Keywords: nerve regeneration, mesenchymal stem cell transplantation, adipose-derived mesenchymal stem cells, recurrent laryngeal nerve, larynx, nerve injury, functional recovery, vocal fold, cell differentiation, neural regeneration
Abstract
Medialization thyroplasty or injection laryngoplasty for unilateral vocal fold paralysis cannot restore mobility of the vocal fold. Recent studies have shown that transplantation of mesenchymal stem cells is effective in the repair of nerve injuries. This study investigated whether adipose-derived stem cell transplantation could repair recurrent laryngeal nerve injury. Rat models of recurrent laryngeal nerve injury were established by crushing with micro forceps. Adipose-derived mesenchymal stem cells (ADSCs; 8 × 105) or differentiated Schwann-like adipose-derived mesenchymal stem cells (dADSCs; 8 × 105) or extracellular matrix were injected at the site of injury. At 2, 4 and 6 weeks post-surgery, a higher density of myelinated nerve fiber, thicker myelin sheath, improved vocal fold movement, better recovery of nerve conduction capacity and reduced thyroarytenoid muscle atrophy were found in ADSCs and dADSCs groups compared with the extracellular matrix group. The effects were more pronounced in the ADSCs group than in the dADSCs group. These experimental results indicated that ADSCs transplantation could be an early interventional strategy to promote regeneration after recurrent laryngeal nerve injury.
Introduction
Recurrent laryngeal nerve (RLN) development follows a long and indirect course after it branches off the vagus nerve to innervate both the abductor and adductor muscles, a process that makes it vulnerable to damage by surgical approaches (Monaco et al., 2015). RLN injury results in vocal fold paralysis and incomplete glottis closure, which compromises the phonic and swallowing functions and affects the quality of life. Medialization thyroplasty first described by Isshiki et al. (1974) and injection laryngoplasty first introduced by Bruening (1911) have been the major treatments for unilateral vocal fold paralysis but do not restore mobility of the vocal fold. Developing novel therapies to enhance RLN regeneration and achieve complete functional recovery have been the focus of attention in recent research.
In the last decade, mesenchymal stem cells (MSCs) have been widely used to promote tissue regeneration, including peripheral nerves. This is due to their inherent high plasticity and low immunogenicity (di Summa et al., 2011). Adipose-derived MSCs (ADSCs) were first isolated around 2001 (Tomita et al., 2013), and their abundance, easy accessibility and ease of isolation has drawn much attention (Bi et al., 2015). Using experimental models of peripheral nerve injury, such as sciatic nerve and facial nerve, many research groups have shown that transplantation of ADSCs enhances nerve regeneration (Sun et al., 2011; Tomita et al., 2013). Kingham et al. (2007) found that ADSCs could differentiate into a phenotype resembling Schwann cells in vitro. Further research confirmed that differentiated Schwann-like ADSCs (dADSCs) expressed a specific peripheral myelin protein (Mantovani et al., 2010) and could form myelin structures with neurons in vitro (Xu et al., 2008). Although dADSCs have displayed the potential to enhance peripheral nerve regeneration (Sun et al., 2011; Tomita et al., 2013), it remains controversial whether dADSCs facilitates the regeneration more effectively than ADSCs. The purpose of this study was to investigate whether transplantation of ADSCs and dADSCs facilitates nerve regeneration and functional recovery after crush injury of RLN in a rat model.
Materials and Methods
Animals
Forty-five adult female Sprague-Dawley rats (8 weeks old, weighing 250–300 g) used in this study were purchased from the Fangyuan Animal Center (license No. SCXK 2009-0014; Beijing, China). Only female rats were selected to reduce the influence of testosterone, an effective neurotherapeutic agent in conditions of nerve injury (Brown et al., 2013; Chan et al., 2014). Animals were maintained on a 12-hour light/dark cycle and had free access to standard rat chow and water.
Forty-five Sprague-Dawley rats were randomly divided into three groups (n = 15 per group) according to the transplanted materials: ADSCs group, dADSCs group, and extracellular matrix (ECM) group. These groups were then subdivided by time points of 2, 4, and 6 weeks post-surgery (n = 5 at each time point). All the surgical procedures were carried out aseptically under general anesthesia by intraperitoneal administration of sodium pentobarbital (60 mg/kg). The ECM (Sigma-Aldrich, St. Louis, MO, USA) was used as the scaffold to keep the transplanted cells in position, owing to its characteristics of displaying thermo-dependent sol-to-gel transition (Kim et al., 2012). The ECM group without viable cells was set as the negative control.
