Abstract
Significance: Damage-associated molecular patterns (DAMPs) and pathogen-associated molecular patterns (PAMPs) emanate from burn-injured tissue and enter systemic circulation. Locally and systemically, they activate pattern-recognition receptors, including toll-like receptors (TLRs), to stimulate cytokine secretion, which in the severest burns typically results in extreme systemic cytokine levels, a dysfunctioning immune system, infection, impaired healing, and excessive scarring. This system-wide disruption of homeostasis can advance to life-threatening, multiorgan dysfunction syndrome. Knowledge of DAMP- and PAMP-TLR signaling may lead to treatments that ameliorate local and systemic inflammation and reduce scarring and other burn injury sequela.
Recent Advances: Many PAMPs and DAMPs, the TLRs they activate, and their downstream signaling molecules have been shown to contribute to local and systemic inflammation and tissue damage following burn injury.
Critical Issues: Whether TLR-pathway-targeting treatments applied at different times postburn injury might improve scarring remains an open question. The evaluation of this question requires the use of appropriate preclinical and clinical burn models carried out until after mature scar has formed.
Future Directions: After TLR-pathway-targeting treatments are evaluated in porcine burn wound models and their safety is demonstrated, they can be tested in proof-of-concept clinical burn wound models.
Keywords: : burns, experimental models, inflammation, scar, tissue death

Kai P. Leung, PhD
Scope and Significance
Toll-like receptor (TLR) signaling is involved in damaged tissue sensing and wound repair, but also contributes to burn wound progression and systemic inflammation. TLR signaling is activated by pathogen-associated molecular patterns (PAMPs) of bacteria present in injured tissue and damage-associated molecular patterns (DAMPs) of injured tissue to produce cytokines. Early after burn injury, DAMPs as well as cytokines are elevated in circulation and contribute to systemic inflammation and secondary tissue damage that increase susceptibility to infection, impair healing, and worsen scarring. The potential of TLR signaling as a therapeutic target for improving burn outcomes is the subject of this review.
Translational Relevance
In animal burn models, suppressing TLR signaling has reduced inflammation in wounds and systemically. During the inflammation phase of healing, suppressing excessive inflammation by a variety of experimental means can mitigate tissue damage and improve healing. Lessening inflammation during the proliferation and remodeling phases can also potentially benefit scar outcomes. However, after the inflammation phase, reducing inflammation has impaired healing in some animal models. Thus, treatments to improve scar outcome must be properly timed and titrated and the risks of stalled healing and infection must be managed.
Clinical Relevance
Hypertrophic scars develop typically after the prolonged inflammation of slow-healing burn wounds.1 In clinical studies, the size of burn injury correlated with the level of circulating DAMPs (e.g., decorin and cell-free nucleic acids) and cytokines. In addition, early (2 weeks postburn) serum levels of decorin, a TLR2 and TLR4 ligand, were a factor (along with early interleukin [IL]-1β and late transforming growth factor [TGF]-β) that was suggested to predict hypertrophic scar.2 Thus, reducing DAMP- and PAMP-TLR signaling can potentially improve deep burn outcomes by mitigating injury progression, systemic inflammation, and the prolonged inflammation and healing associated with hypertrophic scarring.
Background
In the 1960s before early eschar excision and grafting was the standard of care for deep burns and before TLRs were discovered, burned skin extracts injected into the abdomen of mice produced an 80% mortality rate, whereas nonburned skin extracts had no effect. A thermally denatured lipid–protein complex was isolated from the burn extracts that when injected into mice mimicked many effects of burns on the immune system, such as increased susceptibility to Pseudomonas infection, suppressed immune responses to sheep erythrocytes and bacterial endotoxin, and inhibited IL-2-dependent cell growth in culture.3
Since these early studies of burned-tissue signaling, numerous DAMPs and PAMPs have been found to activate TLRs, and the crystal structures of some of these PAMP-TLR complexes have been solved.4 DAMPs and PAMPs are among the numerous signaling molecules that activate the innate immune system, protecting damaged tissue from infection, and participating in the repair of burn-injured skin. Nonetheless, TLR signaling pathways also contribute to tissue-damaging inflammation.
Discussion
The need for anti-scar treatments
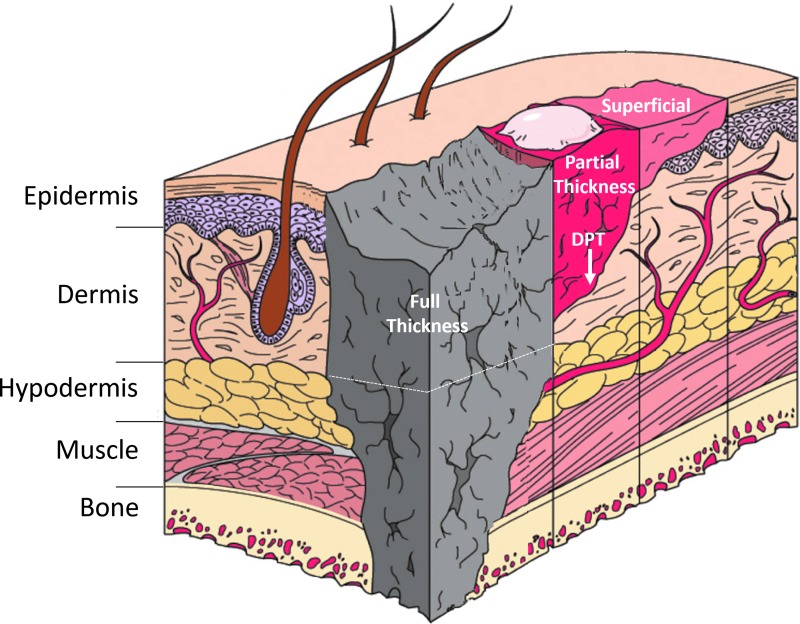
Deep partial-thickness burn wounds destroy blood vessels and skin appendages (Fig. 1). These wounds typically heal in 3–8 weeks with severe scars that can be raised, red, hard, with abnormal sensations, contraction, severe functional impairment, psychological morbidity, and costly long-term healthcare.5 Unlike full-thickness burns that require eschar excision and grafting, deep partial-thickness burn wounds retain some dermal elements that provide regenerative capacity but contribute to hypertrophic scarring. Hypertrophic scars develop in more than half of deep partial-thickness burn wounds, typically after prolonged inflammation, and once formed, treatments are only minimally effective.6 Therefore, treatments are needed that promote regenerative healing before these scars form. TLR signaling pathways are among the candidate molecular targets for such treatments. Hitting these targets can limit inflammation and fibrosis, but risks infection and inhibited healing.
Figure 1.
Depths of cutaneous burn injuries. Shown are superficial, partial-thickness, deep partial-thickness (DPT), and full-thickness (to hypodermis, shown by dotted line, or beyond) cutaneous burn injuries.
Outlines of TLR signaling
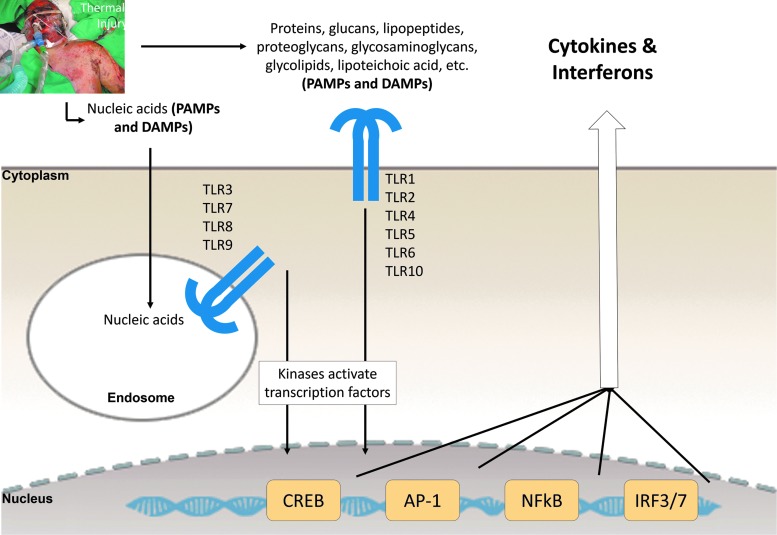
TLR signaling stimulated by DAMPs and PAMPs produces a cytokine-rich milieu for clearing necrotic debris and infection and setting the stage for angiogenesis and granulation tissue formation. TLR signaling activates the expression of many molecules, including cytokines and adhesion molecules that promote leukocyte recruitment and activation.7 There are 13 TLRs in mice and 10 in man. Of the 10 human TLRs, 6 of them are located on the cell surface (TLR1, 2, 4, 5, 6, and 10) where they bind a diversity of molecule types, whereas TLR3, 7, 8, and 9 are in endosomes and sense nucleic acids. Ligand binding to TLRs activates the transcription factors CREB, AP-1, NFκB, IRF3, and IRF7 (Fig. 2).
