Abstract
Alzheimer's disease, a progressive neurodegenerative disease, affects learning and memory resulting from cholinergic dysfunction. Scopolamine has been employed to induce Alzheimer's disease-like pathology in vivo and in vitro through alteration of cholinergic system. N-benzylcinnamide (PT-3), purified from Piper submultinerve, has been shown to exhibit neuroprotective properties against amyloid-β-induced neuronal toxicity in rat cortical primary cell culture and to improve spatial learning and memory of aged rats through alleviating oxidative stress. We proposed a hypothesis that PT3 has a neuroprotective effect against scopolamine-induced cholinergic dysfunction. PT-3 (125–200 nM) pretreatment was performed in human neuroblastoma SH-SY5Y cell line following scopolamine induction. PT-3 (125–200 nM) inhibited scopolamine (2 mM)-induced generation of reactive oxygen species, cellular apoptosis, upregulation of acetylcholinesterase activity, downregulation of choline acetyltransferase level, and activation of p38 and JNK signalling pathways. These findings revealed the underlying mechanisms of scopolamine-induced Alzheimer's disease-like cellular dysfunctions, which provide evidence for developing drugs for the treatment of this debilitating disease.
Keywords: Alzheimer's disease, acetylcholine, apoptosis, acetylcholinesterase inhibitor, oxidative stress, N-benzylcinnamide, natural product, scopolamine, neuronal regeneration
Introduction
Alzheimer's disease (AD), an age-related neurodegenerative disease, is the most common cause of dementia (Kumar et al., 2015). Hyperphosphorylation of tau protein generates neuritic plaques comprising amyloid-β and neurofibrillary tangles, which are characteristics of AD (Anand et al., 2014). A cholinergic deficit, particularly in basal forebrain, together with a decrease in acetylcholine promotes cognitive impairment (Schliebs and Arendt, 2011). In addition, the presence of acetylcholinesterase (AChE), results in a decline in cholinergic transmission (Mesulam, 2013). Currently, primary treatment for AD is a cholinergic replacement therapy based on AChE inhibitors, such as donepezil, galantamine, and rivastigmine (Zemek et al., 2014).
Scopolamine, a muscarinic acetylcholine receptor antagonist used in animal models and in humans (Bajo et al., 2015; Ghumatkar et al., 2015; Alvarez-Jimenez et al., 2016), induces cognitive impairment, which is associated with an attenuation of cholinergic neurotransmission as well as an increase in oxidant stress and inflammation (Kwon et al., 2010; Min et al., 2015; Balaban et al., 2017). Scopolamine has been employed in in vitro testing of new compounds, especially those from natural products, for their potential to restore cognitive impairment. For example, scopolamine causes cytotoxicity and downregulation of neuronal and glial cell markers in neuronal (IMR32) and C6 glioma cells respectively, properties that are reversed upon treatment of an alcoholic extract of Ashwagandha leaves (Konar et al., 2011). Pandareesh and Anand (2013) demonstrated that pre-treatment with Bacopa monniera extract protects against scopolamine-induced damage in rat phochromocytoma PC12 cell line associated with upregulation of AChE and downregulation of brain-derived neurotrophic factor. Lee et al. (2014) reported that sulforaphane (10 or 20 μM), an organosulfur compound present in cruciferous vegetables, attenuates acetylcholine and choline acetyltransferase (ChAT) expression in scopolamine-treated primary mouse cortical neurons.
N-benzylcinnamide (PT-3), purified from Piper submultinerve, has been shown to exhibit neuroprotective properties against amyloid-β-induced neuronal toxicity in rat cortical primary cell culture (Thangnipon et al., 2013). PT-3 treatment also improves spatial learning and memory of aged rats through impacting on parameters associated with oxidant stress, inflammation, apoptosis, and AChE activity (Thangnipon et al., 2015). In addition, PT-3, in combination with bone morphogenetic protein 9, induces neuronal differentiation of human amniotic fluid mesenchymal stem cells by enhancing β-III tubulin-containing cell numbers and ChAT content (Thangnipon et al., 2016).
Hence, in this study, we investigated the neuroprotective mechanisms of PT-3 against scopolamine-induced cholinergic dysfunction in human neuroblastoma SH-SY5Y cell line.
Materials and Methods
Chemicals and reagents
Unless otherwise indicated, media and supplements used for cell culture were obtained from Gibco (Carlsbad, CA, USA). Scopolamine hydrobromide, 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT), 2,7-dichlorodihydrofluorescein diacetate (DCFH-DA), 5,5′-dithio-bis-(2-nitrobenzoic acid) (DTNB), acetylthiocholine iodine and Bradford reagent were from Sigma (St. Louis, MO, USA). Rabbit anti-ChAT antibody was from Abcam (Cambridge, MA, USA) and other primary and secondary antibodies were from Cell Signalling Technology (Cell Signalling Technology Inc., MA, USA).
Cell culture and treatment
Human neuroblastoma SH-SY5Y cell line was a kind gift from Dr. Martin Broadstock (Wolfson Centre for Age Related Diseases, King's College London, UK). Cells were cultured in Dulbecco's modified Eagle's medium (DMEM) supplemented with 10% fetal bovine serum (FBS), 100 U/mL penicillin, and 100 μg/mL streptomycin at 37°C under a humidified atmosphere of 5% CO2 and 95% air. At 80–90% confluence, cells were treated with 0.25% trypsin-ethylenediaminetetraacetic acid (EDTA) and plated onto a 96- or 24-well plate at a density of 2 × 105 cells/mL. Culture medium was changed to serum-free DMEM (Huang et al., 2014) supplemented with 50–200 nM of PT-3 for 1 hour (Thangnipon et al., 2013) prior to incubation with 0.1–3 mM (usually 2 mM) scopolamine hydrobromide for 24 hours (Pandareesh and Anand, 2013). All experiments were performed using cells from passages 18 to 20.
