Abstract
Background
Emerging evidence has shown that downregulation or upregulation of microRNAs (miRNAs) plays an important role in the development and progression of thyroid cancer (TC). However, the potential role of miR-150 and its biological function in TC remains largely unclear.
Material/Methods
Real-time polymerase chain reaction (RT-qPCR) was employed to detect the expression level of miR-150 and RAB11A in human TC tissue and human normal thyroid tissue. MTT assay, colony formation assay, flow cytometry cell cycle, and apoptosis assay were used to investigate the role of miR-150 and RAB11A on the malignant phenotypes in vitro. Nude mouse xenograft assay and western blot assay was used to verify the function of miR-150 in vivo. Western blot assay and immunofluorescence assay were used to detect the activation of WNT/β-catenin pathway mediated by miR-150 and RAB11A. EGFP reporter assay, RT-qPCR assay, and western blot assay were used to validate the regulation relationship.
Results
This study demonstrated that miR-150 expression in human TC tissues was markedly downregulated. Moreover, overexpression of miR-150 markedly inhibited cell proliferation via inducing the cell cycle arrest and promoting cell apoptosis by directly targeting RAB11A in vitro and suppressing tumor growth in vivo. However, overexpression of RAB11A promoted cell malignant phenotypes. In addition, miR-150 restrained the RAB11A mediated WNT/β-catenin activation in TC cells.
Conclusions
miR-150 may function as a suppressor gene in TC cells by inhibiting the RAB11A/WNT/β-catenin pathway.
MeSH Keywords: Apoptosis, Cell Cycle, Cell Growth Processes, MicroRNAs, Wnt Signaling Pathway
Background
Thyroid cancer (TC) is one of the most common malignancies among the endocrine organs [1]; the thyroid is located at the base of the neck [2]. Diverse TCs have been identified and the variability of TCs is also affirmed in molecular, cellular, and clinical characteristics. Some TCs have favorable prognosis, however, aggressive TCs, which were characterized by a less differentiated cellular phenotype and a higher rate of metastasis and cancer-related mortality, have had no effective treatment approach [3]. While the development and progression of TCs are not completely clear, many genetic factors involved in their onset have been reported [4–8]. Increasing evidence has revealed the involvement of microRNAs (miRNAs) in human malignancies, and a number of important direct miRNAs-mRNAs regulations have been described in TCs [9–12].
MiRNAs are a class of short, endogenous single-stranded RNAs that regulate gene expression by base-pairing with target mRNAs, leading to translational repression or mRNA cleavage [13]. During the past decades, many studies have reported that miR-150 is downregulated in juvenile myelomonocytic leukemia [14], human glioma [15], esophageal squamous carcinoma [16], osteosarcoma [17], hepatocellular carcinoma [18], and cholangiocarcinoma [19], suggesting that miR-150 may function as a suppressor gene in cancers. Although miR-150 has been reported to be downregulated in TCs [2,20], its role and underlying functional mechanism is not well studied.
In the present study, we identified that miR-150 was downregulated in TC tissue, and overexpression of miR-150 suppressed cell proliferation in vitro and TC tumor growth in vivo by inducing cell cycle arrest and promoting cell apoptosis. RAB11A was confirmed as a target gene of miR-150 and shown to mediate its tumor suppressor role in TC cells. In addition, miR-150 could inhibit RAB11A mediated WNT/β-catenin activation in TCs cells. Our results provide evidence supporting the tumor suppressor role of miR-150 and suggest a potential mechanism underlying its suppressive function in TCs.
Material and Methods
Materials
Ten pairs of thyroid tissues, consisting of human thyroid cancer tissue and the matched normal thyroid tissue from the same patient were used in this study. The specimens were obtained from patients from the Department of Oncology, Central Hospital of Tai’an City. Written informed consents were obtained from all enrolled patients, and all relevant investigations were performed according to the principles of the declaration of Helsinki. Total RNAs were extracted from the tissue samples and purified using the mirVana miRNA Isolation Kit (Ambion, Austin, TX, USA) according to the manufacturer’s instructions.
Cell lines
Human TC cell lines including K1 and TPC-1 obtained from the American Type Culture Collection (Manassas, VA, USA). K1 and TPC-1 cells were maintained in RPMI 1640 supplemented with 15% FBS, 100 U/mL penicillin, and 100 μg/mL streptomycin in a humidified 5% (v/v) CO2 atmosphere at 37°C.