Figure 2.
Outlines of DAMP- and PAMP-TLR signaling resulting in the production of cytokines and interferons. For the details of these outlines, see O'Neill et al.4 (image modified from4). DAMPs and PAMPs activate similar receptors and converge on similar signaling pathways. The transcription factor–activating kinases are prominent drug targets. Image of thermally injured patient was provided by Rodney K. Chan. DAMP, damage-associated molecular pattern; PAMP, pathogen-associated molecular pattern; TLR, toll-like receptor.
The DAMPs that activate TLRs and other pattern-recognition receptors can be categorized into: (1) proteins secreted through a nonclassical secretion mechanism involving secretory lysosomes—for example, high mobility group box (HMGB)1 and galectin-3; (2) molecules released by necrotic cells—for example, S100 proteins, HMGB1, IL-1α, galectin-3, HSP60, HSP70, HSP72, histones, and nucleic acids; and (3) extracellular matrix molecules—for example, hyaluronan, heparin sulfate, fibronectin, and degraded matrix constituents. Numerous DAMPs have been identified. Theoretically, every molecule that normally resides inside cells and is extruded or is part of the extracellular matrix and is disrupted by tissue damage may potentially function as a DAMP; and hydrophobic surfaces in general have been proposed to act as DAMPs.8 Interestingly, the unique proteins detected in human plasma after trauma mostly reside inside cells.9
PAMPs in burn wounds come from pathogens and skin microbiota that enter the dermis through the breached epidermal barrier. In normal skin, bacterial counts have been quantified using quantitative real-time polymerase chain reaction (16S rRNA gene) to be 1,000,000; 50,000; and 10,000 per square centimeter for the lower epidermal layers (punch biopsies), the intermediate layers (scrapes), and the epidermal surface (swabs), respectively,10 and the bacteria were of diverse species.11 Therefore, once the epidermis is disrupted by burn, these bacterial PAMPs likely activate TLR signaling, possibly most strongly in the microenvironment surrounding hair follicles where bacteria are concentrated.12 With time as the epidermal barrier remains disrupted, ambient external pathogens have opportunity to infiltrate the wound.
TLRs are expressed by circulating leukocytes and a number of cells in the skin, including keratinocytes, Langerhans cells, T and B cells, mast cells, endothelial cells, myofibroblasts, and primary fibroblasts that can release cytokines.13
Burn injury progression
Over the first 2 days after a partial-thickness burn injury, the damaged tissue can expand. This “burn wound progression” is thought to result from progressive ischemia due to thrombosed vessels, increased capillary permeability, hypoperfusion, and oxidative damage, which are exacerbated by locally released signaling molecules from extracellular matrix and ruptured necrotic cells (i.e., DAMPs and PAMPs) that activate surviving proximal cells to produce inflammatory mediators.14 In addition, burn progression likely involves ischemia/reperfusion (I/R) injury because burned-tissue blood flow on the day of injury fluctuated repetitively (Laser Doppler Imaging) in parallel to changes in base deficit.15 In other types of tissue damage involving I/R injury, the importance of TLR signaling, including HMGB1-TLR signaling, has been demonstrated in a large number of in vivo studies (see Ref.16 Tables 1 and 2). In peri-burn tissue during injury progression, necrotic cells released HMGB1 from chromatin into the cytoplasm and extracellular space.17 Extracellular HMGB1 under the highly oxidizing conditions of burns is expected to be in the C23-C45 disulfide form, which activates TLR4 to induce cytokines.18 In addition, oxidized lipoproteins can stimulate TLR2 and TLR4, resulting in inflammation,19 suggesting that oxidized macromolecules in burn tissue may activate this inflammatory pathway.
Although HMGB1-TLR signaling has not been studied in burn-injured tissue per se, in an I/R hepatic injury model (mouse), HMGB1-neutralizing antibody decreased injury and, conversely, administration of recombinant HMGB1 worsened it, but only in TLR4-competent mice, suggesting that HMGB1-TLR4 signaling can mediate tissue damage under I/R redox conditions, as occurred during wound progression.20 Furthermore, when HMGB1 was knocked out specifically in hepatic epithelial cells, there was a profound reduction of infiltrating neutrophils and inflammatory-gene expression,21 suggesting that HMGB1 may be generally required for recruiting neutrophils to necrotic tissue where they may amplify tissue injury.
Burn eschar contributes to inflammatory signaling and scarring
The burn eschar likely contributes to inflammatory signaling and scarring at least partially by acting as a reservoir of DAMPs and PAMPs. Patients with >40% total body surface area (TBSA) burns whose eschars were excised early showed reductions in circulating cytokines, hypermetabolism, and mortality.22 In addition, in a mouse study that compared early (day 1) and late (day 8) excision of 8%-TBSA full-thickness burns (without grafting), early excision of the eschar prevented the extreme inflammation and immune dysfunction occurring after late excision (analyzed on day 2 and 6 post eschar excision) that is associated with susceptibility to infection, organ dysfunction, and scarring.23 In porcine models, early eschar excision accelerated reepithelialization and reduced scarring compared with no excision or delayed excision.24,25 These results suggest that the longer the necrotic eschar remains in situ, the more the inflammation and scarring, which may result at least partially from continuously-released DAMPs and PAMPs within the eschar.
Water loss from breached and immature epidermis signals inflammation through S100A12-TLR4
Evaporative water loss from breached epidermis increases sodium concentration, which can increase inflammation and scarring.26 An increase in sodium concentration of 10% mimics the increase in sodium concentration due to water loss from injured epidermis. Keratinocytes exposed to 10% higher sodium or cultured stratified keratinocytes exposed to reduced hydration secreted increased amounts of the TLR4-activating protein S100A12 (and expressed increased transcripts for COX-2, IL-1β, and IL-8). In addition, reduced hydration caused a sixfold increase in α-smooth muscle actin (SMA) transcripts of fibroblasts in keratinocyte–fibroblast cocultures, which was abolished by RNA interference (RNAi) knockdown of S100A12 in the keratinocytes before coculture. Furthermore, recombinant S100A12 activated fibroblasts alone in culture, and this activation was diminished by specific antagonists of TLR4 or receptor for advanced glycation end product (RAGE), which additively inhibited the fibroblast activation. Moreover, intradermal delivery of recombinant S100A12 to rabbit-ear excision wounds resulted in hypertrophic scar.26
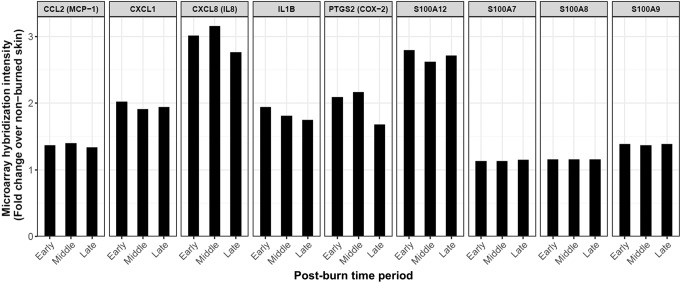
In burn wounds, water loss also likely upregulates the secretion of S100A12 that activates TLR4, which upregulates inflammatory mediators, activating fibroblasts to increase scarring. Water loss stays elevated long after reepithelialization of cutaneous wounds in general; for example, split-thickness skin-graft donor sites completed reepithelialization by postwound day 14, but water loss from the immature epidermis remained elevated for 200–400 days.27 In addition, S100A12 was highly expressed in hypertrophic scar,28 which occurs commonly after deep partial-thickness burns. And, S100A12 expression was elevated from postburn day 0–17 in the margins of partial-thickness burn wounds in a clinical study29 (Fig. 3).