Transdifferentiation of rat ADSCs
ADSCs of Sprague-Dawley rats were purchased from Cyagen Biosciences Inc. (RASMD-01001, Santa Clara, CA, USA). The ADSCs were positive for CD29, CD44 and CD90, but negative for CD34 and CD45. The fourth passage culture of ADSCs was used in this study. For transdifferentiation into Schwann-like cells (Kingham et al., 2007), sub-confluent ADSCs at passage 4 were cultured in serum-free ADSCs growth medium that was supplemented with 1 mM β-mercaptoethanol (Sigma-Aldrich) for 24 hours. After washing with phosphate-buffered saline (PBS; 10 mM, pH 7.4), the cells were incubated in ADSC growth medium containing 35 ng/mL all-trans-retinoic acid (Sigma-Aldrich) for 72 hours. The cells were then washed three times in PBS and incubated in ADSC growth medium. This contained 14 μM forskolin (Sigma-Aldrich), 5 ng/mL platelet-derived growth factor-AA (PeproTech, Rocky Hill, NJ, USA), 10 ng/mL basic fibroblast growth factor (PeproTech) and 200 ng/mL recombinant human heregulin-β1 (PeproTech) for 14 days. The medium was replaced every 3 days.
Immunofluorescence analysis
To confirm transdifferentiation of ADSCs, cultured dADSCs and ADSCs were compared by immunocytochemical analysis. The cells were incubated on cover-slips and fixed with 4% paraformaldehyde in phosphate buffer (0.1 M, pH 7.2) for 10 minutes and washed with PBS before permeabilization in 0.3% Triton X-100. Non-specific binding was blocked with 5% normal goat serum for 20 minutes followed by incubation with rabbit anti-glial fibrillary acidic protein polyclonal antibody (GFAP; 1:100; Abcam, Cambridge, MA, USA) and mouse anti-rat S100β monoclonal antibody (1:10; Abcam) overnight at 4°C. The specimens were then incubated with FITC-conjugated goat anti-rabbit IgG (1:200; ZSGB-BIO, Beijing, China) and TRITC-conjugated goat anti-mouse IgG (1:200; ZSGB-BIO) at room temperature for 2 hours. After counter-staining with 2 μg/mL DAPI for 5 minutes (Molecular Probes, Eugene, USA), the cover-slips were mounted on glass slides with glycerin and examined by spectral laser scanning confocal microscopy (Leica, Wetzlar, Germany).
Rat RLN crush model and cell transplantation
Surgical procedures were performed when a sufficient number of rat ADSCs or dADSCs had been prepared. Surgery was performed under anesthesia while the animal was placed in the supine position on a controlled heating pad. A midline vertical skin incision was made to expose the strap muscles and submaxillary glands. The midline raphe was separated using jeweler forceps, and the RLN on the right side was carefully exposed in the tracheoesophageal groove under a surgical microscope (Zeiss, Oberkochen, Germany). The RLN was crushed for 1 minute using microhemostat forceps (RWD Life Science Co., Shenzhen, China) at the level of the seventh tracheal ring. After the crush injury, the nerve appeared translucent at the crush site.
In the ADSCs or dADSCs group, the crushed RLN was injected at the lesioned area with a suspension of 8 × 105 cells in ECM/growth, 1:1, medium in a total volume of 50 μL, using a sterile syringe. The cell-free ECM/growth medium was injected in the ECM group. After closing the incision, an immediate post-injury laryngoscopy with transoral 0° endoscopy (Richard Wolf, Pforzheimer, Knittlingen, Germany) was performed to confirm complete right-sided vocal fold immobilization. After surgery, all animals were returned to their cages and allowed to recover for 2, 4 or 6 weeks.
