Keywords: nerve regeneration, Schwann cells, nerve injury, small gap conduit therapy, chitin, peripheral nerve injury, Notch signaling, Notch1, Hes1, glial fibrillary acidic protein, S100, neural regeneration
Abstract
Dedifferentiation of Schwann cells is an important feature of the response to peripheral nerve injury and specific negative myelination regulators are considered to have a major role in this process. However, most experiments have focused on the distal nerve stump, where the Notch signaling pathway is strongly associated with Schwann cell dedifferentiation and repair of the nerve. We observed the phenotypic changes of Schwann cells and changes of active Notch signaling on the proximal stump during peripheral nerve repair using small gap conduit tubulization. Eighty rats, with right sciatic nerve section of 4 mm, were randomly assigned to conduit bridging group and control group (epineurium suture). Glial fibrillary acidic protein expression, in myelinating Schwann cells on the proximal stump, began to up-regulate at 1 day after injury and was still evident at 5 days. Compared with the control group, Notch1 mRNA was expressed at a higher level in the conduit bridging group during the first week on the proximal stump. Hes1 mRNA levels in the conduit bridging group significantly increased compared with the control group at 3, 5, 7 and 14 days post-surgery. The change of the Notch intracellular domain shared a similar trend as Hes1 mRNA expression. Our results confirmed that phenotypic changes of Schwann cells occurred in the proximal stump. The differences in these changes between the conduit tubulization and epineurium suture groups correlate with changes in Notch signaling. This suggests that active Notch signaling might be a key mechanism during the early stage of neural regeneration in the proximal nerve stump.
Introduction
Peripheral nerve injuries still present challenges despite progress in laboratory and clinical solutions based on evolving scientific theories. Epineurium neurorrhaphy remains the first choice for treating peripheral nerve injury (Lundborg, 2000), but results in only partial recovery and limited functionality in most patients. Using conduits in therapy for nerve injuries and exploring nerve regeneration is a more recent, alternative experimental model (Kehoe et al., 2012). In previous studies, the small gap sleeve bridging conduit method was confirmed to result in superior functional recovery to that by epineurium suture and nerve autograft method (Zhang et al., 2008a, b; Yu et al., 2009). In these experiments, the nerve conduit was found to not only offer an endoluminal substructure preventing neuroma formation, but also provide a relative closed microenvironment for neurotrophic factors to act (Zhang et al., 2008a, b).
During axonal regeneration, Schwann cells have a very important role because of their intimate biological, developmental and morphological associations with axons (Zujovic et al., 2007; He et al., 2015; Gan and Nan, 2016). Mature Schwann cells, which are also called myelinating Schwann cells and labelled with S100, can dedifferentiate back to an immature state after the loss of axons during peripheral nerve injury and thus regain the ability to facilitate the regeneration of nerve after injury (Jessen et al., 2008). These myelinating Schwann cells elevate their glial fibrillary acidic protein (GFAP) expression after denervation, a feature of non-myelinating Schwann cells. The elevated expression of GFAP triggers a shape change of Schwann cells and extension of the axonal process, enabling the recovery of nerve function (Wang et al., 2010).
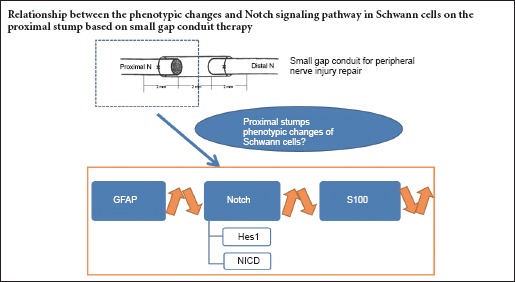
Most experiments working on nerve regeneration by conduit therapy have focused on the distal nerve stump and gaps (Zhang et al., 2008a, b, 2009; Yu et al., 2009). Soon after injury, there are huge metabolic and structural changes in this region, involving macrophage invasion, axon degeneration, endoneurial collagen production and Schwann cell proliferation, as well as other molecular and cellular activities. It is believed that loss of contact with neuronal cell body is critical to these events during Wallerian degeneration (Zhang et al., 2010). In the proximal stump of transected nerves, the axons are still in contact with their cell bodies. The microenvironment of the proximal region might be important because this is the center for the first reconstructive event. However, less attention has been paid to this region in previous research. To redress this we performed conduit therapy for sciatic nerve injury in rats and studied the phenotypic changes of Schwann cells in the proximal nerve stumps.
