Abstract
Background:
Intracranial abscess remains a significant health-care problem. Its causes, diagnosis, treatment, and outcome are changing.
Aim:
This paper reviewed the demography, examined new trends, and compared outcomes with different treatment options.
Methodology:
Retrospective analysis of intracranial abscesses managed at Memfys Hospital, Enugu (2004–2014) and University of Nigeria Teaching Hospital (2009–2014). Patients were followed up for at least 6 months. All patients had neuroimaging before intervention. Microscopy and culture were performed for the specimens. Intravenous antibiotics were given for 2 weeks before conversion to oral.
Results:
Seventy-nine parenchymal abscesses (eight cases per year) were managed. Peak age was the second decade of life. Previous head injury (21.5%) and meningitis (16.5%) were the most common predisposing factors. The frontal lobe was most common anatomical location (32%). Only 24% had positive culture result. Three cases were fungal infections. Seventy percent of patients managed with burr hole drainage and 37.5% of craniotomy made complete recovery. Overall, 58% of patients made complete recovery, whereas 19.0% died. Nine percent of cases died before definitive intervention. Among the 24% of patients that presented in coma, 47% died within 6 months. Most important factor influencing mortality was admission level of consciousness. Abscess recurred in 6% of cases.
Conclusion:
Intraparenchymal abscesses in Enugu were mostly solitary lesions resulting from poorly managed head injury and meningitis. Predisposition from otitis media and systemic diseases has reduced. The proportion of fungal organisms is increasing. A significant proportion of the patients present in coma. Burr hole and aspiration of abscess is less invasive and has very good outcome.
KEYWORDS: Changing pattern, outcome, parenchymal abscess, surgery
INTRODUCTION
Intracranial abscesses remain significant health-care problem worldwide, especially in developing countries.[1,2,3] The mortality and morbidity of brain abscesses range from 10% to 25%.[4,5,6] The pattern of the disease and its outcome however appear to be changing worldwide as influenced by factors such as improvements in the quality of diagnostic and therapeutic medical care, antibiotics that can penetrate the blood brain barrier as well as emerging diseases like the HIV/AIDS epidemic and other congenital and acquired causes of immune deficiency.[1,4,7,8] In developing countries like Nigeria, “over the counter” antibiotics abuse is common and this may also influence the pattern of brain abscesses in these environments. Different modalities of treatment are available including craniotomy, burr hole aspiration, stereotactic aspiration, and nonsurgical options. Opinions still differ on the best treatment modality and this is particularly relevant in resource poor countries where the availability of specialists and other resources for healthcare are challenging. Some studies have highlighted the usefulness of less invasive surgical options for brain abscess.[4,9] The aims of this paper are to review the current epidemiology of brain abscesses in Enugu, Nigeria and compare outcomes of the different treatment options.
METHODOLOGY
This is a collaborative retrospective analysis of intracranial abscesses managed at Memfys Hospital for Neurosurgery (MHN) and the University of Nigeria Teaching Hospital (UNTH) which were the two major neurosurgery centers serving Enugu State and its surrounding during the study. Similar management protocol for brain abscess is used in both hospitals since the both units have academic, training, and clinical collaboration. The data from MHN included 52 cases collected between 2004 and 2014, whereas the remaining 27 cases were managed at UNTH between 2009 and 2014. Case folders, radiology, and operative notes were used. All the patients were admitted following clinical evaluation and postcontrast-enhanced computed tomography (CT) scan. Magnetic resonance imaging (MRI) scan with postcontrast enhancement was also performed for sixteen patients. Following radiology diagnosis, those that needed surgery were worked up for surgery. Intravenous antibiotics were commenced for all patients on admission based on the suspected spectrum of organisms. The protocol was to administer intravenous ceftriaxone and metronidazole initially and subsequently modify based on microscopy and/or sensitivity results of specimens obtained at surgery whenever a positive result was available. Intravenous regimen was continued for a minimum of 2 weeks before conversion to oral medication which was continued for a minimum of 6 weeks. The duration of antibiotic administration was guided by clinical recovery, C-reactive protein, erythrocyte sedimentation rate, and neuroimaging investigation in patients that could afford repeat scans. The protocol for anticonvulsants in both units was to use either phenytoin or carbamazepine as prophylaxis which is tailed off after 3 months in the absence of seizure. Those that presented with seizures were continued on anticonvulsants for a minimum of 1 year before being tailed off the medication.