Plasmid construction
The miR-150 expression vector (pri-miR-150) was amplified from genomic DNA and cloned into the pcDNA3 vector at KpnI and EcoRI sites. The 2′-O-methyl-modified miR-150 antisense oligo nucleotide (ASO-miR-150) was commercially synthesized as an inhibitor of miR-150. The 3′UTRs of RAB11A that contain the miR-150 binding sites and mutant 3′UTR fragments with mutant miR-150 binding sites were obtained by annealing double-strand DNA and inserting it into the pcDNA3/EGFP vector. The pSilencer/shR-RAB11A (shR-RAB11A) plasmid expressing siRNA targeting RAB11A was constructed by annealing double-strand hairpin cDNA and inserting it into the pSilencer 2.1-U6 neo vector (Ambion, Austin, TX, USA) at BamHI and EcoRI sites. The full-length sequence of human RAB11A cDNA (NM_004663.4) was obtained by RT-PCR and was cloned into pcDNA3 at KpnI and XhoI sites. The resulting plasmid was termed pRAB11A. All primers for PCR were followed by: Pri-miR-150-Foward, 5′CGGGGTACCGGTATAAGGCAGGGACTGGG3′; Pri-miR-150-Reverse, 5′ CCGGAATTCGGATATGTGGGACCCGAAGG3′; ASO-miR-150, 5′CACUGGUACAAGGGUUGGGAGA3′; ASO-NC, 5′CAGUACUUUUGUGUAGUACAA3′; RAB11A-3UTR-top, 5′GATCCATGGCTGGATCTTGGGAGAAAGCTTG3′; RAB11A-3UTR-bot, 5′AATTCAAGCTTTCTCCCAAGATCCAGCCATG3′; RAB11A-3UTR-mut-top, 5′GATCCATGGCTGGATCCCAGACAGAAGCTTG3′; RAB11A-3UTR-mut-bot, 5′AATTCAAGCTTCCAGACAGAAGCTTG3′; RAB11A-qPCR-Forward, 5′ CGGGGTACCGCCACCATGGGCA CCCGCGACGACGAG3′; RAB11A-qPCR-Reverse, 5′ CCGCT CGAGTTAGATGTTCTGACAGCACTGCACC3′; shR-RAB11A-S, 5′GATCCGCCTTATTGGT TTATGACATTCTCGAGAA TGTCATAA ACCAATAAGTTTTTGA3′; shR-RAB11A-AS, 5′AGCTTCAAAAA GCCTTATTGGTTTATGACATTCTC GAGAATGTCATAAACCAATAAGG3′.
MTT assay
For cell proliferation, K1 and TPC-1 cells that were treated with pri-miR-150 or ASO-miR-150 and the respective controls were seeded into 96-well plates (4,500 cells per well). Cell viability at 0, 24, 48, and 72 hours post-transfection was determined by MTT assay. Absorbance was measured at a wave length of 570 nm using Benchmark Plus™ microplate spectrometer (Bio-Rad Laboratories Inc.).
Colony formation assay
For the colony formation assay, the TC cells were counted at 24 hour post-transfection and seeded into 12-well plates in triplicate at a density of 500 cells per well. Culture medium was replaced every 72 hour. These plates were incubated at 37°C for two weeks and the cells were stained with crystal violet. Colonies containing 50 cells or more were counted.
EGFP reporter assay
Cells were seeded in 48-well plates one day before transfection and then co-transfected with pri-miR-150 or ASO-miR-150 and pEGFP-RAB11A 3′UTRor pEGFP-RAB11A3′UTR-mut. After transfection for 48 hours, the cells were lysed using radio-immunoprecipitation assay (RIPA) lysis buffer, and the EGFP intensities were measured with a fluorescence spectrophotometer (F4500, Hitachi, Tokyo, Japan).
RT-qPCR assay
First-strand cDNA was generated through reverse transcription of total cellular RNA using M-MLV reverse transcriptase (Promega, Madison, WI, USA). The SYBR Premix Ex Taq™ Kit (TaKaRa, Shiga, Japan) was used according to the manufacturer’s instructions, and qPCR was performed and analyzed using the iQ5 Detection System (Bio-Rad, CA, USA). All the primers were followed by: miR-150-RT, 5′GTCGTATCCAGTGCAGGGTCCGAG GTGCACTGGATACGACCACTGGT3′; miR-150-qPCR-Foward, 5′TGCGGTCTCCCAACCCTTG3′; U6-RT: 5′GTCGTATCCAGT GCAGGGTCCGAGGTATTCGCACTGGATACGACAAAATATGGAAC3′; U6-Foward, 5′TGCGGGTGCTCGCTTCGGCAGC3′; Oligo dT, 5′TTTTTTTTTTTTTTTTTT3′; Universal reverse primer, 5′CCAGTGCAGGGTCCGAGGT3′; β-actin-qPCR-Foward, 5′CGTGACATTAAGGAGAAGCTG3′; β-actin-qPCR-Reverse, 5′CTAGAAGCATTTGCGGTGGAC3′; RAB11A-qPCR-Forward, 5′GGCTCTTGTGGTCATTTC3′; RAB11A-qPCR-Reverse, 5′CCCATCAAGTGCTCCTCT3′.
Western blot analysis
Before protein extraction, TC cells were washed with 1*PBS to remove the culture media. Cells were collected and lysed with RIPA lysis buffer at 4°C, and lysates were centrifuged at 12,000 rpm for five minutes at 4°C. Forty micrograms of cellular proteins were separated by 12% SDS-PAGE, and were transferred to PVDF membrane (Millipore, Bedford, MA, USA). After blocking with 5% nonfat milk at room temperature for two hours, the membranes were probed with primary antibodies at 4°C overnight. Then, the membranes were incubated with corresponding secondary antibodies at room temperature for two hours. The primary antibodies used in this study included RAB11A (Abcam, Cambridge, UK) and GAPDH (Abcam, Cambridge, UK), and the secondary goat anti-rabbit antibody was obtained from Sigma-Aldrich (St. Louis, MO, USA).
Cell cycle and apoptosis flow cytometry analyses
These analyses were conducted according to the protocol provided by Tianjin Sanjian Biotech (http://www.sungenebiotech.com).
