Keywords: nerve regeneration, spinal cord injury, methotrexate, methylprednisolone, gait, gene expression profile, inflammation, oxidative stress, apoptosis, nerve repair, Solexa gene sequencing, secondary lesion, neural regeneration
Abstract
Methylprednisolone is a commonly used drug for the treatment of spinal cord injury, but high doses of methylprednisolone can increase the incidence of infectious diseases. Methotrexate has anti-inflammatory activity and immunosuppressive effects, and can reduce inflammation after spinal cord injury. To analyze gene expression changes and the molecular mechanism of methotrexate combined with methylprednisolone in the treatment of spinal cord injury, a rat model of spinal cord contusion was prepared using the PinPoint™ precision cortical impactor technique. Rats were injected with methylprednisolone 30 mg/kg 30 minutes after injury, and then subcutaneously injected with 0.3 mg/kg methotrexate 1 day after injury, once a day, for 2 weeks. TreadScan gait analysis found that at 4 and 8 weeks after injury, methotrexate combined with methylprednisolone significantly improved hind limb swing time, stride time, minimum longitudinal deviation, instant speed, footprint area and regularity index. Solexa high-throughput sequencing was used to analyze differential gene expression. Compared with methylprednisolone alone, differential expression of 316 genes was detected in injured spinal cord treated with methotrexate and methylprednisolone. The 275 up-regulated genes were mainly related to nerve recovery, anti-oxidative, anti-inflammatory and anti-apoptotic functions, while 41 down-regulated genes were mainly related to proinflammatory and pro-apoptotic functions. These results indicate that methotrexate combined with methylprednisolone exhibited better effects on inhibiting the activity of inflammatory cytokines and enhancing antioxidant and anti-apoptotic effects and thereby produced stronger neuroprotective effects than methotrexate alone. The 316 differentially expressed genes play an important role in the above processes.
Introduction
The incidence of spinal cord injury (SCI) caused by many events, including traffic accidents, mining and construction accidents, seismic and natural disasters, has tended to rise year on year. The pathology of SCI usually includes primary injury and secondary injury (Tator, 1991). Primary injury often occurs in the spine, leading to SCI (Ray et al., 2016; Yu et al., 2016). The secondary damage includes inflammation, oxidative stress, neuronal apoptosis, intracellular and extracellular ion imbalance and a series of pathological reactions (Luo et al., 2013). Therefore, to avoid second injury and to promote the survival of axons and neurons, therapies aim to reduce or eliminate the destructive pathological response and to promote the regeneration, repair and functional reconstruction of nerve tissue in the chronic phase.
Methylprednisolone (MP) acts through a variety of mechanisms to prevent the occurrence of secondary SCI and is currently the only food and drug administration-approved drug for the treatment of acute SCI (Bracken, 2012). However, high doses of MP lead to many side effects, including glucocorticoid-induced infection, diabetic complications, and impaired wound healing, and can endanger the lives of patients. Therefore, many experts suggest that high-dose MP shock therapy should not be used as a conventional treatment, although it can be used as a selective treatment strategy (Zhang et al., 2013).
Methotrexate (MTX) is a common anti-rheumatic drug that can improve the disease state and has anti-inflammatory and immunosuppressive effects (Keith et al., 2012). MTX is generally used to compensate for the poor efficacy of glucocorticoid or other anti-rheumatic drugs. MTX can also be combined with hormones in early rheumatoid arthritis, so as to reduce hormone doses, thereby alleviating hormone side effects (Pincus et al., 2015). MTX can be taken long-term because of low cost, various routes of administration, steady long-term efficacy and high safety; namely, it can be taken as an anchor drug (Saeki et al., 2012). Low doses of MTX can be used in the treatment of SCI to ameliorate inflammation (Sanli et al., 2012), oxidative stress and apoptosis (Bakar et al., 2013; Kertmen et al., 2013), thereby preventing the occurrence and development of secondary injury.
Therefore, large-dose MP pulse therapy has been implemented in the early stage of SCI, followed by low-dose MTX maintenance treatment to compensate for the toxic effects of MP. Under this regimen, MTX can protect the remaining synaptic and neuronal functions. Most studies have explored the therapeutic effect on SCI of MTX or MP alone. This study, however, explored the molecular mechanisms of combined MP and MTX administration for treating SCI by high-throughput sequencing.
Materials and Methods
Animals
Fifty male Sprague-Dawley rats weighing 245 ± 20 g were provided by Hunan Slack Kingma Company, China (license No. 43004700001369). All rats were housed at room temperature (25 ± 2°C) and 65% humidity with a 12 hour light/dark cycle. Bedding was changed every two days. The rats were allowed free access to food and water.
To allow rats to adapt to the treadmill environment, fifty rats were adaptively trained on a ZH-PT computer controlled animal treadmill (Anhui Zhenghua, Huaibei, China) for 5 days before the operation. All rats were randomly assigned to the sham group (sham operation), SCI group (model establishment), MP group (SCI + MP alone), MTX group (SCI + MTX alone) and MP + MTX group (SCI + MP combined with MTX). Ten rats from each group were used for gait analysis, six for histology and the remaining four for Solexa gene sequencing.
All procedures were approved by the Experimental Animal Ethics Committee of Jiangxi University of Traditional Chinese Medicine (approval No. A20150107). The experiment followed the United States National Institutes of Health Guidelines for the Care and Use of Laboratory Animals (NIH Publication No.85-23, revised 1986).
Surgical procedure for SCI
SCI models were prepared using a modified method reported by Gu et al. (2011). Rats were anesthetized with 5% chloral hydrate and fixed on a stereotaxic apparatus in the prone position. After shaving the thoracolumbar back region, the skin was disinfected with iodophor. Taking the T10 spinous process as the center, a 4.0 cm-long median longitudinal incision was made to separate the muscle and fascia from both sides of the spine. The spinous process and lamina were gently removed, and the spinal dura mater was exposed. The PinPointTM Precision Cortex Striker (diameter 3.0 mm, Hatteras Instrument Cary, NC, USA) was moved to the top of the T10 dural surface and centered on the median vessel. When the contact head, which has a high-precision contact sensor, touched the surface of the dura mater, an audible warning was generated. Subsequently, the three-dimensional coordinates were determined. T10 injury was produced with an impact depth of 1.0 mm; an impact velocity of 2.0 m/s; and a residence time of 200 ms. Immediately after rat tail spasm and retraction flutter of lower extremities, cold gauze was used to stop bleeding. Muscles and skin were sutured layer by layer.
The sham group was operated as above, but no impact was performed. The preparation and postoperative nursing of SCI models were in accordance with procedures in a previous study (Gu et al., 2011). After surgery, rats were injected with 5 mL Ringer's lactate solution to compensate for blood loss. The rectal temperature was maintained at 37 ± 1°C with a thermostatically controlled heating pad. Penicillin and gentamicin sulfate (2.5 mL/kg) were intramuscularly injected twice a day for 7 days. If urinary tract infection occurred, the antibiotics were administered for 10 days. At a fixed time, urination was induced 2 to 3 times every day, until spontaneous urination.
Therapeutic schedule
MP (Methylprednisolone Sodium Succinate for Injection; batch number: Z07305) was provided by Pfizer Pharmaceutical Belgian Company (Belgian, Ixelles). MTX (0.05 g/1 mL, batch number: 271021-2) was purchased from the Lingnan Pharmaceutical Co., Ltd. (Guangzhou, China).
The necks of rats in sham and SCI groups were subcutaneously injected with 0.5 mL normal saline 30 minutes after injury. The rats in the MP group were injected with MP (30 mg/kg, 187 μL; Pfizer, Belgian, Ixelles) via the tail vein. The rats in the MTX group were subcutaneously injected with MTX (0.3 mg/kg, 1.5 μL; Lingnan Pharmaceutical Co., Ltd.), once every other day, for 2 weeks. The rats in the MP + MTX group were injected with MP (30 mg/kg, 187 μL) via the tail vein 30 minutes before injury, and were then subcutaneously injected with MTX (0.3 mg/kg, 1.5 μL) at 1 day after injury, once a day, for 2 weeks.
Gait analysis
The hind limb gait, including hind limb swing time, stride time, minimum longitudinal deviation, instant speed, footprint area and regularity index, was recorded using the small animal gait analyzer (Clever Sys. Inc., Reston, VA, USA). The video files were analyzed using Treadscan 4.0 system software (Clever Sys. Inc.) (Zhang et al., 2012). Gaits were tested at 2, 4, and 8 weeks after injury. Before the test, each rat ran freely for 30–45 seconds and was then placed on the walking table of the gait analyzer. The speed of the walking table increased from 7 cm/s to 15 cm/s over 20 seconds. Each rat was tested three times.
Histology
Sections containing injured spinal cord tissue were generated as previously described (Pinzon et al., 2008). Briefly, at 8 weeks, all animals were anesthetized and perfused intracardially with normal saline solution followed by 4% paraformaldehyde solution. Cord segments of about 1 cm surrounding the injured site were dissected, embedded in paraffin, and cut vertically into serial sections. One section at the center of impact (+0 mm) and sections at around 3 and 5 mm from the impact site were selected and stained with Harris’ hematoxylin and eosin for routine pathological examination.
