Keywords: nerve regeneration, neurodegeneration, genistein, Alzheimer's disease, neuroprotection, hippocampus, learning, memory, tau protein, CAMK4, CALM, CAMKK1, neural regeneration
Abstract
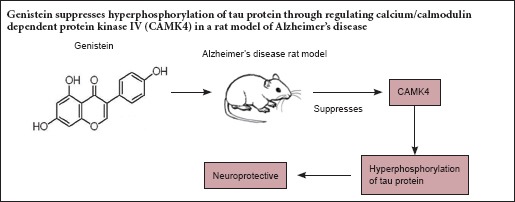
Genistein has a neuroprotective effect in Alzheimer's disease, but its mechanism of action needs further clarification. Accumulating evidence suggests that excessive phosphorylation of tau protein causes production of neurofibrillary tangles, which is one of the main pathological characteristics of Alzheimer's disease, and tau protein can be phosphorylated by calcium/calmodulin dependent protein kinase IV (CAMK4). After 7 days of pre-administration of genistein (90 mg/kg), an Alzheimer's disease rat model was established using an intraperitoneal injection of D-galactose combined with an intracerebral injection of amyloid-β peptide (25–35). The rat was then continuously administered genistein (90 mg/kg) for 42 days. The Morris water maze test, western blotting and hematoxylin-eosin staining results showed that genistein significantly decreased the escape latency and increased the number of times crossing the platform, reduced p-tau, CALM, CAMKK1 and p-CAMK4 protein levels in the hippocampus, and alleviated hippocampal neuron damage. These findings indicate that genistein may play a neuroprotective role in Alzheimer's disease through regulating CAMK4 to modulate tau hyperphosphorylation.
Introduction
Alzheimer's disease (AD) is an age-related chronic progressive degenerative disease of the central nervous system with cognitive dysfunction and mental disorder. Senile plaques, neurofibrillary tangles and loss of a wide range of hippocampal neurons are the main pathological characteristics of AD (Yin et al., 2013; Pike, 2017).
Excessive phosphorylation of the microtubule-associated protein tau (MAPT; commonly known as tau) causes production of neurofibrillary tangles, which are formed by abnormal neuronal fiber aggregation. The main fiber structures in AD are double helices, in which the main component in the core is tau protein. Hyperphosphorylation of tau protein inhibits binding to microtubules and destabilizes the cytoskeletal system, causing the formation of double helix fibers, eventually leading to neuronal death (Hanger et al., 2009; George et al., 2014; Nisbet et al., 2015).
Calcium/calmodulin dependent protein kinase IV (CAMK4) can phosphorylate microtubule-associated protein 2 and tau protein (Miyano et al., 1992). CAMK4 is a multifunctional serine/threonine kinase, coded by the CAMK4 gene in humans, and is highly expressed in the brain and thymus (Heist et al., 1998; Hook et al., 2001). Calmodulin (CALM) and calcium/calmodulin dependent protein kinase kinase 1 (CAMKK1) are necessary for the activation of CAMK4. CALM is a binding protein, which combines with Ca2+ when intracellular Ca2+ increases sufficiently to form calcium-calmodulin complexes (Ca2+/CALM). Ca2+/CALM can activate CAMKK1 by phosphorylation. Then CAMKK can activate CAMK4 aslo by phosphorylation (Soderling, 1999; Fujisawa, 2001; Hook et al., 2001). CAMK4 regulates various cellular activities through the phosphorylation of transcription factors, affecting the immune response, inflammation and memory consolidation (Hanissian et al., 1993; Fukushima et al., 2008).
Genistein (GS) is an 4′,5,7-3 hydroxy isoflavone extracted from soybean, pueraria and other plant species. The chemical structure of GS is similar to endogenous estrogen, and it exhibits effects by binding to the cell membrane and intracellular estrogen receptors (Wang et al., 1996; Kuiper et al., 1997). Increasing evidence has revealed that GS can prevent tumors, cardiovascular disease, osteoporosis and menopausal symptoms, and also improve the immune function of organisms (Chinigarzadeh et al., 2015; Gupta et al., 2015; Shafiee et al., 2016; Yazdani et al., 2016; Odle et al., 2017). Furthermore, several studies have found that GS has a neuroprotective effect in the prevention and treatment of AD, and can resist toxicity, reduce the deposition of Aβ, inflammatory damage and calcium levels (Luo et al., 2012; Ma et al., 2015; Bonet-Costa et al., 2016). However, the mechanism of action of GS is not clear. Studies have also found that GS can significantly improve learning and memory in AD model rats and reduce apoptosis in the hippocampus. It has been suggested that its mechanism may involve inhibition of the mitochondrial apoptosis pathway (Wang et al., 2016). However, the neuroprotective effect of GS in AD needs further investigation.
