Abstract
Background:
Several authors have demonstrated regional and temporal changes in the demographics of urethral stricture and its management.
Objectives:
To assess the changes in the demographics of the patients and the evolution of the management of urethral stricture in this institution.
Subjects and Methods:
This is a retrospective study. The files of all the men who were diagnosed with urethral stricture from May 2006 to April 2016 were retrieved from the database of the records department of the hospital. The predictor variables assessed included age at presentation, occupation, etiology, presenting symptoms, stricture site, length of stricture, treatment method, year of treatment, complications of treatment, result of urine microscopy and sensitivity, comorbidities, and social habits of the patients. The outcome variables were the proportion of men in relation to the predictor variables and the test of correlation (P = 0.05 and below significant). Analysis was done using the Statistical Package for the Social Sciences (SPSS) version 21.
Results:
Forty-six patients were diagnosed as having urethral stricture within the study period. All were males. The mean age was 53.11 years (standard deviation 17.63852) with a range from 19 to 96. There were 4 (8.7%) students, 11 (23.9%) civil servants, 4 (8.7%) businessmen, 3 (6.5%) military men, and 24 (52.2%) others who were essentially artisans. Majority of them (68.9%) presented with lower urinary tract symptoms while Escherichia coli was the most commonly cultured organism from their urine (17.4%). The most common single etiology was urethritis (30.4%). From 2013 onward, there was an abrupt transition from conservative treatment using dilatation which dropped from 38.9% to 17.9%. More complex surgeries such as buccal mucosal graft urethroplasty for bulbar strictures and two-stage repair for penile strictures increased from 11.1% to 57.1%.
Conclusion:
Urethritis is still the most common single etiological factor in urethral stricture disease in this rural community. Artisans such as drivers and mechanics were the most commonly afflicted. There was an abrupt transition from the old conservative methods of treatment to complex urethroplasties within the study period.
KEYWORDS: Changes, demographics, treatment, urethral stricture
INTRODUCTION
The urethra measures about 16 to 22 cm in the adult males and 4 cm in the adult females.[1] In males, it is anatomically divided into posterior and anterior segments. The posterior segment, traditionally referred to as the posterior urethra, measures about 4 cm and traverses the prostate and pelvic membrane beginning at the bladder neck. The anterior urethra which is in continuity with the posterior includes the bulbar and penile urethra.[2] This subdivision is important for clinical reasons related to the treatment of urethral diseases.
The urethra has the dual purpose of being a conduit for urine and semen, both functions of which are a sine qua non for a good-quality life.[3] Narrowing of the urethra is a common cause of presentation at both the Urological Outpatient and the Accident and Emergency unit.[4] According to the World Health Organization consensus,[5] stricture refers to such disease of the anterior urethra. Following pelvic fracture, there may be associated distraction injury of the urethra, the ensuing gap of which is usually replaced by fibrous tissue (pelvic fracture urethral distraction defect). Other types of posterior urethral disease are referred to as stenosis. In the 1960s, gonococcal urethritis was the most common cause of urethral stricture until effective antibiotics became available. Currently, the causes of urethral stricture include trauma,[6] instrumentation, catheterization, transurethral resection of the prostate (TURP), open prostatectomy, posthypospadias repair, lichen sclerosis, and urethritis.[7]
Urethral stricture is one of the oldest known diseases of the urethra. It is rare in females as the female urethra is more capacious than the male counterpart. Therefore, the term “urethral stricture” often refers to the male urethra. The management begins with a good history. Affected men usually, in uncomplicated cases, present with lower urinary tract symptoms (LUTSs) while confirmation is with a retrograde urethrography,[8] sometimes in combination with micturating cystourethrography. The oldest known method of treatment is dilatation which is often palliative.[9] Over the years, the treatment has evolved[10] through urethrotomy and urethroplasty of various techniques. Often, the method used depends on the expertise of the urologist and the available tools.
This work was conducted in Irrua Specialist Teaching Hospital, a Federal establishment located in a semi-urban area of Edo state and subserving a population of about four million people. The aim was to assess the demographics and evolution of the management of urethral stricture in this institution.