Total RNA extraction and Solexa gene sequencing
Rats were decapitated 8 weeks after surgery. Four rats were selected from each group for RNA extraction. An approximately 10 mm spinal cord section surrounding the damage center was isolated. Total RNA extraction and cDNA synthesis were performed as previously described (Liu et al., 2016). Briefly, total RNA from each sample was isolated using an RNeasy Protect Mini Kit (Qiagen, Dusseldorf, Germany). RNA purity and quantity were assessed with a SmartSpec Plus (Bio-Rad, Hercules, CA, USA). cDNA synthesis was performed using an iScript cDNA Synthesis Kit (Bio-Rad). The amplification procedure was: pre-denaturation for 30 seconds at 98°C; 18 cycles of denaturation for 10 seconds at 98°C, annealing for 30 seconds at 65°C, extension for 15 seconds at 72°C; extension for 7 minutes at 72°C; holding at 8°C. The amplification products were sequenced after gel purification by HiSeq2000 high-throughput sequencing (Illumina, San Diego, CA, USA). The obtained image files were converted into digital signals. The results were analyzed and processed after removing impurities and linker sequences.
Quality evaluation and RNA-Seq bioinformatic analysis
Original HiSeq2000 Single-end sequencing images were transformed into raw data. Adapters and low quality, contaminated data were removed from the raw data and the length distribution was analyzed. Sequences were then matched against the rat genome. The ratios of sequence to the genome and unique gene were calculated using SOAP software (BGI, Shenzhen, China). To annotate and classify gene functions for each sequence, all assembled unique sequences were searched against GenBank's non-redundant database, the Swiss-Prot protein database (http://www.expasy.ch/sprot), the KEGG pathways database (Kanehisa et al., 2008), and the COG database (http://www.ncbi.nlm.nih. gov/COG/) using BLASTx and against nucleotide (nt) using BLASTn with a threshold of E <1.0E-5. According to protein (nr) annotation information, we used Blast2GO software (Conesa et al., 2005) to obtain GO comment information from Unigene. AGRIGO and WEGO software were used to conduct GO functional classification (Ye et al., 2006). R program was used to identify differentially expressed genes from different samples. Differential expression was analyzed between two groups (SCI and sham groups, MP and SCI groups, and MTX + MP and MP groups) using DEGseq software (Wang et al., 2010). The abundance of gene expression was measured using per million mapped fragments (Mortazavi et al., 2008). The differentially expressed genes in different treatment groups were screened using a digital gene expression profile difference gene detection method (Audic and Claverie, 1997). The P-value of each differentially expressed gene was computed. The Benjamini–Hochberg false discovery rate (Pang et al., 2013) was applied to correct P-values. The genes suppressed at an estimated |log2(Fold change)|≥1 and with a false discovery rate adjusted P-value ≤ 0.05 were considered to be differentially expressed (Wu et al., 2010). All differentially expressed genes were mapped to each term of the Gene Ontology database (http://www.geneontology.org/, release date: Aug 1st, 2012) and the gene numbers were calculated from each GO term. The rigorous Bonferroni correction method was used for P value correction (Chen et al., 2014). When the corrected GO term had a cutoff value of no more than 0.05, it was defined as a significant enrichment.
Quantitative polymerase chain reaction (qPCR)
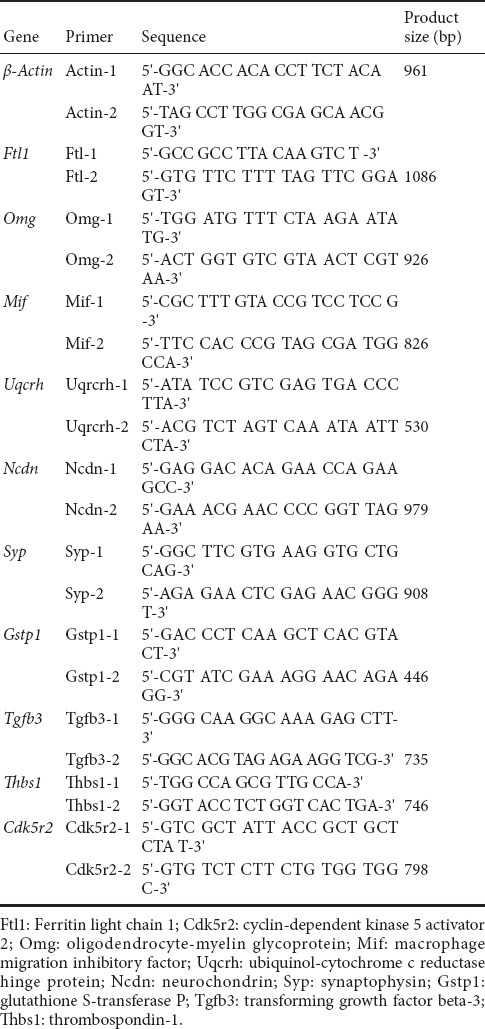
To detect the expression level of selected unigenes, quantitative real-time PCR was performed as described previously (Chen et al., 2014). A 5 μL aliquot of cDNA was blended with Super Mix (Invitrogen) and the PCR primers in a 20 μL reaction volume. The iQ5 instrument (Bio-Rad, Hercules, CA, USA) was used to perform quantitative PCR under the following conditions: 95°C for 30 seconds, followed by 40 cycles of 95°C for 15 seconds and 60°C for 35 seconds. Relative expression was calculated by the 2–ΔΔCt method using β-actin as an internal control (Chen et al., 2011). Gene-specific primers used are listed in Table 1. The RNA library used was generated from a separate sample from that used for RNA-seq. Three replicates were performed for each sample.
Table 1.
Primer sequences used in quantitative polymerase chain reaction
Statistical analysis
Data analysis was performed using SPSS 16.0.2 statistical software (SPSS, Chicago, IL, USA). All data are presented as the mean ± SD. Comparisons among groups were performed by one-way analysis of variance followed by Dunnett's test. Gene matching randomness was analyzed using the Kolmogorov-Smirnov test (Lee et al., 2017). A value of P < 0.05 was considered statistically significant.
Results
Effects of MP and MTX on motor function in rats with SCI
Swing time
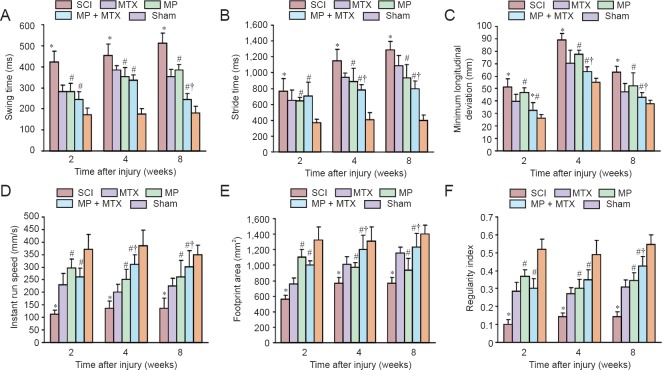
As shown in Figure 1A, the swing time in the MP group was significantly shorter than that of the SCI group (P < 0.05) from 2 to 8 weeks post-injury. At 8 weeks post-injury, improvement was significantly enhanced by combined MP and MTX compared with the use of MP alone (P < 0.05).
Figure 1.
Comparison of gait parameters among groups at 2, 4 and 8 weeks after SCI.
(A–F) Comparison of swing time, stride time, minimum longitudinal deviation, instant run speed, footprint area and regularity index. Data are expressed as the mean ± SD (n = 10 per group, one-way analysis of variance followed by Dunnett's test). *P < 0.05, vs. SCI group; #P < 0.05, vs. MP group; †P < 0.05, vs. sham group. SCI: Spinal cord injury; MP: methylprednisolone; MTX: methotrexate.
Stride time
As shown in Figure 1B, the stride time was significantly longer between SCI and sham groups after injury (P < 0.05). The MP + MTX group achieved a significant better therapeutic effect between 4 and 8 weeks post-injury compared with the MP group (P < 0.05).
Minimum longitudinal deviation
As shown in Figure 1C, the minimum longitudinal deviation in the MP group was significantly decreased compared with the SCI group (P < 0.05). The restorative effects in the MP + MTX group were significantly better at each time point compared with the MP group (P < 0.05).
Instant run speed
As shown in Figure 1D, the instant run speed was significantly decreased in the SCI group at each time point after injury compared with the sham group (P < 0.05). The instant run speed in the MP group was significantly improved compared with the SCI group (P < 0.05). The instant run speed in the MP + MTX group at 4 and 8 weeks post-injury was significantly increased compared with the MP group (P < 0.05).
Footprint area
As shown in Figure 1E, at 2–8 weeks after injury, the footprint area in the SCI group was significantly decreased compared with the sham group (P < 0.05). The footprint area in the MP group was significantly increased compared with the SCI group at each time point (P < 0.05). The improvements in the MP + MTX group were significantly more at 4 and 8 weeks post-injury compared with the MP group (P < 0.05).
Regularity index
As shown in Figure 1F, 2–8 weeks after injury, the regularity index in the SCI group was significantly lower than that of the sham group (P < 0.05). The regularity index was significantly improved in the MP group compared with the SCI group 2 weeks post-injury (P < 0.05). The MP + MTX group and the MP group showed similar restorative effects at 2 and 4 weeks, but the MP + MTX group was significantly more improved at 8 weeks compared with MP group (P < 0.05).
Effects of MP and MTX on histology in SCI rats
The results of hematoxylin-eosin staining are shown in Figure 2. Eight weeks after injury, white matter and gray matter structures were complete in the sham group. The number of neurons was decreased and the vacuole was very severe in the SCI group. In the MP + MTX and MP groups, there were various degrees of tissue loss and cyst formation, and all the damage had a tendency to spread to the adjacent area. Myelin sheath swelling and the loose structure of the white matter and the white matter bundle were improved in the MP + MTX group. Total glial cell proliferation, tissue voids, and the size of cysts at the injury center and in adjacent areas were significantly smaller in the MP + MTX group compared with those in the MP group.
Figure 2.