Tau can be phosphorylated by CAMK4 and its hyperphosphorylation causes the formation of neurofibrillary tangles (Miyano et al., 1992). Therefore, we focus on whether the neuroprotective effect of GS in AD occurs through alleviation of tau hyperphosphorylation by regulating CAMK4. Here, we used the Morris water maze experiment, hematoxylin-eosin staining and western blot assays to explore the neuroprotective effects of GS in AD model rats and the expression of p-tau, tau, p-CAMK4, CALM and CAMKK1, to further elucidate the mechanisms of GS on the prevention of AD.
Materials and Methods
Animals
A total of 80 female Sprague-Dawley rats aged 10 months and weighing 250–350 g were sourced from the Animal Experiment Center, Anhui Medical University, China (certificate No. SXCK (Wan) 2011-002).
The study protocol was approved by the Animal Management Center of Anhui University of Chinese Medicine, China. The experimental procedure followed the United States National Institutes of Health Guide for the Care and Use of Laboratory Animals (NIH Publication No. 85-23, revised 1986).
The rats were randomly divided into four groups: sham-operated group (sham group), AD group, GS group and estradiol valerate (EV) group as positive control group (n = 20 per group).
Drug intervention
GS (Sigma, St. Louis, MO, USA) was suspended in 0.5% sodium carboxymethyl cellulose (CMC-Na) (Zhiyuan Chemical Reagent, Tianjin, China) at a concentration of 9 mg/mL, and EV (Delpharm Lille SAS, ZI de Roubaix-Est, France) was dissolved in distilled water to a concentration of 0.03 mg/mL for further use. Seven-day pretreatments with GS, EV and CMC-Na were intragastrically administered (1 mL/100 g body weight) before the establishment of the AD model, which was followed for 49 days.
Establishment of rat models
We intraperitoneally injected 100 mg/kg D-galactose (Sinopharm Chemical Reagent, Shanghai, China) in combination with a bilateral hippocampal injection of 10 μg Aβ25–35 (Sigma) to establish a rat model of AD.
The GS group received 90 mg/kg GS, which in a previous study had the best effect on the prevention of AD (Wang et al., 2016). The EV group received 0.3 mg/kg EV.
After 7 days of pretreatment with drugs, rats in the AD, GS and EV groups were intraperitoneally injected with 100 mg/kg D-galactose once a day, for 42 days. After treatment with D-galactose for 21 days, the rats were fastened to the brain stereotaxic instrument (Stoelting, Wood Dale, IL, USA), injected with 5 μL (10 μg) Aβ25–35 with a microsyringe (Anting Scientific Instrument, Shanghai, China) in the bilateral hippocampi respectively. The injection point was located 4.4 mm posterior to and 2.2 mm from to the anterior fontanelle as origin point, and the needle was 3.0 mm from the rat hippocampus (George et al., 1997). The left hippocampus was localized first, then the right hippocampus. The sham group was injected with an equal volume of saline. Increased escape latencies and decreased frequency of crossing the original platform in Morris water maze test, which were statistically significant compared with the sham group, demonstrated that the AD rat model was established successfully.
Sample preparation
After drug treatment, 10 rats were randomly selected from each group, and sacrificed by anesthesia with intraperitoneal injection of 10% chloral hydrate (380 mg/kg). The brain tissues were quickly removed on saline ice. Hippocampal tissues were isolated from both sides of the brain and stored at −80°C for western blotting to detect the protein levels of p-tau, tau, p-CAMK4, CALM and CAMKK1.
The remaining 10 rats in each group underwent cardiac perfusion and fixation. After the rats were narcotized as above and the chest was opened to expose the heart. The infusion needle was inserted from the apex and the right atrial appendage was cut. After perfusion with 100–200 mL normal saline through the heart, 250–300 mL 4% paraformaldehyde was perfused until the body was rigid. The brain was removed carefully and fixed in 4% paraformaldehyde for hematoxylin-eosin staining.