Figure 3.
Elevated gene expression in the margins of clinical partial-thickness burn wounds. These data were obtained from a clinical study by Greco and Nanney and colleagues that used the Affymetrix U133 plus 2.0 GeneChip™29 to evaluate global gene expression in the wound edge of burns at postburn time periods: Early (0–3 days), Middle (4–7 days), and Late (7–17 days). The significantly elevated genes graphed were extracted from Gene Expression Omnibus accession record GSE8056 (using R69). The genes shown were upregulated in cultured keratinocytes in response to high sodium, dependent on the activity of the sodium channel Nax (scn7a).28
These studies suggest that pharmacological targeting of pathways downstream of water loss could potentially improve scarring, and they also support the use of occlusive water-impermeable barriers (e.g., silicone sheets) applied after reepithelialization to reduce scarring (as discussed26). In addition, topical treatments that reduce signaling resulting from water loss before reepithelialization might possibly benefit scar outcome, consistent with the benefits of moist wound healing.
PAMP-TLR signaling in burn wounds
Although infections impair healing, low-level bacterial colonization of wounds can sometimes help healing, depending on the level of colonization and the wound type.13 The normal inflammation at the edges of full-thickness incision wounds was reduced in Tlr3−/− mice, suggesting that double-stranded RNA (dsRNA) from damaged cells normally activates TLR3 signaling. However, Staphylococcus epidermidis lipoteichoic acid (LTA) applied to the wounds inhibited the increase in IL-6 and tumor necrosis factor (TNF)-α at the wound edge of wild-type but not Tlr3−/− mice.30 In vitro, in primary human keratinocytes, S. epidermidis LTA activated TLR2 signaling that inhibited dsRNA-TLR3 signaling. Mechanistically, LTA-TLR2 signaling induced TRAF1, which inhibits TRIF, an adaptor protein required for TLR3 signaling. Thus, S. epidermidis appears to balance the normal inflammatory dsRNA-TLR3 signaling during healing, and this mechanism might possibly function in the microenvironment of the wound edge where local inflammation may be downregulated when keratinocytes touch S. epidermidis.
Although LTA-TLR2 signaling inhibited dsRNA-TLR3 inflammatory signaling of keratinocytes in vitro and inhibited cytokine production at the incision wound edge, LTA-TLR2 signaling conversely stimulated cytokine production by other cell types in vitro: macrophages and dendritic and endothelial cells.30 Thus, the activation of TLR2 signaling by skin flora might benefit or impair wound healing depending on the wound locale.
Deep partial-thickness burn wounds, compared to incision wounds, have a large surface area where LTA-TLR2 signaling might be inflammatory. At the wound perimeter and at appendage stubs,31 such as hair follicles where bacteria are concentrated,12 LTA-TLR2 signaling might be predominantly anti-inflammatory—hypothetically, to protect sites of regenerative growth.
Such speculative beneficial effects of skin microbiota, however, might be eliminated by anti-microbial standard-of-care treatments, such as silver sulfadiazine cream, possibly explaining at least partially why this treatment has both retarded healing in several studies and increased hypertrophic scarring (rabbit ear excisional wound model).32
The importance of PAMP-TLR signaling in noninfected wounds has also been suggested by studies of germ-free mice. Never exposed to bacterial products, these mice expressed much less TRAF1 in their skin,30 as expected since they lack Staphylococcal LTA to stimulate TLR2 and increase TRAF1 (possibly to balance dsRNA-TLR3 inflammatory signaling). However, despite lacking this anti-inflammatory mechanism, the wounds of germ-free mice had fewer neutrophils, more mast cells, more macrophages expressing healing genes, increased angiogenesis, accelerated healing, and reduced scar—which were reversed when germ-free mice were “conventionalized” by receiving the conventional-mouse microbiota.33 Thus, the improved wound healing of germ-free mice might result from their complete absence of PAMP signaling in wounds and/or to an alternatively configured immune system resulting from the lifetime absence of microbiota.
Conversely, bacterial colonization benefited wound healing in some studies (as described in13), suggesting that heightened inflammation may aid in clearing necrotic debris and increasing blood flow. But these studies used rodents that are million-fold more resistant to endotoxin or bacterial loads compared to humans.9 In human partial-thickness burn wounds that already have high inflammation, bacterial colonization may not be beneficial.
Large burns disrupt homeostasis systemically
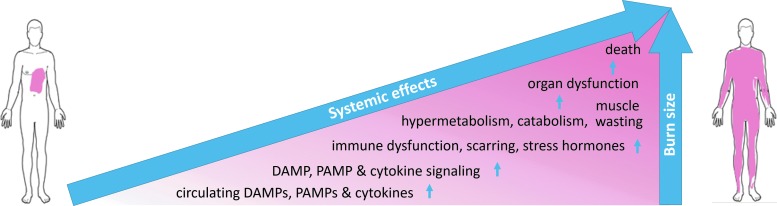
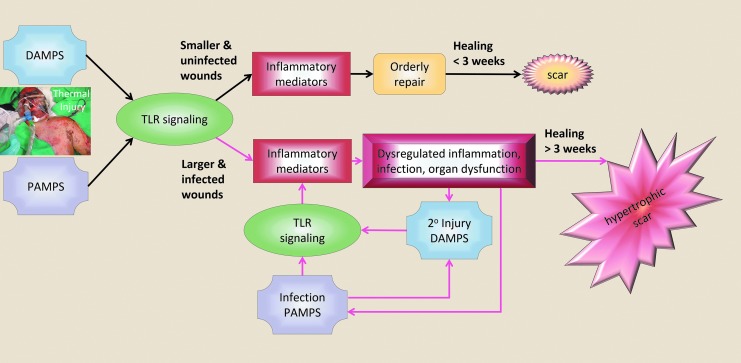
As with other traumatic injury, large burn injury, ∼20% TBSA and greater, can hyperactivate inflammatory cascades that can result in systemic cytokine storm with a paralyzed immune system that can progress to multiorgan dysfunction syndrome (MODS) in the most severe cases.34 The inflammatory cascade is initiated in the wound as signals, including DAMPs, ignite local inflammation. The inflammation, as well as damaged and clogged blood vessels, cause local edema, increased hydrodynamic pressure, I/R injury, and redox imbalance, which contribute to local spreading of tissue damage (burn injury progression) and an immune response that can spillover into the circulation to result in system-wide capillary leak, edema, and the release of large amounts of oxygen and nitrogen radical species. Stress hormones surge, up to more than 10-fold baseline, and can persist together with hypermetabolism and catabolism for up to and beyond 2 years after severe burn injury.35 Circulating cytokine levels are altered before the metabolic abnormalities, and larger burns produce greater and more persistent perturbations in circulating inflammatory mediators, immune functions, and stress hormones, as well as more severe catabolism and hypermetabolism to compensate for the evaporative water and heat loss due to the denuded skin.22 The systemic effects of large burns include increased susceptibility to infection, organ damage and dysfunction, disrupted healing, hypertrophic scarring, and at the extreme end, MODS and mortality (Figs. 4 and 5).
Figure 4.
Correlation between burn size and systemic effects. The relationship is not necessarily linear.
Figure 5.
Disruption of systemic homeostasis following burn injury. Larger, deeper, and infected wounds produce uncontrolled inflammation with dysregulated systemic inflammation, susceptibility to infection, organ damage and dysfunction, impaired healing, and high risk for hypertrophic scar (red arrows).