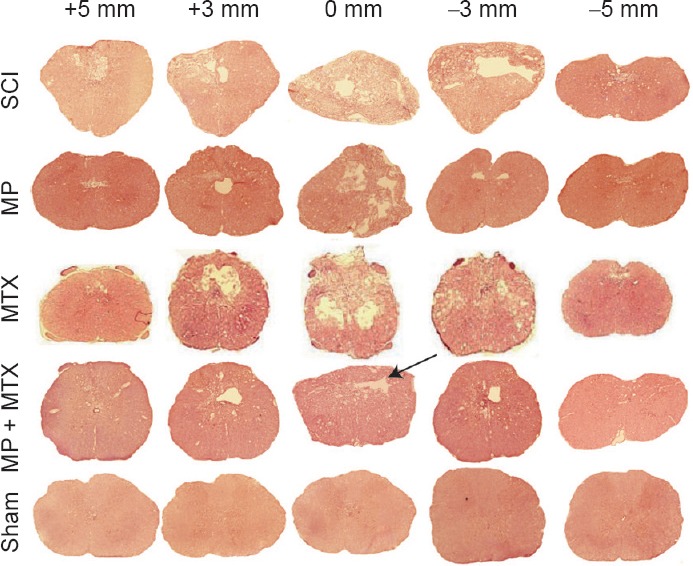
Histopathological changes of the spinal cord at 8 weeks post-trauma (hematoxylin-eosin staining, × 20).
Vertical cross-sections were taken from the injured site (+0 mm), and 3 and 5 mm rostral (+) and caudal (–) to the epicenter. Eight weeks after injury, the SCI, MP + MTX and MP groups had different degrees of tissue loss and cyst occurrence, and there was a tendency of damage to spread to the adjacent area. After MP + MTX combination therapy, fibrous scar formation was present at the lesion; total glial cell proliferation, tissue voids, and cyst area were significantly less compared with the use of MP or MTX alone (as indicated by the arrow). MP: Methylprednisolone; MTX: methotrexate; SCI: spinal cord injury.
Gene sequencing
Evaluation of sequencing results
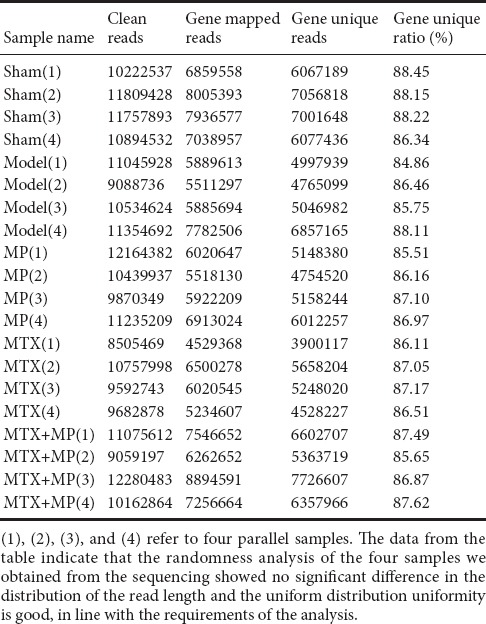
Quality evaluation and RNA-Seq bioinformatic analysis showed that linker sequences were removed from the original data. Reads with a length of < 20 bp were filtered out. Sequences containing an unknown base ratio of > 4% were removed. Retention of valid data was > 70%, which was in line with requirements for subsequent analyses. The gene matching ratio was up to > 50% (Table 2). Matching randomness analysis of all reads showed no significant differences in length distribution and uniform distribution; the randomness and uniformity were in line with the expected requirements.
Table 2.
The matching of each sample sequence with the gene and the unique matching statistics
Differential gene expression after SCI injury
The difference in gene expression between two groups (SCI and sham, MP and SCI, MTX+MP and MP) showed that overall gene expression was roughly similar between groups (Figure 3). Thus, the scatters overlapped and showed a diagonal line.
Figure 3.
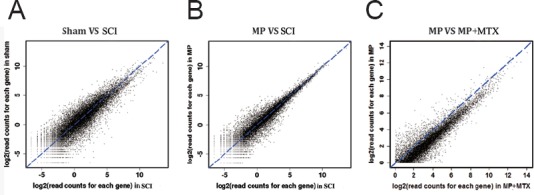
Scatter plots of gene expression differences between two groups after normalization.
Differential expression was analyzed between two groups (SCI and sham groups, MP and SCI groups, MTX + MP and MP groups) using DEGseq software (Wang et al., 2010). (A) Comparison of the sham group and SCI group. (B) Comparison of the MP group and SCI group. (C) Comparison of MP group (30 mg/kg MP) and MP + MTX group (30 mg/kg MP + 0.3 mg/kg MTX). Sham group and SCI groups were subcutaneously injected with 0.5 mL saline. MP: Methylprednisolone; MTX: methotrexate; SCI: spinal cord injury.
According to GO classification, differentially expressed genes were split into three categories: biological processes, cellular components and molecular functions; each category contains a large number of subgroups. All gene GO classifications included reproduction, transcription factor activity, cell killing, immune process, catalytic activity, transport activity, metabolism, and anti-oxidation. Fifty-four regulatory gene subclasses were related to life activities.
There were 443 differentially expressed genes between the SCI and sham groups (269 genes were up-regulated and 174 genes were down-regulated). GO enrichment analysis showed that the upregulated genes in the SCI group were mainly involved in wound repair, ionic equilibrium, stress response, organ development, biosynthesis, signal transduction, inflammation, oxidation and cell apoptosis, suggesting that pathological manifestations after injury might be related to the regulation and control of these genes. Among them, Dcn [Q01129, log2(Fold change) = 2.44, P = 0.0074] can regulate and control neurons and glial cells in the central nervous system to produce decorin (Jungmann et al., 2012). Decorin has anti-fibrosis and anti-inflammation effects (Esmaeili et al., 2014), which are involved in the wound repair process. The upregulation of Dcn expression might be associated with activation of the wound repair mechanism. The Ftl1 [P29391, log2(Fold change) = 2.944, P = 0.00151], Ftl2 [P49945, log2(Fold change) = 2.678, P = 1.091E-81] and Tcirg1 genes [Q13488, log2(Fold change) = 2.278, P = 0.00481] are responsible for regulating the intracellular and extracellular ion balance (Capecci and Forgac, 2013; Tao et al., 2014). The upregulation of this kind of gene might reflect the activation of the ion balance regulatory pathway in response to ion imbalance.
Compared with the sham group, genes related to regulation of neuronal regeneration were down-regulated in the SCI group (Table 3), which corresponded to neurophysiological dysfunction after SCI, and might be the most direct reason for sense and motor function-related disorders. Genes with greater differential expression included Hsp90aa1, encoding heat shock protein 90, [P82995, log2(Fold change) = −4.21, P = 2.21E-05], which corrects the misfolding of synuclein and regulates the neuroprotective effect in neurodegenerative diseases (Sundaramurthi et al., 2012). The gene encoding cyclin-dependent kinase, Cdk5r2, [Q13319, log2(Fold change) = −3.31, P = 2.55E-09] plays a crucial role in the oligodendrocyte differentiation and myelination repair process (Bankston et al., 2013). The gene encoding contactin-2, Cntn2, [P22063, log2(Fold change) = −3.38, P = 0.0116] is a member of the neural recognition family. It participates in the formation and maintenance of the nervous system (Mohebiany et al., 2014). The Omg gene [Q63912, log2 (−Fold change) = −2.85, P = 2.89E–12] can regulate neurons in the central nervous system and oligodendrocytes to express the myelin protein. Myelin is strongly associated with development and maturation of the nervous system (Vourc’h and Andres, 2004). In addition, genes down-regulated in the SCI group compared with the sham group involved biological oxidation, substance and energy metabolism, organ development, ion transportation, and signal transduction, indicating that the down-regulation of these genes might be related to the pathological process after injury.
Table 3.
Examples of down-regulated GO items of differentially expressed genes associated with regeneration of neurons in spinal cord injury and sham groups
Effect of drug therapy on differential gene expression
(1) Differential gene expression between MP and SCI groups
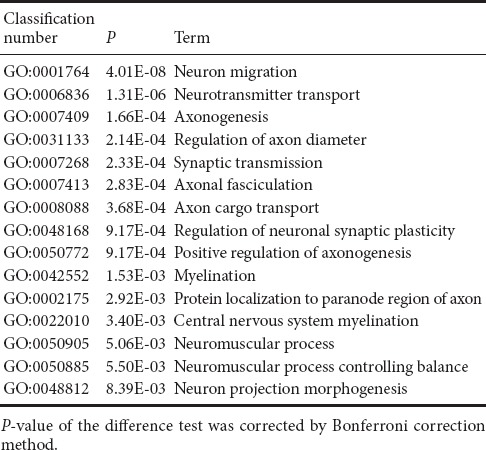
There were 52 differentially expressed genes (33 up-regulated and 18 down-regulated) between MP and SCI groups. GO enrichment analysis showed that the upregulated genes associated with SCI injury and repair mainly reflected nervous system activities, inflammatory processes, biological oxidation, and apoptosis (Table 4).
Table 4.
Examples of up-regulated GO items of differentially expressed genes associated with secondary damage mechanisms in spinal cord injury and methylprednisolone groups
The up-regulated genes associated with nervous system activities were involved with positive regulation of axonal regeneration, synaptic transportation, and oligodendrocyte generation, which directly reflected the promoting role of MP in nerve regeneration after SCI. Among them, Gstp1 [P04906, log2 (Fold change) = 2.11, P = 0.00285], encodes glutathione-S-transferase, an isoenzyme that marks oligodendrocyte maturation in mammals (Tamura et al., 2007). The Mif gene [P34884, log2 (Fold change) = 2.02, P = 0.0163)] encodes macrophage migration inhibition factor, a pluripotent cytokine that participates in inflammation and immune responses, and can promote the regeneration of peripheral nerves and inhibit Schwann cell apoptosis (Nishio et al., 2002).
