Abstract
Purpose: POSITIVE (Pregnancy Outcome and Safety of Interrupting Therapy for women with endocrine responsIVE breast cancer) is a prospective clinical trial assessing safety and pregnancy outcomes in premenopausal hormone receptor-positive breast cancer survivors (age ≤42) who interrupt endocrine therapy (ET) to attempt pregnancy. We sought to assess interest in this study and perspectives on fertility preservation (FP) among United States medical oncologists who had previously enrolled women at their institutions on select premenopausal endocrine studies.
Methods: From August 2015 to December 2015, 301 investigators were invited to complete a web-based survey on behalf of their institution. We assessed FP practices and attitudes, barriers to discussing FP, and willingness to open/enroll women on POSITIVE.
Results: Of 93 respondents (31%), most were affiliated with an National Cancer Institute (NCI)-designated comprehensive cancer center (44%). Almost all said they usually or always discussed the issue of future fertility with patients (98%) and referred patients with fertility questions to specialists (97%). Over half of respondents cited discomfort with recommending women to stop ET, as well as perceived patient concern regarding ET interruption, as factors seen as influencing POSITIVE enrollment; however, 84% were willing to recommend trial participation for selected patients.
Conclusions: Most providers reported discussing fertility with their young patients, indicating awareness of FP guidelines for cancer patients. While most oncologists said that they would be willing to recommend POSITIVE, many also expressed discomfort in endorsing women to stop ET temporarily, underscoring the need for prospective data regarding the safety of ET interruption. High willingness to recommend POSITIVE suggests the potential for successful accrual to this study, which addresses a critical issue for young breast cancer survivors.
Keywords: : endocrine therapy, breast cancer, pregnancy, fertility
Introduction
In recent decades, an increasing number of women have delayed childbearing for both personal and societal reasons. As a result, young women diagnosed with breast cancer may be less likely than in the past to have started or completed their planned childbearing and may be interested in future fertility at diagnosis.1–6 For young women with breast cancer, there are numerous potential challenges that can impact both interest in and planning for future fertility, including an increased risk of permanent or premature menopause due to the direct effects of chemotherapy and/or ovarian suppression,7 as well as concerns about disease recurrence.6,8 Furthermore, for women with hormone-receptor positive (HR+) breast cancer who desire pregnancy, there is the added complexity of how a full course of endocrine therapy (ET) may affect fertility plans. For these women, the recommended 5–10 years9 of ET may not only decrease their natural fertility but also may be intolerable from a psychosocial standpoint, resulting in some women who may choose to forgo a full duration of treatment to pursue pregnancy.
POSITIVE (Pregnancy Outcome and Safety of Interrupting Therapy for women with endocrine responsIVE breast cancer) is an ongoing, prospective international clinical trial designed to address this clinical conundrum.10 While prior studies have not found women who become pregnant following a breast cancer diagnosis to be at increased risk of recurrence,11,12 the impact of a shorter duration of ET on disease outcomes has not been evaluated prospectively. POSITIVE is expected to enroll 500 women and will assess the safety and feasibility of pregnancy in premenopausal HR+ breast cancer survivors who interrupt ET to attempt pregnancy. Eligibility criteria include a desire to become pregnant, documented premenopausal status at diagnosis, histologically confirmed HR+ breast cancer, no evidence of locoregional or distant disease, and completion of 18–30 months of ET before enrollment. The primary outcome of POSITIVE is the risk of breast cancer recurrence; secondary endpoints include pregnancy and offspring outcomes, menstruation recovery patterns, and ET adherence after treatment resumption. Given the challenging goals addressed in this study (optimizing reproductive goals, as well as disease outcomes), we conducted a survey to evaluate interest in opening the POSITIVE trial in the United States, including representative cancer center interest and potential factors that may affect enrollment. In addition, we sought to describe fertility preservation (FP) practices, attitudes, and barriers, including practices that are relevant in the pre-treatment setting, which likely are related to intentions regarding interest in the post-treatment POSITIVE trial, among select medical oncologists from academic and community practices across the United States.