Morris water maze tests
The Morris water maze test (Fukuda et al., 2007; Tan, 2009) was used to measure the learning and memory abilities of the rats. After establishment of the AD model on day 36, the rats were trained once a day, for 6 days. In the place navigation test, the rats were put into the water facing the pool wall (back to platform) with an entry point, to observe and record the time needed to find and climb onto the platform (escape latency). If the rats did not find the platform within 90 seconds, they were guided to the platform, and the escape latency was recorded as 90 seconds. The escape latencies of rats were recorded on day 5. In the spatial probe test, the platform was removed on day 6, and the number of times of crossing the original platform location in the pool within 90 seconds was recorded. Data collection and processing were performed using a Morris water maze image automatic monitoring system (ZH, Huaibei, Anhui Province, China).
Hematoxylin-eosin staining
Hematoxylin-eosin staining was used to observe morphological changes in the rat hippocampus. Brain tissue was dehydrated with ethanol, permeabilized with xylene, embedded with paraffin, and sliced into 5 μm sections with a microtome. The sliced specimens were then dewaxed using dimethyl benzene, and dehydrated by ethanol. The morphological changes in the hippocampal CA1 area were observed by hematoxylin-eosin staining and photographs were taken under an optical microscope (BX51; Olympus, Tokyo, Japan).
Western blot assays
Western blot assays were used to detect the protein expression of CALM, CAMKK1, p-CAMK4, tau and p-tau in the rat hippocampus. First, rat hippocampus total protein was extracted using a lysis buffer in the presence of a phosphatase inhibitor followed by centrifugation, and quantified using a bicinchoninic acid kit (Best Bio, Shanghai, China). Protein samples (50 μg) were separated by 10% sodium dodecyl sulfate polyacrylamide gel electrophoresis. The total protein was transferred onto the membrane by a wet method for 2 hours at 120 V. The membrane was blocked in 5% skimmed milk for 2 hours, and washed twice with Tris-buffered saline with Tween (TBST), each for five minutes. The membrane was incubated with rabbit monoclonal antibodies for CALM, CAMKK1, p-CAMK4, tau and p-tau (1:1,000; Cell Signaling Technology, Danvers, MA, USA) at 4°C overnight, and washed 5 times (3 minutes each) with TBST. The membrane was incubated with goat anti-rabbit IgG (1:20,000; ZSGB-BIO, Beijing, China) for 2 hours at room temperature, washed for 3 minutes five times with TBST. Enhanced chemiluminescence was used to visualize the protein bands. The membrane was assessed in a flow cytometry gel imaging system (ProteinSimple, Hercules, CA, USA) on automatic exposure. The image was processed with a gel image processing system (ProteinSimple). The gray value of the protein expression was analyzed by Alpha View Software (ProteinSimple) and the quantities of each protein were calculated as the averages of three replicates with β-actin as an internal reference.
Statistical analysis
Experimental data were expressed as the mean ± SD, and statistically analyzed using SPSS 17.0 software (SPSS, Chicago, IL, USA). Differences between groups were compared using one-way analysis of variance followed by the least significant difference test. A value of P = 0.05 was set as the level of statistical significance.
Results
GS improved learning and memory in AD model rats
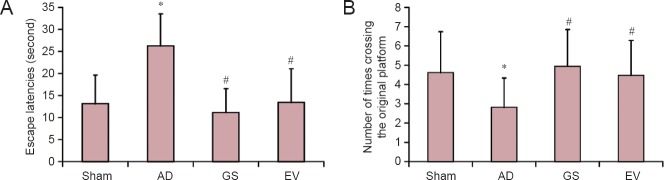
As shown in Figure 1, compared with the sham group, the escape latency of AD rats significantly increased and the number of times crossing the original platform location significantly reduced (P < 0.01). However, compared with the AD group, the escape latencies of the GS and EV groups were significantly reduced (P < 0.01), and the number of times of crossing the original platform location was significantly increased (P < 0.01). This indicates that GS may improve learning and memory in AD model rats.
Figure 1.
Effect of GS on learning and memory in AD model rats (Morris water maze test).
(A, B) Effect of GS on escape latencies (A) and number of times of crossing the original platform location (B) of AD model rats. Data are expressed as mean ± SD (n = 20; one-way analysis of variance and the least significant difference test). *P < 0.01, vs. Sham group; #P < 0.01, vs. AD group. Sham: sham-operated group (injected with normal saline, normal feed); AD: Alzheimer's disease group (intraperitoneal injection of D-galactose in combination with bilateral hippocampal injection of amyloid-β peptide (25–35), 0.5% sodium carboxymethyl cellulose); GS: genistein group (90 mg/kg GS); EV: estradiol valerate group (0.3 mg/kg EV).