Among the upregulated genes were multiple GO items associated with the inflammatory reaction. Among them, the factors that directly reflect the anti-inflammatory effect of MP were negative regulation of leukocyte proliferation, generation of monocyte chemoattractant protein (MCP-1), interleukin (IL)-1β, and tumor necrosis factor (TNF), and T cell proliferation. The anti-inflammatory effect might be an important mechanism of MP for promoting nerve function restoration after SCI. The RT1-Ba gene [P20037, log2(Fold change) = 3.24, P = 0.00186], encoding class II tissue antigen, participates in the genetic control and regulation of the immune response, and can decrease inflammation after injury by inhibiting the proliferation of T cells (Havik et al., 2007). The Mif gene, encoding macrophage migration inhibitory factor, can also inhibit the inflammatory reaction by limiting the excessive phagocytosis of macrophages that occurs in the pathological process (Muller et al., 2014).
In addition, genes associated with oxidation process and cell apoptosis were up-regulated. GO enrichment analysis showed that genes reflecting inhibition of oxidative stress by MP were mainly involved in positively regulating the biosynthesis of inducible nitric oxide synthase (iNOS) and negatively regulating the binding activities of riboflavin and glutathione. We speculated that MP could regulate oxidation in damaged tissue and play a role in the inhibition of oxidative stress. The Rfk gene [8CFV9, log2 (Fold change) = 2.54, P = 0.00695], encoding riboflavin kinase that catalyzes riboflavin to produce a coenzyme of antioxidase, regulates the biological oxidation process to ensure normal metabolism (Schramm et al., 2014). Uqcrh [P99028, log2 (Fold change) = 3.34, P = 0.00742] is a target gene of peroxisome proliferator activated receptor-1α (PGC-1α) in normal mitochondrial metabolism. The expression of Uqcrh was significantly down-regulated by oxidative stress and other pathological states (Zaza et al., 2013). The regulation of apoptosis by MP was reflected in the negative regulation of myeloid cell apoptosis and necrosis.
Compared with the SCI group, the genes down-regulated in the MP group were associated with neuronal differentiation and regeneration, inflammatory reaction and biological oxidation (Table 5). The genes participating in the regulation of neuronal differentiation and regeneration were involved in neural genesis and dendritic morphogenesis. The down-regulation of this kind of gene might reflect MP therapy having inhibitory effects on nerve regeneration; however, the ultimate effect on the restoration of neural function is the sum of positive and negative effects. The down-regulated genes associated with inflammatory reaction mainly reflected the activation of chemotactic factors. Pf4 [P06765, log2(Fold change) = −2.31, P = 2.93E-05] is an important marker gene of the inflammatory reaction (Liu et al., 2014). It encodes platelet factor-4, which can increase the chemotaxis of immune cells, and promote their migration to inflammatory sites, so as to aggravate the inflammatory reaction. The down-regulation of this kind of gene further illustrated that MP had an anti-inflammatory effect. Compared with the SCI group, there were associated oxidation processes highlighted in the MP group, but they did not directly affect oxidative stress.
Table 5.
Examples of down-regulated GO items of differentially expressed genes associated with secondary damage mechanisms in spinal cord injury and methylprednisolone groups
(2) Analysis of differential gene expression between MP + MTX and MP groups
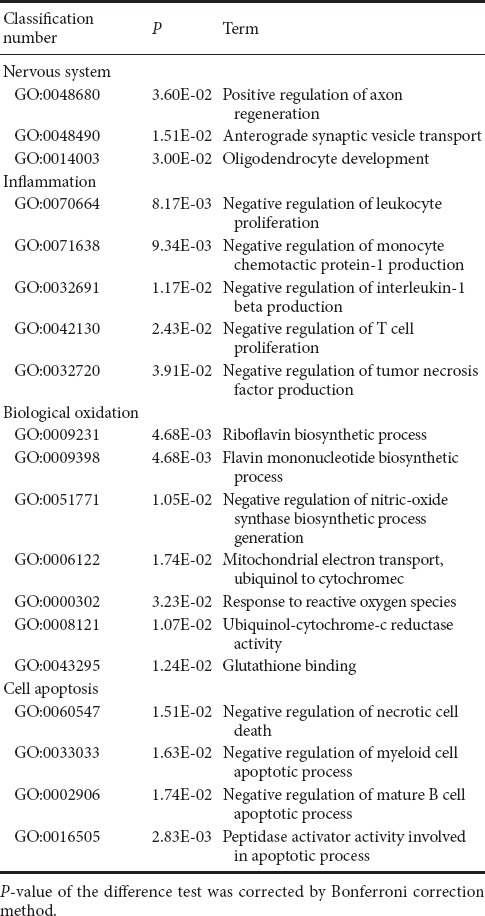
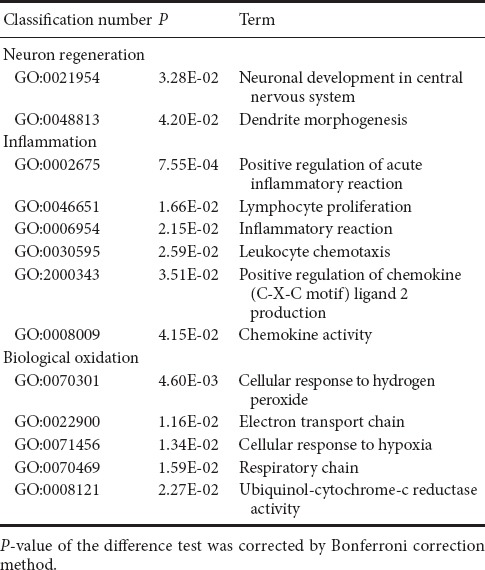
There were 316 genes that were differentially expressed between MP + MTX and MP groups (275 genes were up-regulated and 41 were down-regulated). GO enrichment analysis showed that there were a large number of associated items that promote nerve restoration among the up-regulated genes that also involve anti-inflammation, anti-oxidation and anti-apoptosis (Table 6). The down-regulated genes were mainly involved in pro-inflammatory and pro-apoptotic processes (Table 7).
Table 6.
Examples of up-regulated GO items of differentially expressed genes associated with secondary damage mechanisms in methylprednisolone and methylprednisolone + methotrexate groups
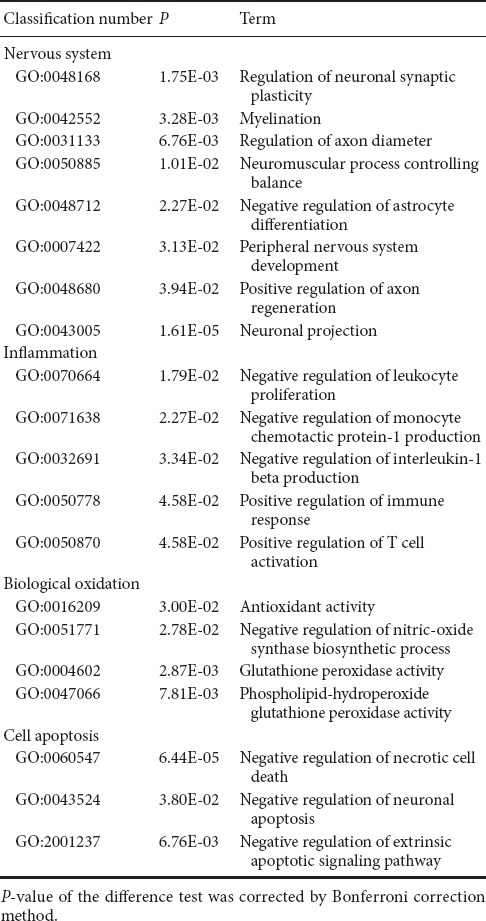
Table 7.
Examples of down-regulated GO items of differentially expressed genes associated with secondary damage mechanisms in methylprednisolone and methylprednisolone + methotrexate groups
Compared with the MP group, the up-regulation of genes that promote neural restoration in the MP + MTX group was related to the regulation of neuronal synaptic plasticity, myelin formation, control of neuromuscular balance, and regeneration of neurons and axons. The Ndcn gene [O35095, log2 (Fold change) = 2.47, P = 0.0161], encoding neural cartilage protein, and the Syp gene [P07825, log2 (Fold change) = 2.72, P = 0.0143], encoding synaptic vesicle protein, play neurotrophic effects on promoting axonal growth and modulating synaptic plasticity in the central nervous system (Oku et al., 2013; Park et al., 2015). The Mal gene [Q64349, log2 (Fold change) = 2.39, P = 0.00377], encoding myelin and lymphocyte protein, is mainly produced by oligodendrocytes and Schwann cells. It regulates the peripheral nervous system to produce myelin. It might promote myelin wrapping by regulating the formation of membrane components (Buser et al., 2009).
Among the up-regulated genes, the anti-inflammatory GO genes mainly negatively regulate the proliferation of leukocytes, and the generation of IL-1β and chemokines. Among them, the Mif gene [P34884, log2 (Fold change) = 2.84, P = 0.014] is involved in inhibiting excessive phagocytosis of macrophages. The Gstp1 gene [P04906, log2 (Fold change) = 2.70, P = 0.000151], encoding glutathione-S-transferase, is also an important marker of the inflammatory reaction (Iorio et al., 2015).