Evaluation of vocal fold mobility
At 2, 4 or 6 weeks post-surgery (i.e., n = 5 rats at every time point), the movement of the right vocal fold was evaluated during spontaneous respiratory cycles under anesthesia using a transoral 0° Storz endoscope. The animals were placed in the supine position and the tongues were retracted using a 3-0 silk suture that was placed midline in the anterior one third of the tongue. Vocal fold mobility was independently graded by two investigators using a scale from 0 to 4. Scoring of vocal fold movement and position was guided by the following criteria: 0: no vocal fold movement; 1: slight vocal fold movement; 2: ≤ 50% abduction of the vocal fold; 3: ≥ 50% abduction of the vocal fold, and unsmooth vocal fold movement; 4: vocal fold movement and positioning indistinguishable from the normal situation (Tessema et al., 2009). Scores from both investigators were combined and assigned a single average value.
Evoked electromyography (EMG)
After evaluating vocal fold movement, laryngeal evoked EMG was performed at postoperative 2, 4 or 6 weeks (n = 5 rats at each time point), recording from the thyroarytenoid muscles on the injured side. Bipolar concentric needle electrodes (Nicolet, Pleasanton, CA, USA) were placed percutaneously into the thyroarytenoid muscles. Monopolar needle electrodes were used to stimulate the RLN at the eighth tracheal ring level. The stimulation intensity was 8 mA. The latency, amplitude and duration of the evoked muscle response potentials were then evaluated.
Morphological evaluation of regenerated nerve segments
Following EMG evaluation, animals were sacrificed with an intraperitoneal lethal dose injection of pentobarbital at postoperative 2, 4 or 6 weeks. Immediately thereafter, the right RLN distal from the crush site was harvested. All specimens were immediately fixed with 2.5% glutaraldehyde for 2 hours at room temperature, then post-fixed with 1% osmium tetroxide in 0.1 M PBS for 2 hours and dehydrated in graded ethanol and embedded in epoxy resin. Semi-thin transverse sections (1 μm) were cut, stained with 1% toluidine blue solution and examined under a light microscope (Olympus, Tokyo, Japan). The density of myelinated fibers (i.e., fibers/mm2) was calculated by dividing the total number of myelinated nerve fibers within the total cross-sectional area by the whole nerve cross-section area using Image-Pro Plus software (Media Cybernetics, Rockville, MD, USA). For electron microscopy, ultrathin sections (50 nm) were stained with lead citrate and uranyl acetate, followed by examination under a transmission electron microscope (Hitachi, Tokyo, Japan). Photographs were taken from five random fields of view from each ultrathin section. The myelin thickness was analyzed at 5,000× final magnification using Image Pro Plus software.
Morphological evaluation of thyroarytenoid muscle
The larynx was harvested from the superior epiglottis to the first tracheal ring level and post-fixed in 4% paraformaldehyde overnight at room temperature at 2, 4 or 6 weeks after crush injury. The larynx was then paraffin embedded and cut into 5 μm sections. Masson trichrome staining was applied to the sections before photographs were taken under a light microscope with a color digital camera. For each of the Masson trichrome stained sections of every specimen, photographs were taken from three random fields at 200× final magnification and analyzed with Image-Pro Plus software. The area percentage of the muscle fiber was calculated according to the following formula: muscle fiber area/area of the visual field at 200× final magnification.
Statistical analysis
The data were exhibited as the mean ± SEM and analyzed by one-way analysis of variance with SPSS 19.0 software (IBM Analytics, Armonk, New York, USA). If there was a significant overall difference among groups, pair-wise comparisons were legitimately conducted using Scheffe's post hoc test. Alpha values of P < 0.05 were considered statistically significant differences between groups of data.
Results
Differences between ADSCs and dADSCs in vitro
Fourteen days after transdifferentiation, rat ADSCs were transformed from a flat fibroblast-like to a spindle-shaped morphology that resembled the appearance of Schwann cells and continued to proliferate. Cell immunofluorescence showed that dADSCs were positive for the Schwann cells markers S100β and GFAP (Kingham et al., 2007), while ADSCs were mostly negative for Schwann cells markers S100β and GFAP (Figure 1).
Figure 1.
Surface protein expression in differentiated Schwann-like adipose-derived mesenchymal stem cells (dADSCs) and adipose-derived mesenchymal stem cells (ADSCs) (cell immunofluorescence).
dADSCs expressed S100β (red) and glial fibrillary acidic protein (GFAP) (green), confirmed by the merged images. ADSCs mostly failed to express such markers. Nuclei were counter-stained with 4′,6-diamidino-2-phenylindole (DAPI) (blue). Scale bars: 50 μm.
