Abstract
Background
Cryptococcal meningitis (CM) is associated with substantial mortality in HIV-infected patients. Optimal timing of antiretroviral therapy (ART) in persons with CM represents a clinical challenge, and the burden of CM in Latin America has not been well described. Studies suggest that early ART initiation is associated with higher mortality, but data from the Americas are scarce.
Methods
HIV-infected adults in care between 1985–2014 at participating sites in the Latin America (the Caribbean, Central and South America network (CCASAnet)) and the Vanderbilt Comprehensive Care Clinic (VCCC) and who had CM were included. Survival probabilities were estimated. Risk of death when initiating ART within the first 2 weeks after CM diagnosis versus initiating between 2–8 weeks was assessed using dynamic marginal structural models adjusting for site, age, sex, year of CM, CD4 count, and route of HIV transmission.
Findings
340 patients were included (Argentina 58, Brazil 138, Chile 28, Honduras 27, Mexico 34, VCCC 55) and 142 (42%) died during the observation period. Among 151 patients with CM prior to ART 56 (37%) patients died compared to 86 (45%) of 189 with CM after ART initiation (p=0.14). Patients diagnosed with CM after ART had higher risk of death (p=0.03, log-rank test). The probability of survival was not statistically different between patients who started ART within 2 weeks of CM (7/24, 29%) vs. those initiating between 2–8 weeks (14/53, 26%) (p=0.96), potentially due to lack of power.
Interpretation
In this large Latin-American cohort, patients with CM had very high mortality rates, especially those diagnosed after ART initiation. This study reflects the overwhelming burden of CM in HIV-infected patients in Latin America.
Keywords: Cryptococcal meningitis, AIDS defining events, HIV, AIDS, Latinamerica, Opportunistic Infections in HIV
Introduction
Cryptococcal meningitis (CM) is associated with substantial morbidity and mortality worldwide among HIV-infected patients. Mortality in settings across low, middle, and high income countries has ranged from 10–43%, despite availability of appropriate treatment1. In studies from Brazil and Argentina, mortality rates among those with CM have been as high as 30–63%2. However, other reports from Peru and Brazil demonstrate that when intracranial pressure is aggressively managed, mortality rates are lower, at 19% and 26%, respectively3. In many resource-constrained settings however, performing serial lumbar punctures or placing ventriculo-peritoneal shunts to manage intracranial pressure may not be possible. Additionally, the gold standard of effective induction therapy for CM requires potent fungicidal drugs such as flucytosine (5-FC) with amphotericin B (lipid formulation)4, which may be unavailable in resource-constrained settings such as some countries in Latin America.
Further, without reconstitution of the immune system in patients with acquired immunodeficiency syndrome (AIDS), as well as consolidation and maintenance therapy after induction, relapse of CM may be as high as 15%4. Moreover, correctly timed introduction of antiretroviral therapy (ART) represents a clinical challenge given the risk for immune reconstitution inflammatory syndrome (IRIS) and fatal outcomes5, 6,7 A prospective randomized controlled trial from Zimbabwe found that patients who received ART within 72 hours of diagnosis of CM had mortality rates of 88% versus 54% in those who delayed ART initiation to 10 weeks; all participants received 800 mg of fluconazole daily for CM induction therapy8. Another randomized controlled trial in Uganda and South Africa demonstrated that patients who received early ART (within 2 weeks of diagnosis of cryptococcal meningitis) were at higher risk of mortality than those in whom ART initiation was deferred until 5 weeks after diagnosis (45% vs 30% at 26 weeks)9. The majority of studies of HIV-associated CM have been conducted in Sub-Saharan Africa, whereas studies of CM outcomes in HIV-infected patients in the Americas (which have a mix of low, middle, and high-income countries) are scarce. We investigated mortality and timing of ART initiation among patients with CM at study sites in South (3), Central (1), and North America (2).
Methods
Data Collaboration
The Caribbean, Central and South America network for HIV epidemiology (CCASAnet) cohort (www.ccasanet.org) has been described elsewhere10. The collaboration was established in 2006 as Region 2 of the International Epidemiologic Databases to Evaluate AIDS (IeDEA; www.iedea.org) with the purpose of collecting retrospective clinical HIV data to describe the unique characteristics of the epidemic in Latin America and the Caribbean. Five CCASAnet sites that routinely collect clinical endpoints contributed patient data to this study: Centro Medico Huesped/Hospital Fernandez, Buenos Aires, Argentina (CMH/HF-Argentina); Instituto de Pesquisa Clinica Evandro Chagas, Fundação Oswaldo Cruz, Rio de Janeiro, Brazil (FC- Brazil); Fundación Arriarán, Santiago, Chile (FA-Chile); Instituto Hondureño de Seguridad Social and Hospital Escuela, Tegucigalpa, Honduras (IHSS/HE-Honduras); and Instituto Nacional de Ciencias Médicas y Nutrición Salvador Zubirán, Mexico City, Mexico (INCMNSZ-Mexico). Data were also obtained from the Vanderbilt Comprehensive Care Clinic in Nashville, Tennessee, U.S.A. (VCCC-US), which provides care to HIV-infected individuals in a large region of the southeastern United States.