Following imaging investigation, patients were assigned treatment groups based on their clinical state as well as the size, location and multiplicity of the abscess to either surgery or nonoperative groups.[10] Those likely to benefit from surgery were either booked for a less invasive burr-hole or a formal craniotomy procedure. After surgery, unstable and unconscious patients were monitored in the Intensive Care Unit until stable. The outcome of management was assessed at discharge, 3 months and 6 months, respectively after surgery. Outcome was assessed clinically and with the 5-scale Glasgow outcome score (GOS).[11] Analysis of data was performed using descriptive statistics.
RESULTS
Demography
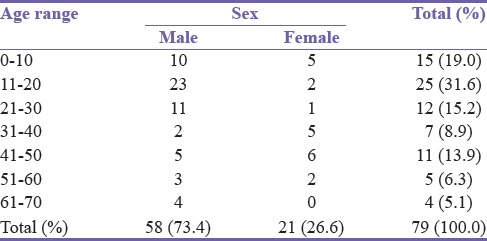
A total of 79 parenchymal abscesses representing eight cases per year presented for management. Male to female ratio was 2.8:1.0. The mean age was 25.6 years. Patients in the second decade of life were most susceptible [Table 1].
Table 1.
Age distribution
Risk factors and clinical presentation
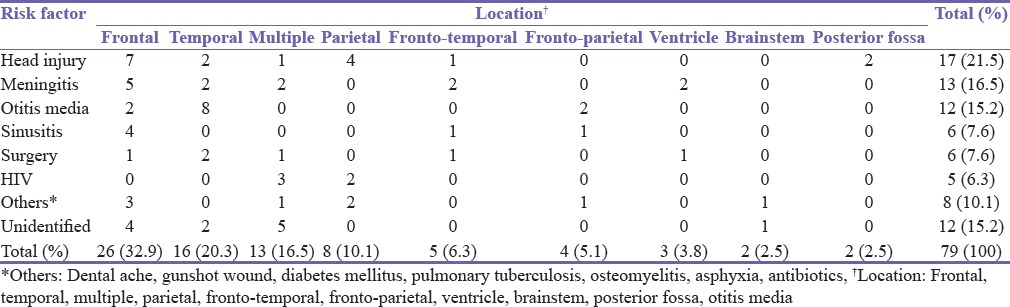
Seizure (54.4%), headache (48.1%) and extremity weakness (34.1%) were the predominant symptoms [Table 2]. However, 24.1% of cases presented in coma and of these, 47.3% died. Mortality was also high among patients that presented with seizure (16.3) [Table 2]. Frontal and temporal lobes were the most common anatomical locations. Previous head injury and meningitis were the most common predisposing factors and mostly associated with frontal lobe abscess. Chronic ear infection was particularly common in temporal lobe abscess. Neurosurgical procedures accounted for 7.6% of brain abscesses. Over 6% of the cases were seen in HIV positive patients [Table 3]. Only 19 (24%) of cases had positive culture result. Seven of these culture results were Staphylococcal organisms, six were Streptococci and three were coliform. Three cases cultured were fungal infections. Sixteen percent of cases had multiple abscesses. HIV associated infection was seen in 23.1% of the multiple abscess. There was no identified risk factor in about 15.2% of all cases of abscess and in 38.5% of the multiple brain abscesses.
Table 2.
Comparison of clinical presentation with outcome at 6 months (n=79)
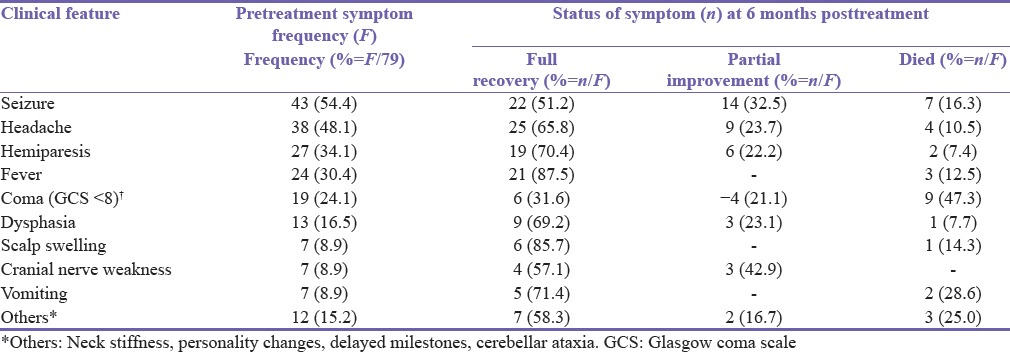
Table 3.