GS reduced hippocampal neuron damage in AD model rats
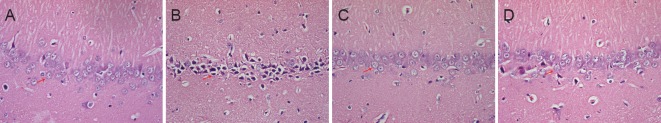
Hippocampal neuron damage in the rats was observed by hematoxylin-eosin staining. As shown in Figure 2, hippocampal neurons in the sham group were strongly packed, uniformly colored (light blue purple) and displayed intact nuclei and hyperchromatic nucleoli (Figure 2A). The hippocampal neuronal cells in the AD group were disordered, the intercellular space became larger, and some cells were dead as characterized by cell shrinkage, pyknosis and dark red staining after lysis (Figure 2B). However, the hippocampal neurons in the GS group were closely packed and uniformly colored (Figure 2C). The number of hippocampal neurons in the EV group increased, and the cytoplasm was rarely darkly stained compared with the AD group (Figure 2D). Compared with the AD group, neuronal damage in the GS and EV groups was rare, which indicated that GS might have neuroprotective effect.
Figure 2.
Effect of GS on morphological changes in the hippocampal CA1 region of AD model rats (hematoxylin-eosin staining, × 400).
(A–D) Morphology in the hippocampal CA1 region in the sham, AD, GS and EV groups, respectively. Compared with the sham group, the number of damaged cells (arrow) was increased in the AD group. Compared with the AD group, the number of damaged cells (arrows) was decreased in the GS and EV groups. Sham: Sham-operated group (injected with normal saline, normal feed); AD: Alzheimer's disease group (intraperitoneal injection of D-galactose in combination with bilateral hippocampal injection of amyloid-β peptide (25–35), 0.5% sodium carboxymethyl cellulose); GS: genistein group (90 mg/kg GS); EV: estradiol valerate group (0.3 mg/kg EV).
GS reduced the level of p-tau in the hippocampus of AD rats
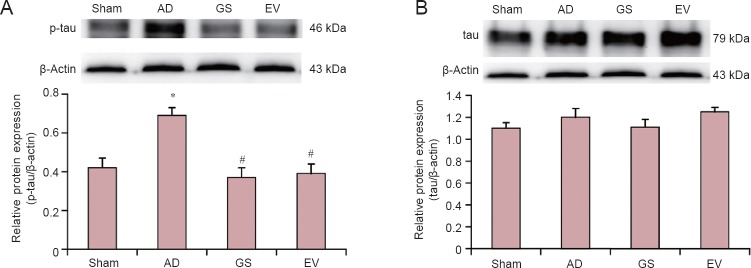
The levels of tau and p-tau in the hippocampus of AD rats were detected by western blot assay. Our results showed that p-tau protein expression was highest in the hippocampus in the AD group, and the levels of p-tau in the hippocampi of the GS and EV groups were lower than in the AD group (Figure 3). Compared with the sham group, the average optical density value of p-tau increased significantly in the AD group (P < 0.01). Compared with the AD group, the protein expression of p-tau significantly decreased in the GS and EV groups (P < 0.01). However, there was no difference in the levels of tau in the hippocampus in the sham, AD, GS and EV groups.
Figure 3.
Changes in p-tau and tau protein expression levels in the hippocampus of AD rats under GS treatment.
(A, B) p-tau and tau protein expression in the hippocampus of AD rats under GS treatment. Results are the gray value ratio of target proteins and β-actin. *P < 0.01, vs. sham group; #P < 0.01, vs. AD group. Data are shown as the mean ± SD (n = 3, one-way analysis of variance and the least significant difference test). Sham: Sham-operated group (injected with normal saline, normal feed); AD: Alzheimer's disease group (intraperitoneal injection of D-galactose in combination with bilateral hippocampal injection of amyloid-β peptide (25–35), 0.5% sodium carboxymethyl cellulose); GS: genistein group (90 mg/kg GS); EV: estradiol valerate group (0.3 mg/kg EV). p-tau: Phosphorylated tau protein.