Recovery of vocal fold movement after dADSCs or ADSCs transplantation in rat models of RLN crush
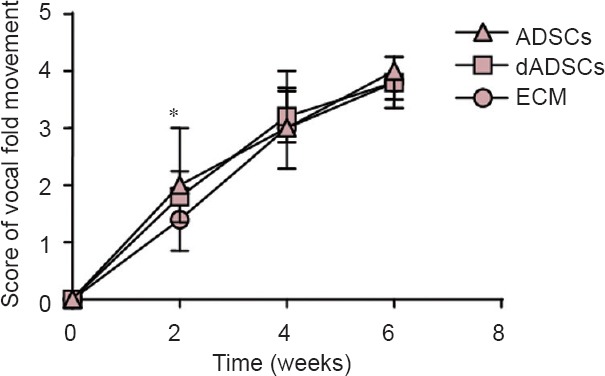
The improvement of vocal fold movement after crush injury as a function of time dependency is shown in Figure 2. Immediately after crush injury, all animals experienced ipsilateral vocal fold immobility. Afterwards, all animals in the three groups demonstrated a time-dependent increase in the score of vocal fold movement.
Figure 2.
Effects of adipose-derived mesenchymal stem cells (ADSCs), differentiated Schwann-like adipose-derived mesenchymal stem cells (dADSCs) and extracellular matrix (ECM) transplantation on vocal fold movement following recurrent laryngeal nerve injury.
The lower scoring of vocal fold movement, the less vocal fold movement is. The post-surgery scores of vocal fold movement at 2, 4 and 6 weeks when compared with all treatments. Data are expressed as the mean ± SEM (n = 5) and analyzed by one-way analysis of variance followed by Scheffe's post hoc test at each time point. *P < 0.05, ADSCs group vs. ECM group.
The vocal fold movement of 80% of the rats (4/5) in each of the three groups had fully recovered at 6 weeks after crush injury. The ADSCs group displayed the fastest improvement in the score of vocal fold movement at 2 weeks post-surgery (P = 0.02, vs. the ECM group), followed by the dADSCs group (P = 0.24, vs. the ECM group). By 4 and 6 weeks post-surgery, the scores of vocal fold movement showed insignificant differences among the three groups (P > 0.05).
Changes of evoked EMG after dADSCs or ADSCs transplantation in rat models of RLN crush
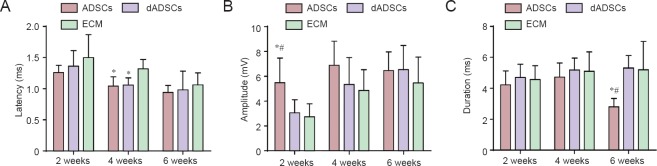
The evoked EMG characteristics recording from the ipsilateral TA muscles as a function of time are shown in Figure 3. At 2 weeks post-surgery, the amplitude of the evoked potential in the ADSCs group was significantly greater than that in dADSCs group and ECM group (P < 0.05; Figure 3B). At 4 weeks post-surgery, the mean latency in the ADSCs and dADSCs groups was significantly shorter than that in ECM group (P < 0.05; Figure 3A). At 6 weeks post-surgery, the duration of the potential in the ADSCs group was significantly shorter than that in dADSCs group and the ECM group (P < 0.05; Figure 3C), while the latency and amplitude of the three groups showed no significant differences (P > 0.05).
Figure 3.
Effects of adipose-derived mesenchymal stem cells (ADSCs), differentiated Schwann-like adipose-derived mesenchymal stem cells (dADSCs) and extracellular matrix (ECM) transplantation on evoked muscle response potentials of the affected thyroarytenoid muscle following recurrent laryngeal nerve injury.
(A) Latency, (B) amplitude, (C) duration of evoked muscle response potentials. Data are expressed as the mean ± SEM (n = 5) and analyzed by one-way analysis of variance followed by Scheffe's post hoc test at each time point. *P < 0.05, vs. ECM group; #P < 0.05, vs. dADSCs group.