Cell viability assay
Cells were incubated with 0.5 mg/mL MTT at 37°C for 3 hours, then supernatant was removed and A570 nm of the blue formazan crystals dissolved in 100 μL of dimethyl sulfoxide (DMSO) was measured with a microplate reader (SpectraMax® 180, Sunnyvale, CA, USA) as described previously (Ivins et al., 1999). Cell viability was presented as the percentage of A570 nm of each sample relative to control.
Reactive oxygen species (ROS) assay
A DCFH-DAdye assay was used to determine levels of intracellular ROS levels (Lin et al., 2000). In brief, following sequential treatment with PT-3 and scopolamine, cells were incubated with 50 μM DCFH-DA in absolute ethanol for 1 hour in the dark at 37°C. Dichlorofluorescein (DCF) fluorescence (485 nm excitation, 530 nm emission) was quantified using a multimode reader (DTX880, Beckman Coulter, Wals, Austria).
Acetylcholinesterase (AChE) activity assay
SH-SY5Y cells (5 × 105/mL) were cultured in complete DMEM medium in a 96-well plate for 24 hours, then incubated with 50–200 nM PT-3 for 1 hour, followed by 2 mM scopolamine in serum-free DMEM for further 24 hours. Then cells were washed twice with 100 μL of phosphate-buffered saline (PBS) and incubated at room temperature with 50 μL of 1.3 mM 5,5′-dithiobis(2-nitrobenzoic acid) (DTNB) and 50 μL of 1.9 mM acetylthiocholine iodide, both dissolved in PBS (Ellman et al., 1961; Gustafsson et al., 2010). A420 nm of TNB2– was measured at 1, 15, and 30 minutes using a multimode reader (DTX880, Beckman Coulter, Fullerton, CA, USA). Absorbance without substrate or cells was subtracted as background.
Western blot analysis
Cell lysate proteins (30 μg; determined using Bradford method) were separated by 10% sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) under sulfhydryl-reducing condition and transferred onto polyvinylidene fluoride membrane. Membranes were incubated with the following primary antibodies in TBS-T overnight at 4°C at the specified dilution: rabbit anti-ChAT (1:1,000 dilution), anti-Bcl-2 (1:1,000 dilution), anti-Bax (1:1,000 dilution), anti-activated caspase-3 (1:500 dilution), anti-phospho-p38 (1:1,000 dilution), anti-p38 (1:1,000 dilution), anti-phospho-ERK1/2 (1:1,000 dilution), anti-ERK1/2 (1:1,000), anti-phospho-JNK (1:1,000), anti-JNK (1:1,000), anti-phospho-Akt (1:1,000 dilution), anti-Akt (1:1,000 dilution) or anti-β-actin (1:2,500 dilution) (latter as internal control) antibodies, and then incubated with horseradish peroxidase (HRP)-conjugated secondary anti-rabbit IgG (1:1,000 dilution) antibodies at room temperature for 1 hour. Immunoreactive protein bands were detected using an ECL western blotting substrate (Bio-Rad, Hercules, CA, USA) and recorded on HyperfilmTM (Amersham Pharmacia Biotech). Band density was determined as scanning units using imageJ software (National Institutes of Health, MA, USA) and expression levels were quantified relative to that of β-actin.
Statistical analysis
One-way analysis of variance (ANOVA) was performed using Prism 5.0a (GraphPad Software, Inc., San Diego, CA, USA) to measure statistical significance of differences, followed by Student-Newman-Keuls test. Data are presented as the mean ± SEM of three independent experiments in triplicate. Results are considered statistically significant at P-value < 0.05.
Results
PT-3 attenuated scopolamine-induced cytotoxicity, ROS generation and apoptosis of SH-SY5Y cells
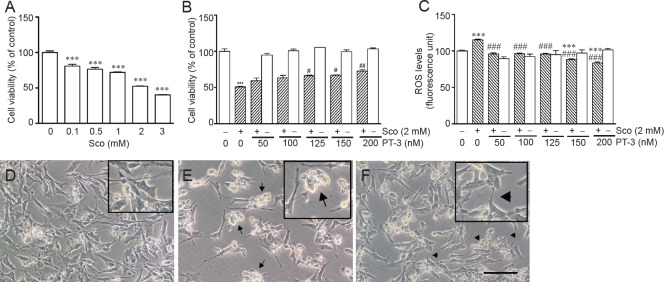
Exposure to scopolamine (1–3 mM) for 24 hours significantly decreased human neuroblastoma SH-SY5Y cell viability in a dose-dependent manner (Figure 1A), which is in accordance with findings from a previous study (Konar et al., 2011). In all subsequent experiments, 2 M scopolamine resulted in 50.6 ± 2.2% loss in cell viability, which was inhibited by pretreatment with 125–200 nM PT-3 for 1 hour in a concentration-dependent manner (Figure 1B). In addition, PT-3 pretreatment ameliorated scopolamine-induced ROS formation over the same concentration range in a dose-dependent manner (Figure 1C). Treatment with PT-3 alone had no apparent effect on cell viability or endogenous ROS level.