Methods
From August 2015 to December 2015, 301 medical oncologists were identified and selected to represent their institutions based on highest enrollment of patients at their institutions to two prior premenopausal endocrine studies (the SOFT and/or TEXT trials13,14) in the United States. Each investigator was emailed an invitation to complete a web-based survey on behalf of their institution, asking them to think about the average patient, as well as the average investigator at their site, for whom the POSITIVE study would be relevant. If the highest enroller was not reached (e.g., email bounce back or nonresponse after re-send), the next highest enroller was invited to participate. Because respondents were asked to answer questions on behalf of their institution/practice, rather than reply based on their personal beliefs or attitudes, this study was deemed nonhuman subject research and was exempt from full review by the Dana-Farber Cancer Institute Institutional Review Board. An introductory letter accompanying the online survey included all elements of informed consent.
The survey included investigator-developed items about practice characteristics, frequency of FP discussion and referral patterns, and willingness to recommend participation in a clinical trial (POSITIVE) in which interested women would stop endocrine treatment after 18–30 months to attempt and potentially carry a pregnancy and would be strongly recommended to resume and complete ET after completion of the pregnancy, as well as anticipated accrual at their site/practice to this trial. Items about barriers and attitudes toward discussing FP were adapted from a prior study of FP in cancer patients15; perceived factors that might influence their cancer center's/practice's ability to enroll patients, as well as influence patient willingness to enroll in POSITIVE, were adapted from a prior survey of oncologists' practices and perceptions regarding patient enrollment in clinical trials.16 Analyses were descriptive, with frequency distributions generated for each response. Sample sizes vary somewhat across items owing to nonresponses. Analyses were conducted in SAS version 9.4 (SAS Institute, Cary, NC).
Results
Participant characteristics
Of 93 respondents (response rate: 31%), 44% were affiliated with an National Cancer Institute (NCI)-designated comprehensive cancer center, 27% another academic center, and 29% a community-based or private practice. Regarding the number of oncologists in their practice who care for breast cancer patients, 42% (39/92) were part of a practice with 2–5 oncologists, 38% (35/92) 6–10 oncologists, and 17% (16/92) were part of a practice that included more than 10 oncologists; 2% (2/92) reported being the only oncologist caring for women with breast cancer in their practice. There was a wide distribution of young breast cancer care patients (age ≤42 years) seen monthly at responding sites: 38% (34/90) reported seeing more than 20 young patients, 26% (23/90) 11–20 patients, 27% (24/90) 6–10 patients, and 10% (9/90) saw 5 or fewer patients.
Participation in the POSITIVE trial
Most physicians (84%, 77/90) said that they would be willing to recommend participation in the POSITIVE trial to women interested in early pregnancy after diagnosis. Approximately 42% of respondents said that they expected to enroll 1–5 patients on the trial; 31%, 6–10 patients; 12%, 11–15 patients, and 8% expected to enroll more than 15 patients, with 8% responding that they did not expect to enroll any patients.
Factors perceived as affecting provider ability to enroll patients on POSITIVE
Table 1 details perceived impact of potential factors that might affect a provider's ability to enroll patients to the POSITIVE trial. Discomfort with recommending that women stop ET was cited as at least somewhat of an influence on enrollment by over half of respondents (52%, 48/92), while 38% (35/92) said lack of time due to clinical or administrative responsibilities would at least somewhat influence enrollment.
Table 1.
Factors Perceived as Affecting Provider Ability to Enroll Patients on POSITIVE
| A significant influence, N (%) | Somewhat of an influence, N (%) | Not at all an influence, N (%) | No opinion, N (%) | |
|---|---|---|---|---|
| Institution/practice sees too few patients ≤42 and therefore unlikely to have any eligible patients | 11 (12) | 15 (16) | 63 (69) | 2 (2) |
| Discomfort recommending women to stop ET after 18–30 months | 9 (10) | 39 (42) | 43 (47) | 1 (1) |
| Lack of time due to clinical/admin responsibilities | 9 (10) | 26 (28) | 56 (61) | 1 (1) |
| Effort/time to consent patients | 4 (4) | 24 (26) | 63 (68) | 1 (1) |
| Lack of adequate support staff to enroll patients | 4 (4) | 24 (26) | 63 (69) | — |
ET, endocrine therapy.
Factors perceived by oncologists as affecting patient willingness to enroll on POSITIVE
Patient concern about interrupting ET (88%, 80/91), concerns and opinions of family, friends, or caregivers (82%, 74/90), and concern about extra laboratory tests/procedures (68%, 61/90) were all frequently perceived by oncologists as potential factors at least somewhat influential in a potential patient's willingness to enroll in the trial (Table 2).