Study Population and Statistical Analysis
The study included all HIV-infected patients ≥18 years of age in care from 1985–2014 at CCASAnet sites in Latin America and VCCC-US sites who were diagnosed with CM. The primary endpoint was all-cause mortality. Mortality rates were compared between patients with CM diagnosis before versus after ART initiation using Kaplan-Meier techniques. A multivariable Cox regression model was used to assess predictors of the hazard of mortality after stratifying by study site. Predictor variables included sex, age at CM diagnosis, route of HIV transmission, CD4 count at CM diagnosis, whether the patient had initiated ART prior to CM diagnosis (yes/no), and years between CM diagnosis and the calendar year of the start of combination ART (cART) era for each site. Additionally, a similar model was used comparing all CCASAnet sites with VCCC-US. The cART era started in 2000 in CMH/FH-Argentina, 1997 in FC-Brazil, 2001 in FA-Chile, 2002 in IHSS/HE-Honduras, 2001 in INMNSZ-Mexico, and 1996 in the VCCC-US. The continuous variables CD4 count, age, and years between CM diagnosis and start of cART era, were included in the models using restricted cubic splines with three knots.
Survival probabilities were estimated using Kaplan-Meier techniques among patients who had CM prior to ART initiation; according to COAT trial9 we divided patients in those who initiated ART within the first two weeks after CM diagnosis were compared with those who initiated ART between 2–8 weeks. Curves were weighted by the inverse probability of starting ART within the first two weeks after CM diagnosis estimated in a logistic model adjusting by site, CD4 count at CM diagnosis, age, sex, route of HIV transmission, and number of years from CM diagnosis to the era of cART in each country.
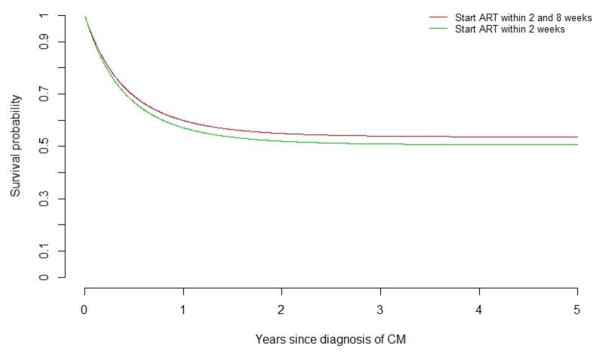
A dynamic marginal structural model was used to compare the treatment strategies: 1) ART start within the first two weeks after CM diagnosis (immediate arm) versus 2) ART start between 2–8 weeks after CM diagnosis (deferred arm). Following standard dynamic marginal structural model methods11 all patients with a CM diagnosis prior to starting ART contributed data to both treatment strategy arms until their data were no longer consistent with a particular treatment strategy, at which time their data were artificially censored from that study arm. Inverse probability weights were used to account for potential bias due to artificial censoring. A pooled logistic model including time in days was used to estimate the probability of starting ART at each week for the first eight weeks following CM diagnosis. This model included study site, age, sex, route of transmission, time-updated CD4 count, and year of CM diagnosis. Inverse probability weights were computed as the inverse probability of not being artificially censored from a given treatment strategy based on the probability of starting (or not starting) ART and the inverse probability of having a CD4 count. Weights were truncated at the 95th percentile (9.75); results were similar truncating weights at the 97.5th percentile (12.3). These truncated weights were then included in a pooled logistic regression model including time from CM diagnosis (both linear and quadratic terms) and treatment strategy indicator (immediate versus defer); confidence intervals were constructed using robust standard errors. The predicted survival probability over time for each treatment strategy was computed using this model for illustration in Figure 3.
Figure 3.
Predicted survival probability over time according to ART initiation strategy.
Dynamic marginal structural models considered the impact of starting ART <2 weeks after CM diagnosis versus starting between 2–8 weeks after diagnosis. In this model, no statistical differences in mortality were seen, with an odds of death for starting ART <2 weeks after CM of 1.09 (95% CI, 0.44–2.67; p=0.84) times higher than for starting between 2–8 weeks.