SUBJECTS AND METHODS
This is a retrospective study. Patients were de-identified. The files of all the men who were diagnosed with urethral stricture from May 2006 to April 2016 were retrieved from the database of the records department of the hospital. Information extracted from the files included age at presentation, etiology, presenting symptoms, confirmatory investigation, number of strictures, length of stricture, complications, treatment method, year of treatment, complications of treatment, preoperative urine microscopy and sensitivity, comorbidity, and social habits of the patients. The main outcome variables assessed were the different methods of treatment used and their correlation with the year of treatment and the proportion of patients in relation to the predictor variables. In addition, the social habits of the men were correlated with the year of treatment. P < 0.05 was considered statistically significant. Information obtained was analyzed using the Statistical Package for Social Sciences (SPSS) Version 21 (SPSS Inc., Chicago, IL, USA).
RESULTS
A total of 46 patients with urethral stricture were analyzed within the study period. Eighteen patients were analyzed from 2006 to 2012 (6 years) while this increased to 28 over the period of 2013–2016 (4 years), indicating a remarkable increase in number and burden of urethral stricture.
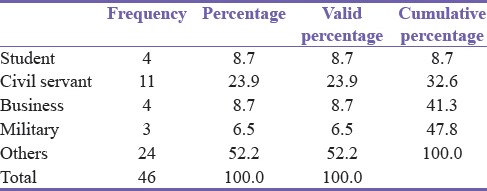
All were males. The mean age was 53.11 years (standard deviation 17.63852) with a range from 19 to 96. The occupation of the patients is shown in Table 1. The group “Others” included artisans such as drivers and mechanics and “Civil Servants” stood for those serving in or retired from white-collar jobs while “Military” represented all men in uniformed service.
Table 1.
Occupation of patients
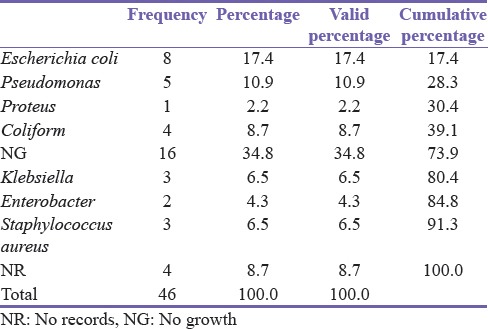
Of the 46 patients, 32 (69.6%) complained of LUTS, 9 (19.6%) had urinary retention while 2 (4.3%) had drainage of urine from the perineum (watering can perineum). The remaining 3 (6.5%) patients presented with uncommon complaints such as hematuria. Clinical findings on examination were urinary retention or suprapubic catheter in 32 (69.6%) patients, induration along the urethra in 5 (10.9%), and perineal fistula in 2 (4.3%). Table 2 shows the organisms cultured from the urine of the patients.
Table 2.
Bacteriology
Hypertension was the most common comorbidity found among these men. While it was present in 17 (37%) patients, it occurred in association with diabetes mellitus in 1 patient. Diabetes mellitus was present in 3 (6.5%) patients.
On the social side, 19 (41.3%) patients admitted to alcohol consumption while 27 (58.7%) did not. This did not have a statistically significant correlation with stricture etiology (P = 0.281) or the year at diagnosis (P = 0.381) though alcohol consumption was more common in men with posttraumatic and posturethritis strictures [Table 3]. Only 3 (6.5%) patients smoked cigarette [Figure 1].
Table 3.
Alcohol consumption and etiology cross-tabulation
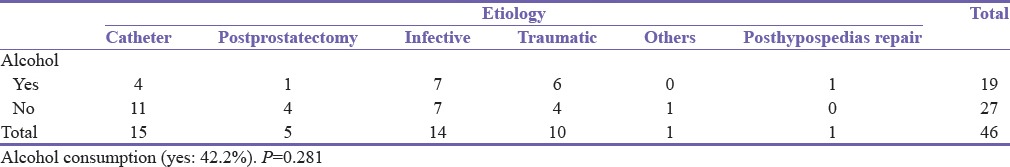
Figure 1.
