Keywords: nerve regeneration, spinal cord injury, Jisuikang, Chinese medicine, prednisolone, microenvironment, axon regeneration, secondary changes, neuronal apoptosis, brain-derived neurotrophic factor, nerve growth factor, neural regeneration
Abstract
The Chinese medicine compound, Jisuikang, can promote recovery of neurological function by inhibiting lipid peroxidation, scavenging oxygen free radicals, and effectively improving the local microenvironment after spinal cord injury. However, the mechanism remains unclear. Thus, we established a rat model of acute spinal cord injury using a modified version of Allen's method. Jisuikang (50, 25, and 12.5 g/kg/d) and prednisolone were administered 30 minutes after anesthesia. Basso, Beattie, and Bresnahan locomotor scale scores and the oblique board test showed improved motor function recovery in the prednisone group and moderate-dose Jisuikang group compared with the other groups at 3–7 days post-injury. The rats in the moderate-dose Jisuikang group recovered best at 14 days post-injury. Hematoxylin-eosin staining and transmission electron microscopy showed that the survival rate of neurons in treatment groups increased after 3–7 days of administration. Further, the structure of neurons and glial cells was more distinct, especially in prednisolone and moderate-dose Jisuikang groups. Western blot assay and immunohistochemistry showed that expression of brain-derived neurotrophic factor (BDNF) in injured segments was maintained at a high level after 7–14 days of treatment. In contrast, expression of nerve growth factor (NGF) was down-regulated at 7 days after spinal cord injury. Real-time fluorescence quantitative polymerase chain reaction showed that expression of BDNF and NGF mRNA was induced in injured segments by prednisolone and Jisuikang. At 3–7 days after injury, the effect of prednisolone was greater, while 14 days after injury, the effect of moderate-dose Jisuikang was greater. These results confirm that Jisuikang can upregulate BDNF and NGF expression for a prolonged period after spinal cord injury and promote repair of acute spinal cord injury, with its effect being similar to prednisolone.
Introduction
After spinal cord injury (SCI) in the adult central nervous system, disruption of the lesioned microenvironment can lead to limited axonal regeneration, which involves inflammation (Wang et al., 2011; Jiang et al., 2012; Yu et al., 2016), glial scar formation (David et al., 2003; Nishimura et al., 2013), neurotrophic factor (NTF) deficiency (Brown et al., 2007; Geng et al., 2010; Xie et al., 2012), and inhibitory factor deposition associated with myelin in the extracellular matrix (Yuan et al., 2011; Alizadeh et al., 2015). Thus, identifying an appropriate approach for intervention of these environmental mechanisms has a promising future as a therapeutic agent for SCI. Nerve growth factor (NGF) and brain-derived neurotrophic factor (BDNF) are the two most prominent members of the NTF family. They activate intrinsic axonal regeneration and neuronal neural apoptosis (Cui, 2006; Kaplan et al., 2010) by influencing neuronal proliferation and differentiation (Lin et al., 2010).
Jisuikang (JSK) is a clinical experiential Chinese herbal formula that inhibits nitric oxide synthase expression, reduces nitric oxide and tumor necrosis factor alpha (TNF-α) levels, decreases superoxide dismutase activity, and suppresses secondary changes at SCI lesion sites (Jian et al., 2008, 2009).
However, there is no evidence determining whether JSK treats SCI by regulating NTF expression. Accordingly, we hypothesized that JSK can prevent secondary spinal cord injury by regulating expression of NGF and BDNF. To verify this hypothesis and determine the effect of JSK on recovery of neurological function, we examined spinal changes by immunohistochemistry and hematoxylin-eosin staining, as well as examining BDNF and NGF expression. Prednisolone (PED) was selected as a positive control, as it has previously been shown to be effective in treating SCI (Jian et al., 2009; Wu et al., 2015).
Materials and Methods
Animals
A total of 180 adult female Sprague-Dawley rats weighing 180–220 g (Zhejiang Animal Experimental Institute, Zhejiang, China; SCXK (Zhe) 2008-0033) were used in this study. The rats were housed with five per cage in the Animal Experimental Institute of Nanjing University of Chinese Medicine (Nanjing, China), with free access to food and water under controlled temperature and humidity conditions.
The study protocol was approved by Animal Ethics Committee of the Affiliated Hospital of Nanjing University of Chinese Medicine. The experimental procedure followed the United States National Institutes of Health Guide for the Care and Use of Laboratory Animals (NIH Publication No. 85-23, revised 1986).
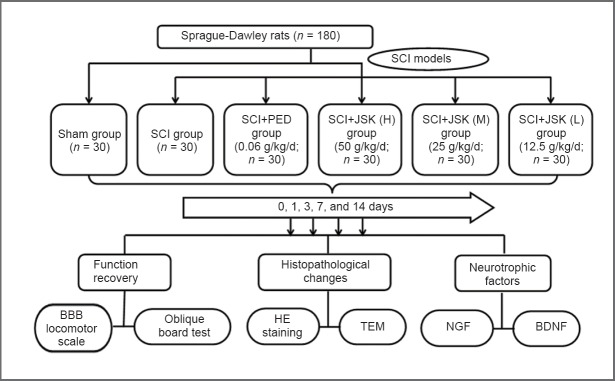
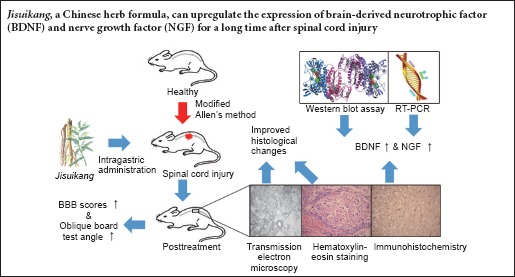
Rats were randomly divided into two groups: sham group (sham surgery) with 30 rats, and SCI group with 150 rats. The SCI group was further divided into five subgroups: SCI, SCI + PED, SCI + JSK (high-dose, H), SCI + JSK (moderate-dose, M), and SCI + JSK (low-dose, L) (n = 30 for each group). Rats were sacrificed two hours after final intragastric administration, and spinal cord samples were obtained at 3, 7, and 14 days after injury (Figure 1).
Figure 1.
Flow chart of the experiment.
PED: Prednisolone; JSK: Jisuikang; BBB: Basso, Beattie, and Bresnahan; HE: hematoxylin-eosin; TEM: transmission electron microscopy; NGF: nerve growth factor; BDNF: brain-derived neurotrophic factor.
Establishment of SCI models
The acute SCI model based on a modified Allen's method was used (Allen, 1911). Briefly, all rats were intraperitoneally anesthetized with 10% chloral hydrate (0.4 g/kg). After a dorsal incision, laminectomy was performed to expose the dorsal dura mater at the T9–11 spinal cord level. Concurrently, a solid metal bar (10 g; diameter, 3 mm) was dropped freely from a height of 25 mm onto T10 of the spinal cord. Tension in the rats’ double posterior limb was immediately abolished, indicating that the model had been successfully established. In the sham group, the spinal cord was exposed but not injured. Muscle and skin were sutured in two layers and erythromycin applied to prevent infection.
