Abstract
Significance: Chronic inflammation and maladaptive repair contribute to the development of fibrosis that negatively impacts quality of life and organ function. The toll-like receptor (TLR) system is a critical node in the tissue response to both exogenous (pathogen-associated) and endogenous (damage-associated) molecular pattern factors (PAMPs and DAMPs, respectively). The development of novel TLR ligand-, pathway-, and/or target gene-specific therapeutics may have clinical utility in the management of the exuberant inflammatory/fibrotic tissue response to injury without compromising the host defense to pathogens.
Recent Advances: DAMP ligands, released upon wounding, and microbial-derived PAMPs interact with several TLRs, and their various coreceptor partners, engaging downstream pathways that include Src family kinases, the epidermal growth factor receptor, integrins and the tumor suppressor phosphatase and tensin homolog (PTEN). Toll-like receptor 4 (TLR4) activation enhances cellular responses to the potent profibrotic cytokine transforming growth factor-β1 (TGF-β1) by attenuating the expression of receptors that inhibit TGF-β1 signaling.
Critical Issues: Common as well as unique pathways may be activated by PAMP and DAMP ligands that bind to the repertoire of TLRs on various cell types. Dissecting mechanisms underlying ligand-dependent engagement of this complex, highly interactive, network will provide for adaptation of new and focused therapies directed to the regulation of pathologically significant profibrotic genes. Inherent in this diversity are therapeutic opportunities to modulate the pathophysiologic consequences of persistent TLR signaling. The recently identified involvement of receptor and nonreceptor kinase pathways in TLR signaling may present novel opportunities for pharmacologic intervention.
Future Directions: Clarifying the identity and function of DAMP-activated TLR complexes or ligand-binding partners, as well as their engaged downstream effectors and target genes, are key factors in the eventual design of pathway-specific treatment modalities. Such approaches may be tailored to address the spectrum of TLR-initiated pathologies (including localized and persistent inflammation, maladaptive repair/fibrosis) and, perhaps, even titrated to achieve patient-unique beneficial clinical outcomes.
Keywords: : TLR4, wound repair, signaling, fibrosis, inflammation, TGF-β1

Paula J. McKeown-Longo, PhD

Paul J. Higgins, PhD
Scope and Significance
Activation of a fibro-inflammatory genomic program accompanies the host response to pathogens and tissue injury mediated by pathogen-associated molecular patterns (PAMPs) and damage-associated molecular patterns (DAMPs), respectively, via the toll-like receptor (TLR) system. Persistent TLR signaling often results in nonresolving inflammation, tissue fibrosis, intravascular coagulation, multiorgan failure, and poor patient outcomes. This review discusses some of the more recent findings that highlight the growing complexity of TLR signaling.
Translational and Clinical Relevance
In response to ligands released from pathogenic microorganisms or upon tissue injury, activation and maintenance of TLR signaling often result in systemic as well as localized inflammation, disseminated coagulopathy, exuberant wound repair, excessive scarring, and fibrosis. The time course and amplitude of the inflammatory phase are largely dictated by the duration of the stimulus, the postreceptor pathways engaged, and the target genes impacted. There is a growing recognition of the involvement of “noncanonical” toll-like receptor 4 (TLR4) signaling effectors (e.g., phosphatase and tensin homolog [PTEN], integrins and the epidermal growth factor receptor [EGFR], and transforming growth factor-β1 [TGF-β1] networks) as important downstream participants. These provide new opportunities to manage the pathophysiologic consequences encountered upon aberrant activation of this important member of the TLR family.
Discussion of the Literature
Introduction
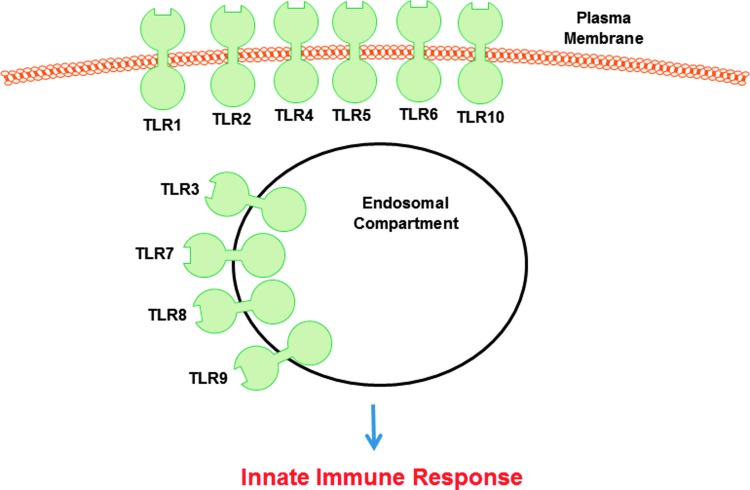
TLR4 is a member of a family of 10 human pattern recognition receptors initially implicated in the regulation of the innate immune system (Fig. 1). In addition to its prototypic PAMP ligand, bacterial lipopolysaccharide (LPS), TLR4 can also be activated by various endogenous components released following an injury.1–3 These DAMP molecules include the heat shock and high-mobility group box 1 proteins (HMGB1) as well as several extracellular matrix (ECM) elements, including hyaluronic acid, proteoglycans, the matricellular protein Tenascin C, and the alternatively spliced extra domain A (EDA) isoform of fibronectin. Activation of TLR4 by ECM-derived DAMPs induces the expression of fibro-inflammatory genes that promote tissue remodeling and normal as well as maladaptive wound repair. When the response becomes exacerbated, DAMPs drive both chronic inflammation and pathological fibrosis.4 There is evidence to suggest possible cooperation between these two classes of TLR “ligands.” In an in vivo model of persistent cutaneous injury using a combination of PAMPs and DAMPs, excessive and sustained inflammation results in impaired healing and increased scarring.5 Dermal fibroblasts isolated from hypertrophic scars (HTS) express transcripts for many TLRs, including TLR4, and their intracellular adaptors at levels considerably greater than that of normal fibroblasts.6 Hypertrophic scarring is a frequent outcome in severe burn injury, and the combination of bacterial colonization and thermal tissue damage, in the context of elevated TLR signaling, may lead to persistent inflammation and exuberant scar formation.6 Indeed, synthesis of proinflammatory cytokines in response to LPS was significantly increased in HTS fibroblasts compared with normal cells. Collectively, it has become apparent that (1) increased TLR2/4 surface levels and/or functional activation result in impaired cutaneous wound healing due, in large part, to prolonged inflammation, (2) TLRs have different roles in injury repair, and (3) outcomes depend on TLR expression, timing of activation, the specific cell type(s) involved, and the adaptor network engaged.7
Figure 1.