In vivo tumor xenograft studies
All animal experiments in this study were approved by the ethics committee of Shandong University, and the guidelines of National Animal Care and Use Committee were followed. TPC-1 cells (200 μL, 1×107 cells) transfected with pri-miR-150 or pcDNA3 were injected into the left and right flank regions of six-week-old male nude mice (Institute of Zoology, Chinese Academy of Sciences, Shanghai, China). Mice were euthanized four weeks after injection and tumor nodules were removed and weighted.
Immunofluorescence
Immunochemical staining was carried out following the manufacturer’s instructions. Briefly, cells on coverslips were fixed with 4% paraformaldehyde at 25°C for 30 minutes and then washed with PBS. Cells were incubated for 10 minutes with PBS containing 10% bovine serum albumin (BSA), washed once with PBS containing 0.2% Triton X-100, and incubated with a primary antibody specific to β-catenin at the proper dilution (1: 2,000, Santa Cruz Biotechnology, Inc.) for 60 minutes at 37°C. Cells were then rinsed three times with PBS and incubated with the corresponding FITC conjugated secondary antibody (Santa Cruz Biotechnology, Inc.) for 60 minutes at 37°C. Cells were rinsed three times with PBS and incubated in 4, 6-diamino-2-phenylindole (DAPI) for 10 minutes to visualize nuclei. Cells were again rinsed three times with PBS before observation.
Statistical analysis
Data are presented as the means ± standard deviation (SD) from at least three independent experiments. Student’s t-test was used to compare differences between two groups; p<0.05 was considered statistically significant: * p<0.05; ** p<0.01;*** p<0.001.
Results
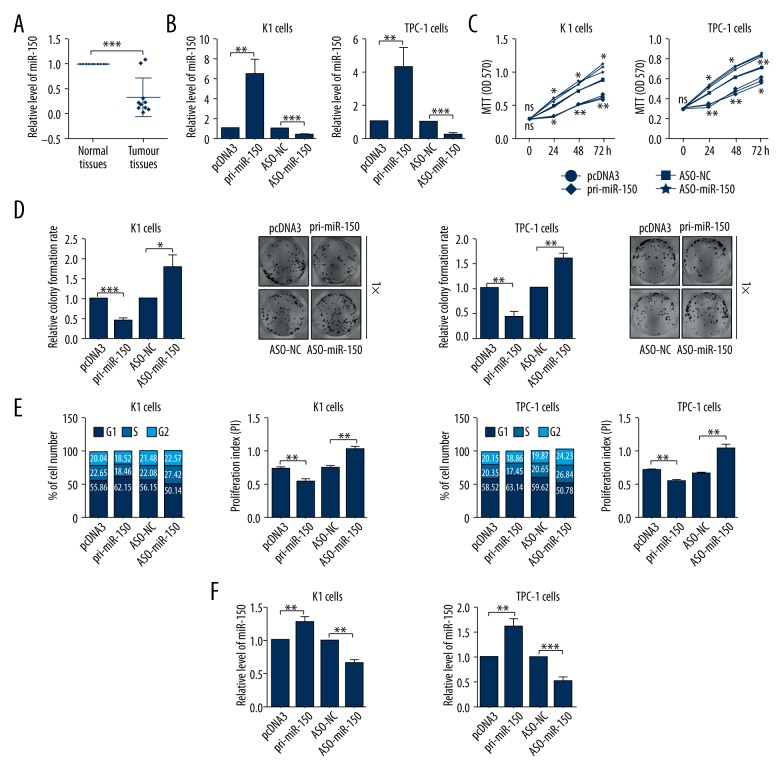
MiR-150 is downregulated in TC tissues
To evaluate the role of miR-150 in TC, we first detected the expression level of miR-150 in 10 pairs of human TC tissues and the matched normal tissues by RT-qPCR assays. As shown in Figure 1A, miR-150 expression levels were significantly downregulated in human TC tissues compared with matched normal tissues (Figure 1A).
Figure 1.
MiR-150 was downregulated in thyroid cancer (TC) tissues and inhibited TC cell proliferation. (A) The expression level of miR-150 in thyroid tissues was determined by RT-qPCR assays. (B) The efficiency of pri-miR-150 expression plasmid or ASO-miR-150 was confirmed by RT-qPCR assays in TC cells. (C) The impacts of miR-150 on K1 and TPC-1 cellular viabilities were determined by MTT assays. (D) The relative colony formation rate of K1 and TPC-1 cells with indicated treatment was determined by colony formation assays. (E) The impacts of miR-150 on cell cycle of K1 and TPC-1 cells were evaluated by flow cytometry cell cycle assays. (F) The impacts of miR-150 on cell apoptosis of K1 and TPC-1 cells were evaluated by flow cytometry cell apoptosis assays; * p<0.05; ** p<0.01; *** p<0.001.
