Abstract
Purpose
The murine double minute (MDM)2 is a critical negative regulator of the p53 tumor suppressor, and MDM2 SNP309G is associated with a higher risk of proliferative vitreoretinopathy (PVR); in addition, the MDM2 T309G created using clustered regularly interspaced short palindromic repeats (CRISPR)/associated endonuclease (Cas)9 enhances normal rabbit vitreous-induced expression of MDM2 and survival of primary human retinal pigment epithelial (hRPE) cells in vitro. The goal of this study was to determine whether this MDM2 T309G contributes to the development of experimental PVR.
Methods
hRPE cells expressing MDM2 T309G or T309T only were treated with vitreous from human PVR donors (HV). The expression of MDM2 and p53 in the treated cells was examined by Western blot. The in vitro vitreous-induced cellular responses, such as contraction were assessed, and PVR was induced by intravitreal injection of the hRPE cells with MDM2 T309G or T309T only into rabbit eyes.
Results
Western blot analyses indicated that treatment of hRPE cells with HV led to a significant increase (1.7 ± 0.2-fold) in the expression of MDM2 and a significant decrease in p53 in the cells expressing MDM2 T309G compared with those with MDM2 T309T. In addition, HV promoted contraction of the hRPE cells expressing MDM2 T309G significantly more than those with MDM2 T309T only. Furthermore, MDM2 T309G in the hRPE cells enhanced the development of PVR in a rabbit model.
Conclusions
The MDM2 SNP309 in RPE cells enhances their potential of PVR pathogenesis.
Keywords: MDM2, CRISPR/Cas9, SNP309, RPE, PVR
The major cause of failure in surgery to correct rhegmatogenous retinal detachment (RRD) is proliferative vitreoretinopathy (PVR),1–3 which is characterized with formation of fibrotic-cellular epiretinal membranes (ERMs) composed of extracellular matrix proteins and cells, including RPE cells, retinal glial cells, fibroblasts, and macrophages. Indeed, RPE and glial cells in these vitreal membranes appear to play an especially critical role in the pathogenesis of PVR.4
PVR occurs in 5% to 10% of patients who undergo the surgical repair of RRD, and accounts for approximately 75% of all primary failures after the surgery.1,5–10 Currently, repeat surgery is the only option for PVR2,3; however, the visual outcome of the surgery is poor because of the retinal damage resulting from recurrent detachment and the PVR process itself.11 Adjuvant pharmacotherapies that have been evaluated thus far have neither blocked the formation of PVR nor reduced the rates of retinal redetachment secondary to PVR consistently.11 Incomplete understanding of the PVR pathogenesis is a major obstacle for developing new therapeutic options for individuals who are afflicted by this potentially blinding disease.
The oncogenic protein murine double minute 2 (MDM2), an E3 ubiquitin-protein ligase, with a human homologue called Hdm2, is an important negative regulator of the p53 tumor suppressor.12–14 We have previously shown that vitreous from experimental rabbits or PVR patients can trigger the signaling pathway of phosphoinositide 3 kinase (PI3K)/Akt, which phosphorylates MDM2. The phosphorylated MDM2 enhances p53 degradation15; in addition, blocking MDM2 binding to p53 with a small molecule nutlin-3 protects rabbits from retinal detachment in a PVR rabbit model.5
Importantly, the G allele of single nucleotide polymorphisms (SNPs) (rs2279744) in the MDM2 intron promoter locus between exons 1 and 2 attenuates the p53 tumor suppressor pathway and accelerates tumor formation in humans.16 Intriguingly, this SNP is also associated with a higher risk of PVR for RRD patients,1 but whether this MDM2 G309 SNP contributes to the development of PVR is still unknown.
Clustered regularly interspaced short palindromic repeats (CRISPR) and CRISPR-associated nucleases (Cas) in bacteria and archaea form their adaptive immune system, in which CRISPR RNAs (crRNAs) can guide the Cas to cleave the foreign nucleic acids.17–19 In Streptococcus pyogenes (Sp) there are two nuclease domains in the SpCas9, RuvC and HNH, each of which can cleave one strand of the double-stranded target DNA when directed by the crRNA and trans-activating crRNA (tracrRNA).19,20 Importantly, this SpCas9 can be engineered to target specific genomic loci in mammalian cells together with the processed single guide RNAs (sgRNAs), which consist of the crRNA and tracrRNA, at a prior protospacer adjacent motif.19,21,22 With this CRISPR/Cas9 technology, we have created the MDM2 T309G in the genomic DNA of human primary RPE (hRPE) cells and found that the MDM2 T309G mutation enhances rabbit vitreous (RV)-induced expression of MDM2 and cell proliferation,21 but whether this mutation contributes to the pathogenesis of PVR is still unclear. The goal of the studies presented in this article aims to resolve this question.