There are 10 members of the TLR family in humans. These are evolutionary-conserved homologs of the Drosophila Toll protein that play a key role in the innate immune response to pathogen-derived and endogenous danger signals (see Chen and DiPietro76 for a complete description of TLR ligands). TLR10 is unique among the repertoire of TLRs as it appears to function to suppress inflammatory signaling and currently has no known ligand. TLRs 1, 2, 4, 5, 6, and likely 10 are found on the cell surface whereas TLRs 3, 7, 8, and 9 localize to intracellular compartments (e.g., endosomes and lysosomes). TLR, toll-like receptor.
Two of the best studied DAMPs are the ECM proteins EDA-fibronectin and Tenascin C. While not normally present at detectable levels in adult tissues, both are upregulated following injury and highly expressed in inflamed and fibrotic tissues as well as in the skin of systemic sclerosis patients. Animal studies, moreover, support a role for these DAMPs in disease progression.8 Elevated EDA-fibronectin levels are evident in keloids and psoriatic lesions,9–11 while EDA-fibronectin-deficient mice exhibit diminished inflammatory and fibrotic responses.12–15 These findings provide the basis for the hypothesis that products of tissue injury and sustained generation of DAMPs activate a TLR4-dependent feed-forward loop that promotes chronic inflammation and persistent fibrosis.12,16,17 TLR4 DAMP-type ligands, however, are structurally diverse and the mechanism(s), by which they initiate TLR4 signaling, are not well understood. In contrast, the molecular events underlying TLR4 activation by LPS are better defined, requiring the two ancillary molecules CD14 and MD-2. CD14 is a glycosyl phosphatidylinositol (GPI)-linked protein which captures LPS from solution and presents it to the TLR4/MD-2 complex. MD-2 is a TLR4 binding secreted protein which cooperates with CD14 to stimulate TLR4 dimerization and downstream signaling.18 Similar to LPS, DAMPs may not interact directly with TLR4, but may bind to accessory factors as part of a larger TLR4 complex.19
Involvement of the EGFR and integrins in TLR4 signaling
TLR4 activation and downstream signaling are also regulated by the cellular context in which TLR4 receptor complexes assemble as well as by the contributions of costimulated parallel pathways. Recent studies implicate both the EGFR and integrins in the TLR response to pathogens; it appears, moreover, that the EGFR is required for LPS to signal via TLR4.20 Although the mechanism is unclear, the EGFR inhibitor erlotinib blocks LPS-induced expression of tumor necrosis factor α (TNF-α) and interleukin-6 (IL-6), while attenuating LPS-induced endotoxicity, indicating that the EGFR is essential for LPS-induced signaling in vivo.20 Perhaps not unexpectedly, since LPS-induced TLR4 activation of NF-κB is impaired by EGFR knockdown or erlotinib, EGFR kinase inhibitors (AG1478, erlotinib, gefitinib) have proven to be effective in treating septic shock in preclinical models. Gefitinib and erlotinib reduce pulmonary damage and promote increased survival in mouse models of sepsis,20,21 while erlotinib blunts the proinflammatory and proliferative responses following hepatic injury.22 AG1478 also reduced fibrosis and inflammation in obesity-related cardiac disease and hyperlipidemia-induced renal injury.23,24 Induction of proinflammatory and profibrotic outcomes upon TLR4 signaling, furthermore, depends on EGFR transactivation through complex pathways involving several kinase networks.22–25 Linkages, however, are unclear and interactions between TLR4 and EGFR may be functional as complexes between the two receptors have not been detected.20,21
Integrins are also implicated in pathogen-induced TLR activation; such cooperation occurs in both immune and nonimmune cells where integrins enhance the innate immune response. Certain integrins may serve as TLR coreceptors, binding directly to ligands to initiate TLR signaling. In human monocytes and 293 cells, complexes between the αvβ3 integrin and TLR2 coordinate an inflammatory response.26,27 In addition to ligand recognition, functional interactions between integrins and TLRs are also evident at the level of intracellular trafficking. Herpes simplex virus/αvβ3 integrin interaction, for example, activates TLR2 signaling by modulating subcellular localization.27–29 Similarly, binding of Borrelia burgdorferi to the α3β1 integrin enhances TLR2-mediated responses by stimulating internalization and endosomal targeting of B. burgdorferi/TLR2 complexes.30 The αmβ2 (CD11b/CD18) integrin regulates both LPS binding to TLR4 as well as downstream signaling by promoting TLR4 trafficking into the endosomal compartment.31
TLR responses are also modulated by integrin-dependent events activated in parallel; such effects on TLR pathways can be either positive or negative as well as bidirectional. Positive regulation occurs in dendritic cells, where α9β1 integrin-stimulated ERK activation enhances TLR2- and TLR4-mediated cytokine production.32 In macrophages, the αvβ3 integrin cooperates with TLR4 in a two-step mechanism resulting in TNF-α expression. In this model, LPS activation of TLR4 induces synthesis of the matricellular protein WISP-1, an αvβ3 ligand that further potentiates TNF-α expression via TLR4 signaling.33 Integrins also negatively regulate TLR function, particularly in monocytes derived from β2−/− mice which exhibit a hyperresponsiveness to TLR agonists due to increased activation of NF-κB.34
Bidirectional cross talk between integrins and TLRs is evident in macrophages where TLR-dependent activation of the αmβ2 integrin, in turn, inhibits further TLR signaling by stimulating the syk kinase-dependent phosphorylation, and subsequent degradation, of the TLR adaptor MyD88.35 Similar cross talk also occurs in leukocytes where TLR2 agonists activate the β2 integrin,36 resulting in positive or negative regulation of the TLR2 and TLR4 pathways.37 These data illustrate the complexity of TLR signaling in response to pathogens and provide a cellular and molecular context for ligand- and cell type-specific TLR responses. The requirements for TLR4 activation by the products of tissue damage (DAMPs) as well as their downstream targets, in contrast, are not well understood; the available data suggest that they are also ligand- and cell type-dependent. The association of TLR4 with distinct accessory proteins or coreceptors may dictate the assembly of ligand-specific TLR4 complexes, thereby tailoring the inflammatory response to achieve specific biological outcomes.38,39 Little is known, however, regarding the impact of integrins and EGFR on DAMP-initiated fibro-inflammation. Based on findings from studies on pathogen-initiated TLR signaling, it appears likely that adhesion receptors and receptor tyrosine kinases may play important roles in the regulation of the fibro-inflammatory responses to tissue damage.