Notch is a transmembrane receptor protein. When it binds to a ligand and is cleaved, it generates an intracellular fragment called the Notch intracellular domain (NICD). In the nucleus, NICD acts as a transcriptional regulator. It is also a key factor in determining whether a cell responds to Notch signal or not (Thomas et al., 2005; Geng et al., 2015). In developing embryonic nerves, Notch signaling regulates the correct timing of the generation of immature Schwann cells for Schwann cell precursors and controls Schwann cell proliferation (Zujovic et al., 2007). Notch is selectively down-regulated in nerves that begin myelination, which is a characteristic of negative myelination regulators. Notch can also be suppressed by Krox-20, a positive myelination regulator, in vitro (Jessen and Mirsky, 2008). In the distal stump of transected nerves, the expression of NICD is strongly elevated and its dysregulation results in demyelination (Woodhoo et al., 2009). In this study, we will measure changes in the Schwann cells and of Notch signaling in proximal stumps during peripheral repair using small gap conduit therapy. The results will provide evidence for the role of Notch signaling in neuronal repair.
Materials and Methods
Animal preparation and surgery
Eighty 3-month-old male Sprague-Dawley rats, weighing 250–350 g, were provided by Shandong University of China (SCXK (Lu) 2013-0009). The study protocol was approved by Animal Ethics Committee of Qilu Hospital of Shandong University of China (No. KLYY-2014-018). The experimental procedure followed the United States National Institutes of Health Guide for the Care and Use of Laboratory Animals (NIH Publication No. 85-23, revised 1986).
The rats were randomly assigned to either the conduit bridging group or the control group (epineurium suture) with 40 rats in each group. In both groups, the right sciatic nerves of rats were exposed and cut off at 1 cm above sciatic nerve bifurcation under pentobarbital anesthesia (30 mg/kg) in accordance with a previous study (Zhang et al., 2010). In the conduit bridging group, the biological conduit of 4 mm was used to bridge both stumps of the nerve defects. About 1 mm of each of the distal and proximal ends of the nerve was inserted into the conduit, leaving a gap of 2 mm between the nerve stumps. Rats in the control group received conventional epineurium suture.
After surgery, a 3–4 mm length of nerve proximal to the transected tip was cut for immunohistochemical staining and real-time polymerase chain reaction at selected time points (1, 3, 5, 7, 14, 21, 28, and 60 days postoperatively) in rats of both groups as a previous study (Zhang, 2010).
The conduit used in this experiment was a de-acetyl chitin conduit with inner diameter of 1.5 mm provided by People's Hospital of Peking University of China (Yu, 2009).
Immunohistochemical staining
At each selected time point, five rats were obtained from each group and intraperitoneally anesthetized with 2% sodium pentobarbital. After exposing the thoracic cavity, an infusion needle was inserted in the cardiac apex. Perfusion of saline was conducted until the liver became white. The proximal stump of injured sciatic nerve was collected and fixed in paraformaldehyde for 5 hours. Three specimens were collected from each rat. Specimens were then immersed in sucrose solution at 4°C overnight, embedded in an optimal cutting temperature compound (OCT, Sakura Finetek, Torrence, CA) and sliced into 7-μm frozen sections. These sections were dried at room temperature for 24 hours and stored in a refrigerator. Before staining, the sections were fixed in acetone at −20°C for 20 minutes and washed three times with 0.3% Triton X-100/phosphate buffered saline (PBS), each for 5 minutes.