Among the up-regulated genes, the antioxidant genes were mainly related to the activation of antioxidase activity and inhibition of peroxidase activity. Prdx1 [P0CB50, log2 (Fold change) = 2.60, P = 0.0484], encoding peroxide reductase-1, is an important antioxidase that scavenges for free radicals and protects against oxidative damage. Gpx4 [O70325, log2 (Fold change) = 2.40, P = 0.00914], encoding glutathione peroxidase, is also a major endogenous antioxidant enzyme in animals. It catalyzes the disproportionation reaction of superoxide anions (Storey, 1996). Gstp1 [P04906, log2 (Fold change) = 2.79, P = 0.00468] was an up-regulated gene. Its expression is inversely proportional to that of iNOS in the pathological process (Raposo et al., 2013).
Among the up-regulated genes, the anti-apoptosis GO genes were associated with the negative regulation of apoptosis and apoptosis signaling pathways. Among them, Tgfb3 [Q07258, log2 (Fold change) = 2.57, P = 0.0114] is important for cell morphology genesis, differentiation and tissue reconstruction. It can enhance the protective effects of various neurotrophic factors and is an important cytokine for neuronal survival (Krieglstein et al., 2002).
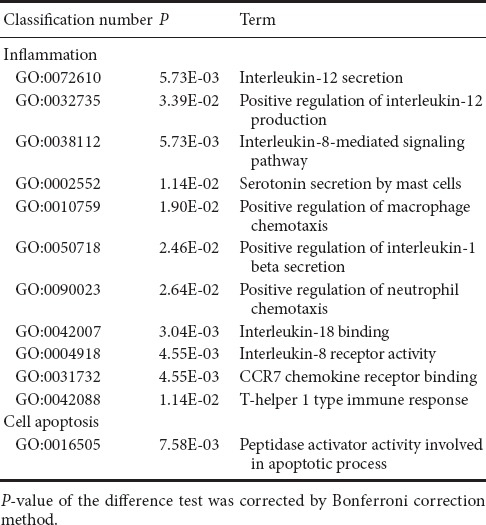
Compared with the MP group, proinflammatory genes were down-regulated in the MTX + MP group. These were mainly related to interleukin secretion, mast cells, macrophages and neutrophils, and inflammatory factor chemotaxis. Among them, Cxcr1 [P70612, log2 (Fold change) = −2.99, P = 0.0447], Il18bp [Q9Z0M9, log2 (Fold change) = −2.01, P = 5.49E-05] and Ccl19 [O70460, log2 (Fold change) = −2.63, P = 0.00230] can regulate secretion of IL-8, IL-18 and IL-19 and binding with their respective receptors. These interleukins can promote the chemotaxis of macrophages in pathological processes and induce inflammation (Galindo et al., 2009; Bishayi et al., 2015; Xuan et al., 2015). Thbs1 [P35441, log2 (Fold change) = −2.99, P = 0.00771] can promote macrophage chemotaxis and its expression was confirmed to be increased in inflammation-associated pathological processes (De Luna et al., 2010). In addition, pro-oxidant and pro-apoptotic genes also appeared among the down-regulated genes. Gapdh [P16858, log2 (Fold change) = −2.57, P = 0.0146] encodes glyceraldehyde-3-phosphate dehydrogenase, an important enzyme in glycolysis and aerobic metabolism. However, a recent study found its expression was strongly associated with the apoptosis of neurons in neurodegenerative diseases (El Kadmiri et al., 2014).
Quantitative PCR results
Ten genes shown to be significantly differentially expressed by Solexa sequencing were randomly selected and their relative mRNA expression determined. The results were consistent with the results of Solexa sequencing (Figure 4). These results indicated that the Solexa sequencing data reflected the real situation of gene expression in each experimental group; i.e., the information obtained by gene expression profile sequencing was of high reliability.
Figure 4.
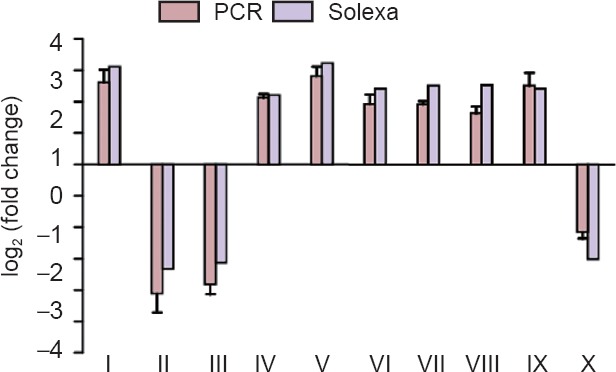
qPCR analysis of 10 genes shown to be differentially expressed by Solexa sequencing.
PCR was performed on 10 genes randomly selected from differentially expressed genes identified by Solexa sequencing. Data are expressed as the mean ± SD (n = 4; one-way analysis of variance followed by Dunnett's t-test). Each data point represents the average from three tests, and error bars represent the standard deviations. Ftl1: Ferritin light chain 1; Cdk5r2: cyclin-dependent kinase 5 activator 2; Omg: oligodendrocyte-myelin glycoprotein; Mif: macrophage migration inhibitory factor; Uqcrh: ubiquinol-cytochrome c reductase hinge protein; Ncdn: neurochondrin; Syp: synaptophysin; Gstp1: glutathione S-transferase P; Tgfb3: transforming growth factor beta-3; Thbs1: thrombospondin-1. The experiment was repeated three times. qPCR: Quantitative polymerase chain reaction. I: Ftl1; II: Cdk5r2; III: Omg; IV: Mif; V: Uqcrh; VI: Ncdn; VII: Syp; VIII: Gstp1; IX: Tgfb3; X: Thbs1.
Discussion
The present study showed that the combination of MP with MTX significantly improved damaged motor function caused by SCI in rats. Both compounds and MP combined with other drugs have been widely studied; however, to our knowledge, this is the first study to show motor function recovery in rats after traumatic SCI by a combination of MP and MTX at the level of the transcriptome. Our study illustrates that the restorative effects of this combination therapy may be through the anti-inflammatory reaction, inhibition of oxidative stress and apoptosis and other common gene regulation pathways.
Gait analysis
Treadscan gait analysis can provide an all-around gait analysis for the assessment of motor function in SCI rats (Beare et al., 2009; Myers et al., 2012; Schira et al., 2012). In our study, the effects of MP and MTX, alone and in combination, were compared by Treadscan gait analysis. Measures included hind limb swing time, stride time, minimum longitudinal deviation, instant run speed, hindlimb footprint area and regularity index. Forgione showed that footprint area was significantly decreased in incomplete spinal cord contusion model rats (Forgione et al., 2014). The results of this study also showed that SCI significantly reduced the area of the posterior footprint, and the treatment of MP combined with MTX significantly improved the posterior surface area after SCI; the regularity index was decreased in the SCI group compared with sham group, which was consistent with a previous study (Chiang et al., 2014).
Gene sequencing
The possible mechanism of different combinations of drugs that promote recovery of motor function in rats was explored through expression profile sequencing analysis. Here, differential gene expression analysis in SCI and sham groups showed that neural development- and function-associated genes were down-regulated after SCI, directly reflecting the damage to nerve structure and function. Moreover, genes associated with energy metabolism, transcription, catalysis, ion exchange, and signal transduction were differentially expressed. These results, at the genetic level, are consistent with a previous study (Tang et al., 2014).
MP treatment
In this study, gene expression profile analysis after MP treatment showed that the expression of genes associated with axon regeneration, synaptic transportation, and oligodendrocyte genesis were up-regulated, confirming that MP had an effect on nerve function recovery after injury. In addition, the expression of genes regulating neurogenesis and dendritic morphogenesis in the central nervous system were down-regulated, indicating that MP treatment had certain side effects on neurological recovery. This may contribute to the reason why severe SCI fails to be completely cured by MP. However, the specific mechanism of action remains to be further examined.
The inflammatory reaction is an important mechanism of secondary SCI. A large number of experimental results show that high-dose MP treatment in the acute stage can decrease the infiltration of inflammatory cells, and effectively control the inflammatory reaction (Alibai et al., 2010; Zhang et al., 2014). Our gene expression profile analysis showed that MP treatment can up-regulate the expression of anti-inflammatory genes and down-regulate the expression of genes promoting the inflammatory reaction, which demonstrated the anti-inflammatory effect of MP. These results explained the anti-inflammatory effects of MP in three ways: (1) negative regulation of immune cell activation and immune response: genes that negatively regulate leukocyte and T cell proliferation were up-regulated. Genes positively regulating the inflammatory and immune responses were down-regulated. (2) Negative regulation of the pro-inflammatory effect of chemokines: The negatively regulating Mcp-1 was up-regulated and genes activating chemokine activity were down-regulated (Hassanshahi et al., 2013; White et al., 2009). (3) Negative regulation of inflammatory factor expression: The genes negatively controlling IL-1β and TNF generation were up-regulated. IL-1β and TNF were mainly produced by activated monocytes/macrophages, and could promote neutrophil phagocytosis, cause an inflammatory reaction, participate in fever, blood coagulation and other pathological processes (Linton and Thoman, 2014). Gene expression analysis, therefore, indicated a trend against the inflammatory reaction after MP treatment.
Oxidative stress plays an important role in secondary spinal cord injury. Boyaci et al. (2015) reported that MP treatment could effectively reduce oxidative stress products after SCI and improve the level of antioxidants. In our study, many oxidation-related genes were differentially expressed after MP treatment. The genes directly reflecting oxidative stress inhibition by MP, such as iNOS (negative regulation of NO biosynthesis), and positive regulation of glutathione binding activity were up-regulated. iNOS overexpression can catalyze the generation of peroxynitrite after injury (Maggio et al., 2012). Glutathione itself is an important antioxidant (Horiyama et al., 2014) and endogenous glutathione can be consumed in a few hours or even a few minutes after injury (Lucas et al., 2002).