Materials and Methods
Major Reagents and Cell Culture
The antibodies against p53 and MDM2 were purchased from Cell Signaling Technology (Danvers, MA, USA) and from Abgent (San Diego, CA, USA), respectively. The primary antibody against β-actin and the secondary antibodies of the horseradish peroxidase (HRP)-conjugated goat anti-rabbit IgG and anti-mouse IgG were purchased from Santa Cruz Biotechnology (Santa Cruz, CA, USA). Enhanced chemiluminescent substrate for detection of HRP was from Thermo Scientific (Waltham, MA, USA).
hRPE cells were purchased from Lonza (Walkersville, MD, USA) and cultured in Dulbecco's modified Eagle's medium (DMEM)/nutrient mixture F-12 medium (F12) (Thermo Scientific, Grand Island, NY, USA) supplemented with 10% fetal bovine serum (FBS) and 1% penicillin/streptomycin. The hRPE cells with MDM2 T309G or T309T only were created using a system of AAV-SpCas9 D10A and AAV-SpGuide (MDM2 or lacZ) and a homology direct recombinant DNA template with MDM2 G309 as described previously.21 All cells were cultured at 37°C in a humidified 5% CO2 atmosphere.
Quantitative PCR
The hRPE cells with MDM2 T309G were plated into six-well plates at a density of 1 × 105 cells per well in DMEM/F12 supplemented with 10% FBS overnight and then serum-starved for 24 hours. Subsequently, the cells were treated with or without vitreous from patients with PVR (HV) (diluted 1:3 in DMEM/F12) for 0.5, 2, 4, and 16 hours. Then total RNA was respectively isolated with a RNeasy mini kit (Qiagen, Germantown, MD, USA), and cDNA was synthesized with an ISCRIPT cDNA synthesis kit (BioRAD, Hercules, CA, USA) in a program (25°C, 5 minutes; 42°C, 30 minutes; 85°C, 5 minutes; 4°C, forever). The cDNA was subjected to quantitative PCR using a Fast Start universal SYBR green Master mix (Roche, Basel, Switzerland) in a Light Cycler 480 II machine (Roche). Primers of quantitative PCR synthesized by Integrated DNA Technology (Coralville, IA, USA) were (forward: 5′-AGAAGGACAAGAACTCTCAGATG-3′, reverse: 5′-GTGCATTTCCAATAGTCAGCTAA-3′) for MDM2 and (forward: 5′-CCTGGCGTCGTGATTAGTGAT-3′, reverse: 5′-AGACGTTCAGTCCTGTCCATAA-3′) for a housekeeping gene hHPRT1.
Western Blot
hRPE cells with MDM2 T309G or T309T cultured to 90% confluence in 24-well plates were switched to serum-free medium for 24 hours and then were treated with or without HV (diluted 1:3 in DMEM/F12) for 16 hours. After rinsing twice with ice-cold PBS, cells were lysed in 1 × sample buffer, which was diluted with extraction buffer (10 mM Tris-HCl, pH 7.4, 5 mM EDTA, 50 mM NaCl, 50 mM NaF, 1% Triton X-100, 20 μg/mL aprotinin, 2 mM Na3VO4, and 1 mM phenylmethylsulfonyl fluoride) from the 5 × protein sample buffer (25 mM EDTA, pH 7.0, 10% SDS, 500 mM dithiothreitol, 50% sucrose, 500 mM Tris-HCl, pH 6.8, and 0.5% bromophenol blue). The samples were heated at 95°C for 5 minutes and then centrifuged for 5 minutes at 13,000g. Proteins in the samples were then separated by 10% SDS-PAGE, transferred to polyvinylidene difluoride (PVDF) membranes, and subjected to Western blot analysis. Signal intensity was determined by densitometry using NIH Image J (http://imagej.nih.gov/ij/; provided in the public domain by the National Institutes of Health, Bethesda, MD, USA).21,22
Cell Proliferation Assay
The hRPE cells with MDM2 T309G or T309T were plated into 24-well plates at a density of 3.0 × 105 cells per well in DMEM/F12 supplemented with 10% FBS. After the cells had attached to the plates, the medium was replaced with DMEM/F12 supplemented with HV (1:3 dilution in DMEM/F12). Three days later, the cells were counted in a hemocytometer; at least three independent experiments were performed.21,23
Apoptosis Assay