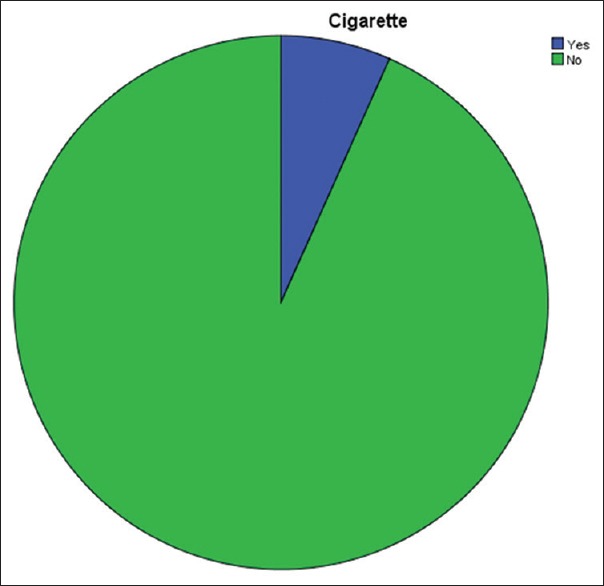
Cigarette smoking (yes: 6.7%)
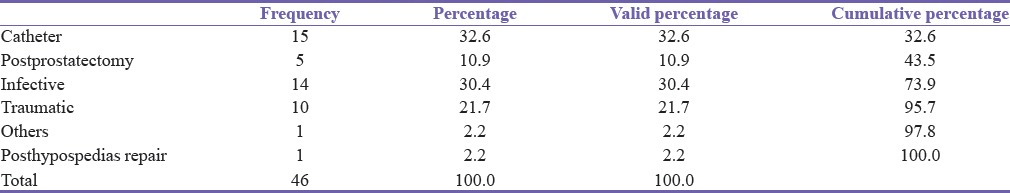
Of the 46 men, urethritis accounted for 14 (30.4%) while 15 (32.6%) were catheter associated (a dual etiology of inflammation or trauma), making urethritis the most common single etiology [Table 4].
Table 4.
Distribution of etiology
Bulbar urethral stricture was the most common in this study, accounting for 32 (69.6%) patients. Figure 2 shows distribution of stricture site, and Table 5 shows stricture site in relation to etiology.
Figure 2.
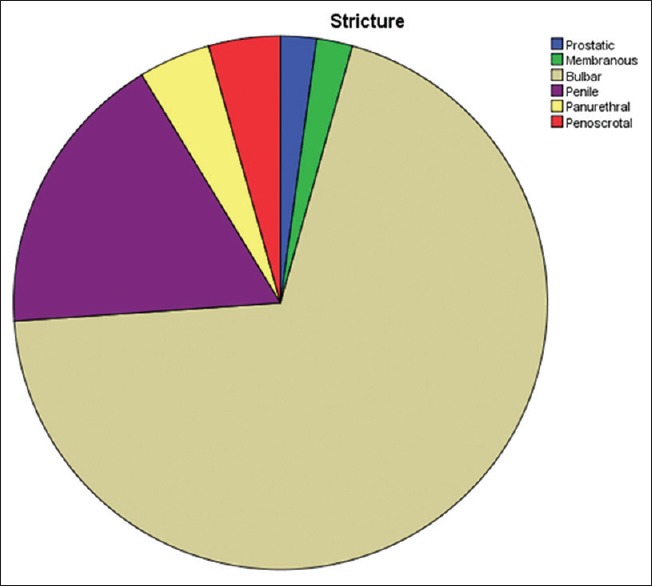
Distribution of stricture site
Table 5.