Drug delivery
PED treatment
PED (Tianjin Chemical Company, Tianjin, China) was dissolved and diluted in saline solution (final concentration 0.3%; stored at 4°C). Rats received intragastric administration of PED (60 mg/kg/d) for 30 minutes after anesthetic recovery, twice a day (09:00 and 15:00).
JSK treatment
JSK was composed of milkvetch root (30 g), Chinese angelica (12 g), red peony root (12 g), earthworm (10 g), Szechwan lovage rhizome (10 g), peach seed (10 g), and safflower (10 g) (Pharmacy, Affiliated Hospital of Nanjing University of Chinese Medicine). High concentration of JSK was prepared (crude drug 2.5 g/mL) by boiling, steam boiling, and then concentrating, before storing at 4°C. JSK was prepared at the Experiment Center of First Clinical Medical College in Nanjing University of Chinese Medicine. Intragastric administration was performed as for PED treatment with JSK (H) at 50 g/kg/d, JSK (M) at 25 g/kg/d, and JSK (L) at 12.5 g/kg/d. JSK (H) was diluted to JSK (M) and JSK (L) with saline water before intragastric administration.
Sham and SCI groups were treated with equal volumes (20 mL/kg/d) of normal saline solution.
Behavioral assessment
Basso, Beattie, and Bresnahan (BBB) locomotor scale
The BBB locomotor scale was performed in an open field, based on observations of hind limb movements, specifically, gait and coordination. All groups were assessed at postoperative days 1, 3, 7, and 14 by double-blind, double independent observation, and recorded according to a previously described method (Basso et al., 1995). Higher BBB locomotor scale scores reflect healthier rat behavior.
Oblique board test
All rats underwent the oblique board test at postoperative days 1, 3, 7, and 14. Rats were placed on a rectangular ramp that was perpendicular to the ramp's longitudinal axis. The height of the board was raised and the largest angle that the rats remained on the board for more than 5 seconds recorded. Each rat was tested three times, with the average value taken as the final result.
Tissue preparation
At postoperative days 3, 7, and 14 after behavioral assessment, six rats were randomly chosen from each group. They were deeply anesthetized by intraperitoneal injection of 10% chloral hydrate (0.4 g/kg) and perfused with 0.01 M phosphate-buffered saline (PBS) followed by 4% paraformaldehyde. Afterwards, 2 cm lengths of spinal cord centered at the lesion site were fixed in the same fixative at 4°C for 24 hours. After gradual dehydration with ethanol and xylene, the spinal cord pieces were embedded in paraffin. Serial coronal sections (4–5 μm thickness) were sliced 5 mm from the lesion site using a microtome, and collected in cold PBS for hematoxylin-eosin staining, transmission electron microscopy, and immunohistochemistry.
At postoperative days 3, 7, and 14 after behavioral assessment, four rats were randomly chosen from each group. They were deeply anesthetized by intraperitoneal injection of 10% chloral hydrate (0.4 g/kg) and perfused with 0.01 M PBS. Next, 2 cm lengths of spinal cord centered at the lesion site were washed with cold saline, and then frozen at −80°C in liquid nitrogen for western blot assay and quantitative real-time polymerase chain reaction (qRT-PCR).
Histomorphological observation and immunohistochemistry
Hematoxylin-eosin staining
Three paraffin sections randomly chosen from each group at 3 and 7 days after intervention were stained by hematoxylin and eosin and viewed by light microscopy (DM1000; Leica, Germany).
Transmission electron microscopy
Spinal cord sections from three cases were randomly chosen from each group at 3 and 7 days after intervention. Ultrastructure of the lesion site was observed by transmission electron microscopy and assessed by Kaptanoglu ultrastructure using the SCI scoring method (Kaptanoglu et al., 2002, 2005). Overall, 20 neurons, 20 neuronal axons, and 20 mitochondria were assessed from each sample.
Immunohistochemistry
Spinal cord sections from three cases were randomly chosen from each group at 3, 7, and 14 days after intervention. Immunohistochemistry was performed according to the Envision method. Briefly, sections were dewaxed and hydrated. After endogenous peroxidase inactivation, sections were blocked in 3% H2O2 for 20 minutes at room temperature, followed by antigen retrieval. Sections were incubated at room temperature for 60 minutes in diluted primary antibodies: anti-BDNF monoclonal antibody (rabbit-anti-rat, 1:250) and anti-NGF polyclonal antibody (rabbit-anti-rat, 1:400; Abcam, Cambridge, MA USA). Polymer toughener was dropped onto each section and incubated at room temperature for 20 minutes, followed by enzyme targeted anti-rat/-rabbit polymer incubation for 30 minutes at room temperature and 3,3′-diaminobenzidine staining. Afterwards, sections were lightly counterstained with hematoxylin, treated with 0.1% hydrochloric acid, dehydrated with gradient alcohol, permeabilized with xylene, mounted, and observed under a 200× light microscope (Leica). Expression of BDNF and NGF was assessed, with light yellow granular staining in cells classified as a positive reaction, with absence of this staining defined as a negative reaction. All slides were blindly reviewed by two pathologists. Image-Pro Plus 6.0 software (Media Cybernetics Inc., Rockville, MD, USA) was used to examine randomly selected images from each slide. Mean density (MD) was represented by relative expression of NGF and BDNF.
Western blot assay
Spinal cord sections from three cases were randomly chosen from each group at 3, 7, and 14 days after intervention. Spinal cord was removed from −80°C storage, and crushed in a mixture of RIPA lysis buffer and phenylmethanesulfonyl fluoride (final concentration, 1 mM) for 30 minutes on ice. Insoluble material was discarded by centrifugation at 15,000 r/min for 30 minutes at 4°C. Protein concentration was assessed using the bicinchoninic acid protein assay (Beyotime Biotechnology, Haimen, China) with bovine serum albumin as the standard. Protein was denatured at 95°C for 5 minutes with 2× sodium dodecyl sulfate polyacrylamide gel electrophoresis sample loading buffer, and stored at −80°C after adjusting to a specific concentration. Protein (26.7 μg) was loaded onto 10% polyacrylamide minigels and transferred to polyvinylidene difluoride membranes (Millipore, Bedford, MA, USA). For immunoblotting, membranes were blocked with 5% nonfat dry milk in Tris-buffered saline for 2 hours at room temperature, and then incubated with primary antibodies: rabbit-anti-rat BDNF monoclonal antibody (1:1,000; Abcam) and anti-beta-actin monoclonal antibody (1:1,000; Cell Signaling, Danvers, MA, USA) at 4°C overnight in 1× TBS, 5% bovine serum albumin, and Tris-buffered saline with 0.1% Tween-20 (TBST). Membranes were washed three times for 5 minutes in TBST and incubated with goat anti-rabbit lgG (1:5,000; Bioworld, Visalia, CA, USA). Proteins were visualized using SuperSignal West Pico (Thermo Fisher Scientific, Waltham, MA, USA) and imaged by Image Quant LAS 4000 mini ultrasensitive chemical luminescence tomography (GE Healthcare, Aurora, OH, USA). Relative BDNF expression (BDNF/beta-actin densitometry) was analyzed using Adobe Photoshop CS5 software (Adobe Systems Inc., San José Canton, CA, USA).