The hRPE cells with MDM2 T309G or T309T were seeded into 6-cm dishes (1 × 105 cells/dish) in DMEM/F12 with 10% FBS. After cell attachment, the medium was replaced with DMEM/F12 supplemented with HV (1:3 dilution in DMEM/F12). On day 3, the cells were harvested and stained with fluorescein isothiocyanate (FITC)-conjugated annexin V and propidium iodide (PI) following the instructions provided with the apoptosis kit (BD Biosciences, Palo Alto, CA, USA). The cells were analyzed using fluorescence-activated cell sorting (FACS) in a Coulter Beckman XL instrument. At least three independent experiments were performed.21,23
Collagen Contraction Assay
The hRPE cells with MDM2 T309G or T309T only were resuspended in 1.5 mg/mL of neutralized collagen I (INAMED, Fremont, CA, USA) (pH 7.2) on ice at a density (1 × 106 cells/mL).24,25 The mixture was transferred into wells of 24-well plates that had been preincubated overnight with 5 mg/mL BSA/PBS. Ninety minutes later at 37°C, 0.5 mL DMEM/F12 or HV (1:3 dilution in DMEM/F12) was added. On day 3 after being photographed, the gel diameter was measured, and the gel area was calculated using a formula 3.14 × r2, where r is the radius of the gel. At least three independent experiments were performed.25–27
PVR Patient Vitreous
As previously described,25,27 human vitreous from patients with PVR (HV) (1.0 to 1.5 mL) was obtained during pars plana vitrectomy before initiating the pars plana infusion at the Vancouver Hospital. An ethical approval was obtained before the initiation of this project from the Vancouver Hospital and University of British Columbia Clinical Research Ethics Board and from Nova Scotia Health Research Ethics Board, Dalhousie University. The University of British Columbia Clinical Research Ethics Board and the Nova Scotia Health Research Ethics Board policies comply with the Tri Council Policy and the Good Clinical Practice Guidelines, which have their origins in the ethical principles in the Declaration of Helsinki. Written informed consent was obtained from patients.
Experimental PVR in Rabbits
As described previously,26–28 PVR was induced in right eyes of Dutch Belted rabbits (6 months old, 2–3 kg, male and female) (Covance, Denver, PA, USA). Briefly, a gas vitrectomy was performed by injecting 0.1 mL perfluoropropane (C3F8) (Alcon, Fort Worth, TX, USA) into the vitreous cavity 4 mm posterior to the corneal limbus. One week later, all rabbits were injected with 0.1 mL of platelet-rich plasma (PRP) from rabbits and 0.1 mL DMEM/F12 containing either 3.0 × 105 cells of hRPE cells with MDM2 T309G or T309T only (10 rabbits per group) under an operative microscope. The retinal status was examined with an indirect ophthalmoscope through a +30 diopter (D) fundus lens on days 1, 3, 5, 7, 14, 21, and 28 by two ophthalmologists without knowing the injected cell types. PVR was graded using a standard of the Fastenberg classification from 0 through 5.27,29 On day 28, the rabbits were killed. All surgeries adhered to the ARVO Statement for the Use of Animals in Ophthalmic and Vision Research, and a protocol for the use of animals was approved by the Schepens Animal Care and Use Committee (Boston, MA, USA).
Statistics
The data were analyzed using an unpaired t-test or a Mann-Whitney test. A power (P) value less than 0.05 was considered statistically significant.
Results
HV-Induced Expression of MDM2 in the hRPE Cells With MDM2 T309G
MDM2 SNP309 is associated with a higher risk of PVR1 and the CRISPR/Cas9-created MDM2 T309G in hRPE cells enhanced RV-induced expression of MDM2 and a reduction in p53.21 Thus, we investigated whether this mutation in hRPE cells rendered the cells responsive to HV from patients with PVR. hRPE cells containing MDM2 T309G or T309T were treated with HV for 16 hours, and the cell lysates were subjected to Western blot analyses. As shown in Figure 1, HV treatment led to a 1.7 ± 0.2-fold increase in the protein expression and a 1.73 ± 0.15-fold increase in the mRNA of MDM2 in the hRPE cells with MDM2 T309G, compared with the cells with MDM2 T309T only. In addition, HV treatment suppressed expression of p53 by 1.75-fold more in the hRPE cells with MDM2 T309G than in the cells with MDM2 T309T only.
Figure 1.