Intersection of the TLR4 and TGF-β1 networks
LPS activation of TLR4 signaling markedly enhances fibroblast responses to the profibrotic cytokine TGF-β1.40 The mechanism appears to involve downregulation of the TGF-β1 inhibitory receptor BAMBI (through a TLR4/MyD88/NF-κB pathway) that, in turn, may sensitize cells to the relatively abundant levels of TGF-β1 at the wound site.41,42 A specific TGF-β1 gene signature that includes the potent profibrotic serine protease inhibitor plasminogen activator inhibitor-1 (PAI-1; SERPINE1), connective tissue growth factor (CTGF, CCN2), EDA-fibronectin, collagen I, TGF-β1, vimentin, p53, p21, miR-21, miR-29, and α-smooth muscle actin (α-SMA)43–46 is consistently associated with fibrosis in human cells and mouse models. Several of these genes are upregulated in the cutaneous tissue of systemic sclerosis patients as well as in the LPS-treated mouse skin, via a TLR4 pathway and downstream MyD88 signaling, and attenuated by TGF-β1 neutralizing antibodies.47 The two most likely members of the TGF-β1-induced repertoire with potential impact on TLR4 signaling are EDA-fibronectin and the SERPIN PAI-1. TGF-β1 upregulates DAMP-type EDA-fibronectin expression in various cell types, including dermal fibroblasts; this fibronectin variant may function as an endogenous activator of the TLR4 pathway in much the same way as does exogenously-delivered EDA-fibronectin.40,48
Recent findings also implicate PAI-1 in this pathway, perhaps as a matricellular DAMP.49,50 Macrophage activation in response to PAI-1 was dose-dependent and LPS-independent and partially blocked by a TLR4 neutralizing antibody. PAI-1-stimulated TNF-α and macrophage inflammatory protein-2 (MIP-2) expression, moreover, was reduced in TLR4−/− compared with wild-type macrophages suggesting that PAI-1 is involved in the regulation of host inflammatory responses via TLR4.51 PAI-1 knockdown attenuated LPS-induced increases in TLR4, MD-2, MyD88, TNF-α, IL-1β, and NF-κB expression in macrophages, while vector-driven PAI-1 overexpression enhanced these responses.52,53 Although the mechanism is unclear, it appears that PAI-1 regulates the endotoxin-stimulated TLR4/MD-2 inflammatory pathway, at least in cells of the macrophage lineage. This is likely to have a significant impact on fibrogenic outcomes following tissue injury as exogenous PAI-1 treatment significantly increased TGF-β1, collagen 1α1, collagen 1α2, and monocyte chemoattractant protein-1 (MCP-1) transcript abundance in cultured cells.54 The TLR4/RAGE DAMP-type ligand HMGB1 also activates a subset of genes in the TGF-β1 profibrotic signature that includes PAI-1, CTGF, and TGF-β1,55 suggesting that DAMPs and LPS may utilize signaling pathways that may be exploited in the design of interventional strategies. Collectively, it appears that TLR4 may function as a molecular “switch,” activated by endogenous DAMPs to initiate repair while upregulating the TGF-β1 pathway promoting the persistent expression of profibrotic genes to create and maintain a progressive fibrotic microenvironment.40,56
Ligand-dependent TLR4 activation leading to EGFR recruitment20 and enhanced fibroblast responses to TGF-β1 via BAMBI downregulation40 has implications with regard to both pathways. Similar to LPS-initiated TLR4 signaling, control of TGF-β1 target gene expression also involves Src kinases and EGFR transactivation.57–59 TGF-β1-stimulated PAI-1 expression, moreover, was preceded by EGFR phosphorylation on Y845 (a Src kinase target residue) and required pp60c-src activity.59 EGFR inhibition or infection with adenoviruses encoding the EGFRY845F or kinase-dead EGFRK721A mutants or dominant-negative pp60c-src overexpression effectively decreased TGF-β1-stimulated PAI-1 induction implicating EGFR activity and, specifically, the pp60c-src EGFRY845 phosphorylation site in the response. Consistent with these findings, TGF-β1 failed to induce PAI-1 synthesis in triple Src kinase-deficient (SYF−/−/−) fibroblasts, while reexpression of a wild-type pp60c-src construct in SYF−/−/− cells rescued the PAI-1 response to TGF-β1.57–59 It appears, therefore, that Src family kinase EGFR activation is a critical event in both TLR4 and TGF-β1 signaling to downstream effector pathways.
Role of PTEN in TLR4 signaling
Recent studies suggest a role for PTEN, the principle negative regulator of the phosphoinositide 3-kinase (PI3K) pathway and protein kinase B (Akt) activation, in the progression of tissue fibrosis. PTEN deficiency is characteristic of idiopathic pulmonary fibrosis, and genetic ablation of PTEN in the alveolar epithelium promotes lung fibrosis via an Akt pathway.60–62 Similarly, PTEN deficiency in dermal fibroblasts drives skin fibrosis in a mouse model.63 PTEN silencing induces dedifferentiation and cell cycle arrest, both biomarkers of maladaptive repair, and cooperates with TGF-β1 to further stimulate expression of the fibrotic signature genes CTGF, PAI-1, vimentin, α-SMA, and EDA-fibronectin.45,46 The mechanism of PTEN downregulation in the context of fibrosis is not known but is likely the result of increased TGF-β1 levels in the injury microenvironment. TGF-β1 reduces, moreover, the levels of the C-terminal SMAD2/3 protein phosphatase PPM1A, a terminator of TGF-β1 signaling.64 While the specific molecular events are unknown, persistent TGF-β1 stimulation reduces PPM1A levels via the Rho/rho-associated, coiled-coil-containing protein kinase (ROCK) pathway enhancing and maintaining, thereby, SMAD3-dependent transcription of this profibrotic signature.43,46,57 Rho-ROCK signaling leads to inhibition of PTEN-PPM1A activity in TGF-β1-stimulated cells resulting in a reduction of nuclear PPM1A, maintaining pSMAD2/3 levels required for profibrotic gene expression.57 Similar to their involvement in EGFR and TGF-β1 signaling, Src kinases are key intermediates in the Rho/ROCK pathway leading to the expression of the profibrotic genes PAI-1 and CTGF.57 These data implicate Src kinases as upstream regulators of the RhoA-ROCK-SMAD2/3 axis leading to control of PPM1A activity impacting downstream expression of specific profibrotic genes.