Apoptosis of neurons is an important factor in irreversible damage and secondary injury. Neuronal apoptosis can not only lead to local nerve degeneration, but can also cause stop nutrition of distal nerve fibers, leading to necrosis. Wang et al. (2013) reported that intrathecal injection and intravenous injection of MP could effectively inhibit the apoptosis of nerve cells and promote the recovery of motion function in the hind limbs of SCI rabbit models. The results of this study showed that the anti-apoptotic effect of MP was the up-regulation of genes that negatively regulate necrosis and myeloid cell apoptosis-associated genes.
MP + MTX combined therapy
MTX is applied in clinical practice as an anti-inflammatory and antitumor drug. Previous studies have shown that low-dose MTX has a protective effect on nerves after SCI (Lee et al., 2008; Sanli et al., 2012; Bakar et al., 2012). The present study also found that MTX might play a neuroprotective role in SCI repair.
The results showed that MTX could reduce the levels of myeloperoxidase and malondialdehyde in injured tissue, suggesting that MTX has a dual function of anti-inflammation and inhibition of oxidative stress. We also demonstrated that MTX plays an important role in protecting axon and myelin integrity, and inhibiting the ultrastructural changes of injured spinal cord. Long-term application of MTX could increase uric acid levels in blood and urine (Chen et al., 2012; Aoyama et al., 2011). Uric acid has a neuroprotective effect, suggesting that MTX inhibited the development of secondary injury and protected nerve tissue from SCI.
Combined therapy of two or more drugs has become a hot topic in clinical research. Many studies have adopted other drugs in combination with MP (Luo et al., 2013; Yin et al., 2013; Cavus et al., 2014; Li et al., 2016), which achieved better effects. From our results, MP combined with MTX improved treatment of SCI in several aspects compared with MP alone. First, the expression of many nerve restoration promoting genes was up-regulated, such as genes regulating neuronal synaptic plasticity, myelinogenesis, control of neuromuscular balance, and regeneration of neurons and axons, indicating that combined therapy with two drugs showed significant advantages in the treatment of SCI in protecting the integrity of neuronal structures and in promoting neurons to rebuild functional connectivity compared with MP treatment alone. Second, there were two anti-inflammatory advantages: (1) negative regulation of the proliferation and binding activity of immune cells. Genes negatively regulating leukocyte proliferation were up-regulated, while genes positively regulating mast cell secretion, and macrophage and neutrophil chemotaxis were down-regulated. (2) Negative regulation of the production and activity of pro-inflammatory factors. The genes negatively regulating IL-1β and chemotactic factor production were up-regulated. However, genes that positively regulate positively regulating interleukins (IL-8, IL-18, and IL-19), their receptors, and chemotactic factor activities were down-regulated. These inflammatory factors can promote neutrophil migration to the site of injury and release a series of active products, resulting in local inflammation (Kwon et al., 2011; Khalatbary and Zarrinjoei, 2012). Third, the inhibition of oxidative stress reactions has advantages. Differential gene expression analysis showed that the combined administration could positively regulate the activity of antioxidant enzymes (glutathione peroxidase and peroxiredoxin 1) and negatively regulate the activity of iNOS. Endogenous antioxidase is rapidly depleted after SCI. Therefore, the up-regulation of genes regulating antioxidase activity could replenish the antioxidase and inhibit peroxidation. In addition, genes regulating iNOS activity were down-regulated, also suggesting that the combined therapy had a better inhibitory effect on oxidative stress. Nitric oxide is produced from L-arginine by iNOS, and can react with hydroxyl free radicals to produce highly toxic free radicals (Yadav et al., 2014). Studies have shown that high-levels of nitric oxide can also promote cell apoptosis. Finally, the inhibition of nerve cell apoptosis reflected the negative regulation of genes promoting neuronal apoptosis enzymes.
In summary, the effect of MP and MTX combined therapy on the recovery of SCI was verified by gait analysis. The possible inherent mechanism of this treatment was inferred from gene expression profiling. This study selected the anti-inflammatory drug, MTX, with a flexible administration time, in combination with the traditional SCI treatment drug, MP. The results showed that MP and MTX combination therapy could improve the deficiency of MP treatment alone, enhance the neuroprotective effect, inhibit the activities of inflammatory cytokines, strengthen the anti-oxidative and anti-apoptotic effects and increase the clinical therapeutic effect, which could provide new strategies for the control of SCI. However, in-depth research into the mechanism of these therapeutic effects is required owing to the complexity and variety of the differentially expressed genes.
Footnotes
Funding: This research was supported by the National Natural Science Foundation of China, No. 30960448; the Natural Science Foundation of Jiangxi Province, No. 20142BAB205023; the Ph.D. Start-up Fund of Natural Science Foundation of Jiangxi Science & Technology Normal University in China, No. 3000990122.
Conflicts of interest: None declared.
Research ethics: The study protocol was approved by the Animal Ethics Committee of Jiangxi University (approval number A20150107). The experimental procedure followed the United States National Institutes of Health Guide for the Care and Use of Laboratory Animals (NIH Publication No. 85-23, revised 1986).
Data sharing statement: The datasets analyzed during the current study are available from the corresponding author on reasonable request.
Plagiarism check: Checked twice by iThenticate.
Peer review: Externally peer reviewed.
Copyedited by Wang J, Li CH, Qiu Y, Song LP, Zhao M
References
- Alibai E, Zand F, Rahimi A. Erythropoietin plus methylprednisolone or methylprednisolone in the treatment of acute spinal cord injury: a preliminary report. Crit Care Med. 2010;38:U228–228. [PubMed] [Google Scholar]
- Aoyama K, Matsumura N, Watabe M, Wang F, Kikuchi-Utsumi K, Nakaki T. Caffeine and uric acid mediate glutathione synthesis for neuroprotection. Neuroscience. 2011;181:206–215. doi: 10.1016/j.neuroscience.2011.02.047. [DOI] [PubMed] [Google Scholar]
- Audic S, Claverie J.M. The significance of digital gene expression profiles. Genome research. 1997;10:986–995. doi: 10.1101/gr.7.10.986. [DOI] [PubMed] [Google Scholar]
- Bakar B, Kose EA, Kupana Ayva S, Sarkarati B, Kasimcan MO, Kilinc K. Effects of low-dose methotrexate in spinal cord injury in rats. Ulus Travma Acil Cerrahi Derg. 2013;19:285–293. doi: 10.5505/tjtes.2013.65475. [DOI] [PubMed] [Google Scholar]
- Bankston AN, Li WQ, Zhang H, Ku L, Liu GL, Papa F, Zhao LX, Bibb JA, Cambi F, Tiwari-Woodruff SK, Feng Y. p39, the primary activator for cyclin-dependent kinase 5 (Cdk5) in oligodendroglia, is essential for oligodendroglia differentiation and myelin repair. J Biol Chem. 2013;288:18047–18057. doi: 10.1074/jbc.M113.453688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bishayi B, Bandyopadhyay D, Majhi A, Adhikary R. Expression of CXCR1 (interleukin-8 receptor) in murine macrophages after staphylococcus aureus infection and its possible implication on intracellular survival correlating with cytokines and bacterial anti-oxidant enzymes. Inflammation. 2015;38:812–827. doi: 10.1007/s10753-014-9991-1. [DOI] [PubMed] [Google Scholar]
- Boyaci MG, Eser O, Kocogullari CU, Karavelioglu E, Tokyol C, Can Y. Neuroprotective effect of alpha-lipoic acid and methylprednisolone on the spinal cord ischemia/reperfusion injury in rabbits. Br J Neurosurg. 2015;29:46–51. doi: 10.3109/02688697.2014.954986. [DOI] [PubMed] [Google Scholar]
- Bracken MB. Steroids for acute spinal cord injury. Cochrane Database Syst Rev. 2012;1:CD001046. doi: 10.1002/14651858.CD001046.pub2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buser AM, Schmid D, Kern F, Erne B, Lazzati T, Schaeren-Wiemers N. The myelin protein MAL affects peripheral nerve myelination: a new player influencing p75 neurotrophin receptor expression. Eur J Neurosci. 2009;29:2276–2290. doi: 10.1111/j.1460-9568.2009.06785.x. [DOI] [PubMed] [Google Scholar]