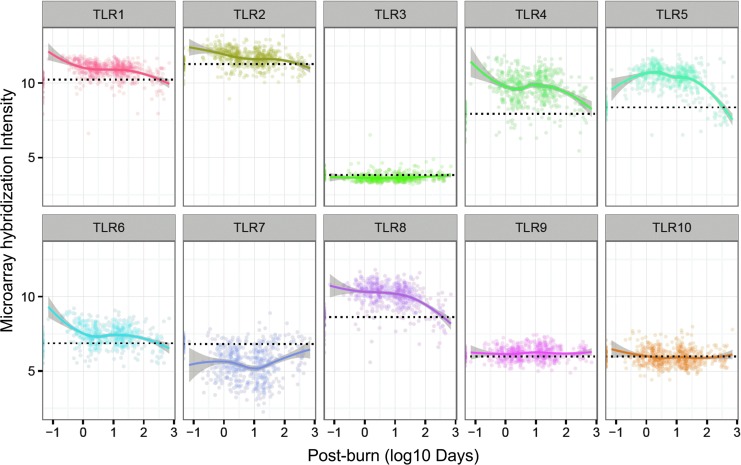
In a large study of >20% TBSA burns, white blood cell (WBC) gene expression was drastically changed (∼80% of genes), referred to as a “genomic storm,” which showed simultaneous upregulation of innate immunity and compensatory anti-inflammatory responses, together with downregulation of adaptive immunity.9 Among the upregulated innate immunity genes were 8 of the 10 human TLRs (Fig. 6), with the two downregulated TLRs being the endosome-localized TLR3 and TLR7 that recognize dsRNA and single-stranded RNA, respectively.9
Figure 6.
TLR gene expression in WBCs of severely burned patients (n = 244) over postburn time and healthy subjects (n = 35). Blood was sampled from patients with severe burns (>20% total body surface area; admitted within 96 h of injury; The Inflammation and the Host Response to Injury Large-Scale Collaborative Research Program).9 Gene expression was analyzed using the Affymetrix U133 plus 2.0 GeneChip. These data were extracted from Gene Expression Omnibus GSE37069. The healthy subject expression values are plotted along the y axis with their mean indicated by a dotted line. The burn-patient TLR expression values were fitted using locally estimated scatterplot smoothing regression, with the 95% confidence interval shown as gray shading. Interestingly, TLRs that heterodimerize (TLR1/TLR2 and TLR4/TLR5) were expressed similarly in WBCs over postburn time. In addition, TLR9 and TLR10 were expressed similarly after burn injury, and both are known to be expressed predominantly in human B cells and to be upregulated with similar kinetics after B cell activation, including activation by CpG DNA.70 WBC, white blood cell.
DAMPs/cytokines at early postburn times
Extensive WBC gene expression changes following severe burns9 likely result at least, in part, from DAMPs elevated in circulation. In a mouse model of ∼30% TBSA full-thickness scald injury, mitochondrial DNA (mtDNA) was elevated threefold in plasma at 3 h postburn, and it remained significantly elevated up to 4 days before returning to baseline by day 10.36 In mice with 25% TBSA full-thickness burn, DAMPs (cytochrome C, HMGB1, fibronectin, and hyaluronan) were elevated in plasma as early as 3 h postburn at the same time that TNF-α, IL-6, and IL-10 were elevated, but these cytokines stayed elevated longer, until 24 h postburn.37 However, by day 3, all the DAMPs and cytokines dropped to baseline, except hyaluronan which remained elevated out to the final time point on day 7.
In a prospective clinical study, cell-free nuclear DNA (nDNA) was elevated ∼10-fold (2,685 genome-equivalents/mL) in the plasma of flame/flash burn patients and ∼2-fold in the plasma of scald burn patients several hours after injury; and in the scald burn cohort, the circulating nDNA quantity correlated with the burn TBSA and the number of operations needed.38 In addition, using a direct rapid fluorometric technique, cell-free DNA at patient admission was elevated 5-fold and correlated with the degree of burn and TBSA.39 Both nDNA and mtDNA can activate TLRs, and condensed complexes of these self-DNAs with proteins such as histones, HMGB1, and LL-37 can protect them from nucleases and may be crucial for their delivery to endosomes and TLR9 (discussed in40).41
In addition, another DAMP, decorin, was elevated in patient serum for 2 weeks after burn injury, correlated with TBSA, and predicted hypertrophic scar better than burn size.2 Decorin is a small leucine-rich proteoglycan that can activate TLR4 (and downstream p38, extracellular signal–regulated kinase [ERK], and NF-κB pathways) and lead to increased secretion of TNF-α, pro-IL-1β, and leukocyte chemoattractants.2
DAMPs from mitochondria, in addition to mtDNA, appear to contribute to systemic and local wound inflammation since they activate neutrophils. In vitro, these DAMPs (supernatants of mitochondrial sonicates), but not purified mtDNA, activated neutrophils, as well as their p38 and ERK1/2 MAPK signaling,42 and inhibition of these TLR-downstream kinases by small molecules blocked the neutrophil activation.43 In addition, mitochondrial DAMPs administered intravenously to rats, in an amount equivalent to 5% of their liver, caused marked injury to lung tissue.42
Overall, the results suggest that DAMPs released from traumatized tissue activate circulating leukocytes, as well as various cell types in peri-burn tissue, to produce excessive amounts of inflammatory mediators that contribute to organ damage. In addition, the paralyzed leukocyte function in the state of genomic storm9 undoubtedly contributes to infection susceptibility and perturbed healing.44
Altered immune functions postburn
Elevated circulating DAMPs and cytokines in burns have been associated with altered functions of immune cells, consistent with the heightened innate immunity and suppressed adaptive immunity shown by the leukocyte genomic storm data. For example, leukocyte responses to TLR agonists were altered in mice at 3–7 days postburn (25% TBSA full thickness): ex vivo leukocytes stimulated with the DAMPs zymosan (TLR2 agonist) or lipopolysaccharide (LPS) (TLR4 agonist) were primed to produce more cytokines.45 In addition, dendritic cells were dysfunctional following burn injury (25% TBSA, mouse model): in the early days postburn, ex vivo treatment with the TLR9 ligand, unmethylated CpG oligodeoxynucleotide, caused splenic conventional dendritic cells to produce a cytokine profile that was anti-inflammatory and could not activate CD4+ T cells to produce Th1 and Th17 cytokines, while plasmacytoid dendritic cells showed impaired ability to secrete pro-inflammatory cytokines and activate T cell proliferation, and both of these defects were associated with low levels of transcripts of TLR9 and several key molecules of the TLR signaling pathway.46 Furthermore, from 1 to 7 days following burn, spleen cells were primed to produce greater amounts of pro-inflammatory cytokines after ex vivo exposure to TLR2 and TLR4 ligands; and burn-injured mice challenged with LPS expressed higher levels of inflammatory cytokines in the lung, liver, spleen, and plasma, primarily due to dendritic cells and macrophages, as judged by intracellular cytokine staining.47 Moreover, increased TLR2 or TLR4 signaling in Kupffer cells has been suggested to be a source of elevated circulating cytokines in burned mice. Kupffer cells isolated from burned rats (30% full thickness) at 24 h postburn and exposed to HMGB1 ex vivo produced more TNF-α and IL-1β proteins than Kupffer cells isolated from sham-burned animals.48 In addition, in these cells, burn injury enhanced HMGB1-induced activation of p38 MAPK, JNK, and NF-κB, and preincubation of the cells with antibody to TLR2 or TLR4 reduced this activation, as well as cytokine production.
These studies suggest that burn injury, within a day, causes priming of the responsiveness of immune cells—in circulation and residing in lymphoid tissues and organs, including lung and liver—to produce greater quantities of inflammatory mediators in response to activation of TLRs by DAMPs, resulting in altered immune system functions.