GS reduced the production of CALM, CAMKK1 and p-CAMK4 in the hippocampus of AD rats
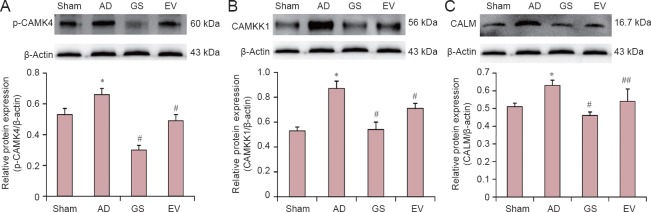
The levels of CALM, CAMKK1 and p-CAMK4 in the hippocampus of AD rats were detected by western blot assay. Our results showed that p-CAMK4, CALM and CAMKK1 levels were highest in the hippocampus in the AD group, and CALM, CAMKK1 and p-CAMK4 were lower in the hippocampus in the GS and EV groups compared with the AD group (Figure 4). Compared with the sham group, the average optical density values of p-CAMK4, CALM and CAMKK1 increased significantly in the AD group (P < 0.01). Compared with the AD group, the protein expression of p-CAMKIV, CALM and CAMKK1 signifcantly decreased in the GS and EV groups (P < 0.05 or P < 0.01). Therefore, our results indicate that GS could reduce the levels of p-CAMK4, CALM and CAMKK1 in the AD model rat hippocampus.
Figure 4.
Changes in p-CAMK4, CAMKK1 and CALM protein expression levels under GS treatment.
(A–C) p-CAMK4, CAMKK1 and CALM protein expression, respectively, in the hippocampus of AD rats under GS treatment. Results are the gray value ratio of target proteins and β-actin. *P < 0.01, vs. sham group; #P < 0.01, ##P < 0.05, vs. AD group. Data are shown as the mean ± SD (n = 3, one-way analysis of variance and the least significant difference test). Sham: Sham-operated group (injected with normal saline, normal feed); AD: Alzheimer's disease group (intraperitoneal injection of D-galactose in combination with bilateral hippocampal injection of amyloid-β peptide (25–35), 0.5% sodium carboxymethyl cellulose); GS: genistein group (90 mg/kg GS); EV: estradiol valerate group (0.3 mg/kg EV). CALM: calmodulin; CAMKK1: calmodulin dependent protein kinase kinase 1; CAMK4: calcium/calmodulin dependent protein kinase IV.
Discussion
AD patients suffer from cognitive obstacles and reduced learning and memory. Senile plaques are one of the main pathological characteristics of AD, and are generally formed by the deposition of Aβ (Galante et al., 2016). In our previous study, intraperitoneal injection of D-galactose in combination with bilateral hippocampal injection of Aβ25–35 was used to establish rat models of AD (Wang et al., 2016). This study indicated that GS could improve the learning and memory abilities of AD rats and reduce neuronal loss in the hippocampus, and had the best effect at a dose of 90 mg/kg. Thus, intraperitoneal injection of D-galactose in combination with bilateral hippocampal injection of Aβ25–35 was chosen to establish an AD rat model, and 90 mg/kg was chosen as the GS dose in this study. Here, we found that the learning ability of AD model rats decreased significantly, and the hippocampal neurons of AD model rats were damaged. These behavioral and pathological results further confirmed the reliability of this animal model. After pre-intervention with GS, we found that GS (90 mg/kg) significantly improved learning and memory, reduced hippocampal neuron damage and had a good neuroprotective effect in AD rats. These results were in accordance with previous findings (Wang et al., 2016).
Neurofibrillary tangles are one of the main pathological characteristics of AD, and hyperphosphorylation of tau protein can cause the production of neurofibrillary tangles (Hanger et al., 2009; Wang, 2016). Tau hyperphosphorylation is induced by many factors, including Aβ deposition in neurons, inflammation and glucose metabolism in the impaired brain (Oddo et al., 2004; Gong et al., 2006). The accumulation of Aβ can exacerbate the hyperphosphorylation of tau in AD (Medeiros et al., 2011). CAMK4 can phosphorylate microtubule-associated protein 2, tau protein, cAMP response element binding protein (CREB) and tyrosine hydroxylase (Miyano et al., 1992; Matthews et al., 1994). CAMK4 is activated by CAMKK1, and CAMKK1 is activated by CALM (Soderling., 1999; Fujisawa., 2001; Hook et al., 2001). In AD, Aβ accumulation causes the constitutive activation of CAMK4 and CREB, and the levels of p-CAMK4, CAMKK1 and p-CREB increase (Marioly et al., 2011). Thus, in this study, p-tau, tau, p-CAMK4, CAMKK1 and CALM were chosen to explore the possible mechanism of GS in preventing AD. Our study found that p-tau, p-CAMK4, CAMKK1 and CALM levels increased significantly in the hippocampi of AD rats, implying that hyperphosphorylation of tau protein is strongly associated with CAMK4.