- Capecci J, Forgac M. The function of vacuolar ATPase (V-ATPase) a subunit isoforms in invasiveness of MCF10a and MCF10CA1a Human breast cancer cells. J Biol Chem. 2013;288:32731–32741. doi: 10.1074/jbc.M113.503771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cavus G, Altas M, Aras M, Ozgur T, Serarslan Y, Yilmaz N, Sefil F, Ulutas KT. Effects of montelukast and methylprednisolone on experimental spinal cord injury in rats. Eur Rev Med Pharmacol Sci. 2014;18:1770–1777. [PubMed] [Google Scholar]
- Chen JH, Xia XL, Yin WL. A poplar DRE-binding protein gene, PeDREB2L, is involved in regulation of defense response against abiotic stress. Gene. 2011;483:36–42. doi: 10.1016/j.gene.2011.05.010. [DOI] [PubMed] [Google Scholar]
- Chen JH, Tian QQ, Pang T, Jiang LB, Wu RL, Xia XL, Yin WL. Deep-sequencing transcriptome analysis of low temperature perception in a desert tree, Populus euphratica. BMC Genomics. 2014;15:326. doi: 10.1186/1471-2164-15-326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen Z, Tu S, Hu Y, Wang Y, Xia Y, Jiang Y. Prediction of response of collagen-induced arthritis rats to methotrexate: an (1)H-NMR-based urine metabolomic analysis. J Huazhong Univ Sci Technolog Med Sci. 2012;32:438–443. doi: 10.1007/s11596-012-0076-9. [DOI] [PubMed] [Google Scholar]
- Conesa A, Gotz S, Garcia-Gomez JM, Terol J, Talon M, Robles M Blast2GO. A universal tool for annotation, visualization and analysis in functional genomics research. Bioinfo. 2005;18:3674–3676. doi: 10.1093/bioinformatics/bti610. [DOI] [PubMed] [Google Scholar]
- De Luna N, Gallardo E, Sonnet C, Chazaud B, Dominguez-Perles R, Suarez-Calvet X, Gherardi RK, Illa I. Role of thrombospondin 1 in macrophage inflammation in dysferlin myopathy. J Neuropath Exp Neurol. 2010;69:643–653. doi: 10.1097/NEN.0b013e3181e0d01c. [DOI] [PubMed] [Google Scholar]
- El Kadmiri N, Slassi I, El Moutawakil B, Nadifi S, Tadevosyan A, Hachem A, Soukri A. Glyceraldehyde-3-phosphate dehydrogenase (GAPDH) and Alzheimer's disease. Pathol Biol. 2014;62:333–336. doi: 10.1016/j.patbio.2014.08.002. [DOI] [PubMed] [Google Scholar]
- Esmaeili M, Berry M, Logan A, Ahmed Z. Decorin treatment of spinal cord injury. Neural Regen Res. 2014;9:1653–1656. doi: 10.4103/1673-5374.141797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Galindo RC, Munoz PM, de Miguel MJ, Marin CM, Blasco JM, Gortazar C, Kocan KM, de la Fuente J. Differential expression of inflammatory and immune response genes in rams experimentally infected with a rough virulent strain of Brucella ovis. Vet Immunol Immunop. 2009;127:295–303. doi: 10.1016/j.vetimm.2008.10.326. [DOI] [PubMed] [Google Scholar]
- Gu Bing, JIN Jian-bo, Hua-nan L. Establishment of traumatic spinal cord injury model in rats. Chin J Clin Pharmacol Ther. 2011;16:7. [Google Scholar]
- Hassanshahi G, Amin M, Shunmugavel A, Vazirinejad R, Vakilian A, Sanji M, Shamsizadeh A, Rafat Panah H, Poor NM, Moosavi SR, Taheri S. Temporal expression profile of CXC chemokines in serum of patients with spinal cord injury. Neurochem Int. 2013;63:363–367. doi: 10.1016/j.neuint.2013.07.012. [DOI] [PubMed] [Google Scholar]
- Havik B, Rokke H, Dagyte G, Stavrum AK, Bramham CR, Steen VM. Synaptic activity-induced global gene expression patterns in the dentate gyrus of adult behaving rats: Induction of immunity-linked genes. Neuroscience. 2007;148:925–936. doi: 10.1016/j.neuroscience.2007.07.024. [DOI] [PubMed] [Google Scholar]
- Horiyama S, Takahashi Y, Hatai M, Honda C, Suwa K, Ichikawa A, Yoshikawa N, Nakamura K, Kunitomo M, Date S, Masujima T, Takayama M. Methyl vinyl ketone, a toxic ingredient in cigarette smoke extract, modifies glutathione in mouse melanoma cells. Chem Pharm Bull. 2014;62:772–778. doi: 10.1248/cpb.c14-00109. [DOI] [PubMed] [Google Scholar]
- Iorio A, Polimanti R, Piacentini S, Liumbruno GM, Manfellotto D, Fuciarelli M. Deletion polymorphism of GSTT1 gene as protective marker for allergic rhinitis. Clinrespir J. 2015;9:481–486. doi: 10.1111/crj.12170. [DOI] [PubMed] [Google Scholar]
- Jungmann O, Nikolovska K, Stock C, Schulz JN, Eckes B, Riethmuller C, Owens RT, Iozzo RV, Seidler DG. The dermatan sulfate proteoglycan decorin modulates alpha2beta1 integrin and the vimentin intermediate filament system during collagen synthesis. PLoS One. 2012;7:e50809. doi: 10.1371/journal.pone.0050809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kanehisa M, Araki M, Goto S, Hattori M, Hirakawa M, Itoh M, Katayama T, Kawashima S, Okuda S, Tokimatsu T, Yamanishi Y. KEGG for linking genomes to life and the environment. Nucleic Acids Res 36(Database issue):D480–D484. Keith MP, Edison JD, Gilliland WR (2012) Progress toward personalized treatment of rheumatoid arthritis. Clin Pharmaco Ther. 2008;92:440–442. doi: 10.1093/nar/gkm882. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kertmen H, Gurer B, Yilmaz ER, Sanli AM, Sorar M, Arikok AT, Sargon MF, Kanat MA, Erguder BI, Sekerci Z. The protective effect of low-dose methotrexate on ischemia-reperfusion injury of the rabbit spinal cord. Eur J Pharmacol. 2013;714:148–156. doi: 10.1016/j.ejphar.2013.05.012. [DOI] [PubMed] [Google Scholar]
- Khalatbary AR, Zarrinjoei GR. Anti-inflammatory effect of oleuropein in experimental rat spinal cord trauma. Iran Red Crescent Med J. 2012;14:229–234. [PMC free article] [PubMed] [Google Scholar]
- Krieglstein K, Strelau J, Schober A, Sullivan A, Unsicker K. TGF-beta and the regulation of neuron survival and death. J Physiol Paris. 2002;96:25–30. doi: 10.1016/s0928-4257(01)00077-8. [DOI] [PubMed] [Google Scholar]
- Kwon BK, Casha S, Hurlbert RJ, Yong VW. Inflammatory and structural biomarkers in acute traumatic spinal cord injury. Clin Chem Lab Med. 2011;49:425–433. doi: 10.1515/CCLM.2011.068. [DOI] [PubMed] [Google Scholar]
- Lee G, Bang L, Kim SY, Kim D, Sohn KA. Identifying subtype-specific associations between gene expression and DNA methylation profiles in breast cancer. BMC medical genomics. 2017;10:28. doi: 10.1186/s12920-017-0268-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee JM, Yan P, Xiao Q, Chen S, Lee KY, Hsu CY, Xu J. Methylprednisolone protects oligodendrocytes but not neurons after spinal cord injury. J Neurosci. 2008;28:3141–3149. doi: 10.1523/JNEUROSCI.5547-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li XG, Lin XJ, Du JH, Xu SZ, Lou XF, Chen Z. Combination of methylprednisolone and rosiglitazone promotes recovery of neurological function after spinal cord injury. Neural Regen Res. 2016;11:1678–1684. doi: 10.4103/1673-5374.193250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Linton PJ, Thoman ML. Immunosenescence in monocytes, macrophages, and dendritic cells: Lessons learned from the lung and heart. Immunol Lett. 2014;162:290–297. doi: 10.1016/j.imlet.2014.06.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu JT, Zhu L, Zhang S, Deng Z, Huang Z, Yuan M, Wu W, Yang K. The Autographa californica multiple nucleopolyhedrovirus ac110 gene encodes a new per os infectivity factor. Virus Res. 2016;221:30–37. doi: 10.1016/j.virusres.2016.05.017. [DOI] [PubMed] [Google Scholar]
- Liu XQ, Yan Y, Bao L, Chen BD, Zhao YY, Qi RM. Ginkgolide B inhibits platelet release by blocking Syk and p38 MAPK phosphorylation in thrombin-stimulated platelets. Thromb Res. 2014;134:1066–1073. doi: 10.1016/j.thromres.2014.08.025. [DOI] [PubMed] [Google Scholar]
- Lucas JH, Wheeler DG, Guan Z, Suntres Z, Stokes BT. Effect of glutathione augmentation on lipid peroxidation after spinal cord injury. J Neurotraum. 2002;19:763–775. doi: 10.1089/08977150260139138. [DOI] [PubMed] [Google Scholar]
- Luo CK, Li WW, Wang XY, Wu P, Pang XY, Xu ZQ, Zeng H, Zhang PH, Peng W. Effect of infliximab combined with methylprednisolone on expressions of NF-kappa B, TRADD, and FADD in rat acute spinal cord injury. Spine. 2013;38:E861–869. doi: 10.1097/BRS.0b013e318294892c. [DOI] [PubMed] [Google Scholar]
- Maggio DM, Chatzipanteli K, Masters N, Patel SP, Dietrich WD, Pearse DD. Acute molecular perturbation of inducible nitric oxide synthase with an antisense approach enhances neuronal preservation and functional recovery after contusive spinal cord injury. J Neurotraum. 2012;29:2244–2249. doi: 10.1089/neu.2012.2371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mohebiany AN, Harroch S, Bouyain S. New insights into the roles of the contactin cell adhesion molecules in neural development. Adv Neurobiol. 2014;8:165–194. doi: 10.1007/978-1-4614-8090-7_8. [DOI] [PubMed] [Google Scholar]
- Mortazavi A, Williams BA, McCue K, Schaeffer L, Wold B. Mapping and quantifying mammalian transcriptomes by RNA-Seq. Nat Methods. 2008;7:621–628. doi: 10.1038/nmeth.1226. [DOI] [PubMed] [Google Scholar]