Results
Clinical Characteristics of Study Population
A total of 340 patients with CM were included. Of these, 58 were from CMH/FH-Argentina, 138 from FC-Brazil, 28 from FA-Chile, 27 from IHSS/HE-Honduras, 34 from INCMNSZ-Mexico, and 55 from VCCC-US (Table 1). The median patient age at enrolment in care was 36 years (interquartile range [IQR] 30–43) and 80% were male. Median CD4 count at diagnosis of CM was 45 cells/mm3 (IQR 16–100). One hundred and fifty-one (44%) patients had CM before initiating ART initiation, and among these, 27 (8% of the study population) never initiated ART. CD4 counts were not statistically different for those who presented with CM after starting ART compared to those with CM before ART (55 vs 39 cells/mm3, respectively; p=0.27), although 39% had missing CD4 data at the time of CM diagnosis. For patients with CM before ART initiation who subsequently started ART, the median time to ART initiation was shorter in the Latin American cohort when compared with the VCCC-US [33 days (IQR 14–73) vs 58 days (IQR 32–214), respectively; p=0.002] (Table 1). Among the 189 individuals who developed CM after starting ART, the median time to CM after ART initiation was 743 days (IQR 98–2274, Table 1). Of these, 79 (42%) had evidence of virological failure at time of CM diagnosis and 93 (76%) had history of virological failure before CM was diagnosed.
Table 1.
Patient characterstics by site
| CMH/F H- Argenti na (n=58) |
FC- Brazil (n=138) |
FA-Chile (n=28) |
IHSS/HE - Hondur as (n=27) |
INMNSZ- Mexico (n=34) |
VCCC- United States (n=55) |
CCASA net (n=28 5) |
Combin ed (n=340) |
p- value * |
|
|---|---|---|---|---|---|---|---|---|---|
| Patient age at enrollment (years) | 35 (31–39) | 35 (29 – 41) | 34 (28–43) | 37 (31 – 43) | 35 (29 – 44) | 41 (36 – 45) | 35 (29 – 41) | 36 (30 – 43) | <0.001 |
| Male sex | 51 (88%) | 105 (76%) | 26 (93%) | 16 (59%) | 29 (85%) | 44 (80%) | 227 (80%) | 271 (80%) | 1.000 |
| CD4 at enrollment | 46 (19–75) | 111 (40 – 242) | 46 (4–104) | 50 (45 – 110) | 37 (13 – 96) | 29 (8 – 58) | 51 (21 – 132) | 46 (15 – 105) | <0.001 |
| Missing CD4 at enrollment, n(%) | 34 (59%) | 92 (67%) | 19 (68%) | 10 (37%) | 1 (3%) | 0 (0%) | 156 (55%) | 156 (46%) | |
| CD4 at diagnosis of CM | 22 (13–45) | 83 (31 – 182) | 86 (10–142) | 65 (45 – 150) | 28 (10 – 51) | 38 (11 – 69) | 47 (17 – 112) | 45 (16 – 100) | 0.065 |
| Missing CD4 at diagnosis of CM, n(%) | 26 (45%) | 61 (44%) | 14 (50%) | 8 (30%) | 1 (3%) | 21 (38%) | 110 (39%) | 131 (39%) | |
| CD4 at ART initiation | 30 (14–65) | 110 (43 – 251) | 30 (7–88) | 50 (32 – 102) | 31 (13 – 51) | 56 (17 – 90) | 51 (24 – 128) | 51 (22 – 124) | 0.495 |
| Missing CD4 at ART initiation, n(%) | 25 (43%) | 68 (49%) | 16 (57%) | 3 (11%) | 9 (26%) | 20 (36%) | 121 (42%) | 141 (41%) | |
| Route of HIV transmission, n(%) | 0.442 | ||||||||
| Heterosexual | 16 (28%) | 50 (36%) | 9 (32%) | 16 (59%) | 13 (38%) | 24 (44%) | 104 (36%) | 128 (38%) | |
| MSM | 18 (31%) | 53 (38%) | 18 (64%) | 1 (4%) | 20 (59%) | 16 (29%) | 110 (39%) | 126 (37%) | |
| Other | 19 (33%) | 9 (7%) | 1 (4%) | 0 (0%) | 0 (0%) | 8 (15%) | 29 (10%) | 37 (11%) | |
| Unknown | 5 (9%) | 26 (19%) | 0 (0%) | 10 (37%) | 1 (3%) | 7 (13%) | 42 (15%) | 49 (14%) | |