Figure 1.
N-benzylcinnamide (PT-3) pretreatment prevented scopolamine (Sco)-induced cytotoxicity and intracellular reactive oxygen species (ROS) generation in SH-SY5Y cells.
Cell viability and intracellular ROS levels were determined using MTT assay and DCFH-DA reagent respectively. (A) Viability of SH-SY5Y cells exposed to 2 mM scopolamine. (B) Viability of SH-SY5Y cells treated with 125–200 nM PT-3 prior to 2 mM scopolamine exposure was significantly increased. (C) Intracellular ROS production of SH-SY5Y cells treated with 125–200 nM PT-3 prior to 2 mM scopolamine exposure was significantly decreased. Results are shown as the mean ± SEM of three independent experiments. ***P < 0.001, vs. control; #P < 0.05, ##P < 0.01, ###P < 0.001, vs. scopolamine-treated group (one-way analysis of variance followed by Student-Newman-Keuls test). (D) Morphology observed under inverted phase-contrast microscopy of untreated SH-SY5Y cells. (E) Presence of moribund SH-SY5Y cells (arrows) following 2 mM scopolamine treatment. (F) Prior treatment with 150 nM PT-3 restored neurites of SH-SY5Y cells (arrowheads) subjected to 2 mM scopolamine treatment. The insets in D–F are higher magnification views of SH-SY5Y cells. Scale bar: 50 μm.
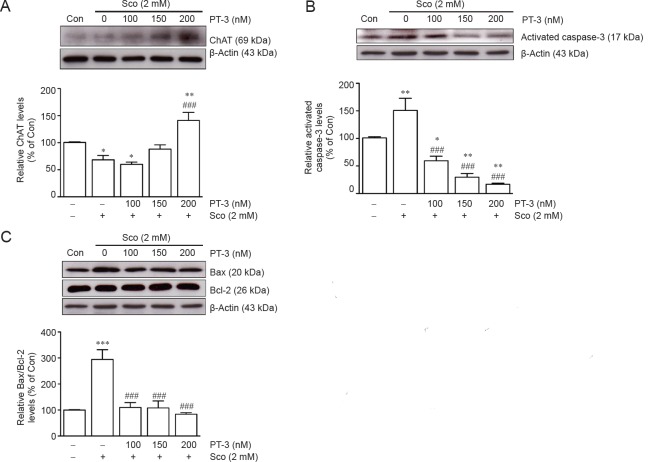
An inverted phase-contrast microscopy revealed that live cells presented shrinkage and cell death (apoptosis) of SH-SY5Y cells following scopolamine exposure, which were prevented by PT-3 pretreatment (Figure 1D–F). Cell apoptosis was confirmed by the presence of activated caspase-3 (Figure 2B) and elevated Bax/Bcl-2 ratio (Figure 2C), which again were diminished by PT-3 (200 nM) pretreatment.
Figure 2.
N-benzylcinnamide (PT-3) pretreatment prevented scopolamine (Sco)-induced reduction of choline acetyltransferase (ChAT) activity and induction of pro-apoptosis events in SH-SY5Y cells.
Cells were treated with PT-3 and Sco as described in legend to Figure 1. ChAT activity, activated caspase-3, Bax and Bcl-2 levels were determined by western blot analysis (upper panels). PT-3 pretreatment increased ChAT activity (A), and reduced activated caspase-3 (B) and Bax/Bcl-2 (C) levels. Results are shown as the mean ± SEM of three independent experiments. *P < 0.05, **P < 0.01, ***P < 0.001, vs. control group; ###P < 0.001, vs. Sco treated group (one-way analysis of variance followed by Student-Newman-Keuls test). Con: Control.
PT-3 suppressed AChE activity but restored ChAT activity in scopolamine-treated SH-SY5Y cells
As scopolamine upregulated AChE activity in rat PC12 cells (Pandareesh and Anand, 2013), a similar phenomenon was observed when SH-SY5Y cells were treated with 2 mM scopolamine, resulting in 150.5 ± 3.5%, 131.7 ± 2.1%, and 113.4 ± 1.5% increase in AChE activity compared to untreated control when assayed for 1, 15 and 30 minutes, respectively (Figure 3), which, as expected, was prevented by PT-3 (50–200 nM) pretreatment. PT-3 restored ChAT activity, reduced by scopolamine, with 1.5 ± 0.16-fold increase in ChAT activity compared to control cells at the highest (200 nM) PT-3 concentration used (Figure 2A).
Figure 3.
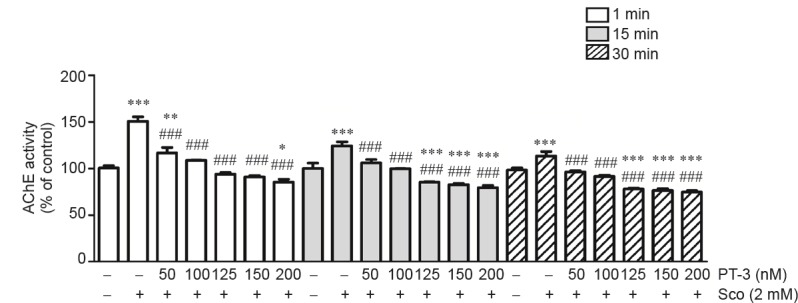
N-benzylcinnamide (PT-3) pretreatment reversed scopolamine (Sco)-induced elevation of acetylcholinesterase (AChE) activity in SH-SY5Y cells.