Axons were labeled with mouse monoclonal anti-neurofilament 200 (NF200) (1:200; Sigma-Aldrich, St. Louis, MO, USA). Schwann cells were labeled with rabbit polyclonal anti-cow GFAP (1:200; Sigma-Aldrich), and mouse monoclonal anti-S100 (1:100). Myelin sheaths were labelled with mouse anti-myelin basic protein (MBP) monoclonal antibody (1:160; Chemicon, Rolling Meadows, IL, USA). Notch-positive cells were labeled with rabbit polyclonal anti-NICD (1:100; Cell Signaling Technology, Danvers, MA, USA). 4′,6-Diamidino-2-phenylindole (DAPI; Wako, Osaka, Japan) was used for nuclear labeling. Secondary antibodies were fluorescein isothiocyanate (FITC)-conjugated goat anti-rabbit immunoglobulin G (IgG) (1:50; Tiangen, Beijing, China), sheep anti-mouse IgG CY2 and CY3 conjugate (1:200; Friendship Biotechnology, Beijing, China).
Cover slips were mounted with bicarbonate-buffered glycerol (pH 8.6) and slides were observed using a fluorescent microscope (Olympus, Tokyo, Japan).
Immunofluorescence images were overlaid using Photoshop software CS2 V9.0 (Adobe, San Jose, CA, USA). Schwann cells and axons were quantified in nerve specimens using Image-pro Plus 6.0 software (Media Cybernetics, Rockville, MD, USA). The number of positive cells was counted in five high-power fields (400×) and five low-power fields (5×), randomly selected.
Quantitative real-time PCR
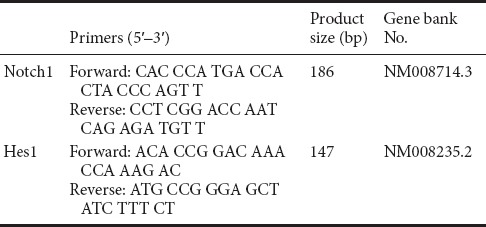
Quantitative real-time PCR was conducted to detect the Notch1 and Hes1 mRNA levels in the proximal stumps of injured sciatic nerve. The primers were purchased from AOGCT Inc., China, and the detailed sequences of primers are displayed in Table 1. Samples were loaded into 1 mL TRIzol reagent (Invitrogen, Waltham, MA, USA) and then homogenized. Total RNA was extracted and purified, then diluted to 500 ng/μL. cDNA was synthesized with Prime-Script1 RT reagent kit (Promega, Madison, WI, USA). Quantitative real-time PCR was performed with SYBRGREEN PCR Master Mix (ABI, New York, NY, USA). Comparative CT method (2–ΔΔCt) was used to calculate the gene expression (Schmittgen and Livak, 2008). β-Actin was used as the control housekeeping gene for normalization of RNA added to reverse transcription reactions.
Table 1.
Oligonucleotide sequences and product sizes of primers
Statistical analysis
All data are presented as the mean ± SD. The results were all analyzed with SPSS 17.0 software (SPSS, Chicago, IL, USA) using independent-sample t-test to examine the difference between two groups. A value of P < 0.05 was considered statistically significant.
Results
Early changes of GFAP-labeled Schwann cells at the proximal stump following injury
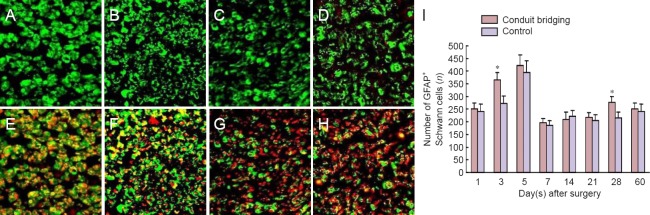
The GFAP expression levels began to rise at 1 day and were evident at 3, 5 and 7 days after surgery in Schwann cells of both groups. On day 5, some rings of GFAP-labeled profiles were observed around myelinated fibers, which were not reported in normal nerve samples (Figure 1). This phenotypic change of myelinating Schwann cells was further confirmed by GFAP and NF200 double-labeling. As displayed in Figure 1, these Schwann cells labeled with GFAP were observed in close contact with myelinated axons labeled with NF200. This upregulated GFAP expression lasted for 5 days, then decreased to a relative steady value until 28 days. The changes of GFAP in Schwann cells and axons of the conduit bridging group were similar to those of the control group during the experiment. The number of GFAP-labeled Schwann cells in the conduit bridging group was slightly higher than that in the control group, but only significantly different at 3 and 28 days (P < 0.05).