While LPS-activated TLR4 signaling enhances cellular responses to TGF-β1 by downregulating BAMBI, PTEN levels are also decreased likely contributing to fibroblast activation in pulmonary fibrosis.41 PTEN deficiency has also been implicated in the profibrotic phenotype of scleroderma fibroblasts as well as in keloid pathophysiology and cutaneous fibrosis.63,65 The impact of DAMP ligands is less clear. PTEN expression is lost in several models of injury-induced fibrosis, and targeted PTEN depletion reduces PPM1A levels while promoting SMAD3 phosphorylation as well as SMAD3 nuclear translocalization.43,46 PPM1A suppression further enhanced TGF-β1-induced SMAD3 phosphorylation and fibrotic gene expression, while PPM1A overexpression inhibited both responses.45,46 Thus, these findings implicate PTEN as an upstream regulator of PPM1A function in dysfunctional tissue repair and establish PPM1A as a novel repressor of the SMAD3 fibrotic pathway. Stable silencing of PTEN, moreover, induced many of the same fibrotic genes as LPS or TGF-β1 (e.g., CTGF, PAI-1, vimentin, α-SMA, and fibronectin).45,46 Relatively, little is known about PPM1A regulation in general or in the context of tissue injury. PTEN, however, interacts with PPM1A in scleroderma65 and PTEN silencing in macrophages increased LPS-induced expression of TNF-α, IL-1β, IL-6, IL-8, and IL-10.66 There are apparently contrary findings, however. LPS activation of TLR4 also stimulates miR-718 expression (in macrophages), which impacts PI3K/Akt signaling by targeting PTEN, promoting Akt phosphorylation leading to a decrease in the production of proinflammatory cytokines.67 pAkt, in turn, downmodulates expression of TLR4 and several of its signaling effectors through let-7e exerting, thereby, multilevel negative regulation to the TLR4 pathway. Thus, depending on the actual magnitude and duration of the stimulus (e.g., LPS and/or TGF-β1), PTEN may function as a rheostat to influence the amplitude and kinetics of the inflammatory response. Increased TGF-β1 sensitivity due to reductions in BAMBI levels, coupled with decreased PTEN expression, may facilitate the transition from the inflammatory to the tissue repair phases of wound healing and, perhaps if persistent over the long term, promote exuberant fibrosis. Since it has recently been determined that PPM1A also regulates NK-κB activation,68 the available data collectively suggest significant interaction between the TLR4 and PTEN pathways.
Implications for cutaneous wound healing
Whether the repair of cutaneous injuries is enhanced or inhibited by TLR engagement depends on, among other factors, the repertoire and levels of TLRs expressed, the involved cell types and their location at the wound site, and the timing of TLR-ligand presentation.7,69 Additional confounders include the time course and amplitude of the inflammatory response, the presence of comorbidities (e.g., diabetes), and microbial colonization of the wound bed, each of which is a significant contributor to wound chronicity. Indeed, persistent activation of TLR2/4 and elevated expression of proinflammatory cytokines typified nonresolving venous ulcers, whereas healing lesions had much reduced TLR2/4 levels.70 In the case of TLR4, both the nature of the ligand (DAMP vs. PAMP) and the cutaneous compartment stimulated (dermis vs. epidermis) appear important in the repair outcome. DAMP-type TLR4 ligands, moreover, appear critical in control of the inflammatory and subsequent fibrotic response in sterile cutaneous wounds.71 Microarray analysis, moreover, confirmed that TLR4 expression is elevated in the wound edge epithelial cohort in a mouse model of skin injury.72 This is likely critical to tissue repair since excisional wound closure was delayed in TLR4 mutant mice compared with their wild-type counterparts, correlating with the attenuated production of IL-1β and IL-6. Consistent with these in vivo results, TLR4 blockade with neutralizing antibodies attenuated in vitro keratinocyte migration following monolayer scratch injury and inhibited wound-induced p38/JNK activation as well as IL-1β expression. These data indicate the requirement for the TLR4-p38/JNK pathway in the regulation of inflammation and wound repair as the presence of a mutated nonfunctional receptor or interference with TLR4 signaling, blunted both processes.72 Collectively, these findings underscore both locational and cell type controls on TLR4 signal transduction and, when coupled with the impact of the expression levels and activation status of specific TLRs, provide some explanation as to discrepancies in the literature regarding the effect of the TLR network on healing of chronic versus acute cutaneous wounds. Patients presenting with difficult-to-heal injuries, moreover, appear to have multilevel deficiencies in TLR pathways. Recent reports suggest that there is increased TLR4 expression, signaling as well as receptor activation in type-1 diabetes, and that streptozotocin-induced diabetic mice upregulate TLR2/4.73 TLR4 downstream effectors were significantly reduced in TLR4−/− animals receiving streptozotocin compared with similarly-treated wild-type controls as were levels of proinflammatory cytokines suggesting that TLR4 is a major contributor to the persistence of the diabetes-associated inflammatory response. Wound site infection further complicates repair events, particularly in diabetic patients. Biofilm-compromised cutaneous wounds in diabetic mice had significantly lower TLR2/4 levels compared with either bacteria-free diabetic or wild-type controls.74 Diabetic wounds failed to upregulate IL-1β or TNF-α expression following infection, whereas wild-type mice exhibited a >140-fold increase in both. By 10 days postinjury, diabetic wound sites had a much greater microbial burden (due to a reduced neutrophil burst) and a significant delay in reepithelialization. Wounds in TLR2−/−, TLR4−/−, and double-knockout TLR2−/−/TLR4−/− mice, moreover, exhibited attenuated healing, reduced numbers of infiltrating macrophages and decreased TGF-β and CCL5 expression relative to wild-type animals.75 Macrophage TGF-β induction by the DAMP ligand hyaluronan was also reduced in the absence of the two TLRs and, in particular, in response to TLR4 deficiency. Topical delivery of TGF-β and CCL5 improved healing in TLR-null mice. Macrophages and the cutaneous tissue of TLR2−/− mice also had reduced TLR4 levels, suggesting that the observed effects of TLR2 loss may be partially due to TLR4 downregulation. These data indicate that TLR4, but perhaps not TLR2, impacts the process of cutaneous wound repair through mechanisms involving TGF-β and CCL5,75 perhaps utilizing the pathways detailed in this review.
Take-Home Messages.
• The TLR4 system regulates the cellular response to both exogenous pathogen-associated (PAMPs) and endogenous damage-associated (DAMPs) factors that can elicit both common and unique outcomes as a function of cell type.
• TLR4 activation by ECM-derived DAMPs induces expression of fibro-inflammatory genes that promote tissue remodeling, maladaptive wound repair, and tissue fibrosis.
• Pathways downstream of TLR4 involving Src family kinases, the EGFR and TGF-β, as well as the RhoA→PTEN/PPM1A network, highlight the growing complexity of TLR4 signaling.
• TLR responses are also modulated, both positively and negatively as well as bidirectionally, by integrin-dependent pathways, in which the integrins function as cofactors in TLR signaling.