Cells were treated with PT-3 and Sco as described in legend to Figure 1. AChE activity was measured by hydrolysis of acetylthiocholine. PT-3 treatment reduced AChE activity determined at 1, 15 and 30 minutes (min), respectively. Results are shown as the mean ± SEM of three independent experiments. *P < 0.05, **P < 0.01, ***P < 0.001, vs. control group; ###P < 0.001, vs. Sco-treated group (one-way analysis of variance followed by Student-Newman-Keuls test).
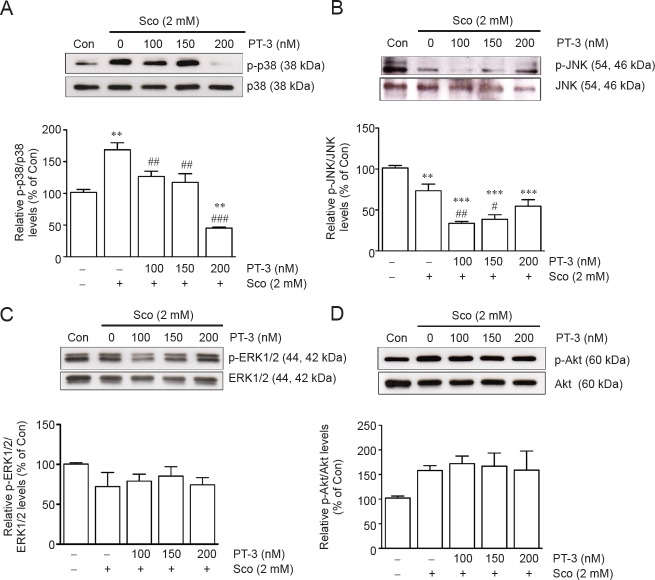
PT-3 modulated p38 and JNK pathways in SH-SY5Y cells exposed to scopolamine
Scopolamine activated redox-responsive cell signaling pathways, such as oxidative stress, inflammation, and cell death (Foyet et al., 2015). In order to identify the pathway(s) involved in the situation of SH-SY5Y cells, western blot analysis was employed to identify key signature factors of activated signaling pathways. Exposure to 2 mM scopolamine resulted in 1.6-fold increase in phospho-p38/p38 ratio over untreated control SH-SY5Y cells, and this was restored to near control value by prior incubation with 100–150 nM PT-3, but only to 50.2 ± 1.8% of control with 200 nM PT-3 (Figure 4A). Whereas 2 mM scopolamine reduced phospho-JNK/JNK ratio to 75.4 ± 2.4% of control cells, which was further reduced by 100 and 150 nM PT-3 pretreatment (Figure 4A, B). However, phosphorylation of ERK1/2 and Akt was not affected (Figure 4C, D).
Figure 4.
N-benzylcinnamide (PT-3) pretreatment reversed scopolamine (Sco)-induced upregulation of phosphorylated p38 (p-p38) and downregulation of phosphorylated JNK (p-JNK), but did not affect phosphorylation of ERK1/2 and Akt in SH-SY5Y cells (western blot analysis).
Cells were treated with PT-3 and Sco as described in legend to Figure 1. Levels of p38, p-p38, JNK, p-JNK, ERK1/2, p-ERK1/2, Akt, and p-Akt were determined by western blot analysis (upper panels). (A) Phospho-p38/p38 ratio. (B) Phospho-JNK/JNK ratio. (C) Phospho-ERK1/2/ERK1/2. (D) p-Akt/Akt ratio. Results are shown as the mean ± SEM of three independent experiments. **P < 0.01, ***P < 0.001, vs. control group; #P < 0.05, ##P < 0.01, ###P < 0.001, vs. Sco-treated group (one-way analysis of variance followed by Student-Newman-Keuls test). Con: Control.
Discussion
SH-SY5Y cells were chosen for study as they are commonly used as models to investigate in vitro neuronal function (Forster et al., 2016) and to assess pharmacological and toxicological potential of AChE inhibitors (Kanhed et al., 2015; Santillo and Liu, 2015). In a previous study (Zhang et al., 2016), scopolamine-induced ROS formation and cellular apoptosis (via the classical Bax/Bcl-2 pathway) in SH-SY5Y cells were ameliorated by pretreatment with PT-3. PT-3 manifests antioxidant property (Thangnipon et al., 2013). C6 glioma cells pre-treated with 0.5–2 μM lactucopicrin, a sesquiterpene lactone derived from Lactuca virosa and Cichorium intybus, have reduced 2′,7′-dichlorofluorescein toxicity induced by 3 mM scopolamine (Venkatesan et al., 2016).
Interestingly, PT-3 pretreatment was capable of suppressing scopolamine-stimulated SH-SY5Y AChE activity. Xian et al. (2015) reported that Honokiol, a lignin from the bark of Magnolia officinalis, decreases AChE activity in brain tissues of scopolamine-treated mice. Honokiol contains two phenolic groups, which can exhibit antioxidant property similar to PT-3 (Dikalov et al., 2008).