Analysis of the anatomical location of abscess with the identified risk factor
Clinical outcomes
Eleven patients were managed conservatively with antibiotics while 61 had surgery and antibiotics. Of the latter, 37 had burr hole evacuation and 24 had craniotomy. Another seven patients that would have benefited from surgery based on brain scan finding died before any intervention. Seventy percent of patients that had burr hole and drainage made complete recovery compared with 37.5% of craniotomy group. The burr hole group however had more mortality (16.2%) compared to the craniotomy group (8.3%). Overall, 63.8% of all the patients that presented made complete recovery, whereas 19.0% died either before definitive treatment or following treatment [Table 4]. There were no deaths in patients assigned to conservative management. The most important factors influencing mortality were the preadmission coma score and presentation with seizure [Table 1]. Of the patients who presented with seizures, 32.5% had persisting seizure at 6 months follow-up but this was not related to any specific modality of intervention, whereas 22% had residual hemiparesis [Table 2]. Recurrence of abscess was experienced in 6% of cases [Table 5].
Table 4.
Glasgow outcome score of intracerebral abscess at 6 months based on treatment group

Table 5.
Complications observed among the 79 cases managed (n=79)

DISCUSSION
In this study, most patients were in the second and third decades of life with a very striking male predominance. This is probably a reflection of the burden of poorly managed head injuries and meningitis[12] which are the most common predisposing factors for brain abscesses identified from this study. Studies from some developing countries have also observed an increasing trend of trauma related brain abscess.[4,6,13] Inadequate management of posttraumatic cerebrospinal fluid (CSF) leaks, chronic osteomyelitis complicating head injury, skull base fractures with air sinus breach, poor wound care of compound skull fractures may be the neglected pathways of brain infections following head injuries. Poorly treated head injuries and meningitis are closely correlated since posttraumatic or even spontaneous CSF leakage may predispose to either meningitis, encephalitis or brain abscess. Better management of head trauma may mitigate these problems.
The proportion of abscesses related to intracranial procedures was 7.6% in this study and is related to the extent of invasive brain procedures carried out in the centers. Postsurgery related abscess appears to be an emerging concern in neurosurgery.[14] The frequency of abscess from chronic suppurative otitis media and sinusitis seems to be reducing significantly in Enugu over the years, unlike findings from other countries.[15,16,17] This may be attributed to increasing number of ear, nose and throat specialists in addition to the unregulated practices that encourage abuse of antibiotics.
The frequency of intracranial abscesses from this study with an average of eight cases per year, seems to be increasing, relative to the frequency from previous studies in Nigeria in the 1970s.[9] Although this may be related to improved patient awareness of the specialty and availability of neuro-diagnostic services,[2,7] the changing trend in the risk factors identified in this study might have also contributed. Similar trend has been reported in neurosurgery centers of other developing countries with 9–15 cases/year.[15,16,17]
The frontal lobe was the most common location of abscesses. One of the patients in this study, who presented very late and died before intervention, was initially erroneously managed as a case of psychiatric illness. Seizure also appears to be prominent clinical manifestation in this study due to the epileptogenic nature of abscesses.[18] The need for neuroimaging in patients presenting with seizures has been argued.[19,20] Since there is no pathognomic clinical feature, the diagnosis of brain abscess should be primarily based on CT or MRI.[4,20]
About 16.5% of the patients had multiple lesions and as expected these were more common in immune-compromised patients. HIV associated infection was seen in 23.1% of multiple abscess. However, significant proportion of cases did not have any identifiable predisposing factor.