MiR-150 inhibits TC cell proliferation in vitro
To investigate the role of miR-150 in TC, we first modulated miR-150 expression level by transient transfection with pri-miR-150 and ASO-miR-150. The upregulation and downregulation of miR-150 in K1 and TPC-1 cells were identified by RT-qPCR assays (Figure 1B). Then, we measured cellular viability using MTT assays after cells were transfected with pri-miR-150 or ASO-miR-150 for 0, 24, 48, and 72 hours. MTT assays demonstrated that miR-150 overexpression significantly inhibited cell viability in K1 and TPC-1 cells and miR-150 knockdown promoted cell proliferation in K1 and TPC-1 cells compared with negative control cells (Figure 1C). The effect of miR-150 on the colony formation abilities of K1 and TPC-1 cells was characterized by a colony formation assay, which showed that overexpression of miR-150 could significantly decrease the relative colony formation rates and knockdown of miR-150 could promote the relative colony formation rates (Figure 1D). The cell cycle was analyzed using flow cytometry cell cycle assay, and the results showed that overexpression of miR-150 induced G1/S arrest and knockdown of miR-150 accelerated the cell cycle process of K1 and TPC-1 cells (Figure 1E). As shown in Figure 1F, cell apoptosis determined by flow cytometry cell apoptosis analysis in pri-miR-150 transfected cells was significantly promoted when compared with the control groups, and knockdown of miR-150 had the opposite effect (Figure 1F). Together, these data indicate that miR-150 was associated with suppressor gene on TC cells.
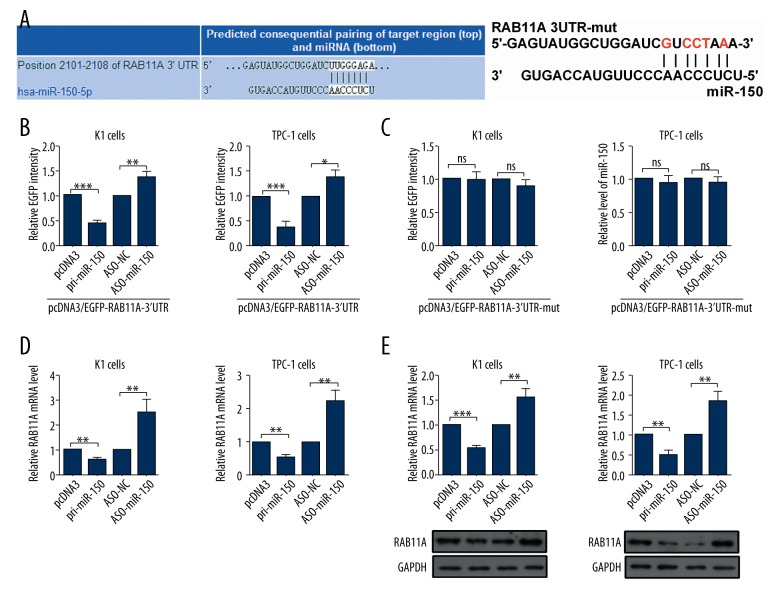
RAB11A is a novel directly target gene of miR-150 in TC cells
To find the underlying mechanisms of miR-150 in TC, we investigated potential targets of miR-150 via the prediction software of TargetScan human 7.1, miRbase and miRanda. Bioinformatic analyses predicted that RAB11A was a hypothetic target gene of miR-150 (Figure 2A). To identify the functional significance of the hypothesis, the 3′UTR of RAB11A mRNA sequences containing putative binding sites of the wild type or mutational type for the seed matching sites were introduced into a EGFP reporter assay and the results showed that pri-miR-150 remarkably reduced and ASO-miR-150 increased the EGFP activity of the RAB11A 3′UTR (Figure 2B), while the EGFP activity of RAB11A 3′UTR-mut in K1 and TPC-1 cells was unchanged (Figure 2C). To further investigate the relationship between miR-150 and RAB11A, we detected the effects of miR-150 on RAB11A mRNA by RT-qPCR assays and protein levels by western blot assays. RT-qPCR assays showed that RAB11A mRNA levels were decreased in K1 and TPC-1 cells transfected with pri-miR-150 (Figure 2D). Furthermore, western blot assays showed a similar relationship: overexpression of miR-150 reduced the protein expression levels of RAB11A (Figure 2E). All the above results demonstrated that RAB11A was a directly target gene of miR-150, and was negative regulated by miR-150 in TC cells.
Figure 2.
MiR-150 negative regulated the expression of RAB11A in thyroid cells. (A) Targeting sites of miR-150 shown in human RAB11A mRNA 3′ UTRs used TargetScan Human 7.1 and the mutational 3′ UTRs of RAB11A. (B, C) EGFP intensity of K1 and TPC-1 cells with indicating treatment was determined by spectrophotometer, and the value of control group (pcDNA3 or ASO-NC) was set to one. (D) RAB11A mRNA levels in K1 and TPC-1 cells with indicating treatment were measured by RT-qPCR assays. (E) RAB11A protein levels in K1 and TPC-1 cells transfected with pri-miR-150 or ASO-miR-150 and respective controls were determined by western blot assays; ns, not significant; * p<0.05; ** p<0.01; *** p<0.001.