Table 2.
Factors Perceived by Oncologists as Affecting Patient Willingness to Enroll on POSITIVE
| A significant influence, N (%) | Somewhat of an influence, N (%) | Not at all an influence, N (%) | No opinion, N (%) | |
|---|---|---|---|---|
| Concern about interrupting ET and the potential effects on disease risks | 44 (48) | 36 (40) | 10 (11) | 1 (1) |
| Patient already has child(ren) | 26 (29) | 44 (48) | 18 (20) | 3 (3) |
| Thoughts and opinions from family/friends/caregiver | 18 (20) | 56 (62) | 15 (17) | 1 (1) |
| Patient desires to carry a pregnancy sooner rather than later | 17 (19) | 46 (51) | 23 (25) | 5 (5) |
| Loved one's desire to have patient carry a pregnancy | 8 (9) | 45 (49) | 30 (33) | 8 (9) |
| Language issues | 5 (6) | 27 (30) | 56 (63) | 1 (1) |
| Lack of interest in being involved in trials | 4 (4) | 56 (62) | 30 (33) | 1 (1) |
| Concern about being treated like a guinea pig | 3 (3) | 40 (43) | 47 (51) | 2 (2) |
| Difficulty reading consent form | 3 (3) | 26 (29) | 60 (66) | 2 (2) |
| Comorbid conditions that would make their trial participation difficult | 3 (3) | 20 (22) | 65 (72) | 2 (2) |
| Concern about extra laboratory tests/procedures | 2 (2) | 59 (66) | 28 (31) | 1 (1) |
| Difficulty understanding scientific concepts of clinical trials such as randomization | 2 (2) | 39 (43) | 48 (53) | 2 (2) |
| Cultural beliefs about cancer | 2 (2) | 37 (41) | 48 (53) | 3 (3) |
| Distrust of health professionals | 1 (1) | 30 (33) | 59 (64) | 2 (2) |
FP practices and attitudes
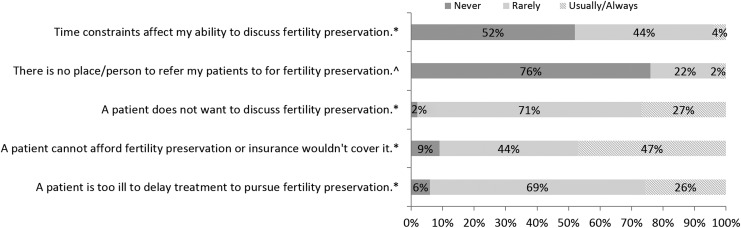
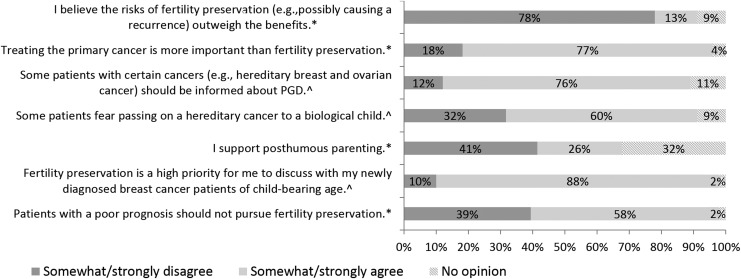
Table 3 describes FP practice behaviors. Almost all oncologists (98%, 90/91) said that they usually or always discussed the issue of future fertility with their breast cancer patients and referred patients with fertility questions to an infertility specialist or reproductive endocrinologist (97%, 87/90). Similarly, most (93%, 84/90) said that they usually or always follow published recommendations when considering FP in patients who are of childbearing age and 74% (66/90) responded that they usually or always provide their patients with resources regarding fertility after cancer. Regarding barriers to discussing FP (Fig. 1), 47% (42/89) responded that cost/insurance was usually or always an issue; almost all (96%, 85/89) said that time constraints were rarely or never an issue. Regarding attitudes (Fig. 2), most agreed that treating the primary cancer is more important than fertility (77%, 70/91); few (13%, 12/91) felt that risks of FP outweighed benefits although 58% (53/91) believed that patients with a poor prognosis should not pursue FP.