The major challenge in this study was with obtaining a positive microbial culture. The spectrum of organisms seen in brain abscess usually depends on the primary source of infection. However, apart from Gram-positive and Gram-negative organisms cultured in the earlier study in the 1970s,[9] fungal infections are emerging important organisms in the current study. The low positive yield of cultures from this study (24%) may be related to the peculiar local problems of late referral of cases, the challenge of anaerobe, atypical bacterial and fungal culture handling as well as abuse of antibiotics. The result of microscopy, culture and sensitivity tests should guide the proper choice of antibiotics so as to minimize abuse, side effects and acquired antibiotic resistance. There is also the need to investigate for fungal brain abscesses in the process of diagnosis.
The choice of treatment depends on many factors like the location of the brain abscess, size, and number of lesions. Majority of our cases were treated with burr hole, aspiration and adjuvant antibiotics which was less invasive, relatively safe and associated with a very good outcome. It is particularly beneficial in emergency settings and for very unstable patients who may not withstand the stress of long procedures like a craniotomy. This option is also very useful in deep seated abscess and selected cases of multiple abscesses. Again, in the deeply unconscious and fully conscious co-operating patients, this procedure can be carried out under local anesthesia. Although opinions still differ, this treatment modality would be very beneficial especially in resource poor countries where there may be dearth of neurosurgeons.[4,9] The result of this study suggests benefits with the use of the minimal procedure whenever possible compared to craniotomy and should be viewed as the first-line option for brain abscess management. Whatever option is adopted, the role of adjuvant antibiotic treatment remains important.
The Glasgow coma score on presentation and seizures were the main factors affecting outcome. The relationship between the level of consciousness and prognosis is also influenced by the anatomical location of abscess. For instance, coma state from a temporal lobe abscess is expected to have a worse outcome than an abscess with comparable reduction in the level of consciousness located within the frontal lobe.[6] Sadly, 24% of the patients in this study presented in deep coma. This was an indication that significant proportions of the patients did not access expert care early enough or there was significant delay in proper investigation of patients. The delay in presentation may also be related to the anatomical location of the lesion in noneloquent brain. Cases that have significant delay in presentation will further impact negatively on the overall outcome. The 9% of cases that died before definitive treatment may thus be a reflection of the burden of late presentation. A proportion of these patients may have been salvageable with improved patient awareness and better networking, inter-professional referral chain and collaboration amongst clinicians.
Overall, 70% of the patients had a good neurological recovery with GOS of at least four on a scale of five; whereas the mortality rate was 19%. Many studies have highlighted the determinants of outcome to depend on the neurological status of the patient at the point of admission.[3,4,5,16] The mortality can be improved if the local problems raised in this study would be addressed. However, the long-term morbidity may still be a significant concern. In this study, residual seizure disorder, nonspecific headache, and focal neurologic deficit were common long-term morbidity. Proper and timely management of patients may help to improve symptoms and overall outcome.
CONCLUSION
Intraparenchymal abscesses in Enugu are most commonly solitary lesions resulting from poorly managed head injury. There is marked male dominance. The frontal lobe is mostly involved. The risk from otitis media has reduced. Bacteria organisms are quite common but the proportion of fungal organisms is increasing. Seizure is the most common presenting feature and a significant proportion present in coma. Adequate treatment for patients with head injury should be emphasized. Burr hole and aspiration of abscess is less invasive and associated with very good outcome.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