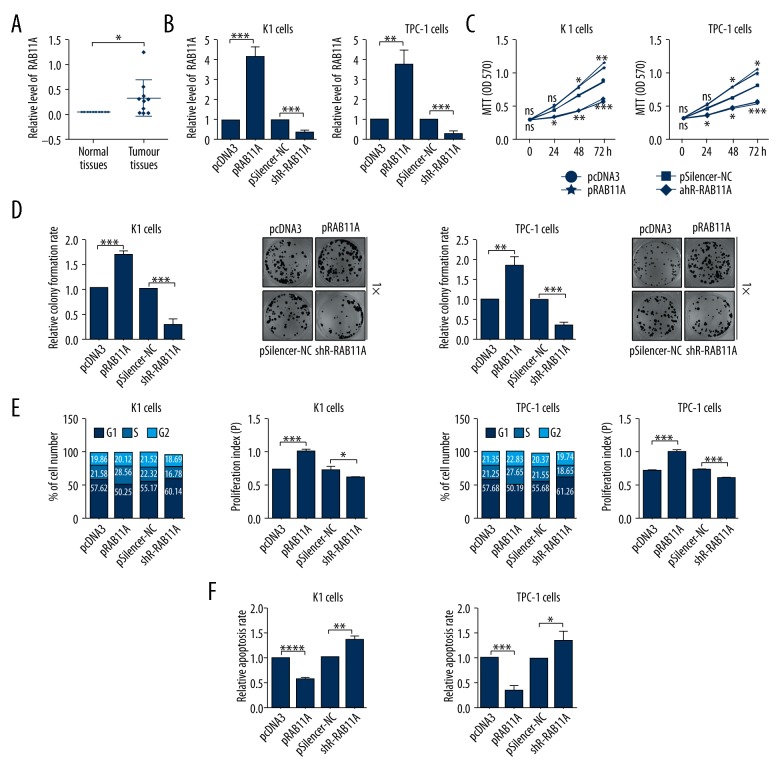
RAB11A promotes TC malignant phenotype in vitro
To confirm the aforementioned findings, the expression levels of RAB11A in human TC tissues were detected by RT-qPCR assay. RT-qPCR analysis showed that the mRNA expression levels of RAB11A were increased in TC tissues compared with the tumor adjacent tissues (Figure 3A). To investigate the effect of RAB11A on TC cell proliferation, we used the pRAB11A or shR-RAB11A and the control plasmids to transfect K1 and TPC-1 cells. The results showed that the expression levels of RAB11A were remarkably upregulated after pRAB11A transfection and the expression levels of RAB11A were markedly downregulated after shR-RAB11A transfection in K1 and TPC-1 cells (Figure 3B). MTT assays demonstrated that overexpression of RAB11A significantly increased cell viability of K1 and TPC-1 cells and knockdown of RAB11A decreased cell viability of K1 and TPC-1 cells compared with negative control groups (Figure 3C). Colony formation assays showed that overexpression of RAB11A could significantly elevate the relative colony formation rate and knockdown of RAB11A could reduce the relative colony formation rate in K1 and TPC-1 cells (Figure 3D). The cell cycle analysis used flow cytometry assay showed that pRAB11A-transfected accelerated the cell cycle process and shR-RAB11A transfected induced G1/S arrest in K1 and TPC-1 cells (Figure 3E). As shown in Figure 3F, cell apoptosis rate used flow cytometry analysis in pRAB11A transfected cells was significantly decreased when compared with the control groups, and shR-RAB11A transfected cells had the opposite effect (Figure 3F). In short, high expression of RAB11A promoted TC malignant phenotype in K1 and TPC-1 cells.
Figure 3.
RAB11A promoted TC malignant phenotype in vitro. (A) The expression level of RAB11A in thyroid tissues was determined by RT-qPCR assays. (B) The efficiency of RAB11A overexpression plasmid or knockdown plasmid was confirmed by RT-qPCR assays. (C) The impacts of RAB11A on K1 and TPC-1 cellular viabilities were determined by MTT assays. (D) The relative colony formation rate of K1 and TPC-1 cells with indicated treatment were determined by colony formation assays. (E) The impacts of RAB11A on cell cycle of K1 and TPC-1 cells were evaluated by flow cytometry cell cycle assays. (F) The impacts of RAB11A on cell apoptosis of K1 and TPC-1 cells were evaluated by flow cytometry cell apoptosis assays; * p<0.05; ** p<0.01; *** p<0.001.