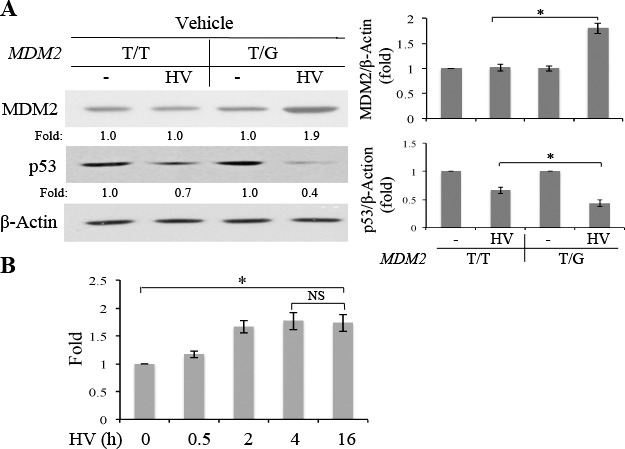
Patient PVR vitreous enhanced expression of MDM2 in the hRPE cells with MDM2 T309G. (A) hRPE cells with MDM2 T309 only (T/T) or MDM2 T309 plus G309 (T/G) were serum-deprived 24 hours and treated with or without patient PVR vitreous (HV) for 16 hours, and the cell lysates were subjected to Western blot analysis with indicted antibodies. “Fold” was calculated by first normalizing to the level of β-actin and then calculating the ratio of the stimulated over the basal (i.e., unstimulated). The data were from three independent experiments, and the asterisk indicates significant difference between the two compared groups. (B) The hRPE cells with MDM2 T309G were serum-starved for 24 hours and then treated with or without HV for 0.5, 2, 4, and 16 hours. Subsequently, the total RNA was isolated and the synthesized cDNA was subjected to quantitative PCR for HV-induced expression of MDM2 mRNA. “Fold” for expression of MDM2 mRNA was calculated by first normalizing to the level of a housekeeping gene hHPRT1 and then calculating the ratio of the stimulated over the basal (i.e., unstimulated). The data normalized by a housekeeping gene were from three independent experiments. The asterisk indicates significant difference between the two compared groups, and NS denotes no significant difference.
MDM2 T309G Enhanced HV-Induced Cell Proliferation and Survival Against Apoptosis
We speculated that an increase in MDM2 and decrease in p53 might enhance cell proliferation and survival.15,16 Thus, we treated the hRPE cells expressing MDM2 T309G or T309T only with HV and assessed their proliferation and survival. As shown in Figure 2A, whereas HV-induced proliferation of hRPE cells with MDM2 T309T only, the proliferative response was greater in hRPE cells with MDM2 T309G than those with MDM2 T309T. Moreover, the two types of hRPE cells were starved for serum for 3 days to induce apoptosis, and the cells were treated with or without HV, and the apoptosis was analyzed using a FITC-conjugated annexin V-based apoptosis kit by FACS. The results (Fig. 2B) showed that HV promoted survival of hRPE cells with MDM2 T309G better than those with MDM2 T309T only.
Figure 2.
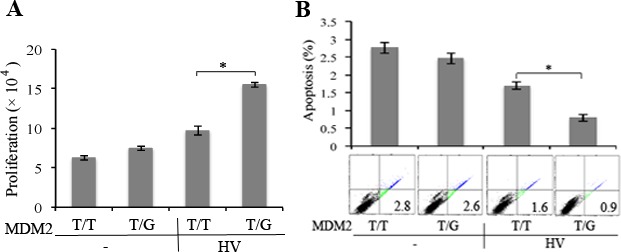
HV enhanced proliferation and survival of hRPE cells with MDM2 T309G compared with those with MDM2 T309T only. (A) The hRPE cells with MDM2 T309 only (T/T) or MDM2 T309 plus G309 (T/G) were plated into a 24-well plate at a density of 3 × 104 cells per well. After the cells had attached the plates, the medium was switched to either DMEM/F12 or HV (diluted 1:3 in DMEM/F12). The media were replaced every day. On day 3, cells were counted with a hemocytometer under a light microscope. Mean ± SD of three independent experiments is shown. *P < 0.05, unpaired t-test. (B) Serum-starved hRPE cells with MDM2 T309G or T309T only were plated into 60-mm dishes at a density of 100,000 cells per dish. After the cells attached to the dishes, the medium was switched to either DMEM/F12 or HV (diluted to 1:3 in DMEM/F12). The media were replaced every day. On day 3, the cells were stained with FITC-conjugated annexin V and PI in an apoptosis assay kit by following the manufacturer's instructions. Cells that were stained with annexin V and/or PI were detected and quantified by flow cytometry in a Beckman Coulter (Brea, CA, USA) XL instrument. The mean ± SD of three independent experiments is shown, and one of the experimental raw data is shown below the bar graphs.
MDM2 T309G Promoted HV-Induced Contraction of hRPE Cells and Enhanced Experimental PVR
One of the major characteristics of PVR is the formation of epiretinal membranes,27 which can contract and cause the retinal redetachment. Thus, we assessed the possible contribution of MDM2 T309G to the development of PVR. In a contraction assay, cells were mixed with collagen to mimic the conditions of an epiretinal membrane. As shown in Figure 3, hRPE cells expressing MDM2 T309G contracted more in response to HV than cells with MDM2 T309T only, indicating that MDM2 T309G in the hRPE cells might contribute to the pathogenesis of PVR.
Figure 3.