Organ dysfunction in severe burns
Larger and deeper burns are at greater risk for MODS and death, and patients with MODS have elevated inflammatory markers.49 A role for TLR4 in postburn systemic inflammation and organ damage has been indicated. First, in a mouse burn model (25%-TBSA full thickness), adhesion of leukocytes to mesenteric venules (i.e., distal to the burn wound) at 30 min postburn was lower in Tlr4−/− mice (C3H.HeJ) as was microvascular leakage at 1–3 h postburn.50 Second, endothelial cell monolayer cultures exposed to burn plasma became permeable, which was attenuated by small interfering RNA (siRNA) to TLR4. Overall, the data suggest that in systemic inflammation after trauma, including burns, TLR-4 plays a role, which in addition does not involve LPS, as LPS-resistant Cd14−/− mice showed the wild-type level of inflammation in response to trauma.50 Furthermore, at 48 h postburn in a rat model of 30% TBSA (no fluid resuscitation), secondary tissue damage was indicated by elevated TNF-α and IL-1β in serum, as well as elevated HMGB1 mRNA and protein in lung, liver, and kidney.51
Altered healing following severe burn injury
In a mouse model of regenerative wound healing in which punch holes in the ear regenerate tissue (MRL/MpJ), a dorsal full-thickness 15% TBSA burn caused an increase in inflammatory mediators in serum, lung, and the earhole wound remote from the burn. In addition, the burn injury caused the earhole wounds to fail to undergo regenerative healing; instead, the wounds were infiltrated with inflammatory cells, ulcerated, and necrotic.52
TLR signaling in hypertrophic scar fibroblasts
Consistent with prolonged inflammation being a known factor contributing to hypertrophic scarring, cultured fibroblasts from hypertrophic scars of burn-injured patients, versus normal skin fibroblasts from the same patients, showed upregulated TLRs and greater expression of cytokines in response to TLR activation.53 The hypertrophic scar fibroblasts had upregulated mRNA for all 10 TLRs and MyD88 (a TLR adaptor), as well as upregulated inflammatory-mediator proteins (prostaglandin E2, IL-6, IL-8, and monocyte chemoattractant protein [MCP]-1). In response to LPS stimulation, the hypertrophic scar fibroblasts produced more MyD88, IL-6, IL-8, and MCP-1 mRNA and protein, but siRNA knockdown of MyD88 decreased these. Thus, hypertrophic scar contains fibroblasts primed for inflammatory reactions, producing greater amounts of cytokines that attract leukocytes which produce pro-fibrotic growth factors such as TGF-β and MCP-1, which stimulate fibroblasts to produce excessive extracellular matrix.53 TLRs of cells of healing wounds with roles in organ fibrosis have been recently reviewed.54
Therapeutic targeting of TLR signaling
Reviewed elsewhere are strategies for reducing burn wound progression,55 dampening cutaneous wound inflammation for improving healing,56 and treating diseases (metabolic) driven by DAMPs57; in this study, we review therapeutic strategies that can potentially ameliorate burn wound progression, systemic inflammation, and hypertrophic scarring through targeting of TLR pathways. Targets of TLR signaling pathways span from the DAMPs and PAMPs that trigger TLRs and extend to downstream kinases that activate the transcription factors driving the expression of inflammatory mediators (Fig. 2).
Within hours after burn injury, topical application of p38 MAPK inhibitors can reduce inflammation, possibly reducing burn wound progression, organ damage, and scarring. For example, in a rat model of partial-thickness burn (30% TBSA), a topical p38 MAPK inhibitor (SB202190) applied immediately after the burn injury reduced dermal inflammatory cytokines, neutrophil infiltration, microvascular damage, and hair follicle cell apoptosis at 6–24 h postburn.58 In addition, in a full-thickness burn (30% TBSA) model in mouse, topical p38 MAPK inhibitors applied 4 h postburn reduced cytokines in the dermis and circulating leukocytes.59 Thus, p38 inhibitor treatment could penetrate the full-thickness burn eschar and be effective within a 4 h window, which suggests the feasibility of using early topical treatments to suppress inflammation that can damage tissue locally and systemically. However, these studies did not evaluate end points past 24 h.
Pointing to the potential efficacy of p38 MAPK inhibitors to reduce scarring, MAPK inhibition reduced fibroblast contraction in vitro (fibroblast-populated collagen-lattice contraction assay) and reduced wound contraction when applied topically to excision wounds, immediately and daily for 10 days, in a rat model.60 Similar results were obtained in red Duroc pig (as described61). In addition, in a preliminary study in Duroc pig, topical p38 inhibitor attenuated inflammation and improved healing of deep partial-thickness excision and burn wounds; importantly, infection of the wounds, which received neither antibiotic nor dressing, when followed out to 20 weeks, was absent.62
Further suggesting a role of p38 MAPK in hypertrophic scarring, a prospective-cohort genome-wide association study of postburn hypertrophic scarring, which tested MAPK-pathway gene single nucleotide polymorphisms for association with the four Vancouver Scar Scale variables in a joint regression model, found a rare missense variant (1.5% minor allele frequency) in the gene for PTPN5, an inhibitor of MAPK, which was associated with decreased severity of postburn hypertrophic scar (p = 1.3 × 10−6), although this result awaits confirmation in an independent clinical cohort.61
Topical MAPK inhibition also protected against severe burn-induced organ damage in a rat model of 30% TBSA partial-thickness burn.63 Topical application of p38 MAPK inhibitor to the burn, immediately and at 8 and 16 h, ameliorated cardiac function deficits at 24 h. This protective effect did not appear to be due to systemic absorption of the inhibitor because p38 MAPK activation in cardiac tissue was not changed. In addition, in vitro, burn-injured skin homogenates or serum added to cardiomyocytes impaired their contractility, but not when the homogenates or serum came from rats whose burns were treated with the topical p38 MAPK inhibitor. Thus, these results suggest that local treatment of burns can reduce systemic mediators of organ dysfunction.
Therapeutic targeting of the signaling involved in water loss–induced inflammation involving TLR4 has shown effectiveness in proof-of-concept studies in a hypertrophic scar model (rabbit ear excision wounds). The water loss–induced increase in sodium concentration is sensed and signaled through two sodium channels, and blocking these channels—Nax by RNAi and ENaC by amiloride—after reepithelialization, reduced scar formation, as did blockade of more downstream molecules, including TLR4 (using TAK-242), RAGE, p38α, COX-2, and IL-1.64 These findings are likely relevant to burn wounds that have high water loss for long times after reepithelialization.
HMGB1 is a candidate therapeutic target for mitigating burn wound sequela. HMGB1 was released from necrotic cells of peri-burn tissue during burn injury progression, likely in the form with C23–C45 disulfide that activates TLR4. HMGB1 can be inhibited by glycyrrhizin—a triterpene glycoconjugate derived from licorice root (Glycyrrhiza glabra) that has been used in Japan for decades to treat patients with hepatitis B and C (up to 140 mg/day).65 Glycyrrhizin directly binds HMGB1 (Kd ∼150 μM), partially explaining its anti-inflammatory properties.65 During diverse types of acute inflammation, glycyrrhizin treatment reduced the activation of downstream molecules (NF-κB, STAT3, and the MAPKs, JNK, p38, and ERK).66
The potent, well-tolerated synthetic TLR4 antagonist Eritoran is a small molecule that has mitigated diverse types of I/R injury67 and therefore may mitigate I/R injury in burn wounds. In addition, antibody to HMGB1 reduced hepatic I/R injury, and knocking out HMGB1 specifically in hepatic epithelial cells profoundly reduced numbers of infiltrating neutrophils and inflammatory gene expression.21 Thus, blocking HMGB1 or its TLR4 receptor may benefit burn wound outcomes.
Suggesting that inhibiting inflammation early after burn can be beneficial, in a clinical study, a monoclonal antibody against ICAM-168 administered intravenously at 6, 12, and 24 h postburn inhibited immune cell extravasation, which was associated with a threefold increase in the number of patients that healed within 21 days. However, scar at 6 months evaluated by laser Doppler and Vancouver scale was not different, possibly because only 40% of the 110 patients could be evaluated.
Collectively, these studies suggest that targeting TLR-signaling pathways to reduce inflammation has potential to improve burn wound outcomes.