qRT-PCR
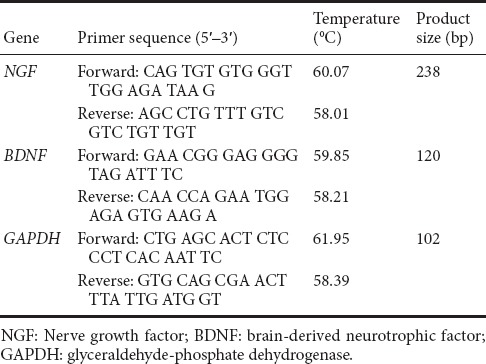
Spinal cord sections from three cases were randomly selected from each group at 3, 7, and 14 days after intervention, and homogenized with 1 mL TRIZOL (Takara, Dalian, China) to prepare total RNA. RNA purity and concentration was determined from optical density values at 260 nm and 280 nm. RNA was reverse-transcribed with PrimeScript RT Master Mix Perfect Real-Time (Takara Biotechnology (Dalian) Co., Ltd., Dalian, China) at the following conditions: 37°C for 15 minutes for reverse transcription into cDNA, 85°C for 15 seconds, and 35°C for 30 seconds to inactivate reverse transcriptase. To identify BDNF, NGF, and glyceraldehyde phosphate dehydrogenase (GAPDH) transcription, PCR reaction mixtures (50 μL) were prepared with 2.0 μL cDNA synthesis mixture, 40 nm dNTPs, 10 pmol sense and antisense primers, and 1.25 U Taq polymerase (Takara, Tokyo, Japan). Two-step fluorescence quantitative PCR was performed with 40 cycles of initial denaturation at 95°C for 30 seconds, denaturation at 95°C for 5 seconds, and annealing at 60°C for 34 seconds. The 2–ΔΔCt method was used to analyze relative expression of NGF and BDNF genes. Primer sequences are shown in Table 1.
Table 1.
Nucleotide primers used for RT-PCR
Statistical analysis
All experimental data were analyzed with SPSS 17.0 software (IBM, Armonk, NY, USA). All quantitative normally distributed data are presented as the mean ± SD, and were analyzed by one-way analysis of variance followed by the least significant difference test for multiple comparisons. Skewed distribution data are presented as median [Q1, Q3], and were analyzed by Kruskal Wallis H test. A value of P < 0.05 was considered statistically significant.
Results
Behavioral assessment
Compared with the sham group, rats in the SCI groups all had lower BBB scores (P < 0.01), with dragging hind legs 24 hours after surgery. From 3 to 7 days post-injury, hind-limb motor function improved, and BBB scores increased, being statistically higher (P < 0.05) in the SCI + PED and all SCI + JSK groups (except SCI + JSK (L) at 3 days) compared with the SCI group. However, there was no significant difference between the SCI + PED and SCI + JSK (M) groups (P = 0.479). No SCI + JSK group had a higher BBB score than the JSK + PED group, although the SCI + JSK (M) group was significantly different at 14 days after surgery (P < 0.05) (Figure 2).
Figure 2.
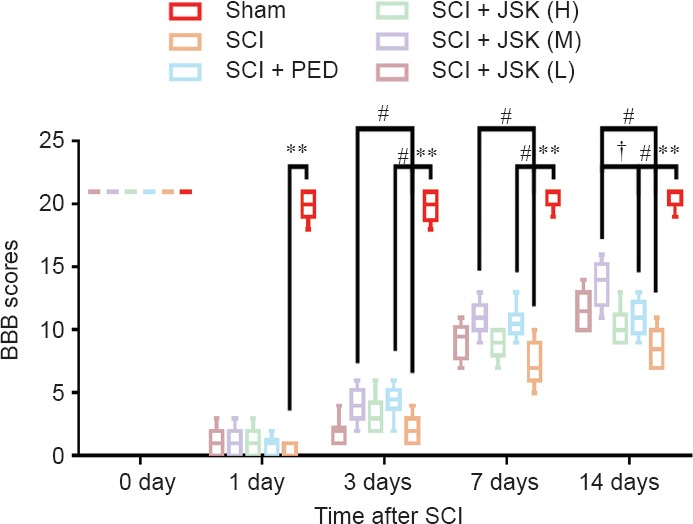
Effect of PED and JSK on recovery of motor function assessed by BBB scores.
Rats subjected to SCI had significant deficits in hind-limb movement. However, there were no significant differences between SCI groups at 24 hours, while significant differences were observed at 3, 7, and 14 days (PED: 60 mg/kg/d, JSK (H): 50 g/kg/d, JSK (M): 25 g/kg/d, JSK (L): 12.5 g/kg/d). Higher BBB scores indicate superior locomotor function. Data are expressed as median [Q1, Q3] (n = 10, Kruskal-Wallis H test). **P < 0.01, #P < 0.05, †P < 0.05. SCI: Spinal cord injury; PED: prednisone; JSK: Jisuikang; BBB: Basso, Beattie, and Bresnahan.
For the oblique board test, smaller angles were observed in all SCI groups compared with the sham group (P < 0.01) at 24 hours after surgery. From 3 to 7 days post-injury, as hind-limb motor function improved, significantly higher angles were observed in the SCI + PED and SCI + JSK (M) groups compared with the SCI group (P < 0.05). It is worth noting that the angles were higher in the SCI + JSK (M) group compared with the SCI + PED group at 7 days after surgery (P < 0.05) (Figure 3).
Figure 3.
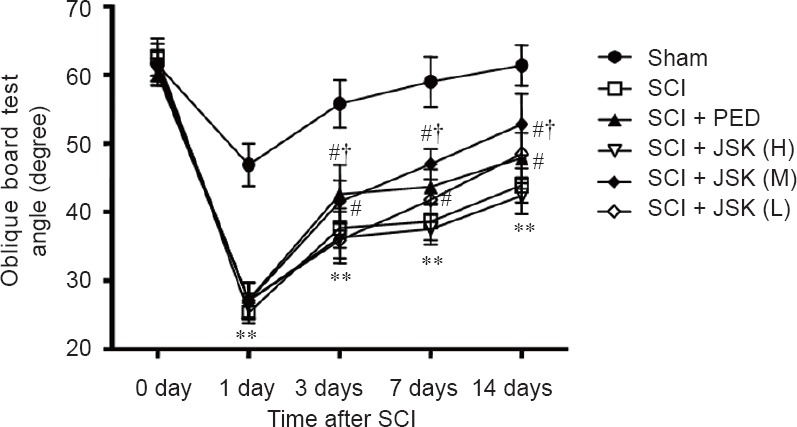
Effect of PED and JSK on recovery of hind-limb motor function assessed by the oblique board test.