There is evidence that undifferentiated or differentiated SH-SY5Y cells contain AChR mRNA (Korecka et al., 2013). In this study, PT-3 restored ChAT activity in SH-SY5Y cells that had been reduced by scopolamine, probably by increasing ChAT expression and thereby restoring cholinergic cell function. This property is not confined to PT-3 as Lim et al. (2016) recently demonstrated that curcumin, a natural antioxidant, increases immunoreactive ChAT level in scopolamine-treated mouse hippocampus and improves learning impaired by scopolamine insult.
The activation of p38 and JNK signalling pathways is associated with cellular stress and pro-inflammatory responses (Davis, 2000). Scopolamine induces a number of pathological phenomena associated with neurodegenerative diseases linked to memory decline, including increased oxidative stress, impaired antioxidative defence system and mitochondrial dysfunction (Wong-Guerra et al., 2017). Additionally, JNK and p38 are stress-activated MAP kinases preferentially activated by cell stress-inducing signals, including oxidative stress, environmental stress and toxic chemical insults. Sustained activation of JNK or p38 is implicated in the induction of many forms of neuronal apoptosis in response to a variety of cellular injuries (Wong-Guerra et al., 2017). In scopolamine-treated SH-SY5Y cells, PT-3 inhibits phosphorylation of p38 and JNK, as have been observed in rat primary cultures and human mesenchymal stem cells (Thangnipon et al., 2013, 2016). Although low doses of PT-3 (100–150 nM) inhibited scopolamine-induced cytotoxicity via inactivation of JNK, surprisingly high dose of PT-3 (200 nM) enhanced JNK activation (but lower than that in control cells) as has been reported by Shi et al. (2009). In that study, low concentrations (50–100 μg/mL) of EGb761, an extract from Ginkgo biloba leaves, inhibits H2O2-induced cell apoptosis via suppression of JNK and caspase-3 activation while high concentrations (250–500 μg/mL) enhance JNK phosphorylation. It is worth noting that pre-treatment with a number of natural phenolic compounds show similar effects on p38 and JNK pathways in oxidant stressed SH-SY5Y cells (Dhanalakshmi et al., 2015; Zhu et al., 2015).
ROS plays a critical role in cell signalling, particularly in redox mechanisms involved in apoptosis, such as the mitochondria-to-cytosol release of cytochrome c, a central event in apoptosis initiation (Circu and Aw, 2010). Interestingly, a Liriope platyphylla extract exerts neuroprotective effects against H2O2 by modulating only p38 and not ERK in SH-SY5Y cells (Park et al., 2015). In addition, p38 inhibitor SB203580 blocks cell loss in H2O2-treated SH-SY5Y cells (Park et al., 2015). Thus, in SH-SY5Y cells, H2O2 requires p38 activation for cytotoxicity.
Lee et al. (2005) suggested that baicalein, a flavonoid from Scutellaria root, induces a significant reduction in phospho-JNK level of 6-hydroxydopamine-induced neuronal cell death. They also found that ERK and Akt pathways are not altered by scopolamine treatment. These pathways are responsible for cell proliferation, cell differentiation and cell survival (Junttila et al., 2008). Similarly, Voleti et al. (2013) found a low dose of scopolamine treatment (25 μg/kg) does not disturb activated ERK level and has a minimal effect on phospho-Akt in cultured rat pyramidal neurons. Gunjima et al. (2014) reported a similar observation of protection by 3,4-dihydroxybenzalacetone, a catechol-containing compound isolated from Inonotus obliquus (persoon) Pilat, against 6-hydroxydopamine toxicity in SH-SY5Y cells that is not affected by ERK inhibitor PD98059. Similarly, Wang et al. (2014) demonstrated that glycyrrhizic acid, a major compound of Glycyrrhiza radix, alone or in combination with 20 mM glutamate, has no effect on phospho-Akt level in PC12 cells.
Additional file: Open peer review report 1 (67KB, pdf) .
“Neuroprotection of N-benzylcinnamide on scopolamine-induced cholinergic dysfunction in human SH-SY5Y neuroblastoma cells”
Additional file: Open peer review report 2 (71.1KB, pdf) .
“Neuroprotection of N-benzylcinnamide on scopolamine-induced cholinergic dysfunction in human SH-SY5Y neuroblastoma cells”
Acknowledgments
The authors are grateful to Professor Prapon Wilairat, Mahidol University, Thailand for critical reading of the manuscript.
Footnotes
Funding: The study was financially supported by a joint Mahidol University and The Thailand Research Fund (TRF) grant (IRG5780009), TRF Royal Golden Jubilee Ph.D. Program (grant No. PHD/0175/2552) and the Office of the Higher Education Commission, Ministry of Education, Thailand.
Conflicts of interest: The authors declare that there are no conflicts of interest.
Data sharing statement: The datasets analyzed during the current study are available from the corresponding author on reasonable request.
Plagiarism check: Checked twice by iThenticate.
Peer review: Externally peer reviewed.
Open peer review reports:
Reviewer 1: Aysegul Yildiz-Unal, Mugla Sitki Kocman University, Turkey.
Comments to authors: There are a number of studies with PT-3 showing its protective effect on degenerating primary neuron cultures. In one of them, Thangnipon W. et al. showed that N-benzylcinnamide protects rat cultured cortical neurons from β-amyloid peptide-induced neurotoxicity. After 2 years, the same research group showed the potential role of N-benzylcinnamide in inducing cholinergic neuronal differentiation. Since scopalamine is also an Alzheimer's disease-like pathology inducer, it makes sense to analyze the protective role of PT-3 on scopalamine -induced Alzheimer's disease-like pathology in a different cell type, SH-SY5Y neuroblastoma cells. See additional file for more details.