Summary
Deep partial-thickness burn wounds that heal over long times with prolonged inflammation typically result in hypertrophic scars. To prevent these scars from forming, treatments are needed that can be applied to wounds early in the repair process to promote regenerative healing. Treatments that target TLR signaling pathways can limit inflammation and fibrosis and can potentially act at multiple stages of wound repair to protect against scarring. In addition, blocking TLR signaling pathways can potentially mitigate systemic inflammation/MODS.
TLR signaling appears to be involved in burn wound progression since from peri-burn tissue, HMGB1 was released from chromatin into the cytoplasm and extracellular space17 on the day of injury where it might activate TLR4 to promote tissue-damaging inflammation. Second, I/R injury occurs during burn wound progression, and TLR signaling is known to be involved in I/R injury of other organs. In addition, oxidative damage is high in burn tissue, and oxidized lipoproteins can stimulate TLR signaling. In addition to HMGB1, other TLR ligands, such as nucleic acids, are released from burn-injured tissue, contributing to inflammatory signaling and burn wound progression.
DAMPs are present in circulation within a few hours after burn injury and correlate with TBSA (e.g., cell-free nuclear and mtDNA, decorin, etc.). At the same time, cytokines in circulation are elevated and leukocyte gene expression changes are pervasive (i.e., genomic storm), indicating simultaneous upregulation of innate immunity and compensatory anti-inflammatory responses, as well as downregulation of adaptive immunity. Consistent with the upregulated innate immunity genes (including 8 of 10 TLRs), immune cells in circulation and residing in lymphoid tissues and organs, including lung and liver, are primed to produce greater quantities of inflammatory mediators in response to TLR activation by DAMPs. In addition, adaptive immune functions are paralyzed. Overall, the results suggest that DAMPs released from traumatized tissue activate various peri-burn skin cells, circulating leukocytes, and cells of distant organs to produce excessive amounts of inflammatory mediators that inhibit healing, activate fibroblasts, increase susceptibility to infection, and contribute to organ dysfunction and risk for mortality.
Immediately after epithelial barrier rupture, water loss increases. Evaporating water leaves a higher sodium concentration, which can increase keratinocyte secretion of S100A12, which can activate fibroblasts (increased α-SMA) through TLR4 and RAGE. In addition, intradermally injected S100A12 increased hypertrophic scar (rabbit ear wound model). Inhibiting molecules in this pathway (sodium channels, TLR4, RAGE, p38 MAPK, COX-2, and IL-1) starting after reepithelialization resulted in reduced hypertrophic scarring in the rabbit ear model of excision wounds; although not a burn wound, these findings are likely relevant to burn wounds in which, like other traumatic skin wounds, water loss remains high for months after reepithelialization.
Take-Home Messages.
• The extent of the systemic sequela of burn injuries, including circulating cytokines, immunosuppression, impaired wound healing, hypertrophic scarring, susceptibility to infection, hypermetabolism, catabolism, muscle wasting, and mortality, is directly related to burn size.
• Deep partial-thickness burn wounds that heal over long times with prolonged inflammation typically result in hypertrophic scars.
• Molecules from burn-injured tissues (DAMPs) are released and interact locally and systemically with TLRs of cells to signal danger. Molecules from microbes, called PAMPs, also activate TLRs. Cells having these receptors overactivated produce excessive amounts of inflammatory mediators that are damaging to organs, stimulate fibrosis, and inhibit healing, which contribute to hypertrophic scarring.
• Inhibitors of TLR signaling pathways are available that can be tested in animal models for efficacy in inhibiting the adverse effects of TLR activation.
• For evaluating treatments to mitigate scarring after burn injury, animal models are used (rodent, rabbit, and pig), and each has strengths and weaknesses; therefore, testing of treatments should be started in clinical studies as soon as their safety is established.
Therapeutic targeting of HMGB1 or TLR4 has mitigated inflammation and injury in several animal models of organ injury (not burn). In addition, topical p38 MAPK inhibitor applied immediately to burn wounds in a mouse model reduced dermal and circulating cytokines, infiltrating neutrophils, and early tissue damage; and when applied up to 4 h postinjury in a rat model, reduced dermal and circulating cytokines. Although these studies did not evaluate long-term outcomes, other preliminary studies suggest that topical p38 MAPK inhibition can inhibit wound contraction in mice and may have had beneficial effects on healing in a pig model. In addition, topical p38 MAPK inhibition protected against severe burn-induced cardiac function deficits at 24 hrs.
To promote regenerative healing, reduce hypertrophic scarring, and mitigate systemic sequela of burn injury, many drugs that target TLR signaling pathway molecules are available for testing. Some of these have demonstrated proof-of-concept efficacy in mitigating short-term end points, mostly in small-animal models. The development of treatments for improving final wound outcome requires evaluating wounds several months after injury, optimally in pigs and then in proof-of-concept clinical models.
Abbreviations and Acronyms
- AP-1
activator protein 1
- COX
cyclooxygenase
- CREB
cAMP response element binding
- DAMP
damage-associated molecular pattern
- DPT
deep partial-thickness
- dsRNA
double-stranded RNA
- ENaC
epithelial sodium channel
- ERK
extracellular signal–regulated kinase
- HMGB
high mobility group box
- HSP
heat shock protein
- I/R
ischemia/reperfusion
- ICAM-1
intercellular adhesion molecule
- IL
interleukin
- IRF
interferon regulatory transcription factor
- JNK
c-Jun N-Terminal Kinase
- LPS
lipopolysaccharide
- LTA
lipoteichoic acid
- MAPK
mitogen-activated protein kinase
- MCP
monocyte chemoattractant protein
- MODS
multiorgan dysfunction syndrome
- mtDNA
mitochondrial DNA
- MyD88
myeloid differentiation primary response gene 88
- Nax
sodium voltage-gated channel type 7
- nDNA
nuclear DNA
- NFκB
nuclear factor kappa-light-chain-enhancer of activated B cells
- PAMP
pathogen-associated molecular pattern
- PTPN5
protein tyrosine phosphatase, non-receptor type 5
- RAGE
receptor for advanced glycation end product
- RNAi
RNA interference
- S100A12
S100 calcium binding protein A12
- siRNA
small interfering RNA
- SIRS
systemic inflammatory response syndrome
- SMA
smooth muscle actin
- TBSA
total body surface area
- TLR
toll-like receptor
- TNF
tumor necrosis factor
- TRAF
TNF receptor-associated factor
- TRIF
TIR domain–containing adapter inducing interferon-β
- WBC
white blood cell
Acknowledgments and Funding Sources
P.D. is supported by the U.S. Army Research Laboratory and the U.S. Army Research Office under contract W911NF1310376 to The Geneva Foundation. K.P.L. is an employee of the U.S. Government. The work presented is part of his official duties and is supported, in part, by the U.S. Army Medical Research and Materiel Command, Combat Casualty Care Research Directorate, Clinical Rehabilitative Medicine Research Directorate, and the Naval Medical Research Center's Advanced Medical Development program (MIPR N3239815MHX040). Title 17 U.S.C. §105 provides that “Copyright protection under this title is not available for any work of the United States Government.” Title 17 U.S.C. §101 defined U.S. Government work as work by a military service member or employee of the U.S. Government as part of that person's official duties. The opinions or assertions contained herein are the private views of the authors and are not to be construed as official or as reflecting the views of the Department of the Army or the Department of Defense.
Author Disclosure and Ghostwriting
The authors do not have any commercial or financial conflicts of interest to declare. This article was written exclusively by the authors without ghostwriters.
About the Authors
Peter D'Arpa is an investigator with The Geneva Foundation, a nonprofit organization that supports and advances medical research and education within the U.S. military. He received his PhD in Pharmacology from The George Washington University and completed postdoctoral fellowships in Cell Biology and Biological Chemistry at Johns Hopkins University School of Medicine. His research focuses on improving health within the U.S. military. Kai P. Leung, PhD, serves as the Director of Science for Dental and Craniofacial Trauma Research and Tissue Regeneration Directorate at the U.S. Army Institute of Surgical Research, Fort Sam Houston, TX. He obtained his PhD in Microbiology from the University of Hawaii at Manoa. He continued postdoctoral studies on neutrophil and macrophage function at the National Jewish Center for Immunology and Respiratory Medicine and the University of Florida. He is a member of the Wound Healing Society. His research focuses on wound infection and repair.