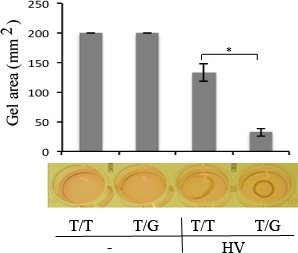
HV promoted more contraction of hRPE cells with MDM2 T309G than those with MDM2 T309T only. The hRPE cells with MDM2 T309G or T309T only were resuspended in 1.5 mg/mL of neutralized collagen (pH 7.2) at a density of 1 × 106 cells/mL and seeded into wells of a 24-well plate that had been preincubated overnight with 5 mg/mL BSA/PBS. The mixture of the collagen and cells was incubated at 37°C for 90 minutes. The collagen gels were overlaid with DMEM/F12 alone (−) or HV. On day 3, the gel diameter was measured and calculated using the formula (3.14 × [diameter/2]2). The mean ± SD of the three independent experiments is shown. *Denotes P < 0.05 using an unpaired t-test. A photograph of the representative experiment is shown at the bottom of the bar graphs.
Thus, we investigated whether the MDM2 T309G polymorphism influenced the pathogenesis of PVR. hRPE cells expressing MDM2 T309T only or the cells with MDM2 T309G were injected intravitreally into rabbit eyes. On the seventh day after cell injection, the cells with MDM2 T309G had begun to form fibrotic membranes, whereas all those with MDM2 T309T remained as cell aggregates in the vitreous (Fig. 4). Observation on weeks 2, 3, and 4 indicated significant differences in the development of PVR in a rabbit model between the two groups of hRPE cells with MDM2 T309T only and those with MDM2 T309G based on the standard of the Fastenberg classification from 0 through 5,27,29 as shown in Figure 4A. On week 4, for instance, the retina detached in 3 of 10 rabbits that were intravitreally injected with the MDM2 T309G hRPE cells, but none of the 10 rabbits containing MDM2 T309T only developed retinal detachment. Histologic examination indicated that there were more cells migrating into the retinas in the vitreous injected with hRPE cells expressing MDM2 T309G than those expressing MDM2 T309T (Fig. 4B). Taken together, these experiments demonstrated that MDM2 T309G polymorphism in the hRPE cells contributes to the pathogenesis of experimental PVR.
Figure 4.
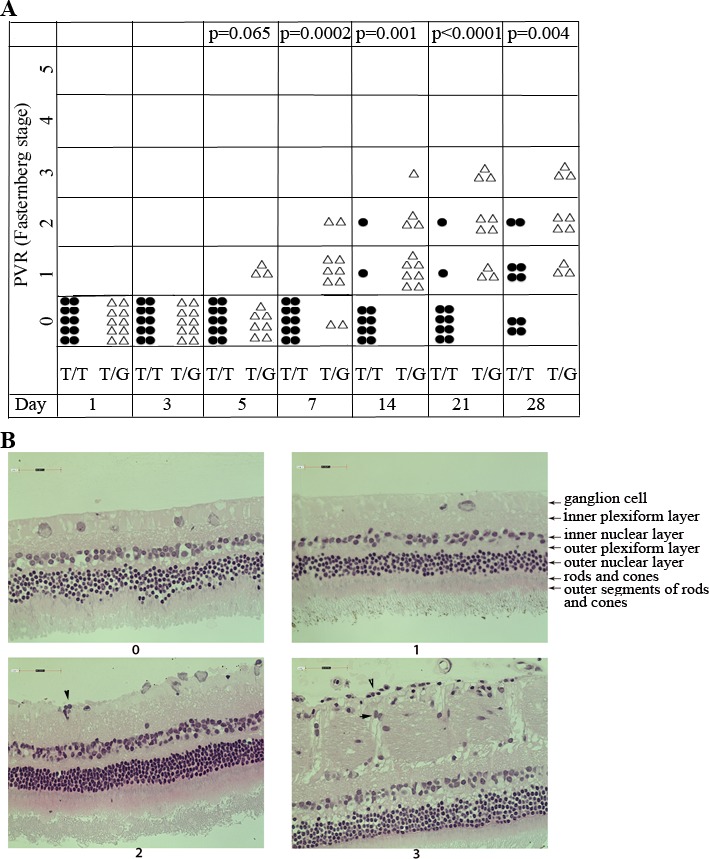
The hRPE cells with MDM2 T309G had more potential to induce PVR in a rabbit model. (A) PVR was induced in the right eyes of Dutch Belted rabbits (10 rabbits per group) as previously described.25,27,28 Briefly, 1 week after gas vitrectomy, rabbits were intravitreally injected with 0.1 ml PRP and hRPE cells (3 × 105) with MDM2 T309 only (T/T) or MDM2 T309 plus G309 (T/G) in 0.1 mL DMEM/F12. The rabbits were examined with an indirect ophthalmoscope and the PVR status for each rabbit was plotted on days 1, 3, 5, 7, 14, 21, and 28. The data were subjected to Mann-Whitney analysis. A P value less than 0.05 is considered a significant difference between the two groups (T/T and T/G). (B) The representative eyeballs from rabbits with PVR stages 0 and 1 (hRPE T309T), and 2 and 3 (hRPE T309G) were fixed, sectioned and stained with hematoxylin-eosin. In the eyes with PVR stages 2 and 3 injected with hRPE expressing MDM2 T309G, there were some cells (arrowheads) attaching to the retina.