| Deaths | 11 (19%) | 85 (62%) | 6 (21%) | 9 (33%) | 13 (38%) | 18 (33%) | 124 (44%) | 142 (42%) | 0.182 |
| Never started on ART | 1 (2%) | 14 (10%) | 3 (11%) | 2 (7%) | 4 (12%) | 3 (5%) | 24 (8%) | 27 (8%) | 0.636 |
| CM before ART initiation | 27 (47%) | 29 (21%) | 9 (32%) | 15 (56%) | 10 (29%) | 34 (62%) | 90 (32%) | 124 (36%) | <0.001 |
| Days from diagnosis of CM to ART initiation (a) | 29 (22 – 72) | 31 (13 – 61) | 59 (24 – 76) | 39 (11 – 106) | 40 (32 – 51) | 58 (32 – 214) | 33 (14 – 73) | 40 (23.5 – 94.5) | 0.002 |
| CM after ART initiation | 30 (52%) | 95 (69%) | 16 (57%) | 10 (37%) | 20 (59%) | 18 (33%) | 171 (60%) | 189 (56%) | <0.001 |
| Days from ART initiation to diagnosis of CM (b) | 1025 (168 – 2383) | 832 (125 – 2404) | 986 (215 – 1822) | 69 (40 – 546) | 363 (80 – 2806) | 734 (122 – 1997) | 743 (97 – 2282) | 743 (98 – 2274) | 0.888 |
| Follow-up after CM (years) | 4 (0.8 – 8) | 3 (0.2 – 6) | 6.6 (3 – 9) | 6.3 (1.6 – 8.4) | 1.3 (0.3 – 4.5) | 3.5 (1.4 – 7) | 3.5 (0.5– 7.5) | 3.5 (0.7–7.5) | 0.39 |
p-values between CCASAnet and Vanderbilt
Continuous variables are reported as medians (interquartile range)
CMH/FH-Argentina- Centro Medico Huesped/Hospital Fernandez, Buenos Aires, Argentina
FC-Brazil- Instituto de Pesquisa Clinica Evandro Chagas, Fundação Oswaldo Cruz, Rio de Janeiro, Brazil
FA-Chile- Fundación Arriarán, Santiago, Chile
IHSS/HE-Honduras- ); Instituto Hondureño de Seguridad Social and Hospital Escuela, Tegucigalpa, Honduras
INMNSZ-Mexico- Instituto Nacional de Ciencias Médicas y Nutrición Salvador Zubirán, Mexico City, Mexico
VCCC- US Vanderbilt Comprehensive Care Clinic, Nashville, Tennessee, U.S.A.
CM Cryptococcal meningitis
MSM- Men who have sex with men
Time to ARV estimated only in patients with a cryptococcosis event reported before ARV initiation.
Time since ARV initiation to cryptococcosis event estimated only in patients with a cryptococcosis event reported after ARV initiation.
Mortality
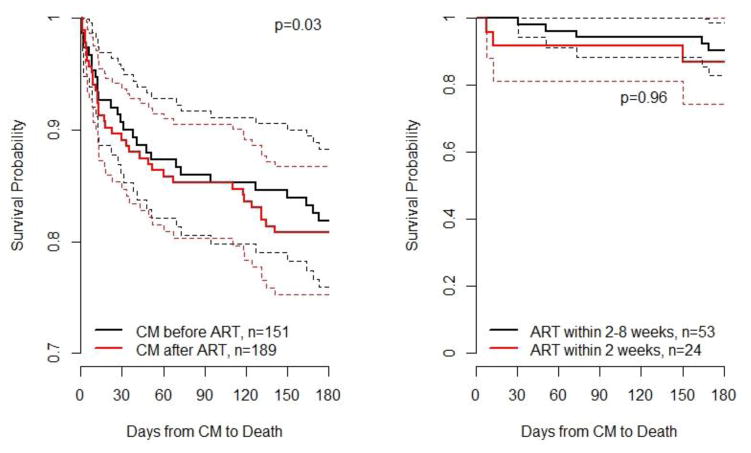
A total of 142 patients with CM (42%) died during the observation period (median follow-up 0.92 years; IQR 0.09–3.61 years), including 124 (44%) in Latin American sites and 18 (33%) in the VCCC-US cohort. Fifty-six (37%) patients who had CM prior to ART initiation died, whereas 86 (45%) of those with CM after ART died, p=0.14. The estimated probability of mortality over time after CM diagnosis according to whether a patient had started ART prior to CM diagnosis is shown in Figure 1A. Patients diagnosed with CM after ART had a slightly higher risk of death (p=0.03, log-rank test).
Figure 1.
A) Estimated survival probability by time of CM and start of ART. B) Estimated survival probability for patients who started ART within 2 weeks of CM vs. those starting between 2–8 weeks.
Figure A) Survival is statistically lower in ART naïve patients with Cryptococcal Meningitis (CM) compared to those with CM after initiating ART. Figure B) In ART naïve patients, no statistical difference was found comparing those who started ART <2 weeks after CM diagnosis with those who started ART 2–8 weeks after CM diagnosis. Both figures show unadjusted Kaplan-Meier estimates; p-values are from log-rank tests.