Our results show that GS shows promise for the reduction of p-tau, p-CAMK4, CAMKK1 and CALM. This indicates that the neuroprotective effects of GS are strongly associated with the alleviation of hyperphosphorylation of tau protein through regulating CAMK4. A possible reason for this is that after GS pre-administration, GS could bind the estrogen receptor to reduce the concentration of Ca2+. This would reduce intracellular calcium overload in the AD model rat, thus decreasing the content of Ca2+/CALM and reducing the activation of CAMKK1. This could then reduce the activation of CAMK4 to alleviate tau hyperphosphorylation, ultimately reducing the formation of neurofibrillary tangles, protecting the normal structure and function of neurons and assisting with the prevention of AD.
In this study, we used the classical Morris water maze test to assess behavior, hematoxylin-eosin staining to assess pathology, and western blot assays to assess protein changes to investigate the influence of GS on the prevention of AD and the possible mechanisms of its effects by alleviating tau hyperphosphorylation through the regulation of CAMK4. Nevertheless, there are some limitations to this study, such as lack of fluorescent quantitative polymerase chain reaction experiments to analyze transcription changes of the MAPT, CAMK4, CAMKK1 and CALM genes. We will investigate this in future experiments.
In conclusion, GS can reduce the hyperphosphorylation of tau protein through regulating CAMK4 in AD model rats. This may be one of the protective mechanisms of GS, and may provide a new experimental basis for AD prevention. Furthermore, future research should focus on the effects of GS on the mechanisms of abnormal tau phosphorylation to further explore the potential prevention mechanisms of GS in AD.
Acknowledgments
We thank Dr. Song Wu from School of Integrated Chinese and Western Medicine, Anhui University of Chinese Medicine, China for his advice on statistical analysis.
Footnotes
Funding: This work was supported by the National Natural Science Foundation of China, No. 81202941 and 81574040; the Key Project Foundation of Oversea Visiting and Research for the Excellent Young and Middle-aged Faculties in Universities of Anhui Province in China, No. gxfxZD2016119; the Key Project Foundation of Natural Science Research in Universities of Anhui Province in China, No. KJ2016A406; the Key Project Foundation of Support Program for the Excellent Young Faculties in Universities of Anhui Province in China, No. gxyq ZD2016138.
Conflicts of interest: None declared.
Research ethics: The study protocol was approved by the Animal Management Center of Anhui University of Chinese Medicine. The experimental procedure followed the United States National Institutes of Health Guide for the Care and Use of Laboratory Animals (NIH Publication No. 85-23, revised 1986).
Data sharing statement: The datasets analyzed during the current study are available from the corresponding author on reasonable request.
Plagiarism check: Checked twice by iThenticate.
Peer review: Externally peer reviewed.
Open peer reviewer: Sage Arbor, Marian University College of Osteo-pathic Medicine, USA.
Copyedited by Wang J, Li CH, Qiu Y, Song LP, Zhao M
References
- Bloom GS. Amyloid-β and tau: the trigger and bullet in Alzheimer disease pathogenesis. JAMA Neurol. 2014;71:505–508. doi: 10.1001/jamaneurol.2013.5847. [DOI] [PubMed] [Google Scholar]
- Bonet-Costa V, Herranz-Pérez V, Blanco-Gandía M, Mas-Bargues C, Inglés M, Garcia-Tarraga P, Rodriguez-Arias M, Miñarro J, Borras C, Garcia-Verdugo JM, Viña J. Clearing amyloid-β through PPARγ/ApoE activation by genistein is a treatment of experimental alzheimer's disease. J Alzheimers Dis. 2016;51:701–711. doi: 10.3233/JAD-151020. [DOI] [PubMed] [Google Scholar]