Discussion
MDM2 T309G, which was generated in the hRPE cells using the CRISPR/Cas9,21 led to enhancement of HV-stimulated expression of MDM2 and cell proliferation, survival, and contraction in response to HV, as well as development of PVR in a rabbit model. Although the cells used in the in vivo PVR model were a mixed population of MDM2 genotypes (20% T309T, 70% T309G, and 10% G309G),21 they demonstrated more significant membrane formation than those expressing only MDM2 T309T.
We chose rabbits for the experimental PVR because of the ease of working in these animals' eyes. The smaller size of the rabbit lens compared with the eyeball permits manipulations to be performed within the eye, without causing any damage to the lens/retina. Rabbits lack a distinct macula; however, the medullary wing detachment simulates a retinal detachment in humans and shows PVR-like features.2 Retinas from rabbits are only partially vascularized, in contrast to those in humans, which are completely vascularized. However, the use of other common laboratory animals such as rats and mice, which have higher retinal vascularization, can be difficult to work with due to the difficulties in isolating and purifying the small volume of the vitreous. Indeed, the mouse lens occupies nearly 75% of the eye.30 It is also important to note that the cell injection into rabbit eyes to induce experimental PVR does not involve any break in the retina. Indeed, RRD models leading to the development of PVR31 may be considered in future studies in an effort to more closely resemble the clinical condition.
Vitreous can induce the transcription factor specific protein (Sp)132,33 to bind the intron promoter of MDM2 in the hRPE cells with MDM T309G and thus increase its expression,21 resulting in a reduction in p53, and enhanced cell proliferation, viability, and contraction, leading to an increased potential of PVR. These findings might be relevant to other eye diseases, such as proliferative diabetic retinopathy (PDR), in which there are similar fibrotic-cellular membranes. Thus, it will be intriguing to determine whether this MDM2 T309G polymorphism also contributes to the development of PDR.
Both normal RV21 and HV (Fig. 1) induce expression of MDM2 as well as proliferation and survival of hRPE cells. Vitreous contains numerous growth factors and cytokines, including epidermal growth factor (EGF), fibroblast growth factor-2 (FGF-2), transforming growth factor (TGF), hepatocyte growth factor (HGF), insulin-like growth factor (IGF), connective tissue growth factor and interleukins (IL)-6 and -8, which are implicated in PVR.34 Some growth factors have been reported to be increased during the development of PVR, such as platelet-derived growth factor (PDGF)35 and TGF-β8 and these vitreal growth factors and cytokines may be essential drivers of the PVR pathogenesis.34 Moreover, the exposure of cells, such as RPE cells, to vitreous containing rich growth factors and cytokines may stimulate them to secrete growth factors and/or cytokines, increasing the local concentrations of these agents.34 A cocktail of agents neutralizing EGF, TGF-α, FGF-2, IL-8, TGF-βs, HGF, IGF-1, and PDGFs blocks experimental PVR induced by fibroblasts,36 but whether the same approach would prevent PVR induced by RPE cells that harbor the MDM2 309SNP is unknown.
Adeno-associated viruses (AAVs) are small viruses that are not at present known to cause any disease, and thus their derived vectors have great potential for human gene therapy.37,38 The eye is an especially ideal target organ for gene therapy, as it is easily accessible and immune privileged.39 In this study, we showed that the AAV-CRISPR/Cas9-created MDM2 T309G contributed to the development of experimental PVR in a rabbit model, further demonstrating that this CRISPR/Cas9 system is a novel powerful tool for the targeted introduction of mutations into eukaryotic genomes for the generation of reproducible disease models.40,41
Acknowledgments
Supported by National Institutes of Health, National Eye Institute Grants R01 EY012509 (HL) and (in part) Core Grant P30EY003790.