Rats with SCI had significant deficits in hind-limb movement. However, there were no significant differences between SCI groups at 24 hours, while significant differences were observed at 3, 7, and 14 days (PED: 60 mg/kg/d, JSK (H): 50 g/kg/d, JSK (M): 25 g/kg/d, and JSK (L): 12.5 g/kg/d). **P < 0.01, vs. sham group; #P < 0.05, vs. SCI group, †P < 0.05, vs. SCI + PED group. Data are expressed as the mean ± SD (n = 8; one-way analysis of variance and the least significant difference test). SCI: Spinal cord injury; PED: prednisone; JSK: Jisuikang.
Histomorphological changes
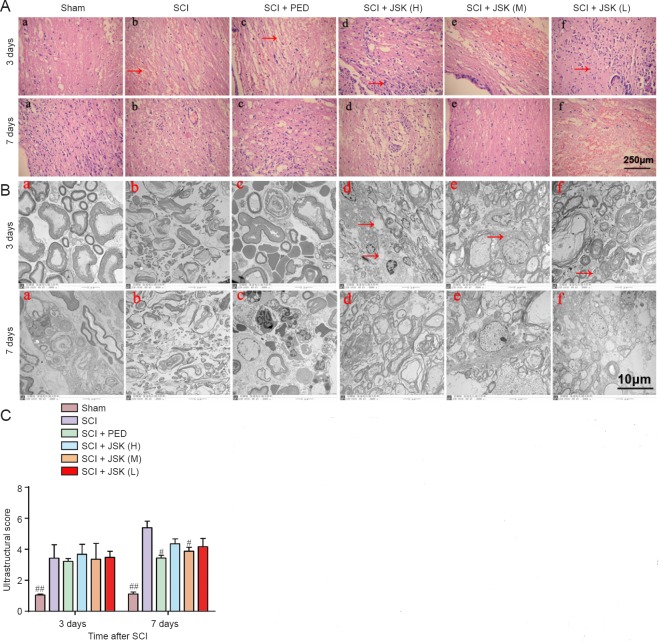
In the sham group, spinal cord neurons showed normal features. From 3 to 7 days post-injury, SCI lesions progressively increased with a few showing irreversible damage. Compared with glial cell infiltration in the sham group at 3 days after surgery, nerve fibers in SCI groups had a disordered arrangement with pyknosis, necrosis, and dissolution visible in some. In the SCI + JSK (H) group, there were many infiltrating glial cells and a few inflammatory cells with little hemorrhage. While in the SCI + JSK (M) and SCI + JSK (L) groups, few glial cells and inflammatory cells had infiltrated with less hemorrhage. In degenerating axons, myelin degeneration, lamellar separation, few mitochondria, and rough endoplasmic reticulum were observed by transmission electron microscopy in SCI groups treated with PED and JSK. Seven days after surgery, more neurons survived in the SCI + PED, SCI + JSK (M), and SCI + JSK (L) groups compared with the SCI group, with less edema and necrosis, white matter and gray matter vacuoles, and inflammatory cell infiltration. Moreover, the moderate dose of JSK had a superior effect compared with the low dose, while the high dose showed little improvement in reducing inflammation. Transmission electron microscopy also showed organelles changes, such as cavities, in SCI groups treated with PED and JSK. Glial cells showed normal structures while neurons had irregular nuclei with mild mitochondrial swelling and rough endoplasmic reticulum expansion (Figure 4A, B).
Figure 4.
Effect of PED and JSK on histomorphological changes in the spinal cord of rats.
(A) Hematoxylin-eosin staining showed normal neuronal and glial morphology in the sham group. Typical necrosis including broad hemorrhage (arrow in b), edema (arrow in c), reactive gliosis (arrow in d), and neuronal apoptosis with condensed nuclei (arrow in f) was observed in the SCI group at 3 days. Compared with the SCI group, only mild glial proliferation, hemorrhage, inflammation, and edema occurred in the SCI + PED, SCI + JSK (M), SCI + JSK (L), and SCI + JSK (M) groups, thereby showing improved features (PED: 60 mg/kg/d, JSK (H): 50 g/kg/d, JSK (M): 25 g/kg/d, and JSK (L): 12.5 g/kg/d). Scale bar: 250 μm, light microscope. (B, C) Transmission electron microscopy showed no apoptotic cells in the sham group, in which nuclear membranes were integrative and nuclei round with no formation of apoptotic bodies. Scale bar: 10 μm. In comparison, many apoptotic cells were found in the SCI group, with shrunken neurons of abnormal morphology and with vacuolated cytoplasm (arrow in d), broken or missing mitochondrial ridges (arrow in d), and dramatically enlarged endoplasmic reticulum (arrow in e) at 3 days. Neuronal ultrastructure in treated groups demonstrated clearer morphology at 7 days, with mildly vacuolated cytoplasm, almost intact nuclear membranes, and mildly enlarged granular endoplasmic reticulum. Ultrastructure scores (proportional to severity of injury) are shown as the mean ± SD (n = 3, one-way analysis of variance and least significant difference test). #P < 0.05, ##P < 0.01, vs. SCI group. SCI: Spinal cord injury; PED: prednisolone; JSK: Jisuikang.
Ultrastructure scores of groups analyzed by one-way analysis of variance and least significant difference test were significantly higher in the SCI group compared with the sham group at 3 to 7 days post-injury (P < 0.01). The score in the SCI group increased with time, while treatment groups showed minimal changes. By 7 days post-injury, ultrastructure scores were significantly lower in the SCI + PED and SCI + JSK (M) groups compared with the SCI group (P < 0.05) (Figure 4C).
NGF and BDNF expression
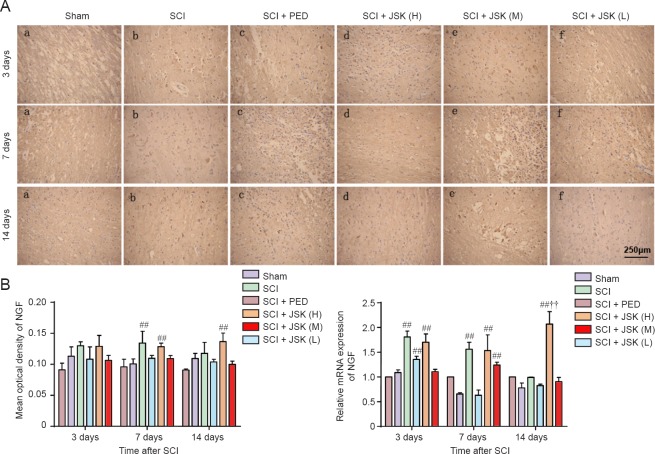
Immunohistochemistry showed low NGF and BDNF expression in the lesioned area of the sham group. Compared with the sham group, the immune response and NGF mRNA expression in the SCI + PED and SCI + JSK (M) groups increased from 3 to 7 days, showing significant differences compared with the SCI group (P < 0.01). The immune response and NGF mRNA expression remained at high levels in the SCI + JSK (M) group 14 days after injury, despite a slight decline in the other treatment groups, which showed significant differences with the SCI + PED group (P < 0.01). NGF expression was still significantly higher in the SCI + PED group compared with the sham group. In addition, apart from the SCI + JSK (M) group, no group showed statistically different immunohistochemistry and RT-PCR results compared the sham group (Figure 5).