Stricture site in relation to etiology
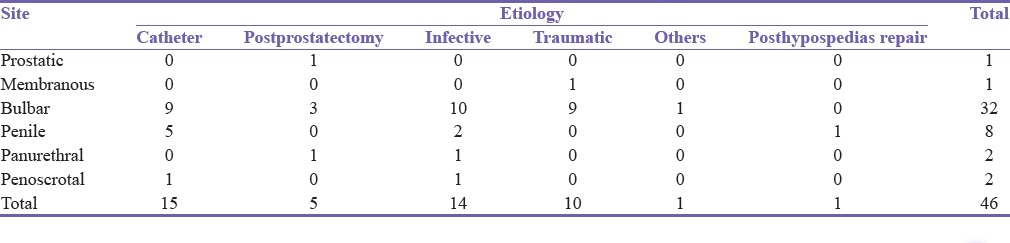
Urethritis was the most common cause of bulbar urethral stricture while penile strictures were most commonly associated with catheter usage. Before 2013, treatment modalities used were dilatation in 7 patients, anastomotic urethroplasty in 5, dilatation and subsequent anastomotic urethroplasty in 2, dilatation, anastomosis, and subsequent buccal mucosal graft (BMG) urethroplasty in 1, and two-stage urethroplasty in 1. From 2013 to 2016, treatment methods used were dilatation in 5 patients, two-stage urethroplasty in 2 [Figure 3a and b], dilatation and subsequent anastomotic urethroplasty in 1, dilatation and subsequent BMG urethroplasty for penile stricture in 1, dorsal onlay BMG urethroplasty for distal bulbar disease in 1, and ventral onlay BMG urethroplasty for proximal bulbar stricture in 12. One of the later had a pan urethral stricture, and a Johanson's two-stage urethroplasty was done for the penile segment. The others are with suprapubic catheter and awaiting urthroplasty. Two of the patients who had BMG urethroplasty for bulbar urethral stricture from 2013 to 2016 had surgical site infection with stricture recurrence in 1 (7.7%) patient. This method of treatment had a statistically significant correlation (P = 0.007) with the year of treatment.
Figure 3.
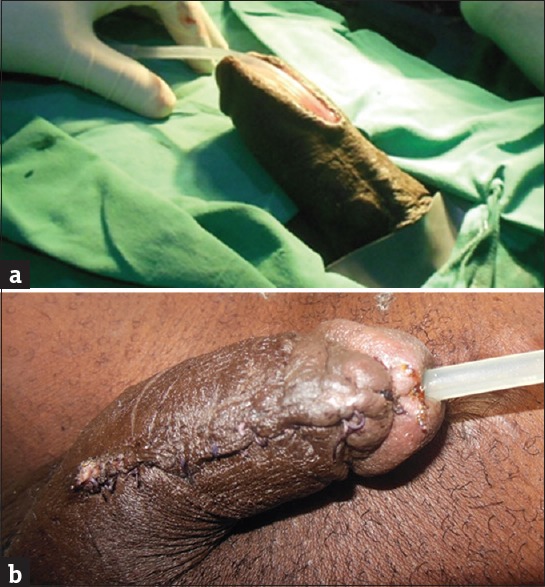
(a) A healthy urethral plate following urethrotomy. (b) Seventh day post second-stage urethroplasty
DISCUSSION
Stricture of the urethra results from narrowing of the urethral lumen due to spongiofibrosis.[11] The consequence of this is a loss of distensibility and compliance, leading to a reduction in the caliber of the urine stream. Urethral stricture disease is prevalent, and it is a common cause of presentation to the urologist worldwide.[12] Over the decades, there have been remarkable changes in the demographics of men with urethral stricture. For instance, in the 1960s, poorly treated gonococcal urethritis accounted for the majority of cases.[13] Currently, trauma, instrumentation, TURP, lichen sclerosis, posthypospadias repair, catheterization, and prostatectomy are common causes[2] while infection has taken the backseat at the global level.[14] There are however geographical variations,[15] and some poor resource communities still have high contribution to stricture etiology from infective urethritis. This is replicated in this study in which urethritis accounted for 14 (30.4%) of the strictures representing the highest single etiological factor. This is similar to the findings of Olajide et al.[16] in Oshogbo and Ibrahim et al.[11] in Maiduguri, Nigeria, and in contrast to Tijani et al.[17] in Lagos, the commercial capital of Nigeria where traumatic cause predominated.