Disclosure: G. Zhou, None; Y. Duan, None; G. Ma, None; W. Wu, None; Z. Hu, None; N. Chen, None; Y. Chee, None; J. Cui, None; A. Samad, None; J.A. Matsubara, None; S. Mukai, None; P.A. D'Amore, None; H. Lei, None
References
- 1. Pastor-Idoate S,, Rodriguez-Hernandez I,, Rojas J,, et al. Genetics on PVRSG: the T309G MDM2 gene polymorphism is a novel risk factor for proliferative vitreoretinopathy. PLoS One. 2013; 8: e82283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Agrawal RN,, He S,, Spee C,, Cui JZ,, Ryan SJ,, Hinton DR. In vivo models of proliferative vitreoretinopathy. Nat Protoc. 2007; 2: 67–77. [DOI] [PubMed] [Google Scholar]
- 3. Di Lauro S,, Kadhim MR,, Charteris DG,, Pastor JC. Classifications for proliferative vitreoretinopathy (PVR): an analysis of their use in publications over the last 15 years. J Ophthalmol. 2016; 2016: 7807596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Fisher SK,, Lewis GP,, Linberg KA,, Verardo MR. Cellular remodeling in mammalian retina: results from studies of experimental retinal detachment. Prog Retin Eye Res. 2005; 24: 395–431. [DOI] [PubMed] [Google Scholar]
- 5. Lei H,, Rheaume MA,, Cui J,, et al. A novel function of p53: a gatekeeper of retinal detachment. Am J Pathol. 2012; 181: 866–874. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Lei H,, Qian CX,, Lei J,, Haddock LJ,, Mukai S,, Kazlauskas A. RasGAP promotes autophagy and thereby suppresses platelet-derived growth factor receptor-mediated signaling events, cellular responses, and pathology. Mol Cell Biol. 2015; 35: 1673–1685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Casaroli-Marano RP,, Pagan R,, Vilaro S. Epithelial-mesenchymal transition in proliferative vitreoretinopathy: intermediate filament protein expression in retinal pigment epithelial cells. Invest Ophthalmol Vis Sci. 1999; 40: 2062–2072. [PubMed] [Google Scholar]
- 8. Connor TB, Jr,, Roberts AB,, Sporn MB,, et al. Correlation of fibrosis and transforming growth factor-beta type 2 levels in the eye. J Clin Invest. 1989; 83: 1661–1666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Leaver PK,, Billington BM. Vitrectomy and fluid/silicone-oil exchange for giant retinal tears: 5 years follow-up. Graefes Arch Clin Exp Ophthalmol. 1989; 227: 323–327. [DOI] [PubMed] [Google Scholar]
- 10. Cui J,, Lei H,, Samad A,, et al. PDGF receptors are activated in human epiretinal membranes. Exp Eye Res. 2009; 88: 438–444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Wubben TJ,, Besirli CG,, Zacks DN. Pharmacotherapies for retinal detachment. Ophthalmology. 2016; 123: 1553–1562. [DOI] [PubMed] [Google Scholar]
- 12. Haupt Y,, Maya R,, Kazaz A,, Oren M. Mdm2 promotes the rapid degradation of p53. Nature. 1997; 387: 296–299. [DOI] [PubMed] [Google Scholar]
- 13. Oliner JD,, Kinzler KW,, Meltzer PS,, George DL,, Vogelstein B. Amplification of a gene encoding a p53-associated protein in human sarcomas. Nature. 1992; 358: 80–83. [DOI] [PubMed] [Google Scholar]
- 14. Vassilev LT,, Vu BT,, Graves B,, et al. In vivo activation of the p53 pathway by small-molecule antagonists of MDM2. Science. 2004; 303: 844–848. [DOI] [PubMed] [Google Scholar]
- 15. Lei H,, Velez G,, Kazlauskas A. Pathological signaling via platelet-derived growth factor receptor {alpha} involves chronic activation of Akt and suppression of p53. Mol Cell Biol. 2011; 31: 1788–1799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Bond GL,, Hu W,, Bond EE,, et al. A single nucleotide polymorphism in the MDM2 promoter attenuates the p53 tumor suppressor pathway and accelerates tumor formation in humans. Cell. 2004; 119: 591–602. [DOI] [PubMed] [Google Scholar]
- 17. Jansen R,, Embden JD,, Gaastra W,, Schouls LM. Identification of genes that are associated with DNA repeats in prokaryotes. Mol Microbiol. 2002; 43: 1565–1575. [DOI] [PubMed] [Google Scholar]
- 18. Barrangou R,, Fremaux C,, Deveau H,, et al. CRISPR provides acquired resistance against viruses in prokaryotes. Science. 2007; 315: 1709–1712. [DOI] [PubMed] [Google Scholar]