Figure 5.
Effect of PED and JSK on NGF protein and mRNA expression determined by immunohistochemistry and qRT-PCR.
(A) Representative images of immunohistochemistry of NGF in the rat spinal cord at 3, 7, and 14 days post-injury. Scale bar: 250 μm. (B) Quantification of NGF immunoreactivity. (C) NGF mRNA analyzed by two-step fluorescent quantitative RT-PCR increased in the SCI + PED and SCI + JSK (M) groups compared with the SCI group. Data are expressed as the mean ± SD (n = 3, one-way analysis of variance and the least significant difference test). ##P < 0.01, vs. SCI group; ††P < 0.01, vs. SCI + PED group. PED: Prednisolone 60 mg/kg/d; JSK: Jisuikang, JSK (H): 50 g/kg/d, JSK (M): 25 g/kg/d, and JSK (L): 12.5 g/kg/d. SCI: Spinal cord injury; qRT-PCR: quantitative real-time polymerase chain reaction.
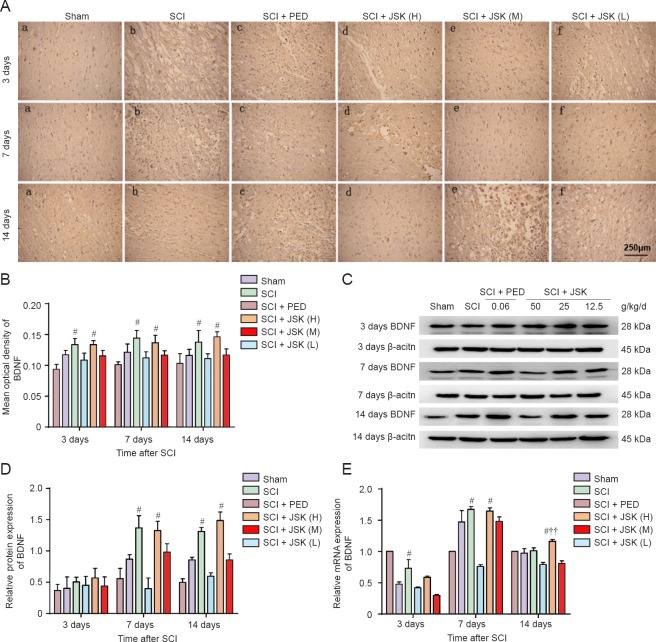
Compared with consistently low BDNF expression in the lesioned area of the sham group, immunohistochemistry showed an increase in BDNF in the SCI group from 3 to 7 days postoperatively, with a slight decline from 7 to 14 days post-injury. This is consistent with the western blot assay and RT-PCR results, except for the continuous increase in the SCI + JSK (M) group by western blot assay. Specifically, no significant consistency was observed at 3 days post-injury. While from 7 to 14 days, both protein and mRNA expression was significantly higher in the SCI + PED and SCI + JSK (M) groups compared with the SCI group (P < 0.05). However, immunohistochemistry and western blot assay results showed no statistical difference between the SCI + PED and SCI + JSK (M) groups (P > 0.05). Conversely, RT-PCR showed statistically significant differences in BDNF mRNA expression between the two groups at 14 days (P < 0.01) (Figure 6).
Figure 6.
Effect of PED and JSK on BDNF protein and mRNA expression by immunohistochemistry, western blot assay, and qRT-PCR.
(A) Representative images of immunohistochemistry of BDNF in the rat spinal cord at 3, 7, and 14 days post-injury. Scale bar: 250 μm. (B) Quantification of BDNF immunoreactivity. (C) Western blot assay of BDNF protein expression. (D) Quantification of BDNF protein densitometry. (E) NGF mRNA expression was quantified by two-step fluorescent quantitative PCR. Data are expressed as the mean ± SD (n = 3, one-way analysis of variance and least significant difference test). #P < 0.05, vs. SCI group; ††P < 0.01, vs. SCI + PED group. PED: Prednisolone 60 mg/kg/d; JSK: Jisuikang, JSK (H): 50 g/kg/d, JSK (M): 25 g/kg/d, and JSK (L): 12.5 g/kg/d. SCI: Spinal cord injury; qRT-PCR: quantitative real-time polymerase chain reaction.
Discussion
The main causes of dysfunction after SCI include primary and secondary injuries, although the effects of the latter are probably more severe (Rossignol et al., 2011; Ray et al., 2016). Secondary injury after SCI includes inflammation, glial cell activation, and scar tissue formation, which directly or indirectly affect the regenerating nerve microenvironment. Therefore, how to effectively improve the microenvironment after SCI has become the key to promoting axonal regeneration.
NTFs are a crucial set of factors in the regenerating axon microenvironment, and aside from nutritive effects on the central nervous system, may also participate in repair of the central nervous system after damage (Novotna et al., 2011; Elkelini et al., 2012; Weishaupt et al., 2012). Among them, NGF and BDNF are the most widely and highly investigated NTFs (Rrice et al., 2007; Keefe et al., 2017). In recent years, NGF has been shown to function in nourishing neurons and regulating various types of neuronal growth, development, differentiation, and regeneration, thereby inducing axons into nerve fibers and reducing neuronal death (Ochodnicky et al., 2011; Elkelini et al., 2012). The specific mechanisms can be attributed to inhibition of the release of toxic amino acids, calcium overload, release of free oxygen radicals, and cell apoptosis. Indeed, recent experiments have confirmed that NGF may be involved in activating signaling pathways to regulate gene expression in target cells through combination with tyrosine kinase receptors and promoting the biological effect of nerve regeneration (Kishibe et al., 2002; Jian et al., 2015; Fan et al., 2016). Chen et al. (2013) found that combined FK506 and NGF treatment had a synergistic effect in treatment of SCI in rats, and effectively promoted neural regeneration and functional recovery. Lin et al., (2012) showed increased expression of growth associated protein 43 and promotion of recovery of motor function in NGF gene-modified Schwann cells after SCI in rats. Alternatively, BDNF induced proliferation and differentiation of NSCs, and reduced free radical accumulation to protect neurons from attack with negative factors (Weishaupt et al., 2012). In addition, BDNF is associated with recovery after SCI (Dougherty et al., 2000), with the local BDNF immune response showing a positive reaction after SCI and the ratio of astrocytes and microglia/macrophages increasing significantly. Thus, testing NGF and BDNF expression may directly provide information on neuronal regeneration and recovery after nerve injury.