Reviewer 2: Siyu Zhang, University of California Berkeley, USA.
Comments to authors: Strengths: the authors tested the changes of the scopolamine induced in SH-SY5Y cells. Weakness: To establish a sound model in SH-SY5Y by scopolamine, the authors did not provide a positive control group which can ameliorate those changes induced by scopolamine. See additional file for more details.
Copyedited by Li CH, Song LP, Zhao M
References
- Alvarez-Jimenez R, Groeneveld GJ, van Gerven JM, Goulooze SC, Baakman AC, Hay JL, Stevens J. Model-based exposure-response analysis to quantify age related differences in the response to scopolamine in healthy subjects. Br J Clin Pharmacol. 2016;82:1011–1021. doi: 10.1111/bcp.13031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anand R, Gill KD, Mahdi AA. Therapeutics of Alzheimer's disease: Past, present and future. Neuropharmacology. 2014;76:27–50. doi: 10.1016/j.neuropharm.2013.07.004. [DOI] [PubMed] [Google Scholar]
- Bajo R, Pusil S, Lopez ME, Canuet L, Pereda E, Osipova D, Maestu F, Pekkonen E. Scopolamine effects on functional brain connectivity: a pharmacological model of Alzheimer's disease. Sci Rep. 2015;5:9748. doi: 10.1038/srep09748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Balaban H, Naziroglu M, Demirci K, Ovey IS. The protective role of selenium on scopolamine-induced memory impairment, oxidative stress, and apoptosis in aged rats: the involvement of TRPM2 and TRPV1 channels. Mol Neurobiol. 2017;54:2852–2868. doi: 10.1007/s12035-016-9835-0. [DOI] [PubMed] [Google Scholar]
- Circu ML, Aw TY. Reactive oxygen species, cellular redox systems, and apoptosis. Free Radic Biol Med. 2010;48:749–762. doi: 10.1016/j.freeradbiomed.2009.12.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davis RJ. Signal transduction by the JNK group of MAP kinases. Cell. 2000;103:239–252. doi: 10.1016/s0092-8674(00)00116-1. [DOI] [PubMed] [Google Scholar]
- Dhanalakshmi C, Manivasagam T, Nataraj J, Thenmozhi A, Essa MM. Neurosupportive role of Vanillin, a natural phenolic compound, on rotenone induced neurotoxicity in SH-SY5Y neuroblastoma cells. Evid Based Complement Alternat Med. 2015;2015:626028. doi: 10.1155/2015/626028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dikalov S, Losik T, Arbiser JL. Honokiol is a potent scavenger of superoxide and peroxyl radicals. Biochem Pharmacol. 2008;76:589–596. doi: 10.1016/j.bcp.2008.06.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ellman GL, Courtney KD, Andres V, Feather-Stone RM. A new and rapid colorimetric determination of acetylcholinesterase activity. Biochem Pharmaco. 1961;l7:88–95. doi: 10.1016/0006-2952(61)90145-9. [DOI] [PubMed] [Google Scholar]
- Forster JI, Koglsberger S, Trefois C, Boyd O, Baumuratov AS, Buck L, Balling R, Antony PM. Characterization of differentiated SH-SY5Y as neuronal screening model reveals increased oxidative vulnerability. J Biomol Screen. 2016;21:496–509. doi: 10.1177/1087057115625190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Foyet HS, Abaissou HH, Wado E, Acha EA, Alin C. Emilia coccinae (SIMS) G extract improves memory impairment, cholinergic dysfunction, and oxidative stress damage in scopolamine-treated rats. BMC Complement Altern Med. 2015;15:333. doi: 10.1186/s12906-015-0864-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ghumatkar PJ, Patil SP, Jain PD, Tambe RM, Sathaye S. Nootropic, neuroprotective and neurotrophic effects of phloretin in scopolamine induced amnesia in mice. Pharmacol Biochem Behav. 2015;135:182–191. doi: 10.1016/j.pbb.2015.06.005. [DOI] [PubMed] [Google Scholar]
- Gunjima K, Tomiyama R, Takakura K, Yamada T, Hashida K, Nakamura Y, Konishi T, Matsugo S, Hori O. 3,4-dihydroxybenzalacetone protects against Parkinson's disease-related neurotoxin 6-OHDA through Akt/Nrf2/glutathione pathway. J Cell Biochem1. 2014;15:151–160. doi: 10.1002/jcb.24643. [DOI] [PubMed] [Google Scholar]
- Gustafsson H, Runesson J, Lundqvist J, Lindegren H, Axelsson V, Forsby A. Neurofunctional endpoints assessed in human neuroblastoma SH-SY5Y cells for estimation of acute systemic toxicity. Toxicol Appl Pharmacol. 2010;245:191–202. doi: 10.1016/j.taap.2010.02.018. [DOI] [PubMed] [Google Scholar]