• The development of novel TLR ligand-, pathway-, and/or target gene-specific therapeutics may have clinical promise in the management of the exuberant inflammatory/fibrotic tissue response to DAMPs released at the site of injury.
Summary
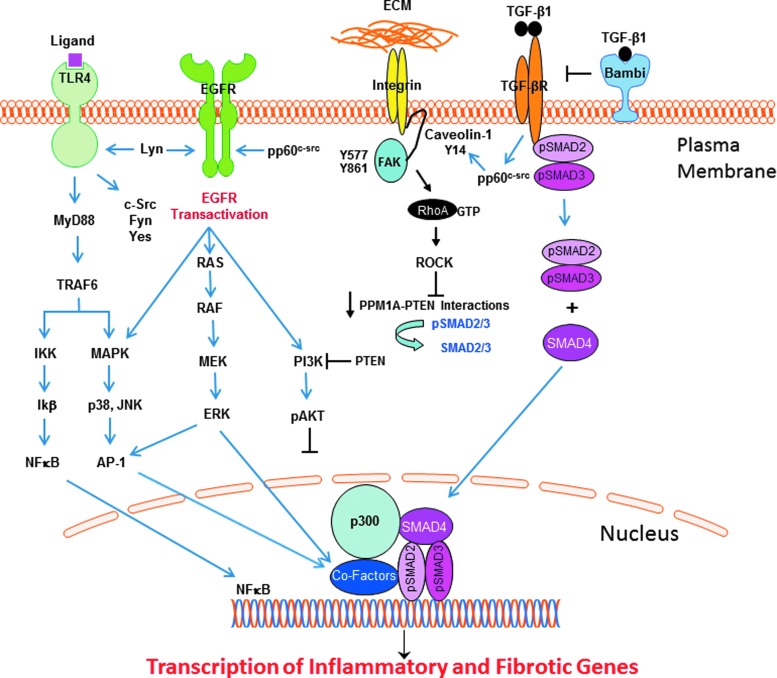
The available data support a model whereby the duration and amplitude of TLR4 activation, and the collateral involved pathways, impacts both normal and maladaptive wound repair. Identification of the mechanisms underlying EGFR, RhoA→PTEN/PPM1A, and TGF-β1 signaling and their involvement in PAMP- and DAMP-induced TLR4 stimulation may provide novel targets for treatment of fibro-inflammatory diseases (Fig. 2). The ongoing clarification of molecular events downstream of TLR4 that dictate the extent of the inflammatory response to microbial pathogens and the endogenous damage-associated factors released upon tissue injury clearly implicate these highly interactive-pathways in TLR4 signaling. Findings suggest, moreover, that different nonreceptor Src A/B-type family tyrosine kinases are involved in activation of the EGFR (Lyn), RhoA/PTEN/PPM1A (Yes), and TGF-β1 (pp60c-src) pathways downstream of TLR4. The growing complexity of TLR4 signaling involving various coreceptors and the integration of both canonical and noncanonical pathways, with each potentially mobilizing a unique set of effectors, may provide new therapeutic targets to differentially manipulate the pathophysiologic consequences of TLR4 activation in response to PAMP versus DAMP ligands.
Figure 2.
TLR4 signaling in response to ligand binding, either directly to the TLR or through cooperative interactions with various coreceptors, mobilizes two major core parallel pathways, resulting in the activation of the transcription factor NF-κB and the p38/JNK MAP kinases via the upstream intermediate MyD88. The collateral or noncanonical pathways that impact TLR4 signaling outcomes involve several Src family kinases, transactivation of the EGFR (and downstream signaling intermediates), integrins, and the caveolin-1/FAK/RhoA/Rock/PTEN axis, as well as the canonical SMAD-dependent TGF-β pathway. The complex interactions among these diverse, although inter-related, pathways positively and negatively regulate the outcome of TLR4 signaling (detailed in this review). The available data suggest that while Akt activation may attenuate the amplitude and duration of the inflammatory response following TLR4 signaling, TGF-β1 regulation of RhoA/ROCK signaling controls PTEN activity that, in turn, affects SMAD2/3 transcription of the profibrotic genes (e.g., PAI-1, CTGF) through the SMAD phosphatase PPM1A. Akt, protein kinase B; CTGF, connective tissue growth factor; ECM, extracellular matrix; EGFR, epidermal growth factor receptor; PAI-1, plasminogen activator inhibitor-1; PTEN, phosphatase and tensin homolog; ROCK, rho-associated, coiled-coil-containing protein kinase; SMAD, Sma+mothers against decapentaplegic; TGF-β, transforming growth factor-β; TLR4, toll-like receptor 4.
Abbreviations and Acronyms
- Akt
protein kinase B
- CD
cluster of differentiation
- CTGF
connective tissue growth factor
- DAMP
damage-associated molecular pattern
- ECM
extracellular matrix
- EDA
extra domain A
- EGFR
epidermal growth factor receptor
- GPI
glycosyl phosphatidylinositol
- HMGB1
high-mobility group box 1 protein
- HTS
hypertrophic scar
- IL
interleukin
- LPS
lipopolysaccharide
- MCP-1
monocyte chemoattractant protein-1
- MD-2
lymphocyte antigen 96
- MIP-2
macrophage inflammatory protein-2
- PAI-1
plasminogen activator inhibitor-1
- PAMP
pathogen-associated molecular pattern
- PI3K
phosphoinositide 3-kinase
- PTEN
phosphatase and tensin homolog
- RAGE
receptor for advanced glycosylation end products
- ROCK
rho-associated, coiled-coil-containing protein kinase
- SERPIN
serine protease inhibitor
- SMA
smooth muscle actin
- SMAD
Sma+mothers against decapentaplegic
- Src
sarcoma
- TGF-β1
transforming growth factor-β1
- TLR
toll-like receptor
- TLR4
toll-like receptor 4
- TNF-α
tumor necrosis factor α
Acknowledgments and Funding Sources
Support for this work was provided by NIH grants R01-CA58626 and R21-AR0667956 (to P.J.M.-L.) and R01-GM057242 (to P.J.H.) as well as by the Graver Family Endowed Fund and the Roach Foundation (to P.J.H.).
Author Disclosure and Ghostwriting
The authors do not have any commercial conflicts of interest. The article was written exclusively by Drs. McKeown-Longo and Higgins; no ghostwriters were involved.