Accumulating evidence has verified that Chinese medicine may provide neurons with an environment that contains the proper proportion of active factors, meeting nerve physiology requirements and being favorable for nerve regeneration (Li et al., 2010; Ke et al., 2012; Lu et al., 2017). For the mechanisms of tonifying Qi, activating blood and collaterals, and nourishing the kidney to promote nerve regeneration, traditional Chinese medicine is strongly associated with inhibiting inflammation, reducing glial scar and syringomyelia formation, improving the regenerating axon microenvironment, improving body immunity, promoting protein synthesis and energy metabolism, and inhibiting neuronal apoptosis caused by nerve injury (Chang et al., 2016; Fang et al., 2017). A previous study based on the Chinese Theory of “treat both Du meridian and kidney”, confirmed that JSK can attenuate nitric oxide synthase expression and reduce nitric oxide, malondialdehyde, and tumor necrosis factor alpha levels (Jian et al., 2008, 2009). Meanwhile, JSK increases superoxide dismutase activity and interleukin-10 expression, removes oxygen free radicals, and improves the regenerating axon microenvironment, thereby suppressing secondary SCI.
Pharmacological research on the main herbs and composition of JSK are as follows: astragalus membranaceus, which can inhibit lipid peroxidation after SCI (Pu et al., 2015), improve microcirculation (Han et al., 2017), and reduce glial scar formation (Jun et al., 2013). Tetramethylpyrazine, which can accelerate functional recovery of traumatized spinal cord in rat models by attenuating inflammation and promoting functional recovery through increased NGF and BDNF at the lesioned site (Yun et al., 2010; Hu et al., 2013). Salvia miltiorrhiza and its preparations, which can inhibit neuronal apoptosis (Meng et al., 2006; Yu et al., 2008), increase blood flow at the SCI site (Rongrong et al., 2016; Fei et al., 2017), adjust water and calcium ion concentration in cells (Tsai et al., 2015; Yan et al., 2015), reduce the inflammatory reaction, and promote gene and neurofilament expression to relieve the series of pathophysiological damage caused by SCI (Kai et al., 2012).
Our results here using behavioral, histological, and molecular biological outcomes demonstrate that compared with PED after SCI, the Chinese herbal formula, JSK, contributes more to promoting regeneration and restoring function. Specifically, NTF members (NGF and BDNF) are upregulated at the injury site, with low expression in normal rats and slight upregulation in SCI models, which may be considered a self-protective reaction (Ji et al., 2011). However, NGF protein and mRNA persisted only briefly, and began to decline on day 7 after SCI. In contrast, JSK (M) and PED slowed down the rate of decline compared with the SCI group. BDNF upregulation was more moderate and long-lasting at high levels, establishing a correlation with motor function recovery after SCI. Thus, we assume that JSK promotes NGF and BDNF expression after SCI, and in synergy improves the regenerating axon microenvironment to promote recovery of neurological function. Overall, long-term use of JSK showed a lasting therapeutic effect versus PED.
However, moderate dose of JSK showed an obvious effect versus the other two doses, but no concentration-response relationship compared with the other groups, which may be related to factors, such as the thickened texture of JSK (H).
Despite progress in this study, the timeline was short and it is difficult to fully evaluate any curative effect. Further, we did not use methylprednisolone, which has a clinical curative effect, as a positive control. Thus, the specific molecular pathway mechanism of JSK that promotes BDNF and NGF expression is not yet clear and is the direction of our further study.
Compared with PED, which visibly promotes motor function recovery and delays pathological damage of SCI rats, JSK had an improved effect at 7 days post-injury. Despite the mild promotion of BDNF and NGF immediately after SCI, the effect of JSK is more lasting than PED, which may be one explanation for JSK's promoting recovery of neurological function and improving the regenerating axon microenvironment.
Acknowledgments
The authors are grateful to Medical Research Center of First Clinical Medical College of Nanjing University of Chinese Medicine for help and support.
Footnotes
Funding: This work was partially supported by the National Natural Science Foundation of China, No. 81573997; the Natural Science Foundation for Colleges and Universities in Jiangsu Province of China, No. 15KJD360001; the Natural Science Foundation of Jiangsu Province of China, No. BK2011180.
Conflicts of interest: None declared.
Research ethics: The study protocol was approved by the Animal Ethics Committee of the Affiliated Hospital of Nanjing University of Chinese Medicine. The experimental procedure followed the United States National Institutes of Health Guide for the Care and Use of Laboratory Animals (NIH Publication No. 85-23, revised 1986).
Data sharing statement: The datasets analyzed during the current study are available from the corresponding author on reasonable request.
Plagiarism check: Checked twice by iThenticate.
Peer review: Externally peer reviewed.
Copyedited by James R, Frenchman B, Wang J, Li CH, Qiu Y, Song LP, Zhao M
References
- Alizadeh A, Dyck SM, Karimi-Abdolrezaee S. Myelin damage and repair in pathologic CNS: challenges and prospects. Front Mol Neurosci. 2015;8:35. doi: 10.3389/fnmol.2015.00035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Allen AR. Surgery of experimental lesion of spinal cord equivalent to crush injury of fracture dislocation of spinal column: a preliminary report. J Am Med Assoc L. 1911;VII:878–880. [Google Scholar]
- Basso DM, Beattie MS, Bresnahan JC. A sensitive and reliable locomotor rating scale for open field testing in rats. J Neurotrauma. 1995;12:1–21. doi: 10.1089/neu.1995.12.1. [DOI] [PubMed] [Google Scholar]
- Brown A, Ricci MJ, Weaver LC. NGF mRNA is expressed in the dorsal root ganglia after spinal cord injury in the rat. Exp Neurol. 2007;205:283–286. doi: 10.1016/j.expneurol.2007.01.025. [DOI] [PubMed] [Google Scholar]