- Huang HC, Tang D, Xu K, Jiang ZF. Curcumin attenuates amyloid-β-induced tau hyperphosphorylation in human neuroblastoma SH-SY5Y cells involving PTEN/Akt/GSK-3β signaling pathway. J Recept Signal Transduct Res. 2014;34:26–37. doi: 10.3109/10799893.2013.848891. [DOI] [PubMed] [Google Scholar]
- Ivins KJ, Ivins JK, Sharp JP, Cotman CW. Multiple pathways of apoptosis in PC12 cells. CrmA inhibits apoptosis induced by β-amyloid. J Biol Chem. 1999;274:2107–2112. doi: 10.1074/jbc.274.4.2107. [DOI] [PubMed] [Google Scholar]
- Junttila MR, Li SP, Westermarck J. Phosphatase-mediated crosstalk between MAPK signaling pathways in the regulation of cell survival. FASEB J. 2008;22:954–965. doi: 10.1096/fj.06-7859rev. [DOI] [PubMed] [Google Scholar]
- Kanhed AM, Sinha A, Machhi J, Tripathi A, Parikh ZS, Pillai PP, Giridhar R, Yadav MR. Discovery of isoalloxazine derivatives as a new class of potential anti-Alzheimer agents and their synthesis. Bioorg Chem. 2015;61:7–12. doi: 10.1016/j.bioorg.2015.05.005. [DOI] [PubMed] [Google Scholar]
- Konar A, Shah N, Singh R, Saxena N, Kaul SC, Wadhwa R, Thakur MK. Protective role of Ashwagandha leaf extract and its component withanone on scopolamine-induced changes in the brain and brain-derived cells. PLoS One. 2011;6:e27265. doi: 10.1371/journal.pone.0027265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Korecka JA, van Kesteren RE, Blaas E, Spitzer SO, Kamstra JH, Smit AB, Swaab DF, Verhaagen J, Bossers K. Phenotypic characterization of retinoic acid differentiated SH-SY5Y cells by transcriptional profiling. PLoS One. 2013;8:e63862. doi: 10.1371/journal.pone.0063862. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kumar A, Singh A, Ekavali E. A review on Alzheimer's disease pathophysiology and its management: an update. Pharmacol Rep. 2015;67:195–203. doi: 10.1016/j.pharep.2014.09.004. [DOI] [PubMed] [Google Scholar]
- Kwon SH, Lee HK, Kim JA, Hong SI, Kim HC, Jo TH, Park YI, Lee CK, Kim YB, Lee SY, Jang CG. Neuroprotective effects of chlorogenic acid on scopolamine-induced amnesia via anti-acetylcholinesterase and anti-oxidative activities in mice. Eur J Pharmacol. 2010;649:210–217. doi: 10.1016/j.ejphar.2010.09.001. [DOI] [PubMed] [Google Scholar]
- Lee HJ, Noh YH, Lee DY, Kim YS, Kim KY, Chung YH, Lee WB, Kim SS. Baicalein attenuates 6-hydroxydopamine-induced neurotoxicity in SH-SY5Y cells. Eur J Cell Biol. 2005;84:897–905. doi: 10.1016/j.ejcb.2005.07.003. [DOI] [PubMed] [Google Scholar]
- Lee S, Kim J, Seo SG, Choi BR, Han JS, Lee KW, Kim J. Sulforaphane alleviates scopolamine-induced memory impairment in mice. Pharmacol Res. 2014;85:23–32. doi: 10.1016/j.phrs.2014.05.003. [DOI] [PubMed] [Google Scholar]
- Lim DW, Son HJ, Um MY, Kim IH, Han D, Cho S, Lee CH. Enhanced cognitive effects of demethoxycurcumin, a natural derivative of curcumin on scopolamine-induced memory impairment in mice. Molecules. 2016;21:E1022. doi: 10.3390/molecules21081022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin JK, Chen PC, Ho CT, Lin-Shiau SY. Inhibition of xanthine oxidase and suppression of intracellular reactive oxygen species in HL-60 cells by theaflavin-3,3′-digallate, (-)-epigallocatechin-3-gallate, and propyl gallate. J Agric Food Chem. 2000;48:2736–2743. doi: 10.1021/jf000066d. [DOI] [PubMed] [Google Scholar]
- Mesulam MM. Cholinergic circuitry of the human nucleus basalis and its fate in Alzheimer's disease. J Comp Neurol. 2013;521:4124–4144. doi: 10.1002/cne.23415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Min AY, Doo CN, Son EJ, Sung NY, Lee KJ, Sok DE, Kim MR. N-palmitoyl serotonin alleviates scopolamine-induced memory impairment via regulation of cholinergic and antioxidant systems, and expression of BDNF and p-CREB in mice. Chem Biol Interact. 2015;242:153–162. doi: 10.1016/j.cbi.2015.09.016. [DOI] [PubMed] [Google Scholar]
- Pandareesh MD, Anand T. Neuromodulatory propensity of Bacopa monniera against scopolamine-induced cytotoxicity in PC12 cells via down-regulation of AChE and up-regulation of BDNF and muscarnic-1 receptor expression. Cell Mol Neurobiol. 2013;33:875–884. doi: 10.1007/s10571-013-9952-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Park HR, Lee H, Park H, Jeon JW, Cho WK, Ma JY. Neuroprotective effects of Liriope platyphylla extract against hydrogen peroxide-induced cytotoxicity in human neuroblastoma SH-SY5Y cells. BMC Complement Altern Med. 2015;15:171. doi: 10.1186/s12906-015-0679-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Santillo MF, Liu Y. A fluorescence assay for measuring acetylcholinesterase activity in rat blood and a human neuroblastoma cell line (SH-SY5Y) J Pharmacol Toxicol Methods. 2015;76:15–22. doi: 10.1016/j.vascn.2015.07.002. [DOI] [PubMed] [Google Scholar]