The above regional differences in etiology, to a large extent, reflect the level of economic and social variations between these regions and in effect, the available health-care resources. In addition, the social awareness, economic well-being, genetic constitution, and lifestyle which contribute to road traffic crash, sexually transmitted diseases, genetic predisposition, and alcohol consumption to a large extent determine the etiology of urethral stricture. This accounts for the remarkable contribution of urethritis to the etiology of urethral stricture in this study as evidenced by the fact that 24 (52.2%) of the men with posturethritis stricture were artisans such as mechanics and commercial drivers. Alcohol consumption was admitted to by 42.2% of the 46 men, 50% of the men with posturethritis stricture, and 60% of those with posttraumatic stricture often from a road traffic crash or a fall astride injury. The role of alcohol consumption on road traffic crash is well known. These factors are immensely important in formulating policies and programs aimed at prevention as advocated by Omisanjo et al.[18]
Few studies are available on Medline on the bacteriology of urethral stricture, of which none was found to be from Nigeria. Most studies document Escherichia coli as the most common organism cultured from the urine of these men. For instance, in the study of Murshidi and Farah,[19] E. coli was the most common organism isolated, accounting for 66.7% of all community-acquired bacterial isolates, which according to him, is well below the findings of Brumfitt and Hamilton-Miller.[20] Urethral stricture was the predisposing diagnosis in 14 (15.4%) of the men with urinary tract infection in Murshidi's study. Although E. coli was the most common isolate in this study (17.4%), the proportion is far lower. While the bacteriology in this study was restricted to urethral stricture, the above authors assessed the predisposing factors to urinary tract infection. In addition to regional, social, and cultural factors,[21] this may explain the difference in the proportion of isolates in the different studies, though the organisms were essentially the same. This is important in surgical prophylaxis for urethroplasty.[22]
The prevalence of hypertension varies between regions and communities. However, what is common to all is that the prevalence and its complications increase with age. This is sometimes the reason for delay or postponement of surgery, and in some cases, settling for less invasive treatment as uncontrolled hypertension may be a cause of intra- and or post-operative hemorrhage. This may jeopardize the graft in substitution urethroplasty. The prevalence of 37% in this study is lower than the 41.6% documented for Niger Delta men of Nigeria by Suleiman et al.,[23] probably because of the younger age of the affected men. Hypertension may complicate obstructive nephropathy in long-standing urethral stricture. Proper care also requires that Diabetes mellitus, second to hypertension in this study, should be controlled before any form of surgery in these men.
The bulbar urethra was the most commonly afflicted by urethral stricture in this study, a finding which is similar to that of many authors.[24] Several anatomical explanations have been given to account for this. The double curve in the bulbar urethra slows urine flow in that segment, and in conjunction with the presence of the periurethral glands and abundant corpus spongiosum, infection is more easily established. The inferior position of the urethra relative to the pubis predisposes it to fall astride crush injury which was common in this study.
The treatment of urethral stricture has evolved rapidly over the years from conservative procedures such as dilatation to direct vision internal urethrotomy and urethroplasty with varying successes claimed.[3,4,25,26] In 2013, the unit established a protocol for the management of urethral stricture, part of which was to de-emphasize the step ladder approach and offer urethroplasty as first-line treatment where feasible. For bulbar urethral stricture, we offer buccal mucosa graft urethroplasty and Johanson's two-stage urethroplasty for penile strictures. The use of two-stage approach in penile urethral stricture was premised on the fact that the patients had preoperative adverse conditions such as urethral stone and diabetes mellitus with a poor urethral plate. The presence of infection therefore meant that a single-stage ventral urethrotomy, a dorsal BMG patch, and closure (Asopa),[27] the other option, was not feasible. The patient who presented with panurethral stricture after the protocol was offered a ventral onlay BMG urethroplasty for the bulbar segment and a two-stage Johanson for the penile stricture as advised by Santucci. The capacity for a single-stage BMG pan urethroplasty of Kulkarni and Barbagli[28] is currently not feasible in this center because of the workforce needed to harvest buccal mucosa bilaterally.
Many authors have jettisoned two-stage repair because of severe infection following the first stage.[11] This is however not the experience in this study. Rather, two-stage repair allowed the preoperative infection to settle and the urethral plate healthier [Figure 3a and b]. On the overall, it has the advantage of avoiding double penile degloving and the associated decreased sensation and penile torsion that sometimes complicate flap procedures. Both complications may affect sexual functions negatively. This approach to urethral stricture treatment from 2013 onward explains the highly significant statistical correlation (P = 0.007) between the treatment method and the year of treatment. Before then, most of the patients had either urethral dilatation or anastomotic urethroplasty or both, the failed dilatation often preceding the urethroplasty. Dilatation, the first treatment for urethral stricture, is palliative[1] and similar to direct vision internal urethrotomy[29] may actually worsen the stricture.[3] The unit currently reserves dilatation for short-segment urethral stricture occurring concurrently with benign prostatic hyperplasia so that both can be treated at the same operating session.