About the Authors
Paula J. McKeown-Longo, PhD and Paul J. Higgins, PhD are Co-Chairs of the Department of Regenerative and Cancer Cell Biology at the Albany Medical College. Dr. McKeown-Longo is an expert in extracellular matrix biology and her work focuses on the role of fibronectin in cellular function and signaling. Research in Dr. Higgins' laboratory centers on the molecular mechanisms underlying transcription of TGF-β1 target genes and their involvement in wound healing and fibrosis.
References
- 1.Gerwirtz AT. Intestinal epithelial toll-like receptors: to protect. And serve? Curr Pharm Des 2003;9:1–5 [DOI] [PubMed] [Google Scholar]
- 2.Hasan UA, Trinchieri G, Vlach J. Toll-like receptor signaling stimulates cell cycle entry and progress in fibroblasts. J Biol Chem 2005;280:20620–20627 [DOI] [PubMed] [Google Scholar]
- 3.Zeuke S, Ulmer AJ, Kusumoto S, Katus HA, Heine H. TLR4-mediated inflammatory activation of human coronary artery endothelial cells by LPS. Cardiovasc Res 2002;56:126–134 [DOI] [PubMed] [Google Scholar]
- 4.O'Neill GM, Golemis EA. Proteolysis of the docking protein HEF1 and implications for focal adhesion dynamics. Mol Cell Biol 2001;21:5094–5108 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Qian LW, Fourcaudot AB, Yamane K, You T, Chan RK, Leung KP. Exacerbated and prolonged inflammation impairs wound healing and increases scarring. Wound Repair Regen 2016;24:26–34 [DOI] [PubMed] [Google Scholar]
- 6.Wang J, Hori K, Ding J, Huang Y, Kwan P, Ladak A, et al. Toll-like receptors expressed by dermal fibroblasts contribute to hypertrophic scarring. J Cell Physiol 2011;226:1265–1273 [DOI] [PubMed] [Google Scholar]
- 7.Dasu MR, Isseroff RR. Toll-like receptors in wound healing: location, accessibility, and timing. J Invest Dermatol 2012;132:1955–1958 [DOI] [PubMed] [Google Scholar]
- 8.Bhattacharyya S, Wang W, Morales-Nebreda L, Feng G, Wu M, Zhou X, et al. Tenascin-C drives persistance of organ fibrosis. Nat Commun 2016;7:11703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Andrews JP, Marttala J, Macarak E, Rosenbloom J, Uitto J. Keloid pathogenesis: potential role of cellular fibronectin with the EDA domain. J Invest Dermatol 2015;135:1921–1924 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.McFadden JP, Baker BS, Powles AV, Fry L. Psoriasis and extra domain A fibronectin loops. Br J Dermatol 2010;163:5–11 [DOI] [PubMed] [Google Scholar]
- 11.Gubán B, Vas K, Balog Z, Manczinger M, Bebes A, Groma G, et al. Abnormal regulation of fibronectin production by fibroblasts in psoriasis. Br J Dermatol 2016;174:533–541 [DOI] [PubMed] [Google Scholar]
- 12.McFadden JP, Basketter DA, Dearman RJ, Kimber IR. Extra domain A-positive fibronectin-positive feedback loops and their association with cutaneous inflammatory disease. Clin Dermatol 2011;29:257–265 [DOI] [PubMed] [Google Scholar]
- 13.Arslan F, Smeets MB, Riem Vis PW, Karper JC, Quax PH, Bongartz LG, et al. Lack of fibronectin-EDA promotes survival and prevents adverse remodeling and heart function deterioration after myocardial infarction. Circ Res 2011;108:582–592 [DOI] [PubMed] [Google Scholar]
- 14.Khan MM, Gandhi C, Chauhan N, Stevens JW, Motto DG, Lentz SR, et al. Alternatively-spliced extra domain A of fibronectin promotes acute inflammation and brain injury after cerebral ischemia in mice. Stroke 2012;43:1376–1382 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Booth AJ, Wood SC, Cornett AM, Dreffs AA, Lu G, Muro AF, et al. Recipient-derived EDA fibronectin promotes cardiac allograft fibrosis. J Pathol 2012;226:609–618 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Goh FG, Piccinini AM, Krausgruber T, Udalova IA, Midwood KS. Transcriptional regulation of the endogenous danger signal tenascin-c: a novel autocrine loop in inflammation. J Immunol 2010;184:2655–2662 [DOI] [PubMed] [Google Scholar]
- 17.Kelsh RM, McKeown-Longo PJ. Topographical changes in extracellular matrix: activation of TLR4 signaling and solid tumor progression. Trends Cancer Res 2013;9:1–13 [PMC free article] [PubMed] [Google Scholar]
- 18.Park BS, Song DH, Kim HM, Choi BS, Lee H, Lee JO. The structural basis of lipopolysaccharaide recognition by the TLR4-MD-2 complex. Nature 2009;458:1191–1195 [DOI] [PubMed] [Google Scholar]
- 19.Triantafilou M, Lepper PM, Olden R, Dias IS, Triantafilou K. Location, location, location: is membrane partitioning everything when it comes to innate immune activation. Mediators Inflamm 2011;2011:Article ID 186093 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.De S, Zhou H, DeSantis D, Croniger CM, Li X, Stark GR. Erlotinib protects against LPS-induced endotoxicity because TLR4 needs EGFR to signal. Proc Nat Acad Sci U S A 2015;112:9680–9685 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Chattopadhyay S, Veleeparambil M, Poddar D, Abdulkhalek S, Bandyopadhyay SK, Fensterl V, et al. EGFR kinase activity is required for TLR4 signaling and the septic shock response. EMBO Rep 2015;16:1535–1547 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Trussoni CE, Tabibian JH, Splinter PL, O'Hara SP. Lipopolysaccharide (LPS)-induced biliary epithelial cell NRas activation requires epidermal growth factor receptor (EGFR). PLoS One 2015;10:e0125793. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Li W, Fang Q, Zhong P, Chen L, Wang L, Zhang Y, et al. EGFR inhibition blocks palmitic acid-induced inflammation in cardiomyocytes and prevents hyperlipidemia-induced cardiac injury in mice. Sci Rep 2016;6:24580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Fang Q, Zou C, Zhong P, Lin F, Li W, Wang L, et al. EGFR mediates hyperlipidemia-induced renal injury via regulating inflammation and oxidative stress: the detrimental role and mechanism of EGFR activation. Oncotarget 2016;7:24361–24373 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.McElroy SJ, Hobbs S, Kallen M, Rejera N, Rosen MJ, Grishin A, et al. Transactivation of EGFR by LPS induces COX-2 expression in enterocytes. PLoS One 2012;7:e38373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Gerold G, Abu Ajak K, Bienert M, Laws HJ, Zychlinsky A, de Diego JL. A Toll-like receptor 2-integrin β3 complex senses bacterial lipopeptides via vitronectin. Nat Immunol 2008;9:761–768 [DOI] [PubMed] [Google Scholar]