Figure 1.
Transverse sections of proximal stumps of injured sciatic nerve labeled with GFAP and NF200 (immunofluorescence staining).
(A–D) Immunohistochemical labeling for Schwann cells with GFAP. (A, B) Images at 5 days of conduit bridging group (A) and control group (B). (C, D) Images at 14 days of conduit bridging group (C) and control group (D). (E–H) Immunohistochemical double labeling for GFAP (CY3, green, for Schwann cells) and NF200 (CY2, red, for axons). (E, F) Images at 5 days of conduit bridging group (E) and control group (F). (G, H) Images at 14 days of conduit bridging group (G) and control group (H). (A–H) Original magnification 400×. (I) Number of GFAP-labeled Schwann cells in conduit bridging and control groups. *P < 0.05, vs. control group (mean ± SD, n = 15, independent-sample t-test). GFAP: Glial fibrillary acidic protein; NF200: neurofilament 200.
Changes of S100-labeled Schwann cells and axon growth
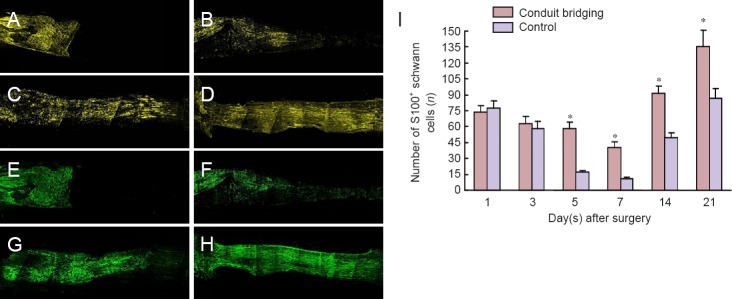
The nerve fibers on the proximal stump near the injury site degraded immediately after surgery and the regeneration into the conduit was only seen on day 7. The number of S100-labeled Schwann cells decreased gradually for 7 days, and then gradually increased. This trend was similar for the two different groups (Figure 2). However, very few S100-positive Schwann cells were detected in the control group at 7 days. In the conduit bridging group, S100-positive Schwann cells decreased slowly but half still remained by 7 days. At 21 days the numbers were almost double that at day 1. There were significant differences in the number of S100-positive cells between the two groups at 7 and 21 days (P < 0.05). The myelin reconstruction, shown by MBP staining, started around 7 days and the myelin sheath reached the center from the proximal stumps at 21 days after injury. In the distal stump, the re-myelination occurred when the regenerating axons reach Schwann cells in the distal stump. The myelin sheath regenerated to the distal end of the conduit at 28 days after surgery (Figure 2) and manifested a mature morphology at 60 days after injury.
Figure 2.
Longitudinal sections of S100 and MBP labeling on the proximal stump of injured sciatic nerve (immunofluorescence staining).
(A–D) Immunohistochemical labeling for Schwann cells with S100 (yellow) in the conduit bridging group at 1, 7, 21 and 28 days. (E–H) Immunohistochemical labeling for MBP (green) in the conduit bridging group at 1, 7, 21 and 28 days. (A–H) Original magnification 5×. (I) The number of S100-labeled Schwann cells in the conduit bridging (orange) and control (blue) groups. *P < 0.05, vs. control group (mean ± SD, n = 15, independent-sample t-test). MBP: Myelin basic protein.
Notch signaling activation during nerve regeneration
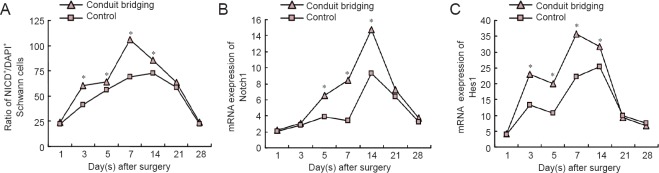
In the proximal stump, the number of NICD+/DAPI+ Schwann cells increased immediately after surgery, reached a high level at 3 days and peaked at 7 days. After that, the number of NICD+/DAPI+ Schwann cells decreased gradually until 28 days (Figure 3). The number of NICD+/DAPI+ cells in the conduit bridging group increased significantly at 3, 5, 7 and 14 days compared with the control group (P < 0.05).