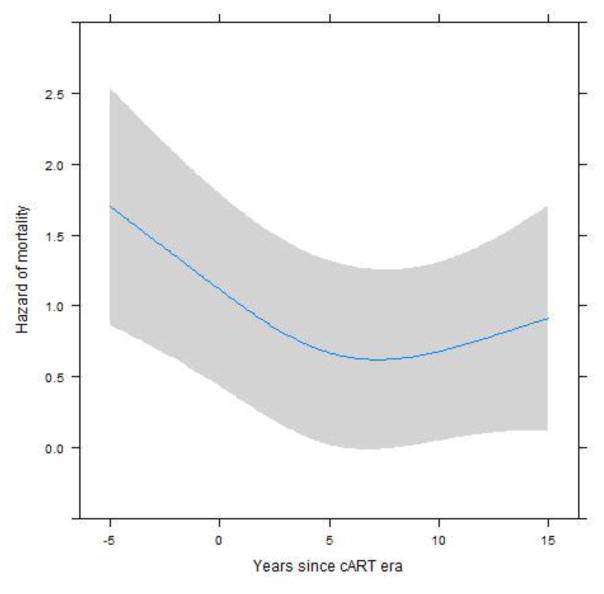
After adjusting for site, age, years between CM diagnosis and the start of the cART era, CD4 count at CM diagnosis, sex, and route of HIV transmission, the hazard of death was 1.28 times higher (95% confidence interval 0.87–1.88, p=0.21) for patients who had CM after ART initiation compared to those who had CM prior to ART (Table 2). In a separate analysis, we compared the Latin American sites versus the US site adjusting for all other variables: the hazard of death was higher in Latin America but did not achieve statistical significance (HR=1.51, 95% CI=0.83–2.72). Forty-two cases of CM occurred in the pre-cART era vs 298 cases in the post-cART era, with 66% mortality rate in the pre-cART era vs 38% in the post-cART era. Higher CD4 count at CM diagnosis (p=0.002) and more recent year of CM diagnosis (relative to the start of the cART era, p=0.01) were associated with lower hazards of mortality (Table 2). Figure 2 shows that the hazard of mortality decreased in the 5 years prior to and the 5 years after the cART era, but has stabilized thereafter. Age, sex, and route of HIV transmission were not statistically associated with mortality.
Table 2.
Factors associated with mortality adjusted by site, years since introduction of cART, CD4 count at CM diagnosis, sex, route of HIVtransmission, age and ART initiation relative to CM diagnosis
| Site | HR (95%CI) | p-value |
|---|---|---|
| Argentina vs Brazil | 0.23 (0.11–0.48) | <0.01 |
| Chile vs Brazil | 0.25 (0.09–0.63) | <0.01 |
| Honduras vs Brazil | 0.40 (0.19–0.84) | 0.01 |
| Mexico vs Brazil | 0.57 (0.30–1.09) | 0.09 |
| VCCC-US vs Brazil | 0.42 (0.23–0.75) | <0.01 |
| Number of years of CM since cART era | ||
| One year before era cART | 1.09 (1.01–1.17) | 0.02 |
| Two years after era cART | 0.84 (0.74–0.97) | 0.02 |
| Five years after era cART | 0.71 (0.54–0.93) | 0.01 |
| Ten years after era cART | 0.69 (0.48–1.00) | 0.05 |
| CD4 at diagnosis of CM | ||
| 100 vs 50 | 0.83 (0.70–0.98) | 0.03 |
| 200 vs 50 | 0.65 (0.47–0.89) | <0.01 |
| 350 vs 50 | 0.51 (0.31–0.84) | <0.01 |
| Male | 1.06 (0.42–2.68) | 0.89 |
| Route of HIV transmission | ||
| Homosexual vs Heterosexual | 1.00 (0.64–1.57) | 0.98 |
| Other vs Heterosexual | 1.19 (0.59–2.39) | 0.61 |
| Unknown vs Heterosexual | 1.60 (0.96–2.68) | 0.07 |
| Age at diganosis of CM | ||
| 30 vs 20 | 0.96 (0.58–1.58) | 0.88 |
| 40 vs 20 | 0.98 (0.48–2.00) | 0.96 |
| 50 vs 20 | 1.08 (0.56–2.10) | 0.80 |
| ART use prior to CM diagnosis | ||
| CM after ART vs CM before ART | 1.28 (0.87–1.88) | 0.21 |
Figure 2.
Adjusted model for mortality risk over time since the beginning of the cART era.
Note: plot adjusted for these values: site=Brazil, Age=20 years, gender=male, Transmission route=Homosexual, CD4 count=50cells/mm3, and CM after ART initiation. Year 0 in x-axis marks the beginning of the cART era.
This figure represents the association between mortality and time from a patient’s CM diagnosis to the year that cART was introduced at the patient’s study site. The highest mortality was observed among patients diagnosed with CM before the introduction of cART; mortality declines in the cART era, although there is a plateau in mortality in later years of the cART era.
Among the 151 patients who had not started ART prior to CM diagnosis, 56 (37%) died during follow-up. Notably, of the 27 who never started ART, 24 (89%) died. The probability of survival between those who started ART within 2 weeks of CM diagnosis and between 2–8 weeks of survival was similar (Figure 1B). However, because of early deaths (i.e., survival bias), these numbers could be misleading if used to determine the optimal timing of ART initiation after CM diagnosis. More appropriately, a dynamic marginal structural model considered the impact of starting ART <2 weeks after CM diagnosis versus starting between 2–8 weeks after diagnosis. In this model, the odds of death for starting ART <2 weeks after CM were 1.09 (95% CI, 0.44–2.67; p=0.84) times higher than for starting between 2–8 weeks (Figure 3).