References
- 1.Tredget EE, Levi B, Donelan MB. Biology and principles of scar management and burn reconstruction. Surg Clin North Am 2014;94:793–815 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Kwan PO, Ding J, Tredget EE. Serum Decorin, IL-1beta, and TGF-beta predict hypertrophic scarring postburn. J Burn Care Res 2016;37:356–366 [DOI] [PubMed] [Google Scholar]
- 3.Allgower M, Schoenenberger GA, Sparkes BG. Pernicious effectors in burns. Burns 2008;34 Suppl 1:S1–S55 [DOI] [PubMed] [Google Scholar]
- 4.O'Neill LA, Golenbock D, Bowie AG. The history of Toll-like receptors—redefining innate immunity. Nat Rev Immunol 2013;13:453–460 [DOI] [PubMed] [Google Scholar]
- 5.Penn JW, Grobbelaar AO, Rolfe KJ. The role of the TGF-beta family in wound healing, burns and scarring: a review. Int J Burns Trauma 2012;2:18–28 [PMC free article] [PubMed] [Google Scholar]
- 6.Finnerty CC, Jeschke MG, Branski LK, Barret JP, Dziewulski P, Herndon DN. Hypertrophic scarring: the greatest unmet challenge after burn injury. Lancet 2016;388:1427–1436 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Newton K, Dixit VM. Signaling in innate immunity and inflammation. Cold Spring Harb Perspect Biol 2012;4:pii: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Bianchi ME. DAMPs, PAMPs and alarmins: all we need to know about danger. J Leukocyte Biol 2007;81:1–5 [DOI] [PubMed] [Google Scholar]
- 9.Tompkins RG. Genomics of injury: the Glue Grant experience. J Trauma Acute Care Surg 2015;78:671–686 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Cundell AM. Microbial ecology of the human skin. Microb Ecol 2016; [Epub ahead of print]; DOI: 10.1007/800248-016-0789-6 [DOI] [PubMed] [Google Scholar]
- 11.Grice EA, Kong HH, Renaud G, et al. . A diversity profile of the human skin microbiota. Genome Res 2008;18:1043–1050 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Lange-Asschenfeldt B, Marenbach D, Lang C, et al. . Distribution of bacteria in the epidermal layers and hair follicles of the human skin. Skin Pharmacol Physiol 2011;24:305–311 [DOI] [PubMed] [Google Scholar]
- 13.Scales BS, Huffnagle GB. The microbiome in wound repair and tissue fibrosis. J Pathol 2013;229:323–331 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Shupp JW, Nasabzadeh TJ, Rosenthal DS, Jordan MH, Fidler P, Jeng JC. A review of the local pathophysiologic bases of burn wound progression. J Burn Care Res 2010;31:849–873 [DOI] [PubMed] [Google Scholar]
- 15.Jaskille AD, Jeng JC, Sokolich JC, Lunsford P, Jordan MH. Repetitive ischemia-reperfusion injury: a plausible mechanism for documented clinical burn-depth progression after thermal injury. J Burn Care Res 2007;28:13–20 [DOI] [PubMed] [Google Scholar]
- 16.Gill R, Tsung A, Billiar T. Linking oxidative stress to inflammation: toll-like receptors. Free Radic Biol Med 2010;48:1121–1132 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Lanier ST, McClain SA, Lin F, Singer AJ, Clark RA. Spatiotemporal progression of cell death in the zone of ischemia surrounding burns. Wound Repair Regen 2011;19:622–632 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Venereau E, Schiraldi M, Uguccioni M, Bianchi ME. HMGB1 and leukocyte migration during trauma and sterile inflammation. Mol Immunol 2013;55:76–82 [DOI] [PubMed] [Google Scholar]
- 19.Chavez-Sanchez L, Madrid-Miller A, Chavez-Rueda K, Legorreta-Haquet MV, Tesoro-Cruz E, Blanco-Favela F. Activation of TLR2 and TLR4 by minimally modified low-density lipoprotein in human macrophages and monocytes triggers the inflammatory response. Hum Immunol 2010;71:737–744 [DOI] [PubMed] [Google Scholar]
- 20.Tsung A, Sahai R, Tanaka H, et al. . The nuclear factor HMGB1 mediates hepatic injury after murine liver ischemia-reperfusion. J Exp Med 2005;201:1135–1143 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Huebener P, Pradere JP, Hernandez C, et al. . The HMGB1/RAGE axis triggers neutrophil-mediated injury amplification following necrosis. J Clin Invest 2015;125:539–550 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Williams FN, Herndon DN, Jeschke MG. The hypermetabolic response to burn injury and interventions to modify this response. Clin Plast Surg 2009;36:583–596 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Fear VS, Poh WP, Valvis S, et al. . Timing of excision after a non-severe burn has a significant impact on the subsequent immune response in a murine model. Burns 2016;42:815–824 [DOI] [PubMed] [Google Scholar]
- 24.Davis SC, Mertz PM, Bilevich ED, Cazzaniga AL, Eaglstein WH. Early debridement of second-degree burn wounds enhances the rate of epithelization—an animal model to evaluate burn wound therapies. J Burn Care Rehabil 1996;17:558–561 [DOI] [PubMed] [Google Scholar]
- 25.Singer AJ, Toussaint J, Chung WT, McClain SA, Raut V, Rosenberg L. Early versus delayed excision and grafting of full-thickness burns in a porcine model: a randomized study. Plast Reconstr Surg 2016;137:972e–979e [DOI] [PubMed] [Google Scholar]
- 26.Zhao J, Zhong A, Friedrich EE, et al. . S100A12 induced in the epidermis by reduced hydration activates dermal fibroblasts and causes dermal fibrosis. J Invest Dermatol 2017;137:650–659 [DOI] [PubMed] [Google Scholar]
- 27.Suetake T, Sasai S, Zhen YX, Ohi T, Tagami H. Functional analyses of the stratum corneum in scars. Sequential studies after injury and comparison among keloids, hypertrophic scars, and atrophic scars. Arch Dermatol 1996;132:1453–1458 [PubMed] [Google Scholar]
- 28.Xu W, Hong SJ, Zhong A, et al. . Sodium channel Nax is a regulator in epithelial sodium homeostasis. Sci Transl Med 2015;7:312ra177. [DOI] [PubMed] [Google Scholar]
- 29.Greco JA, 3rd, Pollins AC, Boone BE, Levy SE, Nanney LB. A microarray analysis of temporal gene expression profiles in thermally injured human skin. Burns 2010;36:192–204 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Lai Y, Di Nardo A, Nakatsuji T, et al. . Commensal bacteria regulate Toll-like receptor 3-dependent inflammation after skin injury. Nat Med 2009;15:1377–1382 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Rittie L, Sachs DL, Orringer JS, Voorhees JJ, Fisher GJ. Eccrine sweat glands are major contributors to reepithelialization of human wounds. Am J Pathol 2013;182:163–171 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Qian LW, Fourcaudot AB, Leung KP. Silver sulfadiazine retards wound healing and increases hypertrophic scarring in a rabbit ear excisional wound model. J Burn Care Res 2017;38:e418–e422 [DOI] [PubMed] [Google Scholar]
- 33.Canesso MC, Vieira AT, Castro TB, et al. . Skin wound healing is accelerated and scarless in the absence of commensal microbiota. J Immunol 2014;193:5171–5180 [DOI] [PubMed] [Google Scholar]
- 34.Jeschke MG, Chinkes DL, Finnerty CC, et al. . Pathophysiologic response to severe burn injury. Ann Surg 2008;248:387–401 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Porter C, Tompkins RG, Finnerty CC, Sidossis LS, Suman OE, Herndon DN. The metabolic stress response to burn trauma: current understanding and therapies. Lancet 2016;388:1417–1426 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Szczesny B, Brunyanszki A, Ahmad A, et al. . Time-dependent and organ-specific changes in mitochondrial function, mitochondrial dna integrity, oxidative stress and mononuclear cell infiltration in a mouse model of burn injury. PLoS One 2015;10:e0143730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Rani M, Nicholson SE, Zhang Q, Schwacha MG. Damage-associated molecular patterns (DAMPs) released after burn are associated with inflammation and monocyte activation. Burns 2017;43:297–303 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Chiu Tor W, Young R, Chan Lisa YS, Burd A, Lo Dennis YM. Plasma cell-free DNA as an indicator of severity of injury in burn patients. Clin Chem Lab Med 2006;44:13–17 [DOI] [PubMed] [Google Scholar]