Figure 3.
Expression of Notch signaling pathway in the proximal stump of injured sciatic nerves.
(A) Ratio of NICD+/DAPI+ Schwann cells. (B) The expression of Notch1 mRNA. (C) The expression of Hes1 mRNA. *P < 0.05, vs. control group (mean ± SD, n = 15, independent-sample t-test). NICD: Notch intracellular domain; DAPI: 4′,6-diamidino-2-phenylindole.
The Notch1 mRNA expression increased gradually in both groups after injury and peaked at 14 days (Figure 3). However, the maximal expression of Notch1 mRNA was significantly higher in the conduit bridging group compared with the control group (P < 0.05).
There was scant expression of Hes1 mRNA in normal nerve but it peaked at 7 days after injury, and then gradually decreased until 28 days in the conduit bridging group (Figure 3). Compared with the control group, the expression of Hes1 mRNA was significantly higher in the conduit bridging group at 3, 5, 7 and 14 days post-operation (P < 0.05). This trend of Hes1 mRNA expression was similar to the change in expression of NICD.
Discussion
Over the past few years, a huge number of studies have been focused on conduit or tube methods for the repair of peripheral nerve injuries (Kehoe et al., 2012). This concept was based on the idea that a short gap between the nerve stumps within the conduit could facilitate the neurotrophic regenerative mechanisms and ease reconstruction. Such materials include resorbable (Jeans et al., 2007) and non-resorbable devices (Oh et al., 2008), seed cells, and growth factors. The conduit used in this work was a deacetylated chitin tube with confirmed safety in vivo (Wan and Tai, 2013). It has been used in a human clinical trial and proved superior to the epineurium method for the recovery of nerve function (Zhang et al., 2013). To further understand the superiority of this therapy, we observed phenotypic changes on the proximal stump of small gap bridging suture after surgery and explored the role of Notch signaling in this process.
Schwann cells undergo different stages during peripheral nerve development, from the early neural crest stem cells to two mature phenotypes: myelination and non-myelinating Schwann cells. During this process, GFAP can be first detected at embryo day 18 in rat embryo, when nearly all Schwann cell precursors have become immature Schwann cells (Zujovic et al., 2007). After that, GFAP expression can only be detected in non-myelinating Schwann cells. During peripheral nerve injury, axons distal to the injury site undergo Wallerian degeneration. After losing contact with axons, the myelinating Schwann cells re-expressed GFAP and shift to a phenotype resembling non-myelinating Schwann cells. This process is called dedifferentiation, which is considered as a reversal of differentiation (Wang et al., 2010). In this experiment, the proximal nerve stump was within the conduit and degenerating axons in the distal stumps had little influence on the proximal stump. However, we also observed elevated expression of GFAP in myelinating Schwann cells as well as non-myelinating Schwann cells in the proximal stumps. GFAP expression began to up-regulate at 1 day after surgery and was evident at 5 days in myelinating Schwann cells, even when they were still in contact with axons. This was confirmed by the decrease of S100-positive Schwann cells. This evidence was clear in the conduit bridging group, which may suggest a possible reason for the favorable therapeutic results of small gap conduit method observed in former experiments (Zhang et al., 2010; Xue et al., 2015). These observed changes occurred immediately after injury and lasted for a relative short period, suggesting that primary suture surgery should be performed as soon as possible after peripheral nerve injury. The sooner surgery could be performed, the shorter the initial regrowth delay period of axons would be.