- 19. Jinek M,, Chylinski K,, Fonfara I,, Hauer M,, Doudna JA,, Charpentier E. A programmable dual-RNA-guided DNA endonuclease in adaptive bacterial immunity. Science. 2012; 337: 816–821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Wang T,, Wei JJ,, Sabatini DM,, Lander ES. Genetic screens in human cells using the CRISPR-Cas9 system. Science. 2014; 343: 80–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Duan Y,, Ma G,, Huang X,, D'Amore PA,, Zhang F,, Lei H. The clustered, regularly interspaced, short palindromic repeats-associated endonuclease 9 (CRISPR/Cas9)-created MDM2 T309G mutation enhances vitreous-induced expression of MDM2 and proliferation and survival of cells. J Biol Chem. 2016; 291: 16339–16347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Huang X,, Zhou G,, Wu W,, et al. Editing VEGFR2 blocks VEGF-induced activation of Akt and Ttbe formation. Invest Ophthalmol Vis Sci. 2017; 58: 1228–1236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Lei H,, Kazlauskas A. Growth factors outside of the PDGF family employ ROS/SFKs to activate PDGF receptor alpha and thereby promote proliferation and survival of cells. J Biol Chem. 2009; 284: 6329–6336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Lei H,, Velez G,, Hovland P,, Hirose T,, Gilbertson D,, Kazlauskas A. Growth factors outside the PDGF family drive experimental PVR. Invest Ophthalmol Vis Sci. 2009; 50: 3394–3403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Lei H,, Velez G,, Cui J,, et al. N-acetylcysteine suppresses retinal detachment in an experimental model of proliferative vitreoretinopathy. Am J Pathol. 2010; 177: 132–140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Lei H,, Rheaume MA,, Velez G,, Mukai S,, Kazlauskas A. Expression of PDGFR{alpha} is a determinant of the PVR potential of ARPE19 cells. Invest Ophthalmol Vis Sci. 2011; 52: 5016–5021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Ma G,, Duan Y,, Huang X,, et al. Prevention of proliferative vitreoretinopathy by suppression of phosphatidylinositol 5-phosphate 4-kinases. Invest Ophthalmol Vis Sci. 2016; 57: 3935–3943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Andrews A,, Balciunaite E,, Leong FL,, et al. Platelet-derived growth factor plays a key role in proliferative vitreoretinopathy. Invest Ophthalmol Vis Sci. 1999; 40: 2683–2689. [PubMed] [Google Scholar]
- 29. Fastenberg DM,, Diddie KR,, Sorgente N,, Ryan SJ. A comparison of different cellular inocula in an experimental model of massive periretinal proliferation. Am J Ophthalmol. 1982; 93: 559–564. [DOI] [PubMed] [Google Scholar]
- 30. Cehofski LJ,, Mandal N,, Honore B,, Vorum H. Analytical platforms in vitreoretinal proteomics. Bioanalysis. 2014; 6: 3051–3066. [DOI] [PubMed] [Google Scholar]
- 31. Mandal N,, Lewis GP,, Fisher SK,, et al. Proteomic analysis of the vitreous following experimental retinal detachment in rabbits. J Ophthalmol. 2015; 2015: 583040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Milanini-Mongiat J,, Pouyssegur J,, Pages G. Identification of two Sp1 phosphorylation sites for p42/p44 mitogen-activated protein kinases: their implication in vascular endothelial growth factor gene transcription. J Biol Chem. 2002; 277: 20631–20639. [DOI] [PubMed] [Google Scholar]
- 33. Tan NY,, Khachigian LM. Sp1 phosphorylation and its regulation of gene transcription. Mol Cell Biol. 2009; 29: 2483–2488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Pennock S,, Haddock LJ,, Eliott D,, Mukai S,, Kazlauskas A. Is neutralizing vitreal growth factors a viable strategy to prevent proliferative vitreoretinopathy? Prog Retin Eye Res. 2014; 40: 16–34. [DOI] [PubMed] [Google Scholar]
- 35. Lei H,, Hovland P,, Velez G,, et al. A potential role for PDGF-C in experimental and clinical proliferative vitreoretinopathy. Invest Ophthalmol Vis Sci. 2007; 48: 2335–2342. [DOI] [PubMed] [Google Scholar]
- 36. Pennock S,, Rheaume MA,, Mukai S,, Kazlauskas A. A novel strategy to develop therapeutic approaches to prevent proliferative vitreoretinopathy. Am J Pathol. 2011; 179: 2931–2940. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Deyle DR,, Russell DW. Adeno-associated virus vector integration. Curr Opin Mol Ther. 2009; 11: 442–447. [PMC free article] [PubMed] [Google Scholar]
- 38. Pillay S,, Meyer NL,, Puschnik AS,, et al. An essential receptor for adeno-associated virus infection. Nature. 2016; 530: 108–112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Wu W,, Tang L,, D'Amore PA,, Lei H. Application of CRISPR-Cas9 in eye disease. Exp Eye Res. 2017; 161: 116–123. [DOI] [PubMed] [Google Scholar]
- 40. Swiech L,, Heidenreich M,, Banerjee A,, et al. In vivo interrogation of gene function in the mammalian brain using CRISPR-Cas9. Nat Biotechnol. 2015; 33: 102–106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Tabebordbar M,, Zhu K,, Cheng JK,, et al. In vivo gene editing in dystrophic mouse muscle and muscle stem cells. Science. 2016; 351: 407–411. [DOI] [PMC free article] [PubMed] [Google Scholar]