Discussion
Latin America has the third highest rate of cryptococcal meningitis associated with HIV/AIDS in the world, with nearly 55,000 cases estimated annually3. In our analysis, dynamic marginal structural models did not show an increased risk of death for patients who started ART within 2 weeks after diagnosis of cryptococcal meningitis compared with those who started during weeks 2–8. Our results are consistent with a systematic review that found no difference in mortality for early versus delayed ART initiation; this review included patients in Puerto Rico, Zimbabwe, the U.S., and South Africa14. However, this differs from the findings of the COAT trial, which found that patients randomized to start ART within 2 weeks after diagnosis of CM had a higher risk of mortality compared to those assigned to defer ART until 5 weeks after diagnosis of CM9.
There are several reasons why our study may have failed to detect a difference in mortality between early and delayed ART initiation. First, it is probable that our study lacked statistical power to detect such a difference. Although our study is one of the largest to date, analyses of this nature are known to require very large sample sizes11,12. Second, variations in treatment across sites and regions in our real-world clinic setting may have affected outcomes. The COAT trial was performed in low income Sub-Saharan African countries with a fixed clinical treatment protocol, whereas our study was conducted retrospectively in a mix of low, middle, and high income countries in the Americas with substantial variation in treatment practices and resources, including the availability of ART regimens and treatments for CM (data.worldbank.org/maps2015, World Bank 2015). For example, sites had varying access to amphotericin products, and none of the sites in our analysis, except for VCCC-US, currently have access to flucytosine. There may also be variation across sites in diagnostic capacity, ability to perform lumbar puncture or place ventriculoperitoneal shunts, or access to neurosurgical specialists or imaging modalities. Further, local guidelines for the treatment of CM and provider adherence to standards of care could vary within the region. It is possible that our cohort heterogeneity may have made it more difficult to detect the impact of the timing of ART initiation on outcomes. Given that patients were not randomized to immediate or deferred ART therapy in our study, those who deferred ART may have been different from those who started ART shortly after CM diagnosis. Although we attempted to control for patient characteristics, as with all observational studies, there may be unmeasured variables that are also associated with patient mortality that affected our results.
Our study results (and limitations) are similar to those from a large collaborative study in North America and Europe that implemented similar analysis techniques using observational data from 235 ART-naïve patients diagnosed with CM between 1998 and 2009, during the combination ART era13. Dynamic marginal structural models were used to compare ART initiation within 14 days of CM to deferring ART until 14–56 days after CM diagnosis. The crude and adjusted hazard ratios comparing early to deferred treatment were 1.29 (95% CI 0.68–2.43) and 1.30 (95% CI 0.66–2.55), respectively, with a total of 15 deaths in the early ART arm and 25 deaths in the deferred arm. The overall mortality rate in this study, 18% at 6 months, was substantially lower than in our study or the COAT trial.
In our analysis, mortality was high, regardless of the clinical site. Even in the cART era overall mortality was 38% (vs 66% pre-cART era). Mortality in the post cART-era was 33% at the VCCC-US site vs 39% at CCASAnet sites (p-value 0.50). Mortality at our US site was higher than observed in the North America/Europe collaborative study13, despite similar access to state of the art therapeutic and diagnostic modalities. However, our results are consistent with mortality figures previously cited elsewhere in Africa, Asia, and the U.S1,2,15. The findings in our study highlight the poor outcomes of HIV-associated CM, regardless of resources or cART availability. The devastating nature of CM in HIV, even during the cART era was further highlighted in a recent study of 283 episodes of CM in Botswana from 2012–2014; this study showed a one year mortality of 65%, despite the use of amphotericin-B plus fluconazole for induction treatment16.
Our analysis straddles the cART era, and the timing of availability of effective cART in each of the six countries was variable, ranging from 1996/1997 in US and Brazil to 2002 in Honduras. Data from Brazil showed a significant reduction in reported cases of CM from 1992 to 2010, attributed to the scale-up of ART access in the region3. Despite improved access to ART, late testers and late presenters are frequent in Latin America, with one study reporting that 45% of those recently diagnosed with HIV had CD4<200 cells/mm3 17. With late testers and presenters comprising such a large proportion of the newly-diagnosed, the risk for CM remains high and prevention of CM must be linked with efforts at earlier HIV diagnosis and linkage to care in the region.