There might be several explanations for the increased expression of GFAP in Schwann cells that still had contact with myelinated axons. Axons would degenerate by several intermodal segments after transection, especially in the distal tip of the proximal stump (Maki et al., 2005). Such retrograde axonal degeneration might trigger the phenotypic change of myelin-forming Schwann cells. In this study, we selected the part of the nerve most proximal to such an area, aiming to minimize its effect. We still detected GFAP expression at a remarkable distance proximal to the tip of the stump. Based on these results, we believed there might be an alternative signal that could trigger the phenotypic changes of Schwann cells we observed. Previously quiescent Schwann cells might be exposed to humoral proteins and signals because of the breakdown of blood-nerve barrier after injury (Echeverry et al., 2011). The signals from axotomized cells might trigger phenotypic change and proliferation of Schwann cells without the loss of contact with its own axons. It was shown in recent studies that this dedifferentiation of Schwann cells also depends on the activation of several negative regulators. These regulators are specific intracellular signaling molecules that should be up-regulated prior to myelination during development, suppressed when myelination began and reactivated after injury (Jessen et al., 2008).
There is evidence suggesting Notch signaling is a powerful regulator of dedifferentiation (Jessen et al., 2008). Elevated NICD expression hinders myelination and myelin gene induction through cAMP (Arthur-Farraj et al., 2011). Myelination is also delayed when NICD expression in Schwann cells is increased shortly after birth (Woodhoo et al., 2009). In injured nerves, NICD also rises rapidly and the demyelination is promoted in nerves overexpressing NICD by gene engineering. However, demyelination after injury is suppressed when NICD elevation is hindered (Jessen et al., 2008). Notch1 belongs to the receptors of Notch signaling system, and Hes1 is a Notch target gene that acts in downstream notch signaling. In this experiment, we detected the NICD level and Hes1 mRNA expression, both of which have a very low level of expression in normal nerves. The level of Notch1 mRNA increased gradually and peaked at 14 days after injury. Compared with the control group, the expression of Notch1 mRNA in the conduit bridging group was significantly higher in the first 14 days. The levels of Hes1 mRNA in the conduit bridging group were significantly increased as compared with the control group at 3, 5, 7 and 14 days post-surgery. The change of NICD, a marker of active Notch signaling, shared a similar trend with Hes1 mRNA expression, as their higher expression occurred mainly in the first 14 days. These results might serve as a mechanism for the phenotypic change we observed in our research. However, the expression of Notch1 mRNA was not so consistent with these two factors, suggesting a more complex relationship between Notch1 and Hes1. Recent studies have suggested that during nerve injury, endogenous molecules such as the peptide PACAP are secreted from the proximal axonal stump to promote Schwann cell dedifferentiation whilst stimulating the intracellular accumulation of components of the myelin sheath (Castorina et al., 2015). The same group also mentioned that these same peptides act in an autocrine fashion in Schwann cell lines to trigger the production and release of enzymes that promote clearance of cellular debris. These results might suggest some new targets on the mechanisms of Schwann cell dedifferentiation.
In summary, the small gap conduit tubulization therapy was more effective than epineurium suture in producing phenotypic changes of Schwann cells in the proximal stump leading to faster neural regeneration. The change of active Notch signaling might be the main mechanism during the early stage of this phenomenon. We hope this study will provide an experimental basis for further work on engineering repair of peripheral nerve injury.
Footnotes
Funding: This study was supported by the Shandong Science and Research Foundation for Youth Scientists in China, No. BS2012YY019.
Conflicts of interest: None declared.
Research ethics: The study protocol was approved by Animal Ethics Committee of Qilu Hospital of Shandong University (approval No. KLYY-2014-018). The experimental procedure followed the United States National Institutes of Health Guide for the Care and Use of Laboratory Animals (NIH Publication No. 85-23, revised 1986).
Data sharing statement: The datasets analyzed during the current study are available from the corresponding author on reasonable request.
Plagiarism check: Checked twice by iThenticate.
Peer review: Externally peer reviewed.