- Chang IA, Lim HD, Kim KJ, Shin H, Namgung U. Enhanced axonal regeneration of the injured sciatic nerve by administration of Buyang Huanwu decoction. J Ethnopharmacol. 2016;194:626–634. doi: 10.1016/j.jep.2016.10.053. [DOI] [PubMed] [Google Scholar]
- Chen G, Zhang Z, Wang S, Lv D. Combined treatment with FK506 and nerve growth factor for spinal cord injury in rats. Exp Ther Med. 2013;6:868–872. doi: 10.3892/etm.2013.1254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cui Q. Actions of neurotrophic factors and their signaling pathways in neuronal survival and axonal regeneration. Mol Neurobiol. 2006;33:155–179. doi: 10.1385/MN:33:2:155. [DOI] [PubMed] [Google Scholar]
- David S, Lacroix S. Molecular approaches to spinal cord repair. Annu Rev Neurosci. 2003;26:411–440. doi: 10.1146/annurev.neuro.26.043002.094946. [DOI] [PubMed] [Google Scholar]
- Dougherty KD, Dreyfus CF, Black IB. Brain-derived neurotrophic factor in astrocytes, oligodendrocytes, and microglia/macrophages after spinal cord injury. Neurobiol Dis. 2000;7:574–585. doi: 10.1006/nbdi.2000.0318. [DOI] [PubMed] [Google Scholar]
- Elkelini MS, Bagli DJ, Fehlings M, Hassouna M. Effects of intravesical onabotulinumtoxinA on bladder dysfunction and autonomic dysreflexia after spinal cord injury: role of nerve growth factor. BJU Int. 2012;109:402–407. doi: 10.1111/j.1464-410X.2011.010362.x. [DOI] [PubMed] [Google Scholar]
- Fan YM, Huang QY, Wu YA, Harvey AR, Cui Q, Gao YQ. Nogo-p4 suppresses TrkA signaling induced by low concentrations of nerve growth factor through NgR1 in differentiated PC12 cells. Neurosignals. 2016;24:25–39. doi: 10.1159/000442609. [DOI] [PubMed] [Google Scholar]
- Fang L, Wang Y, Zheng Q, Yang T, Zhao P, Zhao H, Zhang Q, Zhao Y, Qi F, Li K, Chen Z, Li J, Zhang N, Fan Y. Effects of Bu Shen Yi sui capsule on NogoA/NgR and its signaling pathways RhoA/ROCK in mice with experimental autoimmune encephalomyelitis. BMC Complement Altern Med. 2017 doi: 10.1186/s12906-017-1847-4. doi: 10.1186/s12906-017-1847-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fei YX, Wang SQ, Yang LJ, Qiu YY, Li YZ, Liu WY, Xi T, Fang WR, Li YM. Salvia miltiorrhiza Bunge (Danshen) extract attenuates permanent cerebral ischemia through inhibiting platelet activation in rats. J Ethnopharmacol. 2017 doi: 10.1016/j.jep.2017.06.023. doi: 10.1016/j.jep.2017.06.023. [DOI] [PubMed] [Google Scholar]
- Geng SJ, Liao FF, Dang WH, Ding X, Liu XD, Cai J, Han JS, Wan Y, Xing GG. Contribution of the spinal cord BDNF to the development of neuropathic pain by activation of the NR2B-containing NMDA receptors in rats with spinal nerve ligation. Exp Neurol. 2010;222:256–266. doi: 10.1016/j.expneurol.2010.01.003. [DOI] [PubMed] [Google Scholar]
- Han JY, Li Q, Ma ZZ, Fan JY. Effects and mechanisms of compound Chinese medicine and major ingredients on microcirculatory dysfunction and organ injury induced by ischemia/reperfusion. Pharmacol Ther. 2017 doi: 10.1016/j.pharmthera.2017.03.005. doi: 10.1016/j.pharmthera.2017.03.005. [DOI] [PubMed] [Google Scholar]
- Hu JZ, Huang JH, Xiao ZM, Li JH, Li XM, Lu HB. Tetramethylpyrazine accelerates the function recovery of traumatic spinal cord in rat model by attenuating inflammation. J Neurol Sci. 2013;324:94–99. doi: 10.1016/j.jns.2012.10.009. [DOI] [PubMed] [Google Scholar]
- Ji PZ, Zhi BW, Ai HL. Effect of Buyang Huanwu Decotion on the content of platelet activating factor in spinal cord tissue of rats with spinal cord injury. Zhongguo Zhongyiyao Xinxi Zazhi. 2011;18:46–48. [Google Scholar]
- Jian Q, Li Y, Yin ZQ. Rat BMSCs initiate retinal endogenous repair through NGF/TrkA signaling. Exp Eye Res. 2015;132:34–47. doi: 10.1016/j.exer.2015.01.008. [DOI] [PubMed] [Google Scholar]
- Jian WW, Mao W, Gui CH. Effect of Jisuikang on kinetic dysfunction in patients after spinal injury. Chin J Integr Med. 2008;14:190–193. doi: 10.1007/s11655-008-9006-x. [DOI] [PubMed] [Google Scholar]
- Jian ZZ, Yong M, Shao JY. Experiment study of Jisuikang on treatment of rat after spinal cord injury. Jiangxi Zhongyiyao Daxue Xuebao. 2009;21:71–74. [Google Scholar]
- Jiang MH, Chung E, Chi GF, Ahn W, Lim JE, Hong HS, Kim DW, Choi H, Kim J, Son Y. Substance P induces M2-type macrophages after spinal cord injury. Neuroreport. 2012;23:786–792. doi: 10.1097/WNR.0b013e3283572206. [DOI] [PubMed] [Google Scholar]
- Jun PL. Laboratory study and clinical observation of astragalusroot on influence of nerve function after spinal cord injury. Fuzhou: Fujian Zhongyiyao Daxue Xuebao; 2013. [Google Scholar]
- Kai C, Li Z. Research on the study of protective effects and mechanism of Salvia and its preparations on spinal cord injury. Zhonghua Zhongyiyao Zazhi. 2012;27:2887–2890. [Google Scholar]
- Kaplan GB, Vasterling JJ, Vedak PC. Brain-derived neurotrophic factor in traumatic brain injury, post-traumatic stress disorder, and their comorbid conditions: role in pathogenesis and treatment. Behav Pharmacol. 2010;21:427–437. doi: 10.1097/FBP.0b013e32833d8bc9. [DOI] [PubMed] [Google Scholar]
- Kaptanoglu E, Palaoglu S, Surucu HS, Hayran M, Beskonakli E. Ultrastructural scoring of graded acute spinal cord injury in the rat. J Neurosurg. 2002;97:49–56. doi: 10.3171/spi.2002.97.1.0049. [DOI] [PubMed] [Google Scholar]
- Kaptanoglu E, Solaroglu I, Surucu HS, Akbiyik F, Beskonakli E. Blockade of sodium channels by phenytoin protects ultrastructure and attenuates lipid peroxidation in experimental spinal cord injury. Acta Neurochir (Wien) 2005;147:405–412. doi: 10.1007/s00701-004-0447-5. [DOI] [PubMed] [Google Scholar]
- Ke Y, Chi L, Xu R, Luo C, Gozal D, Liu R. Early response of endogenous adult neural progenitor cells to acute spinal cord injury in mice. Stem Cells. 2012;24:1011–1019. doi: 10.1634/stemcells.2005-0249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keefe KM, Sheikh IS, Smith GM. Targeting neurotrophins to specific populations of neurons: NGF, BDNF, and NT-3 and their relevance for treatment of spinal cord injury. Int J Mol Sci. 2017;18:3. doi: 10.3390/ijms18030548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kishibe K, Yamada Y, Ogawa K. Production of nerve growth factor by mouse hepatocellular carcinoma cells and expression of TrkA in tumor-associated arteries in mice. Gastroenterology. 2002;122:1978–1986. doi: 10.1053/gast.2002.33581. [DOI] [PubMed] [Google Scholar]