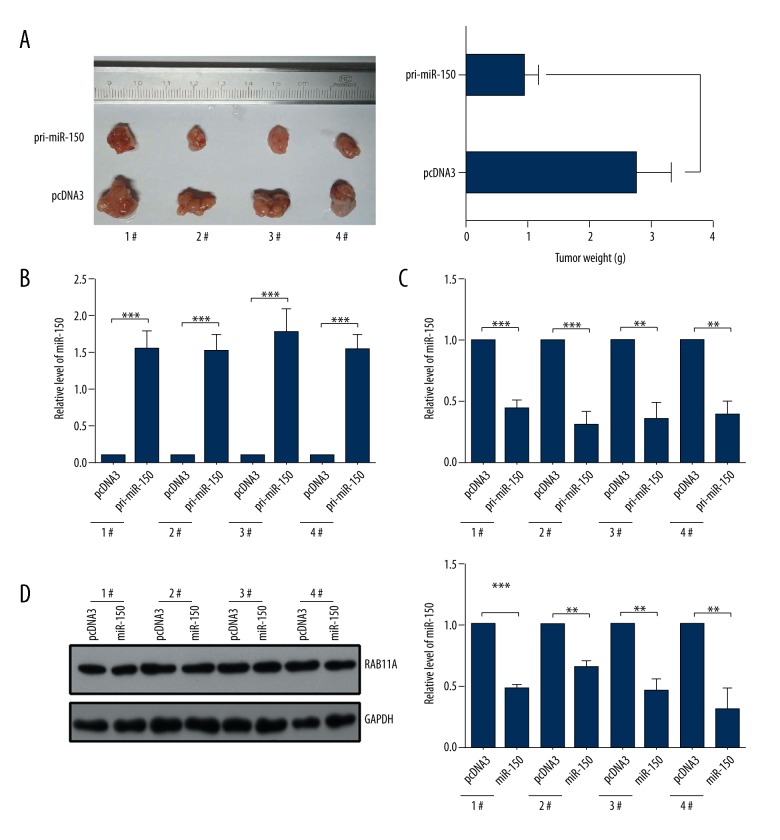
Overexpression of miR-150 inhibits the growth of K1-engrafted tumors in vivo
To further explore the role of miR-150 on tumor growth in vivo, we injected K1 cells of pcDNA3 transfected and pri-miR-150 transfected into nude mice. As shown in Figure 4A, tumors grew slower in the pri-miR-150 transfected group than in the control groups (Figure 4A). In addition, we detected the expression levels of miR-150 and RAB11A by RT-qPCR assays in K1-engrafted tumors. The results showed that the expression levels of miR-150 in the pri-miR-150 transfected groups were higher than in the pcDNA3 transfected groups (Figure 4B), and the expression levels of RAB11A in the pri-miR-150 transfected groups were markedly downregulated compared with the negative control groups (Figure 4C). Finally, we examined the RAB11A protein levels by western blot assay in K1-engrafted tumors and the results showed that the expression level of RAB11A protein was lower in the pri-miR-150-transfected groups (Figure 4D). In general, overexpression of miR-150 inhibited the tumors growth in vivo.
Figure 4.
MiR-150 inhibited the growth of K1-engrafted tumors by downregulating the expression of RAB11A in vivo. (A) The pri-miR-150 treatment markedly repressed the growth of xenograft thyroid cancers derived from K-1 cells. (B) The expression level of miR-150 in xenograft was detected by RT-qPCR assays. (C) The mRNA level of RAB11A in xenograft was detected by RT-qPCR assays. (D) The protein level of RAB11A in xenograft was detected by western blot assays; * p<0.05; ** p<0.01;*** p<0.001.
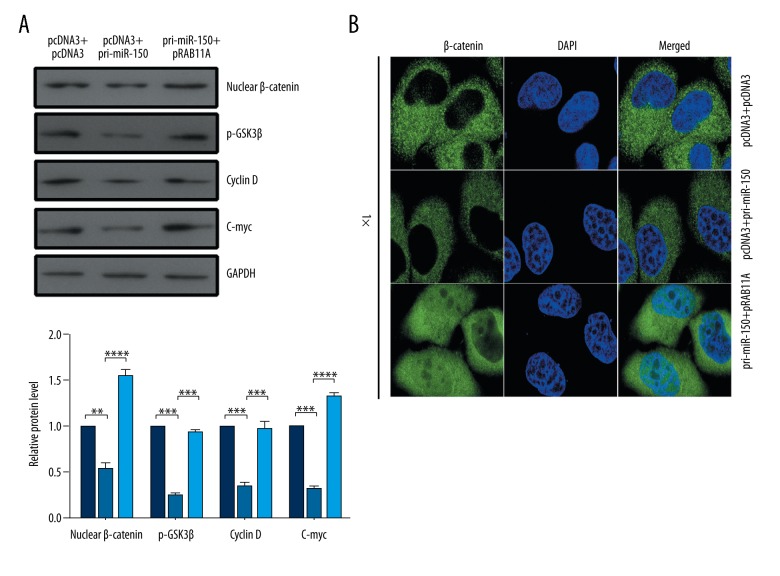
Upregulation of RAB11A partially attenuates the effect of miR-150 on WNT/β-catenin pathway in TC cells
To address the mechanism of miR-150 inhibiting tumor growth, the expression of WNT gene and the activity of β-catenin were analyzed in the context of miR-150 overexpression or miR-150 overexpression in conjunction with pRAB11A in TC cells. Western blot assays showed that the expression of the nuclear β-catenin, p-GSK3β, C-myc, and cyclin D, which are the effectors of the WNT/β-catenin pathway, were reduced by pri-miR-150 treatment and significantly increased by RAB11A restoration (Figure 5A). The localization of β-catenin in K1 cells was examined by immunofluorescence after transfection with pri-miR-150 or pRAB11A or co-transfection with pri-miR-150 and pRAB11A. The nuclear distribution of β-catenin was reduced in the pri-miR-150-transfected cells and increased in the pRAB11A-restored cells (Figure 5B).
Figure 5.
MiR-150 negatively regulated the WNT/β-catenin pathway. (A, B) K1 cells were transfected with the indicated combinations of pcDNA3 and pcDNA3, pcDNA3 and pri-miR-150, pri-miR-150, and pRAB11A. Western blot assay (A) was used to detect the expression level of β-catenin, p-GSK3β, C-myc, and cyclin D. Immunofluorescence assay (B) was used to examine the nuclear distribution of β-catenin; * p<0.05; ** p<0.01; *** p<0.001.