- Muller II, Chatterjee M, Schneider M, Borst O, Seizer P, Schonberger T, Vogel S, Muller KAL, Geisler T, Lang F, Langer H, Gawaz M. Gremlin-1 inhibits macrophage migration inhibitory factor-dependent monocyte function and survival. Int J Cardiol. 2014;176:923–929. doi: 10.1016/j.ijcard.2014.08.051. [DOI] [PubMed] [Google Scholar]
- Myers SA, DeVries WH, Gruenthal MJ, Andres KR, Hagg T, Whittemore SR. Sildenafil improves epicenter vascular perfusion but not hindlimb functional recovery after contusive spinal cord injury in mice. J Neurotraum. 2012;29:528–538. doi: 10.1089/neu.2011.2036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nishio Y, Nishihira J, Ishibashi T, Kato H, Minami A. Role of macrophage migration inhibitory factor (MIF) in peripheral nerve regeneration: anti-MIF antibody induces delay of nerve regeneration and the apoptosis of Schwann cells. Mol Med. 2002;8:509–520. [PMC free article] [PubMed] [Google Scholar]
- Oku S, Takahashi N, Fukata Y, Fukata M. In silico screening for palmitoyl substrates reveals a role for DHHC1/3/10 (zDHHC1/3/11)-mediated neurochondrin palmitoylation in its targeting to rab5-positive endosomes. J Biol Chem. 2013;288:19816–19829. doi: 10.1074/jbc.M112.431676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pang T, Ye C-Y, Xia X, Yin W. De novo sequencing and transcriptome analysis of the desert shrub, Ammopiptanthus mongolicus, during cold acclimation using Illumina/Solexa. BMC Genomics. 2013;1:488. doi: 10.1186/1471-2164-14-488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Park SW, Lee JG, Seo MK, Cho HY, Lee CH, Lee JH, Lee BJ, Baek JH, Seol W, Kim YH. Effects of mood-stabilizing drugs on dendritic outgrowth and synaptic protein levels in primary hippocampal neurons. Bipolar Disorders. 2015;17:278–290. doi: 10.1111/bdi.12262. [DOI] [PubMed] [Google Scholar]
- Pincus T, Sokka T, Cutolo M. The past versus the present, 1980-2004: reduction of mean initial low-dose, long-term glucocorticoid therapy in rheumatoid arthritis from 10.3 to 3.6 mg/day, concomitant with early methotrexate, with long-term effectiveness and safety of less than 5 mg/day. Neuroimmunomodulat. 2015;22:89–103. doi: 10.1159/000362735. [DOI] [PubMed] [Google Scholar]
- Pinzon A, Marcillo A, Quintana A, Stamler S, Bunge MB, Bramlett HM, Dietrich WD. A re-assessment of minocycline as a neuroprotective agent in a rat spinal cord contusion model. Brain Res. 2008;1243:146–151. doi: 10.1016/j.brainres.2008.09.047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raposo C, Nunes AKD, Luna RLD, Araujo SMD, da Cruz-Hofling MA, Peixoto CA. Sildenafil (viagra) protective effects on neuroinflammation: the role of inos/no system in an inflammatory demyelination model. Mediat Inflamm. 2013;19:321460. doi: 10.1155/2013/321460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ray SK, Samntaray S, Banik NL. Future directions for using estrogen receptor agonists in the treatment of acute and chronic spinal cord injury. Neural Regen Res. 2016;11:1418–1419. doi: 10.4103/1673-5374.191212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saeki Y, Matsui T, Saisho K, Tohma S. Current treatments of rheumatoid arthritis: from the ‘NinJa’ registry. Exp Rev Clin Immunol. 2012;8:455–465. doi: 10.1586/eci.12.35. [DOI] [PubMed] [Google Scholar]
- Sanli AM, Serbes G, Sargon MF, Caliskan M, Kilinc K, Bulut H, Sekerci Z. Methothrexate attenuates early neutrophil infiltration and the associated lipid peroxidation in the injured spinal cord but does not induce neurotoxicity in the uninjured spinal cord in rats. Acta Neurochir. 2012;154:1045–1054. doi: 10.1007/s00701-012-1302-8. [DOI] [PubMed] [Google Scholar]
- Schramm M, Wiegmann K, Schramm S, Gluschko A, Herb M, Utermohlen O, Kronke M. Riboflavin (vitamin B2) deficiency impairs NADPH oxidase 2 (Nox2) priming and defense against Listeria monocytogenes. Eur J Immunol. 2014;44:728–741. doi: 10.1002/eji.201343940. [DOI] [PubMed] [Google Scholar]
- Storey KB. Oxidative stress: animal adaptations in nature. Braz J Med Biol Res. 1996;29:1715–1733. [PubMed] [Google Scholar]
- Sundaramurthi H, Manavalan A, Ramachandran U, Hu JM, Sze SK, Heese K. Phenotyping of tianma-stimulated differentiated rat neuronal b104 cells by quantitative proteomics. Neurosignals. 2012;20:48–60. doi: 10.1159/000331492. [DOI] [PubMed] [Google Scholar]
- Tamura Y, Kataoka Y, Cui Y, Takamori Y, Watanabe Y, Yamada H. Intracellular translocation of glutathione S-transferase pi during oligodendrocyte differentiation in adult rat cerebral cortex in vivo. Neuroscience. 2007;148:535–540. doi: 10.1016/j.neuroscience.2007.06.026. [DOI] [PubMed] [Google Scholar]
- Tang Y, Ling ZM, Fu R, Li YQ, Cheng X, Song FH, Luo HX, Zhou LH. Time-specific microRNA changes during spinal motoneuron degeneration in adult rats following unilateral brachial plexus root avulsion: ipsilateral vs. contralateral changes. BMC Neurosci. 2014;15:92. doi: 10.1186/1471-2202-15-92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tao YL, Wu Q, Guo X, Zhang ZZ, Shen YY, Wang FD. MBD5 regulates iron metabolism via methylation-independent genomic targeting of Fth1 through KAT2A in mice. Brit J Hamatol. 2014;166:279–291. doi: 10.1111/bjh.12863. [DOI] [PubMed] [Google Scholar]
- Tator CH FM. Review of the secondary injury theory of acute spinal cord trauma with emphasis on vascular mechanisms. J Neurosurg. 1991;32:107–115. doi: 10.3171/jns.1991.75.1.0015. [DOI] [PubMed] [Google Scholar]
- Vourc’h P, Andres C. Oligodendrocyte myelin glycoprotein (OMgp): evolution, structure and function. Brain Res Rev. 2004;45:115–124. doi: 10.1016/j.brainresrev.2004.01.003. [DOI] [PubMed] [Google Scholar]
- Wang KF, Liu HY, B W. Effects of intrathecal injection of methylprednisolone sodium succinate in acute spinal cord injury rabbits. Zhonghua Wai Ke Za Zhi. 2013;51:5. [PubMed] [Google Scholar]
- Wang L, Feng Z, Wang X. DEGseq: an R package for identifying differentially expressed genes from RNA-seq data. Bioinformatics. 2010;26:136–138. doi: 10.1093/bioinformatics/btp612. [DOI] [PubMed] [Google Scholar]
- White FA, Feldman P, Miller RJ. Chemokine Signaling and the Management of Neuropathic pain. Mol Interv. 2009;9:188–195. doi: 10.1124/mi.9.4.7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu J, Zhang Y, Zhang H, Huang H, Folta KM, Lu J. Whole genome wide expression profiles of Vitis amurensis grape responding to downy mildew by using Solexa sequencing technology. BMC Plant Biol. 2010;10:234. doi: 10.1186/1471-2229-10-234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xuan WJ, Qu Q, Zheng BA, Xiong SD, Fan GH. The chemotaxis of M1 and M2 macrophages is regulated by different chemokines. J Leukocyte Biol. 2015;97:61–69. doi: 10.1189/jlb.1A0314-170R. [DOI] [PubMed] [Google Scholar]
- Yadav R, Goldstein S, Nasef MO, Lee W, Samuni U. Synergistic activity of acetohydroxamic acid on prokaryotes under oxidative stress: the role of reactive nitrogen species. Free Radical Bio Med. 2014;77:291–297. doi: 10.1016/j.freeradbiomed.2014.09.020. [DOI] [PubMed] [Google Scholar]
- Ye J, Fang L, Zheng H, Zhang Y, Chen J, Zhang Z, Wang J, Li S, Li R, Bolund L. WEGO: a web tool for plotting GO annotations. Nucleic Acids Res. 2006;34:W293–297. doi: 10.1093/nar/gkl031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yin Y, Sun WF, Li ZW, Zhang B, Cui H, Deng LX, Xie P, Xiang J, Zou J. Effects of combining methylprednisolone with rolipram on functional recovery in adult rats following spinal cord injury. Neurochem Int. 2013;62:903–912. doi: 10.1016/j.neuint.2013.03.005. [DOI] [PubMed] [Google Scholar]
- Yu YQ, Hu NC, Duan JA, Li DP, Liu C. Neuroprotective effects of sufentanil preconditioning on spinal cord injury in mouse models. Zhongguo Zuzhi Gongcheng Yanjiu. 2016;20:5966–5972. [Google Scholar]
- Zaza G, Granata S, Masola V, Rugiu C, Fantin F, Gesualdo L, Schena FP, Lupo A. Downregulation of nuclear-encoded genes of oxidative metabolism in dialyzed chronic kidney disease patients. PLoS One. 2013;8:e77847. doi: 10.1371/journal.pone.0077847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang Si, Gu B, Wang SY. Application of treadscan gait analysis system in the evaluation of rat spinal cord contusion model. Acta Neuropharmacologica. 2012;2:10. [Google Scholar]
- Zhang YL, Zhang LH, Shen J, Chen C, Mao Z, Li W, Gan WB, Tang PF. Two-photon-excited fluorescence microscopy as a tool to investigate the efficacy of methylprednisolone in a mouse spinal cord injury model. Spine. 2014;39:E493–499. doi: 10.1097/BRS.0000000000000218. [DOI] [PubMed] [Google Scholar]
- Zhang ZC, Li F, Sun TS. An expert consensus on the evaluation and treatment of acute thoracolumbar spine and spinal cord injury in China. Neural Regen Res. 2013;8:3077–3086. doi: 10.3969/j.issn.1673-5374.2013.33.001. [DOI] [PMC free article] [PubMed] [Google Scholar]