REFERENCES
- 1.Donaldson G, Webster D, Crandon IW. Brain abscess at the University Hospital of the West Indies. West Indian Med J. 2000;49:212–5. [PubMed] [Google Scholar]
- 2.Levy RM. Brain abscess and subdural empyema. Curr Opin Neurol. 1994;7:223–8. doi: 10.1097/00019052-199406000-00008. [DOI] [PubMed] [Google Scholar]
- 3.Lu CH, Chang WN, Lin YC, Tsai NW, Liliang PC, Su TM, et al. Bacterial brain abscess: Microbiological features, epidemiological trends and therapeutic outcomes. QJM. 2002;95:501–9. doi: 10.1093/qjmed/95.8.501. [DOI] [PubMed] [Google Scholar]
- 4.Emejulu JK, Shokunbi MT, Malomo AO. Intracerebral abscesses: Outcome following management in the CT era. West Afr J Med. 2004;23:54–7. doi: 10.4314/wajm.v23i1.28083. [DOI] [PubMed] [Google Scholar]
- 5.Sharma R, Mohandas K, Cooke RP. Intracranial abscesses: Changes in epidemiology and management over five decades in Merseyside. Infection. 2009;37:39–43. doi: 10.1007/s15010-008-7359-x. [DOI] [PubMed] [Google Scholar]
- 6.Char G, West K, Jaggon J. Intracranial abscesses: Epidemiological trend over a 39-year period at the University of the West Indies. Internet J Third World Med. 2009;8:5. [Google Scholar]
- 7.Bensalem MK, Berger JR. HIV and the central nervous system. Compr Ther. 2002;28:23–33. doi: 10.1007/s12019-002-0039-3. [DOI] [PubMed] [Google Scholar]
- 8.Goodkin HP, Harper MB, Pomeroy SL. Intracerebral abscess in children: Historical trends at Children's Hospital Boston. Pediatrics. 2004;113:1765–70. doi: 10.1542/peds.113.6.1765. [DOI] [PubMed] [Google Scholar]
- 9.Ohaegbulam SC, Saddeqi NU. Experience with brain abscesses treated by simple aspiration. Surg Neurol. 1980;13:289–91. [PubMed] [Google Scholar]
- 10.Rosenblum ML, Mampalam TJ, Pons VG. Controversies in the management of brain abscesses. Clin Neurosurg. 1986;33:603–32. [PubMed] [Google Scholar]
- 11.Jennett B, Bond M. Assessment of outcome after severe brain damage. Lancet. 1975;1:480–4. doi: 10.1016/s0140-6736(75)92830-5. [DOI] [PubMed] [Google Scholar]
- 12.Emejulu JK, Ekweogwu C, Nottidge T. The burden of motorcycle-related neurotrauma in South-East Nigeria. J Clin Med Res. 2009;1:13–7. [Google Scholar]
- 13.Carpenter J, Stapleton S, Holliman R. Retrospective analysis of 49 cases of brain abscess and review of the literature. Eur J Clin Microbiol Infect Dis. 2007;26:1–11. doi: 10.1007/s10096-006-0236-6. [DOI] [PubMed] [Google Scholar]
- 14.Dashti SR, Baharvahdat H, Spetzler RF, Sauvageau E, Chang SW, Stiefel MF, et al. Operative intracranial infection following craniotomy. Neurosurg Focus. 2008;24:E10. doi: 10.3171/FOC/2008/24/6/E10. [DOI] [PubMed] [Google Scholar]
- 15.Menon S, Bharadwaj R, Chowdhary A, Kaundinya DV, Palande DA. Current epidemiology of intracranial abscesses: A prospective 5 year study. J Med Microbiol. 2008;57(Pt 10):1259–68. doi: 10.1099/jmm.0.47814-0. [DOI] [PubMed] [Google Scholar]
- 16.Prasad KN, Mishra AM, Gupta D, Husain N, Husain M, Gupta RK. Analysis of microbial etiology and mortality in patients with brain abscess. J Infect. 2006;53:221–7. doi: 10.1016/j.jinf.2005.12.002. [DOI] [PubMed] [Google Scholar]
- 17.Babu ML, Bhasin SK, Kanchan Pyogenic brain abscess and its management. JK Sci. 2002;4:21–3. [Google Scholar]
- 18.Okaro AO, Eze CU, Ohagwu CC. Computed tomography and magnetic resonance diagnosis of brain infections: A clinical study with laboratory correlates. Eur J Sci Res. 2010;39:457–61. [Google Scholar]
- 19.Ndubuisi CA, Mezue WC, Ohaegbulam SC, Chikani MC, Ekuma M, Onyia E. Neuroimaging findings in pediatric patients with seizure from an institution in Enugu. Niger J Clin Pract. 2016;19:121–7. doi: 10.4103/1119-3077.173712. [DOI] [PubMed] [Google Scholar]
- 20.Mezue WC, Ndubuisi CA, Chikani MC, Onyia E, Iroegbu L, Ohaegbulam SC. Epilepsy in primary intracranial tumors in a neurosurgical hospital in Enugu, South-East Nigeria. Niger J Clin Pract. 2015;18:681–6. doi: 10.4103/1119-3077.158980. [DOI] [PubMed] [Google Scholar]