Copyedited by Wang J, Li CH, Qiu Y, Song LP, Zhao M
References
- Arthur-Farraj P, Wanek K, Hantke J, Davis CM, Jayakar A, Parkinson DB, Mirsky R, Jessen KR. Mouse schwann cells need both NRG1 and cyclic AMP to myelinate. Glia. 2011;59:720–733. doi: 10.1002/glia.21144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Castorina A, Waschek JA, Marzagalli R, Cardile V, Drago F. PACAP interacts with PAC1 receptors to induce tissue plasminogen activator (tPA) expression and activity in schwann cell-like cultures. PLoS One. 2015;10:e117799. doi: 10.1371/journal.pone.0117799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Echeverry S, Shi XQ, Rivest S, Zhang J. Peripheral nerve injury alters blood-spinal cord barrier functional and molecular integrity through a selective inflammatory pathway. J Neurosci. 2011;31:10819–10828. doi: 10.1523/JNEUROSCI.1642-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gan X, Nan WL. Heparan sulfate/collagen nerve tissue-engineered scaffolds repair peripheral nerve injury. Zhongguo Zuzhi Gongcheng Yanjiu. 2016;20:3744–3749. [Google Scholar]
- Geng X, Sun T, Li JH, Zhao N, Wang Y, Yu HL. Electroacupuncture in the repair of spinal cord injury: inhibiting the Notch signaling pathway and promoting neural stem cell proliferation. Neural Regen Res. 2015;10:394–403. doi: 10.4103/1673-5374.153687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- He B, Tao HY, Wei AL, Li XH, Chen R. Carboxymethylated chitosan effect on cyclic adenosine monophosphate/protein kinase A signaling pathway in rat Schwann cells cultured in vitro. Zhongguo Zuzhi Gongcheng Yanjiu. 2015;19:6930–6934. [Google Scholar]
- Jeans LA, Gilchrist T, Healy D. Peripheral nerve repair by means of a flexible biodegradable glass fibre wrap: a comparison with microsurgical epineurial repair. J Plast Reconstr Aesthet Surg. 2007;60:1302–1308. doi: 10.1016/j.bjps.2006.06.014. [DOI] [PubMed] [Google Scholar]
- Jessen KR, Mirsky R. Negative regulation of myelination: relevance for development, injury, and demyelinating disease. Glia. 2008;56:1552–1565. doi: 10.1002/glia.20761. [DOI] [PubMed] [Google Scholar]
- Kehoe S, Zhang XF, Boyd D. FDA approved guidance conduits and wraps for peripheral nerve injury: a review of materials and efficacy. Injury. 2012;43:553–572. doi: 10.1016/j.injury.2010.12.030. [DOI] [PubMed] [Google Scholar]
- Lundborg G. A 25-year perspective of peripheral nerve surgery: evolving neuroscientific concepts and clinical significance. J Hand Surg Am. 2000;25:391–414. doi: 10.1053/jhsu.2000.4165. [DOI] [PubMed] [Google Scholar]
- Maki Y, Yoshizu T, Tsubokawa N. Selective regeneration of motor and sensory axons in an experimental peripheral nerve model without endorgans. Scand J Plast Reconstr Surg Hand Surg. 2005;39:257–260. doi: 10.1080/0284431051006510. [DOI] [PubMed] [Google Scholar]
- Oh SH, Kim JH, Song KS, Jeon BH, Yoon JH, Seo TB, Namgung U, Lee IW, Lee JH. Peripheral nerve regeneration within an asymmetrically porous PLGA/Pluronic F127 nerve guide conduit. Biomaterials. 2008;29:1601–1609. doi: 10.1016/j.biomaterials.2007.11.036. [DOI] [PubMed] [Google Scholar]
- Schmittgen TD, Livak KJ. Analyzing real-time PCR data by the comparative C(T) method. Nat Protoc. 2008;3:1101–1108. doi: 10.1038/nprot.2008.73. [DOI] [PubMed] [Google Scholar]
- Thomas BJ. Cell-cycle control during development: taking it up a notch. Dev Cell. 2005;8:451–452. doi: 10.1016/j.devcel.2005.03.013. [DOI] [PubMed] [Google Scholar]