Discussion
MiR-150 is located in the genomic region of chromosome 19q13. MiRNA expression profiles have highlighted that miR-150 downregulation was a common appearance in human malignancies. For example, Qin et al. reported that overexpression of miR-150 enhanced apoptosis via targeting ELK1 in endothelial cells, whereas knockdown of miR-150 could partly alleviate apoptotic cell death mediated by ox-LDL via targeting ELK1 [21]; in addition, Li et al. reported that exogenous miR-150 inhibited cell proliferation, invasion, and metastasis and stimulated cell apoptosis by regulating the expression of Sp1 in human osteoblast cells [22]. Moreover, Li et al. reported that the expression levels of miR-150 were also decreased in metastatic cancer tissues compared with pair primary tissues, indicating that miR-150-5p may be involved in HCC metastasis [23]. Although miR-150 has been identified to function as a suppressor gene in many cancers, it has also been shown to act as an onco-miRNA in human non-small cell lung cancer [24]. Thus, to investigate the exact functions of miR-150 in TCs development and progression is of significant importance.
Some researchers have demonstrated that miR-150 may be downregulated in TCs [2,20], however, the role and function of miR-150 in TCs remains unclear. In this study, we found that miR-150 was downregulated in TC tissues, and miR-150 suppressed cell proliferation in vitro and tumor growth in vivo by inducing cell cycle arrest and promoting cell apoptosis in K1 and TPC-1 cells. Based on the results of online predictions and experimental analyses, we predicted and identified that RAB11A was a functional target of miR-150 in human TC cells, which was targeted and downregulated by miR-150.
RAB11A belongs to the Rab family of the small GTPase superfamily, which plays a role in the process of protein transport, and is involved in many cellular processes including phagocytosis and cell migration [25]. Previously, RAB11A was shown to be overexpressed in breast cancer [26], pancreatic cancer [27], and bladder cancer [28], suggesting that RAB11A is important in human cancer development. However, the expression level and role of RAB11A in TCs has not been investigated. Our results showed that the expression level of RAB11A in TC tissues was markedly upregulated, and overexpression of RAB11A promoted TCs malignant phenotype. In addition, Yu et al. demonstrated that RAB11A promotes aggressiveness of pancreatic cancer by activing GSK3β/WNT/β-catenin signaling pathway [29]. Our present results also indicated that miR-150 inhibited the WNT/β-catenin pathway activation and upregulation of RAB11A partially attenuates the effect of miR-150 on WNT/β-catenin pathway in TC cells.
Conclusions
In brief, all our findings demonstrated that the miR-150/RAB11A/WNT/β-catenin axis may be involved in the development and progression of TCs. Collectively these findings may provide insight into tumorigenesis and a potential biomarker for TC.
Footnotes
Conflict of interest
None.
Source of support: This work was partially supported by the National Natural Science Foundation of China (Nos.81272181)
References
- 1.Cabanillas ME, Mcfadden DG, Durante C. Thyroid cancer. Lancet. 2016;388(10061):2783–95. doi: 10.1016/S0140-6736(16)30172-6. [DOI] [PubMed] [Google Scholar]
- 2.Forte S, La RC, Pecce V, et al. The role of microRNAs in thyroid carcinomas. Anticancer Res. 2015;35(4):2037–47. [PubMed] [Google Scholar]
- 3.Kondo T, Ezzat S, Asa SL. Pathogenetic mechanisms in thyroid follicular-cell neoplasia. Nat Rev Cancer. 2006;6(4):292–306. doi: 10.1038/nrc1836. [DOI] [PubMed] [Google Scholar]
- 4.Carr FE, Tai PW, Barnum MS, et al. Thyroid hormone receptor β (TRβ) mediates runt-related transcription factor 2 (Runx2) expression in thyroid cancer cells: A novel signaling pathway in thyroid cancer. Endocrinology. 2016;157(8):en20152046. doi: 10.1210/en.2015-2046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Li S, Zhang HY, Du ZX, et al. Induction of epithelial-mesenchymal transition (EMT) by Beclin 1 knockdown via posttranscriptional upregulation of ZEB1 in thyroid cancer cells. Oncotarget. 2016;7(43):70364–77. doi: 10.18632/oncotarget.12217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Verrienti A, Tallini G, Colato C, et al. RET mutation and increased angiogenesis in medullary thyroid carcinomas. Endocr Relat Cancer. 2016;23(8):665–76. doi: 10.1530/ERC-16-0132. [DOI] [PubMed] [Google Scholar]
- 7.Weinberger P, Ponny SR, Xu H, et al. Cell cvycle M-phase genes are highly upregulated in anaplastic thyroid carcinoma. Thyroid. 2017;7(2):236–52. doi: 10.1089/thy.2016.0285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Zhu X, Kim DW, Zhao L, et al. SAHA-induced loss of tumor suppressor Pten gene promotes thyroid carcinogenesis in a mouse model. Endocr Relat Cancer. 2016;23(7):521–33. doi: 10.1530/ERC-16-0103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Aragon HP, Weng CH, Khawaja HT, et al. MicroRNA expression and association with clinicopathologic features in papillary thyroid cancer: a systematic review. Thyroid. 2015;25(12):1322–29. doi: 10.1089/thy.2015.0193. [DOI] [PubMed] [Google Scholar]