- Chinigarzadeh A, Muniandy S, Salleh N. Enhanced expression of sodium hydrogen exchanger (NHE)-1, 2 and 4 in the uteri of rat model for post-menopause under phytoestrogen genistein influence. Environ Toxicol Pharmacol. 2015;40:39–48. doi: 10.1016/j.etap.2015.05.003. [DOI] [PubMed] [Google Scholar]
- Fujisawa H. Regulation of the activities of multifunctional Ca2+/calmodulin-dependent protein kinases. J Biochem. 2001;129:193–199. doi: 10.1093/oxfordjournals.jbchem.a002843. [DOI] [PubMed] [Google Scholar]
- Fukuda MT, Francolin-Silva AL, Hernandes AS, Valadares CT, Almeida SS. Effects of early protein malnutrition and scopolamine on learning and memory in the Morris water maze. Nutr Neurosci. 2007;10:251–259. doi: 10.1080/10284150701723818. [DOI] [PubMed] [Google Scholar]
- Fukushima H, Maeda R, Suzuki R, Suzuki A, Nomoto M, Toyoda H, Wu LJ, Xu H, Zhao MG, Ueda K, Kitamoto A, Mamiya N, Yoshida T, Homma S, Masushige S, Zhuo M, Kida S. Upregulation of calcium/calmodulin-dependent protein kinase IV improves memory formation and rescues memory loss with aging. J Neurosci. 2008;28:9910–9919. doi: 10.1523/JNEUROSCI.2625-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Galante D, Corsaro A, Florio T, Vella S, Pagano A, Sbrana F, Vassalli M, Perico A, D’Arrigo C. Differential toxicity, conformation and morphology of typical initial aggregation states of Aβ1-42 and Aβpy3-42 beta-amyloids. Int J Biochem Cell Biol. 2012;44:2085–2093. doi: 10.1016/j.biocel.2012.08.010. [DOI] [PubMed] [Google Scholar]
- Gong CX, Liu F, Grundke-Iqbal I, Iqbal K. Impaired brain glucose metabolism leads to Alzheimer neurofibrillary degeneration through a decrease in tau O-GlcNAcylation. J Alzheimers Dis. 2006;9:1–12. doi: 10.3233/jad-2006-9101. [DOI] [PubMed] [Google Scholar]
- Gupta SK, Dongare S, Mathur R, Mohanty IR, Srivastava S, Mathur S, Nag TC. Genistein ameliorates cardiac inflammation and oxidative stress in streptozotocin-induced diabetic cardiomyopathy in rats. Mol Cell Biochem. 2015;408:63–72. doi: 10.1007/s11010-015-2483-2. [DOI] [PubMed] [Google Scholar]
- Hanger DP, Anderton BH, Noble W. Tau phosphorylation: the therapeutic challenge for neurodegenerative disease. Trends Mol Med. 2009;15:112–119. doi: 10.1016/j.molmed.2009.01.003. [DOI] [PubMed] [Google Scholar]
- Hanissian SH, Frangakis M, Bland MM, Jawahar S, Chatila TA. Expression of a Ca2+/calmodulin-dependent protein kinase, CaM kinase-Gr, in human T lymphocytes. Regulation of kinase activity by T cell receptor signaling. J Biol Chem. 1993;268:20055–20063. [PubMed] [Google Scholar]
- Heist EK, Schulman H. The role of Ca2+/calmodulin-dependent protein kinases within the nucleus. Cell Calcium. 1998;23:103–114. doi: 10.1016/s0143-4160(98)90108-7. [DOI] [PubMed] [Google Scholar]
- Hook SS, Means AR. Ca(2+)/CaM-dependent kinases: from activation to function. Annu Rev Pharmacol Toxicol. 2001;41:471–505. doi: 10.1146/annurev.pharmtox.41.1.471. [DOI] [PubMed] [Google Scholar]
- Kuiper GG, Carlsson B, Grandien K, Enmark E, Häggblad J, Nilsson S, Gustafsson JA. Comparison of the ligand binding specificity and transcript tissue distribution of estrogen receptors alpha and beta. Endocrinology. 1997;138:863–870. doi: 10.1210/endo.138.3.4979. [DOI] [PubMed] [Google Scholar]
- Luo S, Lan T, Liao W, Zhao M, Yang H. Genistein inhibits Aβ25-35-induced neurotoxicity in PC12 cells via PKC signaling pathway. Neurochem Res. 2012;37:2787–2794. doi: 10.1007/s11064-012-0872-4. [DOI] [PubMed] [Google Scholar]