- Wan AC, Tai BC. CHITIN -A promising biomaterial for tissue engineering and stem cell technologies. Biotechnol Adv. 2013;31:1776–1785. doi: 10.1016/j.biotechadv.2013.09.007. [DOI] [PubMed] [Google Scholar]
- Wang J, Zhang P, Wang Y, Kou Y, Zhang H, Jiang B. The observation of phenotypic changes of schwann cells after rat sciatic nerve injury. Artif Cells Blood Substit Immobil Biotechnol. 2010;38:24–28. doi: 10.3109/10731190903495736. [DOI] [PubMed] [Google Scholar]
- Woodhoo A, Alonso MB, Droggiti A, Turmaine M, D’Antonio M, Parkinson DB, Wilton DK, Al-Shawi R, Simons P, Shen J, Guillemot F, Radtke F, Meijer D, Feltri ML, Wrabetz L, Mirsky R, Jessen KR. Notch controls embryonic Schwann cell differentiation, postnatal myelination and adult plasticity. Nat Neurosci. 2009;12:839–847. doi: 10.1038/nn.2323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xue F, Wu EJ, Zhang PX, Li-Ya A, Kou YH, Yin XF, Han N. Biodegradable chitin conduit tubulation combined with bone marrow mesenchymal stem cell transplantation for treatment of spinal cord injury by reducing glial scar and cavity formation. Neural Regen Res. 2015;10:104–111. doi: 10.4103/1673-5374.150715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu K, Zhang C, Wang Y, Zhang P, Zhang D, Zhang H, Jiang B. The protective effects of small gap sleeve in bridging peripheral nerve mutilation. Artif Cells Blood Substit Immobil Biotechnol. 2009;37:257–264. doi: 10.3109/10731190903360810. [DOI] [PubMed] [Google Scholar]
- Yu K, Zhang C, Wang Y, Zhang P, Zhang D, Zhang H, Jiang B. The protective effects of small gap sleeve in bridging peripheral nerve mutilation. Artif Cells Blood Substit Immobil Biotechnol. 2009;37:257–264. doi: 10.3109/10731190903360810. [DOI] [PubMed] [Google Scholar]
- Zhang C, Zhang P, Wang Y, Yu K, Kou Y, Jiang B. Early spatiotemporal progress of myelinated nerve fiber regenerating through biological chitin conduit after injury. Artif Cells Blood Substit Immobil Biotechnol. 2010;38:103–108. doi: 10.3109/10731191003634836. [DOI] [PubMed] [Google Scholar]
- Zhang P, Xue F, Kou Y, Fu Z, Zhang D, Zhang H, Jiang B. The experimental study of absorbable chitin conduit for bridging peripheral nerve defect with nerve fasciculu in rats. Artif Cells Blood Substit Immobil Biotechnol. 2008a;36:360–371. doi: 10.1080/10731190802239040. [DOI] [PubMed] [Google Scholar]
- Zhang P, Yin X, Kou Y, Wang Y, Zhang H, Jiang B. The electrophysiology analysis of biological conduit sleeve bridging rhesus monkey median nerve injury with small gap. Artif Cells Blood Substit Immobil Biotechnol. 2008b;36:457–463. doi: 10.1080/10731190802375802. [DOI] [PubMed] [Google Scholar]
- Zhang P, Zhang C, Kou Y, Yin X, Zhang H, Jiang B. The histological analysis of biological conduit sleeve bridging rhesus monkey median nerve injury with small gap. Artif Cells Blood Substit Immobil Biotechnol. 2009;37:101–104. doi: 10.1080/10731190902742620. [DOI] [PubMed] [Google Scholar]
- Zhang PX, Han N, Wang TB, Xue F, Kou YH, Wang YH, Yin XF, Lu LJ, Tian GL, Gong X, Chen SL, Dang Y, Peng JP, Jiang BG. Biodegradable conduit small gap tubulization for peripheral nerve mutilation: a substitute for traditional epineurial neurorrhaphy. Int J Med Sci. 2013;10:171–175. doi: 10.7150/ijms.5312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zujovic V, Bachelin C, Baron-Van EA. Remyelination of the central nervous system: a valuable contribution from the periphery. Neuroscientist. 2007;13:383–391. doi: 10.1177/10738584070130041001. [DOI] [PubMed] [Google Scholar]