Table 3.
Fertility Preservation Practice Behaviors
| Always, N (%) | Usually, N (%) | Rarely, N (%) | Never, N (%) | |
|---|---|---|---|---|
| I discuss fertility issues with patients regardless of their insurance status | 66 (73) | 23 (25) | 1 (1) | 1 (1) |
| I refer patients who have questions about fertility to an infertility specialist or reproductive endocrinologist | 66 (73) | 21 (23) | 2 (2) | 1 (1) |
| I discuss the impact of cancer treatment on future fertility with my breast cancer patients | 65 (71) | 25 (27) | 1 (1) | — |
| I'm comfortable discussing FP with my patients | 64 (70) | 26 (29) | 1 (1) | — |
| I follow published recommendations (e.g., American Society of Clinical Oncology [ASCO], NCCN recommendations), when considering FP in my patients who are of childbearing age | 54 (60) | 30 (33) | 6 (7) | — |
| I consult an infertility specialist or reproductive endocrinologist with questions about potential fertility issues in my patients | 51 (57) | 28 (31) | 10 (11) | 1 (1) |
| I discuss fertility issues with patients that have a poor prognosis | 39 (43) | 37 (41) | 14 (15) | 1 (1) |
| The resources I provide to my patients are relevant to their specific cancer diagnosis | 26 (30) | 39 (44) | 17 (19) | 6 (7) |
| I provide my patients with resources regarding fertility after cancer (e.g., educational material about FP, financial resources) | 25 (28) | 41 (46) | 20 (22) | 4 (4) |
| Someone else within my practice discusses FP with my patients | 9 (10) | 12 (13) | 36 (40) | 32 (36) |
FP, fertility preservation.
FIG. 1.
Barriers to discussing fertility preservation. *N = 89 respondents, ^N = 88 respondents.
FIG. 2.
Attitudes regarding fertility preservation. *N = 91 respondents, ^N = 90 respondents. PGD, preimplantation.
Discussion
Concerns about future fertility and the safety of pregnancy following diagnosis are critical survivorship issues for many young breast cancer survivors. While many premenopausal women continue to menstruate through adjuvant chemotherapy treatment or resume menses soon after completing chemotherapy, earlier menopause or premature infertility is a dreaded consequence for some.17 For women with HR+ breast cancer, the window for conception and pregnancy may be further delayed or not even exist following a 5–10 year course of adjuvant ET. Available evidence to date has found that women who become pregnant following a breast cancer diagnosis are not at increased risk for recurrence11,12; however, there is a lack of prospective data to inform the decision to temporarily interrupt ET in women with HR+ disease. While most oncologists in our study said that they would be willing to recommend trial participation, many also expressed some discomfort in endorsing women to stop ET temporarily and perceived patients would feel similarly, underscoring the need for data regarding the safety of interrupting ET. Results from a recent study that assessed patient interest in potentially enrolling in an ET interruption study found that interest was highest in those women who were young (age 30 and younger at diagnosis) and who had been on ET for at least 30 months,18 suggesting some discordance between oncologists' perceptions and patient attitudes. Given this receptivity among young patients about participating in such a trial, oncologists should be encouraged to inform their young patients with HR+ breast cancer who wish to become pregnant of the opportunity to enroll on POSITIVE.