- 10.Boufraqech M, Klubo-Gwiezdzinska J, Kebebew E. MicroRNAs in the thyroid. Best Pract Res Clin Endocrinol Metab. 2016;30(5):603–19. doi: 10.1016/j.beem.2016.10.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Sun D, Han S, Liu C, et al. Microrna-199a-5p functions as a tumor suppressor via suppressing connective tissue growth factor (CTGF) in follicular thyroid carcinoma. Med Sci Monit. 2016;22:1210–17. doi: 10.12659/MSM.895788. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Titov SE, Ivanov MK, Karpinskaya EV, et al. miRNA profiling, detection of BRAF V600E mutation and RET-PTC1 translocation in patients from Novosibirsk oblast (Russia) with different types of thyroid tumors. BMC Cancer. 2016;16(1):1–15. doi: 10.1186/s12885-016-2240-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Davisdusenbery BN, Hata A. Mechanisms of control of microRNA biogenesis. J Biochem. 2010;148(4):381–92. doi: 10.1093/jb/mvq096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Leoncini PP, Bertaina A, Papaioannou D, et al. MicroRNA fingerprints in juvenile myelomonocytic leukemia (JMML) identified miR-150-5p as a tumor suppressor and potential target for treatment. Oncotarget. 2016;7(34):55395–408. doi: 10.18632/oncotarget.10577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Saiselet M, Pita JM, Augenlicht A, et al. miRNA expression and function in thyroid carcinomas: A comparative and critical analysis and a model for other cancers. Oncotarget. 2016;7(32):52475–92. doi: 10.18632/oncotarget.9655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Wang X, Ren Y, Wang Z, et al. Down-regulation of 5S rRNA by miR-150 and miR-383 enhances c-Myc-rpL11 interaction and inhibits proliferation of esophageal squamous carcinoma cells. FEBS Lett. 2015;589(24):3989–97. doi: 10.1016/j.febslet.2015.11.012. [DOI] [PubMed] [Google Scholar]
- 17.Yang Q, Pan S, Kang M, et al. MicroRNA-150 functions as a tumor suppressor in osteosarcoma by targeting IGF2BP1. Tumor Biol. 2016;37(4):5275–84. doi: 10.1007/s13277-015-4389-8. [DOI] [PubMed] [Google Scholar]
- 18.Sun W, Zhang Z, Wang J, et al. MicroRNA-150 suppresses cell proliferation and metastasis in hepatocellular carcinoma by inhibiting the GAB1-ERK axis. Oncotarget. 2016;7(10):11595–608. doi: 10.18632/oncotarget.7292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Wu X, Xia M, Chen D, et al. Profiling of downregulated blood-circulating miR-150-5p as a novel tumor marker for cholangiocarcinoma. Tumour Biol. 2016;37(11):15019–29. doi: 10.1007/s13277-016-5313-6. [DOI] [PubMed] [Google Scholar]
- 20.Mancikova V, Castelblanco E, Pineiroyanez E, et al. MicroRNA deep-sequencing reveals master regulators of follicular and papillary thyroid tumors. Mod Pathol. 2015;28(6):748–57. doi: 10.1038/modpathol.2015.44. [DOI] [PubMed] [Google Scholar]
- 21.Qin B, Shu Y, Xiao L, et al. MicroRNA-150 targets ELK1 and modulates the apoptosis induced by ox-LDL in endothelial cells. Mol Cell Biochem. 2017;429(1–2):45–58. doi: 10.1007/s11010-016-2935-3. [DOI] [PubMed] [Google Scholar]
- 22.Li X, Chen L, Wang W, et al. MicroRNA-150 inhibits cell invasion and migration and is downregulated in human osteosarcoma. Cytogenet Genome Res. 2015;146(2):124–35. doi: 10.1159/000437379. [DOI] [PubMed] [Google Scholar]
- 23.Li T, Xie J, Shen C, et al. miR-150-5p inhibits hepatoma cell migration and invasion by targeting MMP14. PLoS One. 2014;9(12):e115577. doi: 10.1371/journal.pone.0115577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Hui L, Ouyang R, Zi W, et al. MiR-150 promotes cellular metastasis in non-small cell lung cancer by targeting FOXO4. Sci Rep. 2016;6:39001. doi: 10.1038/srep39001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kelly EE, Horgan CP, Mccaffrey MW. Rab11 proteins in health and disease. Biochem Soc Trans. 2012;40(6):1360–67. doi: 10.1042/BST20120157. [DOI] [PubMed] [Google Scholar]
- 26.Wang B, Yang Z, Wang H, et al. MicroRNA-320a inhibits proliferation and invasion of breast cancer cells by targeting RAB11A. Am J Cancer Res. 2015;5(9):2719–29. [PMC free article] [PubMed] [Google Scholar]
- 27.Li Y, Xin L, Li H, et al. Rab11a sustains GSK3β/WNT/β-catenin signaling to enhance cancer progression in pancreatic cancer. Tumor Biol. 2016;37(10):13821–29. doi: 10.1007/s13277-016-5172-1. [DOI] [PubMed] [Google Scholar]
- 28.Ho JR, Chapeaublanc E, Kirkwood L, et al. Deregulation of Rab and Rab effector genes in bladder cancer. PLoS One. 2012;7(6):e39469. doi: 10.1371/journal.pone.0039469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Yu L, Li X, Li H, et al. Rab11a sustains GSK3β/WNT/β-catenin signaling to enhance cancer progression in pancreatic cancer. Tumour Biol. 2016;37(10):13821–29. doi: 10.1007/s13277-016-5172-1. [DOI] [PubMed] [Google Scholar]