Interestingly, in our study when looking at the unadjusted time from CM diagnosis to death (Figure 1A) mortality was higher in those who developed CM after ART start (p=0.03). However, there was no difference in mortality when looking at the adjusted time from CM diagnosis to death (table 2; HR-1.28, p=0.21). Our results suggest that perhaps people diagnosed with CM after ART initiation may have higher rates of mortality than those diagnosed before ART, but the evidence of such an association is not strong. On average, the individuals who developed CM after ART did so in a median time of 2–3 years after ART initiation, yet still had CD4 counts that were well below 100 cells/mm3, putting them at risk for CM and other opportunistic infections. In the Botswana study of CM in the cART era, 51% of individuals who developed CM were already on ART16. The development of CM after ART initiation could reflect an immune reconstitution syndrome (IRIS) or “unmasking” of CM6, in which case mortality is higher than in those without IRIS; however, one would expect these cases to have developed in the first weeks to months after ART initiation. Alternatively, the CM cases that developed several years after ART initiation could be attributed to poor adherence and/or virological failure indicating perhaps poor prognostic factors overall.; this is supported in our study by the fact that 76% who developed CM after ART start had evidence of preceding virologic failure.
There were several limitations to our study. As mentioned above, our study was not a randomized controlled trial and observational cohort data were used. In an attempt to simulate a randomized controlled trial, dynamic marginal structural models were used. Further, our study period straddles the combination ART era and although we attempted to adjust for these differences, those patients with HIV and CM before the combination ART era may have fundamentally different outcomes than those after the combination ART era. We did not have data on clinical presentation at the time of CM diagnosis, nor do we have data on CSF parameters or other laboratory data on diagnosis. Finally, Latin America is comprised of countries that span low to high income levels18. Our sites represent a heterogeneous group with varying access to diagnostic and treatment resources and differing standards of care, and therefore these results may not be generalizable across other settings in Latin America.
In summary, this is one of the largest studies to investigate mortality among HIV-infected patients after CM diagnosis, and one of very few studies of CM outcomes across the Americas. We found that mortality rates remain high, in spite of location or cART era. In contrast to randomized trials performed in sub-Saharan Africa, there was no statistical difference in mortality according to timing of cART initiation, perhaps due to lack of power, heterogeneity in real-world clinical practice, or unmeasured confounding. For those who started cART and then went on to develop CM, there may be a higher rate of death, which appeared to be often associated with virologic failure. Our study suggests that in resource-mixed settings such as South, Central, and North America, cryptococcal meningitis remains a serious and often fatal complication of HIV/AIDS.
Highlights.
This is a description of clinical outcomes of cryptococcal meningitis (CM) in persons with HIV-infection in a large Latin-American cohort;
Mortality in HIV-infected patients with cryptococcal meningitis varies across sites, and is overall very high (42%);
Many people in the cohort developed cryptococcal meningitis months to years after ART start (median time 743 days), suggesting possible ART failure or noncompliance;
Acknowledgments
Funding: This work was supported in part by the NIH-funded Caribbean, Central and South America network for HIV epidemiology (CCASAnet), a member cohort of the International Epidemiologic Databases to Evaluate AIDS (leDEA) (U01AI069923). This award is funded by the following institutes: Eunice Kennedy Shriver National Institute Of Child Health & Human Development (NICHD), National Cancer Institute (NCI), National Institute Of Allergy And Infectious Diseases (NIAID), National Institute Of Mental Health (NIMH), and Office Of The Director, National Institutes Of Health (OD). This work was also supported in part by the NIH-funded Tennessee Center for AIDS Research (P30 AI110527).
We gratefully acknowledge all patients, caregivers, and data managers involved in the CCASAnet cohort. We also thank Rocío Velazquez Pastrana, Jose Manuel Esquivel Sánchez, Carlos Armando Madrigal Iberri, Sally Bebawy and James Logan for their assistance in data collection and management.
Footnotes
Ethics Statement
Institutional review board approval waiving the requirement for individual patient informed consent was obtained locally for each participating site, and at the VCCC-US and the CCASAnet Data Coordinating Center at Vanderbilt University, Nashville, Tennessee, U.S.A.
Conflits of Interest
B. Crabtree Ramírez, Y. Caro Vega, B.E. Shepherd, C. Le, M. Turner, P. Cahn, B. Grinsztejn, C. Cortes, D. Padgett, T.R. Sterling, C.C. McGowan and A.K. Person have no conflicts of interest to declare.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Jarvis JN, Harrison TS. HIV-associated cryptococcal meningitis. AIDS (London, England) 2007;21:2119–2129. doi: 10.1097/QAD.0b013e3282a4a64d. Editorial Review. [DOI] [PubMed] [Google Scholar]
- 2.Bamba S, Lortholary O, Sawadogo A, et al. Decreasing incidence of cryptococcal meningitis in West Africa in the era of highly active antiretroviral therapy. AIDS (London, England) 2012;26:1039–1041. doi: 10.1097/QAD.0b013e328352d1d8. [DOI] [PubMed] [Google Scholar]
- 3.Vidal JE, Penalva de Oliveira AC, Dauar RF, et al. Strategies to reduce mortality and morbidity due to AIDS-related cryptococcal meningitis in Latin America. Braz J Infect Dis. 2013;17:353–362. doi: 10.1016/j.bjid.2012.10.020. Research Support, N.I.H., Extramural Review. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Perfect JR, Dismukes WE, Dromer F, et al. Clinical practice guidelines for the management of cryptococcal disease: 2010 update by the infectious diseases society of america. Clin Infect Dis. 2010;50:291–322. doi: 10.1086/649858. Practice Guideline Research Support, U.S. Gov’t, P.H.S. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.WHO. [accessed November, 2016];Rapid advice: Diagnosis, prevention and management of cryptococcal disease in HIV-infected adults, adolescents and children. 2011 http://www.who.int/hiv/pub/cryptococcal_disease2011/en/ [PubMed]
- 6.Bisson GP, Molefi M, Bellamy S, et al. Early versus delayed antiretroviral therapy and cerebrospinal fluid fungal clearance in adults with HIV and cryptococcal meningitis. Clin Infect Dis. 2013;56:1165–1173. doi: 10.1093/cid/cit019. Randomized Controlled Trial Research Support, Non-U.S. Gov’t. [DOI] [PubMed] [Google Scholar]
- 7.Department of Health and Human Services. Panel on Antiretroviral Guidelines for Adults and Adolescents. [accessed November, 2016];Guidelines for the use of antiretroviral agents in HIV-1-infected adults and adolescents. http://aidsinfo.nih.gov/contentfiles/lvguidelines/AdultandAdolescentGL.pdf.