Morphological changes of RLN after dADSCs or ADSCs transplantation in rat models of RLN crush
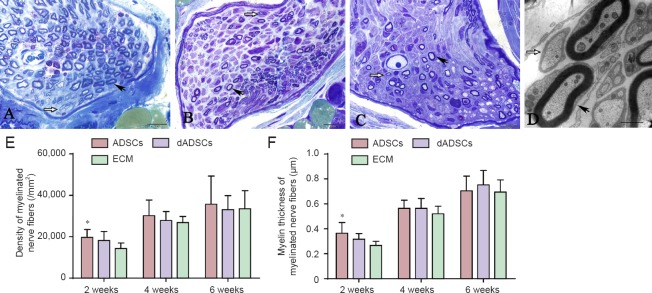
At 2 weeks post-surgery, the transverse section of the RLN displayed unmyelinated axons and thinly myelinated axons. As the recovery time progressed, there were fewer unmyelinated or under-myelinated axons. The density of myelinated fibers and the myelin thickness were quantitatively evaluated and compared as shown in Figure 4. At 2 weeks post-surgery, the density and average myelin thickness of myelinated nerve fibers in the ADSCs group was significantly higher than that in the ECM group (P < 0.05). While that in the dADSCs group was higher than that shown in the ECM group the means were statistically insignificant (P > 0.05). At 4 and 6 weeks post-surgery, the density and myelin thickness of myelinated nerve fibers showed no significant difference between the three groups (P > 0.05).
Figure 4.
Effects of adipose-derived mesenchymal stem cells (ADSCs), differentiated Schwann-like adipose-derived mesenchymal stem cells (dADSCs) and extracellular matrix (ECM) transplantation on the morphological changes of axons following recurrent laryngeal nerve (RLN) injury.
(A–C) RLN in the ADSCs group, the dADSCs group and the ECM group at 2 weeks post-surgery, respectively (toluidine blue staining, light microscopy). (D) The ultrathin transverse section of the RLN as visualized by transmission electron microscopy (ADSCs group). Black arrows point to myelinated axons, while white arrows point to unmyelinated axons. (E, F) For stereological analysis, the density of myelinated fibers (E) and the myelin thickness (F) were quantitatively evaluated and compared by statistical analyses. Data are expressed as the mean ± SEM (n = 5) and analyzed by one-way analysis of variance followed by Scheffe's post hoc test at each time point. *P < 0.05, vs. ECM group. Scale bars A–C = 10 μm; D = 1 μm.
Morphological changes of thyroarytenoid muscle after dADSCs or ADSCs transplantation in rat models of RLN crush
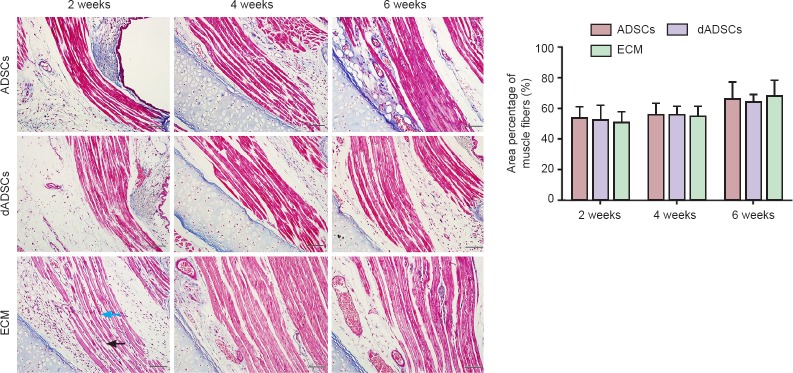
In all three groups, vocal folds on the operated side did not display obvious atrophy. Morphometric analyses were performed on specimens of the thyroarytenoid muscle following Masson's trichrome staining. Muscle cell atrophy was observed, which gradually decreased in a time-dependent fashion. Muscle fiber breakage was observed, especially at 2 weeks post-surgery. However, quantitative analysis showed that the area percentage of muscle fibers of the affected thyroarytenoid muscle displayed insignificant differences among the three groups at 2, 4 and 6 weeks post-surgery (P > 0.05; Figure 5).
Figure 5.
Effects of adipose-derived mesenchymal stem cells (ADSCs), differentiated Schwann-like adipose-derived mesenchymal stem cells (dADSCs) and extracellular matrix (ECM) transplantation on the morphological changes of affected thyroarytenoid muscles following recurrent laryngeal nerve injury (Masson's staining).