- Li LY, Lian SJ, San HG. The effect of the Chinese medicine Huangqi on the immunological regulation of the peripheral blood lymphocyte after acute spinal cord injuries in rats. Zhongguo Gu yu Guanjie Waike. 2010;3:142–148. [Google Scholar]
- Lin J, Wang C, Wu Z. Preliminary study on effects of human brain-derived neurotrophic factor gene-modified bone marrow mesenchymal stem cells by intravenous transplantation on structure and function of rat injured spinal cord. Zhongguo Xiufu Chongjian Waike Zazhi. 2010;24:982–987. [PubMed] [Google Scholar]
- Lin QT, Gang C, Cai HY. The experimental study of human nerve growth factor gene modified schwann cells for therapy of spinal cord injury. Shiyong Yixue Zazhi. 2012;28:2693–2696. [Google Scholar]
- Lu Z, Lei D, Jiang T, Yang L, Zheng L, Zhao J. Nerve growth factor from Chinese cobra venom stimulates chondrogenic differentiation of mesenchymal stem cells. Cell Death Dis. 2017 doi: 10.1038/cddis.2017.208. doi: 10.1038/cddis.2017.208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meng XF, Zou XJ, Peng B, Shi J, Guan XM, Zhang C. Inhibition of ethanol-induced toxicity by tanshinone IIA in PC12 cells. Acta Pharmacol Sin. 2006;27:659–664. doi: 10.1111/j.1745-7254.2006.00324.x. [DOI] [PubMed] [Google Scholar]
- Nishimura S, Yasuda A, Iwai H, Takano M, Kobayashi Y, Nori S, Tsuji O, Fujiyoshi K, Ebise H, Toyama Y. Time-dependent changes in the microenvironment of injured spinal cord affects the therapeutic potential of neural stem cell transplantation for spinal cord injury. Mol Brain. 2013;6:3. doi: 10.1186/1756-6606-6-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Novotna I, Slovinska L, Vanicky I, Cizek M, Radonak J, Cizkova D. IT delivery of ChABC modulates NG2 and promotes GAP-43 axonal regrowth after spinal cord injury. Cell Mol Neurobiol. 2011;31:1129–1139. doi: 10.1007/s10571-011-9714-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ochodnický P, Cruz CD, Yoshimura N, Michel MC. Nerve growth factor in bladder dysfunction: contributing factor, biomarker, and therapeutic target. Neurourol Urodyn. 2011;30:1227–1241. doi: 10.1002/nau.21022. [DOI] [PubMed] [Google Scholar]
- Price RD, Milne SA, Sharkey J, Matsuoka N. Advances in small molecules promoting neurotrophic function. Pharmacol Ther. 2007;115:292–306. doi: 10.1016/j.pharmthera.2007.03.005. [DOI] [PubMed] [Google Scholar]
- Pu X, Fan W, Yu S, Li Y, Ma X, Liu L, Ren J, Zhang W. Polysaccharides from Angelica and Astragalus exert hepatoprotective effects against carbon-tetrachloride-induced intoxication in mice. Can J Physiol Pharmacol. 2015;93:39–43. doi: 10.1139/cjpp-2014-0331. [DOI] [PubMed] [Google Scholar]
- Ray SK, Samntaray S, Banik NL. Future directions for using estrogen receptor agonists in the treatment of acute and chronic spinal cord injury. Neural Regen Res. 2016;11:1418–1419. doi: 10.4103/1673-5374.191212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rongrong W, Li D, Guangqing Z, Xiaoyin C, Kun B. The Effects of iontophoretic injections of salvia miltiorrhiza on the maturation of the arteriovenous fistula: a randomized, Controlled Trial. Altern Ther Health Med. 2016;22:18–22. [PubMed] [Google Scholar]
- Rossignol S, Frigon A. Recovery of locomotion after spinal cord injury: some facts and mechanisms. Annu Rev Neurosci. 2011;34:413–440. doi: 10.1146/annurev-neuro-061010-113746. [DOI] [PubMed] [Google Scholar]
- Tsai CC, Chang LC, Huang SC, Tey SL, Hsu WL, Su YT, Liu CW, Tsai TR. Salvia miltiorrhiza induces tonic contraction of the lower esophageal sphincter in rats via activation of extracellular Ca2+ influx. Molecules. 2015;20:14504–14521. doi: 10.3390/molecules200814504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang CY, Chen JK, Wu YT, Tsai MJ, Shyue SK, Yang CS, Tzeng SF. Reduction in antioxidant enzyme expression and sustained inflammation enhance tissue damage in the subacute phase of spinal cord contusive injury. J Biomed Sci. 2011;18:13. doi: 10.1186/1423-0127-18-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weishaupt N, Blesch A, Fouad K. BDNF: the career of a multifaceted neurotrophin in spinal cord injury. Exp Neurol. 2012;238:254–264. doi: 10.1016/j.expneurol.2012.09.001. [DOI] [PubMed] [Google Scholar]
- Wu LY, Ya FZ, Jian FC, Heng Y, Jun FY. Effects of Jisuikang on Nogo-NgR gene expression in spinal cord rats with injury. Zhongguo Gushang. 2015;28:235–239. [PubMed] [Google Scholar]
- Xie H, Yung WH. Chronic intermittent hypoxia-induced deficits in synaptic plasticity and neurocognitive functions: a role for brain-derived neurotrophic factor. Acta Pharmacol Sin. 2012;33:5–10. doi: 10.1038/aps.2011.184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yan X, Jiang Z, Bi L, Yang Y, Chen W. Salvianolic acid A attenuates TNF-α- and D-GalN-induced ER stress-mediated and mitochondrial-dependent apoptosis by modulating Bax/Bcl-2 ratio and calcium release in hepatocyte LO2 cells. Naunyn Schmiedebergs Arch Pharmacol. 2015;388:817–830. doi: 10.1007/s00210-015-1116-3. [DOI] [PubMed] [Google Scholar]
- Yu XQ, Xue CC, Zhou ZW, Li CG, Zhou SF. Tanshinone IIB, a primary active constituent from Salvia miltiorrhiza, exerts neuroprotective effect via inhibition of neuronal apoptosis in vitro. Phytother Res. 2008;22:846–850. doi: 10.1002/ptr.2404. [DOI] [PubMed] [Google Scholar]
- Yu YQ, Hu NC, Duan JA, Li DP, Liu C. Neuroprotective effects of sufentanil preconditioning on spinal cord injury in mouse models. Zhongguo Zuzhi Gongcheng Yanjiu. 2016;20:5966–5972. [Google Scholar]
- Yuan Y, Su Z, Pu Y, Liu X, Chen J, Zhu F, Zhu Y, Zhang H, He C. Ethyl pyruvate promotes spinal cord repair through ameliorating the glial microenvironment. Br J Pharmacol. 2011;164:1573–1576. doi: 10.1111/j.1476-5381.2011.01804.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yun Z, Jian ZH, Hong BL. Influence of Tetramethylpyrazine on the expression of neurotrophic factors in rats withacute spinal cord injury. Xiandai Zhongxiyi Jiehe Zazhi. 2010;19:1058–1060. [Google Scholar]