- 39.Shoham Y, Krieger Y, Perry ZH, et al. . Admission cell free DNA as a prognostic factor in burns: quantification by use of a direct rapid fluorometric technique. Biomed Res Int 2014;2014:306580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Celhar T, Magalhaes R, Fairhurst AM. TLR7 and TLR9 in SLE: when sensing self goes wrong. Immunol Res 2012;53:58–77 [DOI] [PubMed] [Google Scholar]
- 41.Holl EK, Shumansky KL, Borst LB, et al. . Scavenging nucleic acid debris to combat autoimmunity and infectious disease. Proc Natl Acad Sci U S A 2016;113:9728–9733 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Zhang Q, Raoof M, Chen Y, et al. . Circulating mitochondrial DAMPs cause inflammatory responses to injury. Nature 2010;464:104–107 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Hazeldine J, Hampson P, Opoku FA, Foster M, Lord JM. N-Formyl peptides drive mitochondrial damage associated molecular pattern induced neutrophil activation through ERK1/2 and P38 MAP kinase signalling pathways. Injury 2015;46:975–984 [DOI] [PubMed] [Google Scholar]
- 44.Leliefeld PH, Wessels CM, Leenen LP, Koenderman L, Pillay J. The role of neutrophils in immune dysfunction during severe inflammation. Crit Care 2016;20:73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Schwacha MG, Zhang Q, Rani M, Craig T, Oppeltz RF. Burn enhances toll-like receptor induced responses by circulating leukocytes. Int J Clin Exp Med 2012;5:136–144 [PMC free article] [PubMed] [Google Scholar]
- 46.Shen H, de Almeida PE, Kang KH, Yao P, Chan CW. Burn injury triggered dysfunction in dendritic cell response to TLR9 activation and resulted in skewed T cell functions. PLoS One 2012;7:e50238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Paterson HM, Murphy TJ, Purcell EJ, et al. . Injury primes the innate immune system for enhanced Toll-like receptor reactivity. J Immunol 2003;171:1473–1483 [DOI] [PubMed] [Google Scholar]
- 48.Chen XL, Sun L, Guo F, et al. . High-mobility group box-1 induces proinflammatory cytokines production of Kupffer cells through TLRs-dependent signaling pathway after burn injury. PLoS One 2012;7:e50668. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Kraft R, Herndon DN, Finnerty CC, Shahrokhi S, Jeschke MG. Occurrence of multiorgan dysfunction in pediatric burn patients: incidence and clinical outcome. Ann Surg 2014;259:381–387 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Breslin JW, Wu MH, Guo M, Reynoso R, Yuan SY. Toll-like receptor 4 contributes to microvascular inflammation and barrier dysfunction in thermal injury. Shock 2008;29:349–355 [DOI] [PubMed] [Google Scholar]
- 51.Shen L, Cui Z, Lin Y, Wang S, Zheng D, Tan Q. Anti-inflammative effect of glycyrrhizin on rat thermal injury via inhibition of high-mobility group box 1 protein. Burns 2015;41:372–378 [DOI] [PubMed] [Google Scholar]
- 52.Zins SR, Amare MF, Anam K, Elster EA, Davis TA. Wound trauma mediated inflammatory signaling attenuates a tissue regenerative response in MRL/MpJ mice. J Inflamm (Lond) 2010;7:25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Wang J, Hori K, Ding J, et al. . Toll-like receptors expressed by dermal fibroblasts contribute to hypertrophic scarring. J Cell Physiol 2011;226:1265–1273 [DOI] [PubMed] [Google Scholar]
- 54.Huebener P, Schwabe RF. Regulation of wound healing and organ fibrosis by toll-like receptors. Biochim Biophys Acta 2013;1832:1005–1017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Salibian AA, Rosario AT, Severo Lde A, et al. . Current concepts on burn wound conversion-A review of recent advances in understanding the secondary progressions of burns. Burns: journal of the International Society for Burn Injuries 2016;42:1025–1035 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Rohl J, Zaharia A, Rudolph M, Murray RZ. The role of inflammation in cutaneous repair. Wound Pract Res 2015;23:8–15 [Google Scholar]
- 57.Garcia-Martinez I, Shaker ME, Mehal WZ. Therapeutic opportunities in damage-associated molecular pattern-driven metabolic diseases. Antioxid Redox Signal 2015;23:1305–1315 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Ipaktchi K, Mattar A, Niederbichler AD, et al. . Topical p38MAPK inhibition reduces dermal inflammation and epithelial apoptosis in burn wounds. Shock 2006;26:201–209 [DOI] [PubMed] [Google Scholar]
- 59.Carter D, Warsen A, Mandell K, Cuschieri J, Maier RV, Arbabi S. Delayed topical p38 MAPK inhibition attenuates full-thickness burn wound inflammatory signaling. J Burn Care Res 2014;35:e83–e92 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Hirano S, Rees RS, Gilmont RR. MAP kinase pathways involving hsp27 regulate fibroblast-mediated wound contraction. J Surg Res 2002;102:77–84 [DOI] [PubMed] [Google Scholar]
- 61.Sood RF, Arbabi S, Honari S, Gibran NS. Missense variant in MAPK inactivator PTPN5 is associated with decreased severity of post-burn hypertrophic scarring. PLoS One 2016;11:e0149206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Arbabi S, Warsen A, Shubin N, et al. . Wound infection after attenuating a key inflammatory signaling pathway. Surg Infect 2014;15:O27 [Google Scholar]
- 63.Hoesel LM, Mattar AF, Arbabi S, et al. . Local wound p38 MAPK inhibition attenuates burn-induced cardiac dysfunction. Surgery 2009;146:775–785; discussion 785–776 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Hu X, Wang H, Liu J, et al. : The role of ERK and JNK signaling in connective tissue growth factor induced extracellular matrix protein production and scar formation. Arch Dermatol Res 2013;305:433–445 [DOI] [PubMed] [Google Scholar]
- 65.Mollica L, De Marchis F, Spitaleri A, et al. . Glycyrrhizin binds to high-mobility group box 1 protein and inhibits its cytokine activities. Chem Biol 2007;14:431–441 [DOI] [PubMed] [Google Scholar]
- 66.Yoshida S, Lee JO, Nakamura K, et al. . Effect of glycyrrhizin on pseudomonal skin infections in human-mouse chimeras. PLoS One 2014;9:e83747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.McDonald KA, Huang H, Tohme S, et al. . Toll-like receptor 4 (TLR4) antagonist eritoran tetrasodium attenuates liver ischemia and reperfusion injury through inhibition of high-mobility group box protein B1 (HMGB1) signaling. Mol Med 2015;20:639–648 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Mileski WJ, Burkhart D, Hunt JL, et al. . Clinical effects of inhibiting leukocyte adhesion with monoclonal antibody to intercellular adhesion molecule-1 (enlimomab) in the treatment of partial-thickness burn injury. J Trauma 2003;54:950–958 [DOI] [PubMed] [Google Scholar]
- 69.Team RC. R: A Language and Environment for Statistical Computing. Vienna, Austria: R Foundation for Statistical Computing, 2016 [Google Scholar]
- 70.Bourke E, Bosisio D, Golay J, Polentarutti N, Mantovani A. The toll-like receptor repertoire of human B lymphocytes: inducible and selective expression of TLR9 and TLR10 in normal and transformed cells. Blood 2003;102:956–963 [DOI] [PubMed] [Google Scholar]