For quantitative analysis, the area percentage of muscle fibers in the affected thyroarytenoid among the three groups was not statistically significant at any time point post-surgery. Scale bars are 50 μm. Data are expressed as the mean ± SEM (n = 5) and analyzed by one-way analysis of variance followed by Scheffe's post hoc test. Black arrow points to atrophy of muscle fiber and widening of intermuscular space, while blue arrow points to muscle fiber breakage.
Discussion
In this study, the local transplantation of ADSCs induced a significant acceleration of the recovery of the vocal fold movement in rats with a crushed RLN 2 weeks post-surgery. Further electrophysiological and histological outcomes also demonstrated an improvement, indicating that ADSC transplantation has potential as an early interventional treatment for RLN injury. However, dADSCs transplantation did not demonstrate improved outcomes compared with ECM transplantation alone.
In our study, ADSC transplantation accelerated the recovery of vocal fold movement at 2 weeks post-surgery as compared with the ECM group. The histological outcomes of the ADSC group also showed a maximal difference from those of the ECM group at 2 weeks post-surgery. The reason may be that the survival time of mesenchymal stem cells after transplantation is limited. Erba et al. (2010) suggested that the effects of transplanted ADSCs are more likely to be mediated by an initial boost of growth factors since they found a lack of significant quantities of viable cells 14 days after transplantation. This may explain the early effects seen in our study at 2 weeks post-surgery under conditions where ADSCs promoted functional recovery and nerve regeneration. In all three treatment groups nearly all rats fully recovered by the end of the experiment. A previous study showed that 50% of the animals had reached full recovery by 4 weeks post-surgery and 100% had reached full recovery by 6 weeks post-surgery in the RLN crush rat model without any intervention (Monaco et al., 2015).
Administration of Schwann cells is an effective strategy for nerve regeneration (Nishiura et al., 2004; Keilhoff et al., 2006; Torii and Yamauchi, 2016). Unfortunately, obtaining a sufficient number of Schwann cells for clinical use is currently a time-consuming task, requiring the sacrifice of the donor nerves (Nishiura et al., 2004). However, Kingham et al. (2007) found that adipose-derived stem cells could differentiate into a phenotype resembling Schwann cells in vitro. Therefore, dADSCs are a suitable alternative to Schwann cells, displaying therapeutic effects on axonal regrowth and myelin formation that are superior to that of ADSCs (Sun et al., 2011; Tomita et al., 2013). However, in the present study, dADSCs transplantation did not significantly improve vocal fold movement and histological outcomes, which is concordant to a previous study (Orbay et al., 2012). It has been known that mesenchymal stem cells may improve peripheral nerve regeneration through multiple mechanisms (the bystander effect): trophic factor secretion, microenvironment stabilization and immune modulation, while the transdifferentiation may only play a limited role (Marconi et al., 2012). In our study, we applied the ADSCs or dADSCs directly on top of the crush site, rather than injected them into the crushed nerve. The ADSCs may accelerate the regeneration process better through the bystander effect.
Gradual recovery of vocal fold movement after RLN crush injury has been observed over a period of 6 weeks, even in the ECM only group, which presents a similar time course of recovery as seen in other studies using the RLN crush injury model (Tessema et al., 2009; Monaco et al., 2015). However, our study observed that 1 in 5 (20%) rats in all three groups did not achieve full recovery of vocal fold movement 6 weeks post-surgery, which confirms another report (Monaco et al., 2015). The reason for this might be laryngeal synkinetic reinnervation, which has been noted by electromyographic studies in rats (Tessema et al., 2009).
It is known that denervation of a target muscle induces a shift of protein metabolism from protein synthesis toward protein degradation. Consequently muscle fibers decrease in size and finally results in muscle atrophy (Wang et al., 2005). When the target muscle is re-innervated, the atrophy ceases and its function can be restored (Wang et al., 2005). The present study showed some atrophy of the thyroarytenoid muscle that lessened over time but there were no significant differences over time or between the three groups. This may be due to the relatively mild degree of crush injury and early reinnervation of the laryngeal muscles.
In our study, ADSCs or dADSCs were injected directly onto the crushed site. The cells may leak away during the transplantation and cause the minor differences in the cell quantity. However, we used ECM as a stem cell carrier owing to its biocompatibility and thermosensitive characteristics. It can easily be modified for liquid-to-gel transition, which depends on the applied temperature. The liquid state at low temperature makes it easy to mix with ADSCs and is easily injected, whereas the gel state may decrease cell leakage post-transplantation. The fates and functions of the transplanted ADSCs and dADSCs at the crush injury site during nerve regeneration remain unclear. To understand the mechanism underlying the process, further investigations are planned.