- 8.Makadzange AT, Ndhlovu CE, Takarinda K, et al. Early versus delayed initiation of antiretroviral therapy for concurrent HIV infection and cryptococcal meningitis in sub-saharan Africa. Clin Infect Dis. 2010;50:1532–1538. doi: 10.1086/652652. Randomized Controlled Trial Research Support, Non-U.S. Gov’t. [DOI] [PubMed] [Google Scholar]
- 9.Boulware DR, Meya DB, Muzoora C, et al. Timing of antiretroviral therapy after diagnosis of cryptococcal meningitis. The New England journal of medicine. 2014;370:2487–2498. doi: 10.1056/NEJMoa1312884. Multicenter Study Randomized Controlled Trial Research Support, N.I.H., Extramural Research Support, Non-U.S. Gov’t Research Support, U.S. Gov’t, Non-P.H.S. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.McGowan CC, Cahn P, Gotuzzo E, et al. Cohort Profile: Caribbean, Central and South America Network for HIV research (CCASAnet) collaboration within the International Epidemiologic Databases to Evaluate AIDS (IeDEA) programme. Int J Epidemiol. 2007;36:969–976. doi: 10.1093/ije/dym073. Review. [DOI] [PubMed] [Google Scholar]
- 11.Cain LE, Saag MS, Petersen M, et al. Using observational data to emulate a randomized trial of dynamic treatment-switching strategies: an application to antiretroviral therapy. Int J Epidemiol. 2015 doi: 10.1093/ije/dyv295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Shepherd BE, Liu Q, Mercaldo N, et al. Comparing results from multiple imputation and dynamic marginal structural models for estimating when to start antiretroviral therapy. Stat Med. 2016;35:4335–4351. doi: 10.1002/sim.7007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ingle SMea. Impact of ART on Mortality in Cryptococcal Meningitis Patients: High Income Settings. CROI 2015. Poster presentation; Seatle, WA. Feb 23–26 2015. [Google Scholar]
- 14.Njei B, Kongnyuy EJ, Kumar S, et al. Optimal timing for antiretroviral therapy initiation in patients with HIV infection and concurrent cryptococcal meningitis. Cochrane Database Syst Rev. 2013:CD009012. doi: 10.1002/14651858.CD009012.pub2. Meta-Analysis Research Support, Non-U.S. Gov’t Review. [DOI] [PubMed] [Google Scholar]
- 15.Anekthananon T, Manosuthi W, Chetchotisakd P, et al. Predictors of poor clinical outcome of cryptococcal meningitis in HIV-infected patients. Int J STD AIDS. 2011;22:665–670. doi: 10.1258/ijsa.2011.010538. Research Support, N.I.H., Extramural Research Support, Non-U.S. Gov’t. [DOI] [PubMed] [Google Scholar]
- 16.Tshepo BL, Raju KP, Azzo C, et al. Mortality Due to HIV-Associated Cryptococcal Meiningitis in Botswana in the ART ERA CROI 2017. Poster presentation; Seatle, WA. 2017. p. 745. [Google Scholar]
- 17.Crabtree-Ramirez B, Caro-Vega Y, Shepherd BE, et al. Cross-sectional analysis of late HAART initiation in Latin America and the Caribbean: late testers and late presenters. PLoS ONE. 2011;6:e20272. doi: 10.1371/journal.pone.0020272. Research Support, N.I.H., Extramural. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Park BJ, Wannemuehler KA, Marston BJ, et al. Estimation of the current global burden of cryptococcal meningitis among persons living with HIV/AIDS. AIDS (London, England) 2009;23:525–530. doi: 10.1097/QAD.0b013e328322ffac. Review. [DOI] [PubMed] [Google Scholar]