It is well established that young women are often concerned about their fertility and the potential negative consequences of treatment following a breast cancer diagnosis. In a recent study inclusive of premenopausal women with early-stage HR+ breast cancer, fertility concerns were cited by approximately one-third of women as a reason for not starting tamoxifen as indicated.4 When asked about failure to start tamoxifen or stopping tamoxifen early, 28% of women did not know that resuming tamoxifen following an interruption could be considered and be potentially beneficial,4 highlighting a need for education about ET resumption following cessation. Similarly, other studies have documented concern about future fertility as potentially affecting treatment decision-making in young breast cancer patients. In a large study of over 600 women diagnosed with breast cancer at age 40 and younger, approximately a quarter reported that fertility concerns impacted their treatment decisions,2 consistent with an earlier web-based survey study of young breast cancer survivors, in which 29% said that fertility concerns affected their decisions about treatment.6
Issues related to future fertility in cancer patients, including FP, have historically been under-addressed at diagnosis or before beginning treatment.1,6,19,20 Importantly, referral to fertility specialists soon after diagnosis and before treatment begins can enhance FP outcomes in young women with breast cancer.21 Before starting therapy, women should be advised as to the infertility risks associated with breast cancer treatment and presented with FP options.22 Prior studies have reported relatively lower rates of referrals or visits of young cancer patients to fertility doctors or reproductive endocrinologists.6,15,23 Encouragingly, almost all providers in our study reported addressing fertility concerns and referring women as needed to fertility specialists, suggesting an increased awareness of recommendations surrounding FP for cancer patients since the release of the first American Society of Clinical Oncology FP guidelines over a decade ago.24 Of note, many physicians in our sample perceived cost as a significant FP barrier suggesting a need to educate both providers and patients about the availability of resources to address this barrier. LIVESTRONG Fertility is one such resource which both provides comprehensive fertility information and aims to assist young patients financially.25
We acknowledge that our study has some limitations. Because the primary aim was to evaluate interest and ability in enrolling women in a clinical trial, we only surveyed oncologists who had enrolled patients on prior studies focused on ET for premenopausal women and were the highest accruers at their institutions. Thus, this is a select group of providers and their FP practices and attitudes might differ in substantial ways from medical oncologists who care for fewer premenopausal patients or who do not enroll women on to clinical trials. Given that the oncologists in our sample might not be representative of providers who see very few young breast cancer patients in their practice, the frequency of discussion and counseling surrounding fertility issues and concerns is likely still under-addressed in many settings. In addition, although we asked physicians to answer on behalf of themselves and other investigators at their institution, many of the questions we asked were subjective in nature and may not reflect the experiences of all oncologists in a given practice. While only one-third of oncologists we contacted responded to our survey, survey response rates among physicians are often suboptimal26 and our response rate was similar to that of another survey of oncology providers about FP practices.15
Overall, the results of our study indicate high provider interest in enrolling patients on POSITIVE and suggest the potential for successful accrual in the United States to this study aimed at improving our understanding of an important issue for young breast cancer survivors and providing information that can help guide fertility decisions both before and after treatment. Increasing awareness of the study among oncologists who treat premenopausal breast cancer patients is essential to the successful completion of the study and the greater goal of enhancing care and ultimately improving outcomes in this population.
Acknowledgments
Support for this study was provided by the Centers for Disease Control and Prevention and NIH P50CA168504-02 (Partridge). Dr. Rosenberg is supported, in part, by AHRQ K01HS023680 and the Pink Agenda.
Disclaimer
The findings of this study were presented at the eighth Biennial Cancer Survivorship Research Conference, June 16–18, 2016, Washington, DC and the third ESO-ESMO Breast Cancer in Young Women International Conference (BCY3), November 10–12, 2016, Lugano, Switzerland. Dr. Partridge, Dr. Pagani, and Dr. Korde are POSITIVE trial investigators.
Author Disclosure Statement
No competing financial interests exist.
References
- 1.Goldfarb SB, Kamer SA, Oppong BA, et al. Fertility preservation for the young breast cancer patient. Ann Surg Oncol. 2016;23(5):1530–6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Ruddy KJ, Gelber SI, Tamimi RM, et al. Prospective study of fertility concerns and preservation strategies in young women with breast cancer. J Clin Oncol. 2014;32(11):1151–624567428 [Google Scholar]