After administration of ADSCs, functional and histological improvements were observed in the rats with an RLN crush injury 2 weeks post-surgery. However, dADSCs transplantation did not facilitate functional and histological recoveries. Therefore, we considered that local ADSC transplantation may be a beneficial early interventional strategy that could promote regeneration following RLN injury.
Footnotes
Funding: This study was supported by the National Natural Science Foundation of China, No. 81470680, 81170901; the Natural Science Foundation of Beijing of China, No. 7132053; the Beijing Health Foundation of High-level Technical Personnel in China, No. 2014-2-004.
Conflicts of interest: None declared.
Research ethics: The study protocol was approved by the Animal Ethics Committee of Capital Medical University of China (approval number: 37817). The experimental procedure followed the National Institutes of Health Guide for the Care and Use of Laboratory Animals (NIH Publications No. 8023, revised 1978).
Data sharing statement: The datasets analyzed during the current study are available from the corresponding author on reasonable request.
Plagiarism check: Checked twice by iThenticate.
Peer review: Externally peer reviewed.
Open peer reviewers: Nady Braidy, University of New South Wales, Australia; Christian Bjerggaard Vaegter, Aarhus University, Denmark.
Copyedited by Yu J, Li CH, Qiu Y, Song LP, Zhao M
References
- Bi XJ, Guo CM, Li L, Duan XL, Jiang M. Three-dimensional culture method induces the chondrogenic differentiation of human adipose-derived mesenchymal stem cell microspheres. Zhongguo Zuzhi Gongcheng Yanjiu. 2015;19:24–29. [Google Scholar]
- Brünings W. Über eine neue Behandlungsmethode der Rekurrenslähmung. Ver Deutsch Laryng. 1911;18:93–151. [Google Scholar]
- Brown TJ, Pittman AL, Monaco GN, Benscoter BJ, Mantravadi AV, Akst LM, Jones KJ, Foecking EM. Androgen treatment and recovery of function following recurrent laryngeal nerve injury in the rat. Restor Neurol Neurosci. 2013;31:169–176. doi: 10.3233/RNN-120287. [DOI] [PubMed] [Google Scholar]
- Chan KM, Gordon T, Zochodne DW, Power HA. Improving peripheral nerve regeneration: from molecular mechanisms to potential therapeutic targets. Exp Neurol. 2014;261:826–835. doi: 10.1016/j.expneurol.2014.09.006. [DOI] [PubMed] [Google Scholar]
- di Summa PG, Kalbermatten DF, Pralong E, Raffoul W, Kingham PJ, Terenghi G. Long-term in vivo regeneration of peripheral nerves through bioengineered nerve grafts. Neuroscience. 2011;181:278–291. doi: 10.1016/j.neuroscience.2011.02.052. [DOI] [PubMed] [Google Scholar]
- Erba P, Mantovani C, Kalbermatten DF, Pierer G, Terenghi G, Kingham PJ. Regeneration potential and survival of transplanted undifferentiated adipose tissue-derived stem cells in peripheral nerve conduits. J Plast Reconstr Aesthet Surg. 2010;63:e811–817. doi: 10.1016/j.bjps.2010.08.013. [DOI] [PubMed] [Google Scholar]
- Isshiki N, Morita H, Okamura H, Hiramoto M. Thyroplasty as a new phonosurgical technique. Acta Otolaryngol. 1974;78:451–457. doi: 10.3109/00016487409126379. [DOI] [PubMed] [Google Scholar]
- Keilhoff G, Goihl A, Stang F, Wolf G, Fansa H. Peripheral nerve tissue engineering: autologous Schwann cells vs. transdifferentiated mesenchymal stem cells. Tissue Eng. 2006;12:1451–1465. doi: 10.1089/ten.2006.12.1451. [DOI] [PubMed] [Google Scholar]