- 27.Gianni T, Leoni V, Chesnokova LS, Hutt-Fletcher LM, Campadelli-Fiume G. αvβ3-integrin is a major sensor and activator of innate immunity to herpes simplex virus-1. Proc Natl Acad Sci U S A 2012;109:19792–19797 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Casiraghi C, Gianni T, Campadelli-Fiume G. αvβ3 integrin boosts the innate immune response elicited by epithelial cells through plasma membrane and endosomal Toll-like receptors. J Virol 2016;90:4243–4248 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Gianni T, Campadelli-Fiume G. The epithelial αvβ3-integrin boosts the MYD88-Dependent TLR2 signaling in response to viral and bacterial components. PLoS Pathog 2014;10:e1004477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Marre ML, Petnicki-Ocwieja T, DeFrancesco AS, Darcy CT, Hu LT. Human integrin α3β1 regulates TLR2 recognition of lipopeptides from endosomal compartments. PLoS One 2010;5:e12871. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Ling GS, Bennett J, Woollard KJ, Szajna M, Fossati-Jimack L, Taylor PR, et al. Integrin CD11b positively regulates TLR4-induced signalling pathways in dendritic cells but not in macrophages. Nat Commun 2014;5:3039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Kanayama M, Morimoto J, Matsui Y, Ikesue M, Danzaki K, Kurotaki D, et al. α9β1 integrin-mediated signaling serves as an intrinsic regulator of pathogenidc Th17 cell generation. J Immunol 2011;187:5851–5864 [DOI] [PubMed] [Google Scholar]
- 33.Chen Z, Ding X, Jin S, Pitt B, Zhang L, Billiar T, et al. WISP1-αvβ3 integrin signaling positive regulates TLR-triggered inflammation response in sepsis induced lung injury. Sci Rep 2016;6:28841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Yee NK, Hamerman JA. β2 integrins inhibit TLR responses by regulating NF-κB pathway and p38 MAPK activation. Eur J Immunol 2013;43:1–20 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Han C, Jin J, Xu S, Liu H, Li N, Cao X. Integrin CD11b negatively regulates TLR-triggered inflammatory responses by activating Syk and promoting degradation of MyD88 and TRIF via Cbl-b. Nat Immunol 2010;11:734–742 [DOI] [PubMed] [Google Scholar]
- 36.Kagan JC, Medzhitov R. Phosphoinositide-mediated adaptor recruitment controls Toll-like receptor signaling. Cell 2006;125:943–955 [DOI] [PubMed] [Google Scholar]
- 37.Wang L, Gordon RA, Huynh L, Su X, Park Min KH, Han J, et al. Indirect inhibition of Toll-like receptor and type I interferon responses by ITAM-coupled receptors and integrins. Immunity 2010;32:518–530 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Kelsh R, You R, Horzempa C, Zheng M, McKeown-Longo PJ. Regulation of the innate immune response by fibronectin: synergism between the III-1 and EDA domains. PLoS One 2014;9:e102974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Piccinini AM, Zulliani-Alvarez L, Lim JMP, Midwood KS. Distinct microenvironment cues stimulate divergent TLR4-mediated signaling pathways in macrophages. Sci Signal 2016;9:ra86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Bhattacharyya S, Kelley K, Melichian D, Tamaki Z, Fang F, Su Y, et al. Toll-like receptor 4 signaling augments transforming growth factor-β responses: a novel mechanism for maintaining and amplifying fibrosis in scleroderma. Am J Pathol 2013;182:192–205 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.He Z, Gao Y, Deng Y, Li W, Chen Y, Xing S, et al. Liposaccaride induces lung fibroblast proliferation through toll-like receptor 4 signaling and the phosphoinositide3-kinase-AKT pathway. PLoS One 2012;7:e35926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Seki E, De Minicis S, Österreicher CH, Kluwe J, Osawa Y, Brenner DA, et al. TLR4 enhances TGF-β signaling and hepatic fibrosis. Nat Med 2007;13:1324–1332 [DOI] [PubMed] [Google Scholar]
- 43.Overstreet JM, Samarakoon R, Cardona-Grau D, Goldschmeding R, Higgins PJ. Tumor suppression ataxia telangiectasia mutated functions downstream of TGF-β1 in orchestrating profibrotic responses. FASEB J 2015;29:1258–1268 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Overstreet JM, Samarakoon R, Meldrum KK, Higgins PJ. Redox control of p53 in the transcriptional regulation of TGF-β1 target genes through SMAD cooperativity. Cell Signal 2014;26:1427–1436 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Samarakoon R, Helo S, Dobberfuhl AD, Khakoo N, Falke L, Overstreet JM, et al. Loss of tumor suppressor PTEN expression in renal injury initiates SMAD3- and p53-dependent fibrotic responses. J Pathol 2015;236:421–432 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Samarakoon R, Rehfuss A, Khakoo N, Falke LL, Dobberfuhl AD, Helo S, et al. Loss of expression of the protein phosphatase magnesium dependent 1A (PPM1A) during kidney injury promotes fibrotic maladaptive repair. FASEB J 2016;30:3308–3320 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Stifano G, Affandi AJ, Mahes AL, Rice LM, Nakerakanti S, Nazari B, et al. Chronic toll-like receptor 4 stimulation in skin induces inflammation, macrophage activation, transforming growth factor β signature gene expression and fibrosis. Arthritis Res Ther 2014;16:R136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Bhattacharyya S, Tamaki Z, Wang W, Hinchcliff M, Hoover P, Getsios S, et al. FibronectinEDA promotes chronic cutaneous fibrosis through toll-like receptor signaling. Sci Transl Med 2014;6:232ra50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Maquerlot F, Galliacy S, Malo M, Guignabert C, Lawrence DA, d'Ortho MP, et al. Dual role for plasminogen activator inhibitor type 1 as soluble and as matricellular regulator of epithelial alveolar cell wound healing. Am J Pathol 2006;169:1624–1632 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Cartier-Michaud A, Malo M, Charrière-Bertrand C, Gadea G, Anguille C, Supiramaniam A, et al. Matrix-bound PAI-1 supports cell blebbing via RhoA/ROCK1 signaling. PLoS One 2012;7:e32204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Gupta KK, Xu Z, Castellino FJ, Ploplis VA. Plasminogen-activator inhibitor-1 stimulates macrophage activation through Toll-like Receptor-4. Biochem Biophys Res Commun 2016;477:503–508 [DOI] [PubMed] [Google Scholar]