- 3.Thewes B, Meiser B, Taylor A, et al. Fertility- and menopause-related information needs of younger women with a diagnosis of early breast cancer. J Clin Oncol. 2005;23(22):5155–65 [DOI] [PubMed] [Google Scholar]
- 4.Llarena NC, Estevez SL, Tucker SL, Jeruss JS. Impact of fertility concerns on tamoxifen initiation and persistence. J Natl Cancer Inst. 2015;107(10) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Gorman JR, Malcarne VL, Roesch SC, et al. Depressive symptoms among young breast cancer survivors: the importance of reproductive concerns. Breast Cancer Res Treat. 2010;123(2):477–85 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Partridge AH, Gelber S, Peppercorn J, et al. Web-based survey of fertility issues in young women with breast cancer. J Clin Oncol. 2004;22(20):4174–83 [DOI] [PubMed] [Google Scholar]
- 7.Partridge AH, Ruddy KJ. Fertility and adjuvant treatment in young women with breast cancer. Breast. 2007;16(Suppl 2):S175–81 [DOI] [PubMed] [Google Scholar]
- 8.Gorman JR, Usita PM, Madlensky L, Pierce JP. Young breast cancer survivors: their perspectives on treatment decisions and fertility concerns. Cancer Nurs. 2011;34(1):32–40 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Burstein HJ, Temin S, Anderson H, et al. Adjuvant endocrine therapy for women with hormone receptor-positive breast cancer: American Society of Clinical Oncology clinical practice guideline focused update. J Clin Oncol. 2014;32(21):2255–69 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Pregnancy Outcome and Safety of Interrupting Therapy for Women with Estrogen Responsive Breast Cancer (POSITIVE). Accessed March22, 2017 from https://clinicaltrials.gov/show/NCT02308085
- 11.Azim HA, Jr., Santoro L, Pavlidis N, et al. Safety of pregnancy following breast cancer diagnosis: a meta-analysis of 14 studies. Eur J Cancer. 2011;47(1):74–83 [DOI] [PubMed] [Google Scholar]
- 12.Azim HA, Jr., Kroman N, Paesmans M, et al. Prognostic impact of pregnancy after breast cancer according to estrogen receptor status: a multicenter retrospective study. J Clin Oncol. 2013;31(1):73–9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Pagani O, Regan MM, Walley BA, et al. Adjuvant exemestane with ovarian suppression in premenopausal breast cancer. N Engl J Med. 2014;371(2):107–18 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Francis PA, Regan MM, Fleming GF, et al. Adjuvant ovarian suppression in premenopausal breast cancer. N Engl J Med. 2015;372(5):436–46 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Quinn GP, Vadaparampil ST, Lee JH, et al. Physician referral for fertility preservation in oncology patients: a national study of practice behaviors. J Clin Oncol. 2009;27(35):5952–7 [DOI] [PubMed] [Google Scholar]
- 16.Somkin CP, Ackerson L, Husson G, et al. Effect of medical oncologists' attitudes on accrual to clinical trials in a community setting. J Oncol Pract. 2013;9(6):e275–83 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ruddy KJ, Partridge AH. Fertility (male and female) and menopause. J Clin Oncol. 2012;30(30):3705–11 [DOI] [PubMed] [Google Scholar]
- 18.Pagani O, Ruggeri M, Manunta S, et al. Pregnancy after breast cancer: are young patients willing to participate in clinical studies? Breast. 2015;24(3):201–7 [DOI] [PubMed] [Google Scholar]
- 19.Shnorhavorian M, Harlan LC, Smith AW, et al. Fertility preservation knowledge, counseling, and actions among adolescent and young adult patients with cancer: a population-based study. Cancer. 2015;121(19):3499–506 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Benedict C, Thom B N F.riedman D, et al. Young adult female cancer survivors' unmet information needs and reproductive concerns contribute to decisional conflict regarding posttreatment fertility preservation. Cancer. 2016;122(13):2101–9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Lee S, Ozkavukcu S, Heytens E, et al. Value of early referral to fertility preservation in young women with breast cancer. J Clin Oncol. 2010;28(31):4683–6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Loren AW, Mangu PB, Beck LN, et al. Fertility preservation for patients with cancer: American Society of Clinical Oncology clinical practice guideline update. J Clin Oncol. 2013;31(19):2500–10 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Letourneau JM, Ebbel EE, Katz PP, et al. Pretreatment fertility counseling and fertility preservation improve quality of life in reproductive age women with cancer. Cancer. 2012;118(6):1710–7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Lee SJ, Schover LR, Partridge AH, et al. American Society of Clinical Oncology recommendations on fertility preservation in cancer patients. J Clin Oncol. 2006;24(18):2917–31 [DOI] [PubMed] [Google Scholar]
- 25.LIVESTRONG. Becoming a parent after cancer. Accessed December23, 2016 from www.livestrong.org/we-can-help/livestrong-fertility
- 26.McLeod CC, Klabunde CN, Willis GB, Stark D. Health care provider surveys in the United States, 2000–2010: a review. Eval Health Prof. 2013;36(1):106–26 [DOI] [PubMed] [Google Scholar]