunosuppression fails to improve AAV-based liver gene transfer in non-human primates. J Transl Med 2012;10:122. [DOI] [PMC free article] [PubMed] [Google Scholar]