The limitations of this study are its retrospective nature and the low number of patients. However, it has been able to demonstrate the demographics of patients with urethral stricture in this rural community and the changing trends in its treatment. The demographics here may represent the true state of affair in rural Nigeria and probably rural Sub-Saharan Africa.
CONCLUSION
The demographics of men with urethral stricture appear not to have changed remarkably in this rural community as urethritis still contributes immensely to the etiology. Many urban centers in the country have however reported a change from urethritis to trauma as the leading etiology. Further training and establishment of a protocol with modern treatment methods as its bedrock enables the urologist to follow and adopt the global changes in the treatment of urethral stricture.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
REFERENCES
- 1.Oranusi CK, Nwofor A, Orakwe JC. Urethroplasty practices among reconstructive urologists in Nigeria. Niger J Surg. 2015;21:146–50. doi: 10.4103/1117-6806.162582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Ivaz SL, Bugeja S, Andrich DE. Urethral stricture disease in men. Trends Urol Mens Health. 2015;6:21–4. [Google Scholar]
- 3.Hampson LA, McAninch JW, Breyer BN. Male urethral strictures and their management. Nat Rev Urol. 2014;11:43–50. doi: 10.1038/nrurol.2013.275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Aghaji AE, Odoemene CA. One-stage urethroplasty for strictures: Nigerian experience. Int J Urol. 2001;8:380–5. doi: 10.1046/j.1442-2042.2001.00317.x. [DOI] [PubMed] [Google Scholar]
- 5.Lee YJ, Kim SW. Current management of urethral stricture. Korean J Urol. 2013;54:561–9. doi: 10.4111/kju.2013.54.9.561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Wessels H. Ventral onlay graft bulbar urethroplasty using buccal mucosa. Afr J Urol. 2016;22:40–6. [Google Scholar]
- 7.Wein AJ, Kavoussi LR, Novick AC, Partin AW, Peters AC, Ramchandani P, et al. Cambell-Walsh Urology Tenth Edition. 10th Revised Edition. Philadelphia: Saunders; 2011. [Google Scholar]
- 8.Javali TD, Katti A, Nagaraj HK. Management of recurrent anterior urethral strictures following buccal mucosal graft-urethroplasty: A single center experience. Urol Ann. 2016;8:31–5. doi: 10.4103/0974-7796.162217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Buckley JC, Heyns C, Gilling P, Carney J. SIU/ICUD consultation on urethral strictures: Dilation, internal urethrotomy, and stenting of male anterior urethral strictures. Urology. 2014;83(3 Suppl):S18–22. doi: 10.1016/j.urology.2013.08.075. [DOI] [PubMed] [Google Scholar]
- 10.Granieri MA, Webster GD, Peterson AC. The evolution of urethroplasty for bulbar urethral stricture disease: Lessons learned from a single center experience. J Urol. 2014;192:1468–72. doi: 10.1016/j.juro.2014.05.085. [DOI] [PubMed] [Google Scholar]
- 11.Ibrahim AG, Ali N, Aliyu S, Bakari AA. One-stage urethroplasty for strictures in Maiduguri, North Eastern Nigeria. ISRN Urol 2012. 2012:847870. doi: 10.5402/2012/847870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Attah CA, Mbonu O, Anikwe RM. Treatment of urethral strictures in University of Nigeria Teaching Hospital. Urology. 1982;20:491–4. doi: 10.1016/0090-4295(82)90120-0. [DOI] [PubMed] [Google Scholar]
- 13.Alwaal A, Blaschko SD, McAninch JW, Breyer BN. Epidemiology of urethral strictures. Transl Androl Urol. 2014;3:209–13. doi: 10.3978/j.issn.2223-4683.2014.04.07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Heyns CF, van de Werwe J, Basson J, va de Werwe A. Etiology of male urethral stricture-evaluation of temporal changes at a single center and a review of the literature. Afr J Urol. 2012;18:4–9. [Google Scholar]