- Ma W, Ding B, Yu H, Yuan L, Xi Y, Xiao R. Genistein alleviates β-amyloid-induced inflammatory damage through regulating Toll-like receptor 4/nuclear factor κB. J Med Food. 2015;18:273–279. doi: 10.1089/jmf.2014.3150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marioly Müller, César Cárdenas, Lijuan Mei, King-Ho Cheung, Kevin Foskett J. Constitutive cAMP response element binding protein (CREB) activation by Alzheimer's disease resenilin-driven inositol trisphosphate receptor (InsP3R) Ca2+ signaling. Proc Natl Acad Sci U S A. 108:13293–13298. doi: 10.1073/pnas.1109297108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matthews RP, Guthrie CR, Wailes LM, Zhao X, Means AR, McKnight GS. Calcium/calmodulin-dependent protein kinase types II and IV differentially regulate CREB-dependent gene expression. Mol Cell Biol. 1994;14:6107–6116. doi: 10.1128/mcb.14.9.6107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Medeiros R, Baglietto-Vargas D, LaFerla FM. The role of tau in Alzheimer's disease and related disorders. CNS Neurosci Ther. 2011;17:514–524. doi: 10.1111/j.1755-5949.2010.00177.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miyano O, Kameshita I, Fujisawa H. Purification and characterization of a brain-specific multifunctional calmodulin-dependent protein kinase from rat cerebellum. J Biol Chem. 1992;267:1198–1203. [PubMed] [Google Scholar]
- Nisbet RM, Polanco JC, Ittner LM, Götz J. Tau aggregation and its interplay with amyloid-β. Acta Neuropathol. 2015;129:207–220. doi: 10.1007/s00401-014-1371-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oddo S, Billings L, Kesslak JP, Cribbs DH, LaFerla FM. Abeta immunotherapy leads to clearance of early, but not late, hyperphosphorylated tau aggregates via the proteasome. Neuron. 2004;43:321–332. doi: 10.1016/j.neuron.2004.07.003. [DOI] [PubMed] [Google Scholar]
- Odle B, Dennison N, Al-Nakkash L, Broderick TL, Plochocki JH. Genistein treatment improves fracture resistance in obese diabetic mice. BMC Endocr Disord. 2017;17:1. doi: 10.1186/s12902-016-0144-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paxinos G, Watson C. The rat brain in stereotaxic coordinates. Third edition. Elsevier; 1997. [DOI] [PubMed] [Google Scholar]
- Pike CJ. Sex and the development of Alzheimer's disease. J Neurosci Res. 2017;95:671–680. doi: 10.1002/jnr.23827. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shafiee G, Saidijam M, Tavilani H, Ghasemkhani N, Khodadadi I. Genistein induces apoptosis and inhibits proliferation of HT29 colon cancer cells. Int J Mol Cell Med. 2016;5:178–191. [PMC free article] [PubMed] [Google Scholar]
- Soderling TR. The Ca-calmodulin-dependent protein kinase cascade. Trends Biochem Sci. 1999;24:232–236. doi: 10.1016/s0968-0004(99)01383-3. [DOI] [PubMed] [Google Scholar]
- Tan SE. Activation of hippocampal nitric oxide and calcium/calmodulin-dependent protein kinase II in response to Morris water maze learning in rats. Pharmacol Biochem Behav. 2009;92:260–266. doi: 10.1016/j.pbb.2008.12.006. [DOI] [PubMed] [Google Scholar]
- Wang GF. Effects of Bushen Yizhi Decoction on Alzheimer's disease model rats induced by D-galactose combined with amyloid-beta 25-35 and the underlying mechanism. Zhongguo Zuzhi Gongcheng Yanjiu. 2016;20:7307–7313. [Google Scholar]
- Wang TT, Sathyamoorthy N, Phang JM. Molecular effects of genistein on estrogen receptor mediated pathways. Carcinogenesis. 1996;17:271–275. doi: 10.1093/carcin/17.2.271. [DOI] [PubMed] [Google Scholar]
- Wang Y, Cai B, Shao J, Wang TT, Cai RZ, Ma CJ, Han T, Du J. Genistein suppresses the mitochondrial apoptotic pathway in hippocampal neurons in rats with Alzheimer's disease. Neural Regen Res. 2016;11:1153–1158. doi: 10.4103/1673-5374.187056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yazdani Y, Sharifi Rad MR, Taghipour M, Chenari N, Ghaderi A, Razmkhah M. Genistein suppression of matrix metalloproteinase 2 (MMP-2) and vascular endothelial growth factor (VEGF) expression in mesenchymal stem cell like cells isolated from high and low grade gliomas. Asian Pac J Cancer Prev. 2016;17:5303–5307. doi: 10.22034/APJCP.2016.17.12.5303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yin CH, Li SO, Zhao WN, Feng JC. Brain imaging of mild cognitive impairment and Alzheimer's disease. Neural Regen Res. 2013;8:435–444. doi: 10.3969/j.issn.1673-5374.2013.05.007. [DOI] [PMC free article] [PubMed] [Google Scholar]