- 52.Ren W, Wang Z, Hua F, Zhu L. Plasminogen activator inhibitor-1 regulates LPS-induced TLR4/MD-2 pathway activation and inflammation in alveolar macrophages. Inflammation 2015;38:384–393 [DOI] [PubMed] [Google Scholar]
- 53.Wang Z-H, Ren W-Y, Zhu L, Hu L-J. Plasminogen activator inhibitor-1 regulates LPS induced inflammation in rat macrophages through autophagy activation. ScientificWorldJournal 2014;2014:Article ID 189168 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Jeong BY, Uddin MJ, Park JH, Lee JH, Lee HB, Miyata T, et al. Novel plasminogen activator inhibitor-1 inhibitors prevent diabetic kidney injury in a mouse model. PLoS One 2016;11:e0157012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Cheng M, Liu H, Zhang D, Liu Y, Wang C, Liu F, et al. HMGB1 enhances AGE-induced expression of CTGF and TGF-β via RAGE-dependent signaling in renal tubular epithelial cells. Am J Nephrol 2015;41:257–266 [DOI] [PubMed] [Google Scholar]
- 56.Bhattacharyya S, Varga J. Emerging roles of innate immune signaling and toll-like receptors in fibrosis and systemic sclerosis. Curr Rheumatol Rep 2015;17:474. [DOI] [PubMed] [Google Scholar]
- 57.Samarakoon R, Chitnis SS, Higgins SP, Higgins SE, Krepinsky JC, Higgins PJ. Redox-induced src kinase and caveolin-1 signaling in TGF-β1-initiated SMAD2/3 activation and PAI-1 expression. PLoS One 2011;6:e22896. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Samarakoon R, Dobberfuhl AD, Cooley C, Overstreet JM, Patel S, Goldschmeding R, et al. Induction of renal fibrotic genes by TGF-β1 requires EGFR activation, p53 and reactive oxygen species. Cell Signal 2013;25:2198–2209 [DOI] [PubMed] [Google Scholar]
- 59.Samarakoon R, Higgins SP, Higgis SE, Higgins PJ. TGF-β1-induced plasminogen activator inhibitor-1 expression in vascular smooth muscle cells requires pp60c-src/EGFRY845 and Rho/ROCK signaling. J Mol Cell Cardiol 2008;44:527–538 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Geng J, Huang X, Li Y, Xu X, Li S, Jiang D, et al. Phosphatase and tensin homologue deleted on chromosome 10 contributes to phenotype transformation of fibroblasts in idiopathic pumonary fibrosis via multiple pathways. Exp Biol Med 2016;241:157–165 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Kral JB, Kuttke M, Schrottmaier WC, Birnecker B, Warszawska J, Wernig C, et al. Sustained PI3K activation exacerbates BLM-induced lung fibrosis via activation of pro-inflammatory and pro-fibrotic pathways. Sci Rep 2016;6:23034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Lan R, Geng H, Polichnowski AJ, Singha PK, Saikumar P, McEwen DG, et al. PTEN loss defines a TGF-β-induced tubule phenotype of failed differentiation and JNK signaling during renal fibrosis. Am J Renal Physiol 2012;302:F1210–F1223 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Parapuram SK, Shi-wen X, Elliott C, Welch ID, Jones H, Baro M, et al. Loss of PTEN expression by dermal fibroblasts causes skin fibrosis. J Invest Dermatol 2011;131:1996–2003 [DOI] [PubMed] [Google Scholar]
- 64.Lin X, Duan X, Liang YY, Su Y, Wrighton KH, Long J, et al. PPMA1 functions as a SMAD phosphatase to terminate TGF-β signaling. Cell 2006;125:915–928 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Bu S, Kapabadze B, Hsu T, Trojanowska M. Opposite effects of dihydrosphingosine 1-phosphate and sphingosine 1-phosphate on transforming growth factor β/Smad signaling are mediated through the PTEN/PPM1A-dependent pathway. J Biol Chem 2008;283:19593–19602 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Zhang L, Huang C, Guo Y, Gou X, Hinsdalte M, Lloyd P, et al. MicroRNA-26b modulates the NF-κB pathways in alveolar macrophages by regulating PTEN. J Immunol 2015;195:5404–5414 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Kalantari P, Harandi OF, Agarwal S, Rus F, Kurt-Jones EA, Fitzgerald, et al. miR-718 represses pro-inflammatory cytokine production through targeting PTEN. J Biol Chem 2017;292:5634–5644 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Sun W, Yu Y, Dotti G, Shen T, Tan X, Savoldo B, et al. PPM1A and PPM1B act as IK-β phosphatases to terminate TNFα-induced IKKβ-NF-κB activation. Cell Signal 2015;21:95–102 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Huebener P, Schwabe RF. Regulation of wound healing and organ fibrosis by toll-like receptors. Biochim Biophys Acta 2013;1832:1005–1017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Pukstad BS, Ryan L, Flo TH, Stenvik J, Moseley R, Harding K, et al. Non-healing is associated with persistent stimulation of the innate immune response in chronic venous leg ulcers. J Dermatol Sci 2010;59:115–122 [DOI] [PubMed] [Google Scholar]
- 71.Brancato SK, Thomay AA, Daley JM, Crane MJ, Reichner JS, Sabo E, et al. Toll-like receptor 4 signaling regulates the acute local inflammatory response to injury and the fibrosis/neovascularization of sterile wounds. Wound Repair Regen 2013;21:624–633 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Chen L, Guo S, Ranzer MJ, DiPietro LA. Toll-like receptor 4 has an essential role in early skin wound healing. J Invest Dermatol 2013;133:258–257 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Devaraj S, Tobias P, Jialal I. Knockout of toll-like receptor-4 attenuates the proinflammatory state of diabetes. Cyokine 2011;55:441–445 [DOI] [PubMed] [Google Scholar]
- 74.Nguyen KT, Seth AK, Hong SJ, Geringer MR, Xie P, Leung KP, et al. Deficient cytokine expression and neutrophil oxidative burst contribut ti impaired cutaneous wound healing in diabetic, biofilm-containing chronic wounds. Wound Repair Regen 2013;21:833–841 [DOI] [PubMed] [Google Scholar]
- 75.Suga H, Sugaya M, Fujita H, Asano Y, Tada Y, Kadono T, et al. TLR4, rather than TLR2, regulates wound healing through TGF-β and CCL5 expression. J Dermatol Sci 2014;73:117–124 [DOI] [PubMed] [Google Scholar]
- 76.Chen L, DiPietro LA. Toll-like receptor function in acute wounds. Adv Wound Care 2017;6:344–355 [DOI] [PMC free article] [PubMed] [Google Scholar]