- 15.Stein DM, Thum DJ, Barbagli G, Kulkarni S, Sansalone S, Pardeshi A, et al. A geographic analysis of male urethral stricture aetiology and location. BJU Int. 2013;112:830–4. doi: 10.1111/j.1464-410X.2012.11600.x. [DOI] [PubMed] [Google Scholar]
- 16.Olajide AO, Olajide FO, Kolawole OA, Oseni I, Ajayi AI. A retrospective evaluation of challenges in urethral stricture management in a tertiary care centre of a poor resource community. Nephrourol Mon. 2013;5:974–7. doi: 10.5812/numonthly.13053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Tijani KH, Adesanya AA, Ogo CN. The new pattern of urethral stricture disease in Lagos, Nigeria. Niger Postgrad Med J. 2009;16:162–5. [PubMed] [Google Scholar]
- 18.Omisanjo AO, Ikuerowo SO, Esho JO. A etiology of urethral stricture at the Lagos state university Teaching Hospital Ikeja, Lagos. Niger J Urol. 2013;3:15–7. [Google Scholar]
- 19.Murshidi MS, Farah NB. Urinary tract infections in adult and adolescent males of a developing community: Pattern, bacteriology and genito-urinary predisposing factors. Arch Esp Urol. 2002;55:288–93. [PubMed] [Google Scholar]
- 20.Brumfitt W, Hamilton-Miller JM. Shroder FH, editor. Development of bacterial resistance during the treatmentof urinary tract infections: A constant clinical challenge. Recent Advances in the Treatment of Urinary Tract Infections. Royal Society of Medicine Services International Congress and Symposium Series No. 97. 1985:13–24. [Google Scholar]
- 21.Irekpita E, Ogbetere F, Esezobor E. Acute epididymorchitis: A study of the predisposing factors and the immediate management outcome in Irrua, Nigeria. J Clin Aud. 2016;8:1–6. [Google Scholar]
- 22.Eshiobo I, Ehizomen E, Omosofe F, Onuora V. Buccal mucosal graft urethroplasty for proximal bulbar urethral stricture: A revisit of the surgical technique and analysis of eleven consecutive cases. Niger Med J. 2016;57:266–271. doi: 10.4103/0300-1652.190603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Suleiman IA, Amogu EO, Ganiyu KA. Prevalence and control of hypertension in a Niger Delta semi urban community, Nigeria. Pharm Pract (Granada) 2013;11:24–9. doi: 10.4321/s1886-36552013000100005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Ebubedike UR, Nwofor AM, Umeh EO, Obilo KC, Ikegwuonu NC. Pattern of radiographic findings in urethral stricture in Nnewi, Nigeria. Niger J Urol. 2013;3:19–22. [Google Scholar]
- 25.Santucci RA, Mario LA, McAninch JW. Anastomotic urethroplasty for bulbar urethral stricture: Analysis of 168 patients. J Urol. 2002;167:1715–9. [PubMed] [Google Scholar]
- 26.Turner-Warwick R. Urethral stricture surgery. In: Mundy AR, editor. Current Operative Surgery-Urology. London, UK: Bailliere Tindall; 1988. pp. 160–218. [Google Scholar]
- 27.Pisapati VL, Paturi S, Bethu S, Jada S, Chilumu R, Devraj R, et al. Dorsal buccal mucosal graft urethroplasty for anterior urethral stricture by Asopa technique. Eur Urol. 2009;56:201–5. doi: 10.1016/j.eururo.2008.06.002. [DOI] [PubMed] [Google Scholar]
- 28.Kulkarni SB, Barbagli G. Surgical reconstruction of the bulbar urethra. In: Nasim S, editor. Art of Urethral Reconstruction. Gurgaon: Elsevier; 2012. p. 45. [Google Scholar]
- 29.Santucci R, Eisenberg L. Urethrotomy has a much lower success rate than previously reported. J Urol. 2010;183:1859–62. doi: 10.1016/j.juro.2010.01.020. [DOI] [PubMed] [Google Scholar]