- Schliebs R, Arendt T. The cholinergic system in aging and neuronal degeneration. Behav Brain Res. 2011;221:555–563. doi: 10.1016/j.bbr.2010.11.058. [DOI] [PubMed] [Google Scholar]
- Shi C, Zhao L, Zhu B, Li Q, Yew DT, Yao Z, Xu J. Dosage effects of EGb761 on hydrogen peroxide-induced cell death in SH-SY5Y cells. Chem Biol Interact. 2009;180:389–397. doi: 10.1016/j.cbi.2009.04.008. [DOI] [PubMed] [Google Scholar]
- Thangnipon W, Puangmalai N, Chinchalongporn V, Jantrachotechatchawan C, Kitiyanant N, Soi-Ampornkul R, Tuchinda P, Nobsathian S. N-benzylcinnamide protects rat cultured cortical neurons from β-amyloid peptide-induced neurotoxicity. Neurosci Lett. 2013;556:20–25. doi: 10.1016/j.neulet.2013.09.071. [DOI] [PubMed] [Google Scholar]
- Thangnipon W, Suwanna N, Jantrachotechatchawan C, Ngampramuan S, Tuchinda P, Nobsathian S. Protective roles of N-benzylcinnamide on cortex and hippocampus of aged rat brains. Arch Pharm Res. 2015;38:1380–1388. doi: 10.1007/s12272-015-0593-8. [DOI] [PubMed] [Google Scholar]
- Thangnipon W, Puangmalai N, Suwanna N, Soi-Ampornkul R, Phonchai R, Kotchabhakdi N, Mukda S, Phermthai T, Julavijitphong S, Tuchinda P, Nobsathian S. Potential role of N-benzylcinnamide in inducing neuronal differentiation from human amniotic fluid mesenchymal stem cells. Neurosci Lett. 2016;610:6–12. doi: 10.1016/j.neulet.2015.10.050. [DOI] [PubMed] [Google Scholar]
- Venkatesan R, Subedi L, Yeo EJ, Kim SY. Lactucopicrin ameliorates oxidative stress mediated by scopolamine-induced neurotoxicity through activation of the NRF2 pathway. Neurochem Int. 2016;99:133–146. doi: 10.1016/j.neuint.2016.06.010. [DOI] [PubMed] [Google Scholar]
- Voleti B, Navarria A, Liu RJ, Banasr M, Li N, Terwilliger R, Sanacora G, George TE, Aghajanian G, Duman RS. Scopolamine rapidly increases mammalian target of rapamycin complex 1 signaling, synaptogenesis, and antidepressant behavioral responses. Biol Psychiatry. 2013;74:742–749. doi: 10.1016/j.biopsych.2013.04.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang D, Guo TQ, Wang ZY, Lu JH, Liu DP, Meng QF, Xie J, Zhang XL, Liu Y, Teng LS. ERKs and mitochondria-related pathways are essential for glycyrrhizic acid-mediated neuroprotection against glutamate-induced toxicity in differentiated PC12 cells. Braz J Med Biol Res. 2014;47:773–779. doi: 10.1590/1414-431X20143760. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wong-Guerra M, Jiménez-Martin J, Pardo-Andreu GL, Fonseca-Fonseca LA, Souza DO, de Assis AM, Ramirez-Sanchez J, Del Valle RM, Nuñez-Figueredo Y. Mitochondrial involvement in memory impairment induced by scopolamine in rats. Neurol Res. 2017;39:649–659. doi: 10.1080/01616412.2017.1312775. [DOI] [PubMed] [Google Scholar]
- Xian YF, Ip SP, Mao QQ, Su ZR, Chen JN, Lai XP, Lin ZX. Honokiol improves learning and memory impairments induced by scopolamine in mice. Eur J Pharmacol. 2015;760:88–95. doi: 10.1016/j.ejphar.2015.04.013. [DOI] [PubMed] [Google Scholar]
- Zemek F, Drtinova L, Nepovimova E, Sepsova V, Korabecny J, Klimes J, Kuca K. Outcomes of Alzheimer's disease therapy with acetylcholinesterase inhibitors and memantine. Expert Opin Drug Saf. 2014;13:759–774. doi: 10.1517/14740338.2014.914168. [DOI] [PubMed] [Google Scholar]
- Zhang J, Cai S, Li J, Xiong L, Tian L, Liu J, Huang J, Liu Z. Neuroprotective effects of Theaflavins against oxidative stress-induced apoptosis in PC12 cells. Neurochem Res. 2016;41:3364–3372. doi: 10.1007/s11064-016-2069-8. [DOI] [PubMed] [Google Scholar]
- Zhu A, Wu Z, Meng J, McGeer PL, Zhu Y, Nakanishi H, Wu S. The neuroprotective effects of Ratanasampil on oxidative stress-mediated neuronal damage in human neuronal SH-SY5Y cells. Oxid Med Cell Longev. 2015;2015:792342. doi: 10.1155/2015/792342. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
“Neuroprotection of N-benzylcinnamide on scopolamine-induced cholinergic dysfunction in human SH-SY5Y neuroblastoma cells”
“Neuroprotection of N-benzylcinnamide on scopolamine-induced cholinergic dysfunction in human SH-SY5Y neuroblastoma cells”