- Kim SJ, Park SH, Sung YC, Kim SW. Effect of mesenchymal stem cells associated to matrixen on the erectile function in the rat model with bilateral cavernous nerve crushing injury. Int Braz J Urol. 2012;38:833–841. doi: 10.1590/1677-553820133806833. [DOI] [PubMed] [Google Scholar]
- Kingham PJ, Kalbermatten DF, Mahay D, Armstrong SJ, Wiberg M, Terenghi G. Adipose-derived stem cells differentiate into a Schwann cell phenotype and promote neurite outgrowth in vitro. Exp Neurol. 2007;207:267–274. doi: 10.1016/j.expneurol.2007.06.029. [DOI] [PubMed] [Google Scholar]
- Mantovani C, Mahay D, Kingham M, Terenghi G, Shawcross SG, Wiberg M. Bone marrow- and adipose-derived stem cells show expression of myelin mRNAs and proteins. Regen Med. 2010;5:403–410. doi: 10.2217/rme.10.15. [DOI] [PubMed] [Google Scholar]
- Marconi S, Castiglione G, Turano E, Bissolotti G, Angiari S, Farinazzo A, Constantin G, Bedogni G, Bedogni A, Bonetti B. Human adipose-derived mesenchymal stem cells systemically injected promote peripheral nerve regeneration in the mouse model of sciatic crush. Tissue Eng Part A. 2012;18:1264–1272. doi: 10.1089/ten.TEA.2011.0491. [DOI] [PubMed] [Google Scholar]
- Monaco GN, Brown TJ, Burgette RC, Fargo KN, Akst LM, Jones KJ, Foecking EM. Electrical stimulation and testosterone enhance recovery from recurrent laryngeal nerve crush. Restor Neurol Neurosci. 2015;33:571–578. doi: 10.3233/RNN-130334. [DOI] [PubMed] [Google Scholar]
- Nishiura Y, Brandt J, Nilsson A, Kanje M, Dahlin LB. Addition of cultured Schwann cells to tendon autografts and freeze-thawed muscle grafts improves peripheral nerve regeneration. Tissue Eng. 2004;10:157–164. doi: 10.1089/107632704322791808. [DOI] [PubMed] [Google Scholar]
- Orbay H, Uysal AC, Hyakusoku H, Mizuno H. Differentiated and undifferentiated adipose-derived stem cells improve function in rats with peripheral nerve gaps. J Plast Reconstr Aesthet Surg. 2012;65:657–664. doi: 10.1016/j.bjps.2011.11.035. [DOI] [PubMed] [Google Scholar]
- Sun F, Zhou K, Mi WJ, Qiu JH. Combined use of decellularized allogeneic artery conduits with autologous transdifferentiated adipose-derived stem cells for facial nerve regeneration in rats. Biomaterials. 2011;32:8118–8128. doi: 10.1016/j.biomaterials.2011.07.031. [DOI] [PubMed] [Google Scholar]
- Tessema B, Roark RM, Pitman MJ, Weissbrod P, Sharma S, Schaefer SD. Observations of recurrent laryngeal nerve injury and recovery using a rat model. Laryngoscope. 2009;119:1644–1651. doi: 10.1002/lary.20293. [DOI] [PubMed] [Google Scholar]
- Tomita K, Madura T, Sakai Y, Yano K, Terenghi G, Hosokawa K. Glial differentiation of human adipose-derived stem cells: implications for cell-based transplantation therapy. Neuroscience. 2013;236:55–65. doi: 10.1016/j.neuroscience.2012.12.066. [DOI] [PubMed] [Google Scholar]
- Torii T, Yamauchi J. Gas6-Tyro3 signaling is required for Schwann cell myelination and possible remyelination. Neural Regen Res. 2016;11:215–216. doi: 10.4103/1673-5374.177714. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang X, Hu W, Cao Y, Yao J, Wu J, Gu X. Dog sciatic nerve regeneration across a 30-mm defect bridged by a chitosan/PGA artificial nerve graft. Brain. 2005;128:1897–1910. doi: 10.1093/brain/awh517. [DOI] [PubMed] [Google Scholar]
- Xu Y, Liu L, Li Y, Zhou C, Xiong F, Liu Z, Gu R, Hou X, Zhang C. Myelin-forming ability of Schwann cell-like cells induced from rat adipose-derived stem cells in vitro. Brain Res. 2008;1239:49–55. doi: 10.1016/j.brainres.2008.08.088. [DOI] [PubMed] [Google Scholar]