Abstract
Pancreatic cancer is classified as ductal, acinar, neuroendocrine carcinoma or pancreatoblastoma. Ductal and acinar cells derive from exocrine glands and neuroendocrine cells from endocrine glands; however, mixed acinar-neuroendocrine-ductal carcinoma has different histological carcinomas coexisting within a nodule. The mixed pancreatic carcinoma forms from different developmental origins and therefore requires investigation. The current case report presents a 50-year-old male who had a tumor within the body of the pancreas. Pathological examination clarified the tumor as a mixed acinar-neuroendocrine-ductal carcinoma. The ductal and acinar/neuroendocrine tumor components were isolated using laser-capture microdissection, and next-generation sequencing analysis was performed. Consequently, TP53 frameshift (p.N210fs) and KRAS missense (p.G12R) mutations were identified in both ductal and acinar/neuroendocrine tumors. These results suggested a pancreatic mixed acinar-neuroendocrine-ductal carcinoma was derived from a founder tumor clone, and supports the notion that a founder tumor clone may differentiate and transform into a diverse histological type and form a pancreatic mixed carcinoma.
Keywords: acinar, ductal, mixed carcinoma, neuroendocrine, pancreatic carcinoma, next generation sequencing
Introduction
Pancreatic cancer is one of the most lethal malignancies. Approximately 35,000 cases were reported in Japan in 2014, of whom 32,000 died, and the 5-year survival rate has been estimated as 7% (1). Malignant neoplasms of the pancreas are classified based on the cellular direction of differentiation into ductal, acinar, or neuroendocrine carcinomas (NEC), or pancreatoblastoma (2). Pancreatic ductal adenocarcinomas comprise about 90% of cases, NEC comprise 5%, while acinar cell carcinomas (ACC) are the rarest at 1–2% (3). Although most pancreatic tumors arise as a single cell type, either from the endocrine or exocrine pancreas, mixed neoplasms are listed under WHO classifications as carcinomas with mixed differentiation including mixed acinar-neuroendocrine carcinoma, mixed acinar-ductal carcinoma, and mixed acinar-neuroendocrine-ductal carcinoma (4). These mixed carcinomas are extremely rare, so their clinical and genomic features are poorly understood. Furthermore, it is not clear whether mixed carcinomas derive from the same or distinct tumor clones within a nodule.
Here, we report a case of mixed acinar-neuroendocrine-ductal carcinoma for which we performed genetic analysis. We describe the pathological, immunohistochemical, and molecular characterizations of a pancreatic mixed acinar-neuroendocrine-ductal carcinoma.
Subjects and methods
Patient and sample preparation
Written informed consent for the research study and publication was obtained from this case, which was performed in accordance with the protocols approved by the Institutional Review Board at our hospital. The study complied with Declaration of Helsinki principles and its later amendments ethical standards. Peripheral blood samples were obtained and buffy coats were isolated. Buffy coat DNA was extracted using the QIAamp DNA blood mini QIAcube kit (Qiagen, Hilden, Germany) (5).
Immunohistochemical analysis and laser-capture microdissection
The sections were deparaffinized before the antigens were retrieved by heat treatment in an EDTA solution at pH 8.0. Protein expression was evaluated using 3-µm-thick formalin-fixed and paraffin-embedded (FFPE) sections with anti-chromogranin A (1:50 dilution; clone 5H7, NCL-CHROM-430; Novocastra, Newcastle, UK), anti-trypsin (1:400 dilution; Meridian Life Science, Memphis, TN, USA), anti-mucin 1 (MUC1) (1:50, clone DF-6; Novocastra), anti-Ki67 (ready-to-use, clone SP6, Nichirei, Tokyo, Japan), anti-TP53 (1:200, clone DO7; Leica Biosystems, Wetzlar, Germany) antibodies using using the Ventana BenchMark ULTRA system (Roche, Tucson, AZ, USA). A serial section of 10-µm FFPE tissue was stained with haematoxylin-eosin and then microdissected using an ArcturusXT laser-capture microdissection system (Thermo Fisher Scientific, Inc., Waltham, MA, USA). The ductal component and mixed NEC/ACC component were microdissected from FFPE tumor tissue. FFPE DNA was extracted using the QIAamp DNA FFPE Tissue Kit (Qiagen) (6).
Next-generation sequencing
Tumor and buffy coat samples were subjected to next-generation sequencing analysis using the Ion AmpliSeq™ Cancer Hotspot Panel v2 (Thermo Fisher Scientific, Inc.), targeting the hotspot regions of 50 oncogenes and tumor suppressor genes. Sequencing library was prepared by multiplex PCR with Ion AmpliSeq Library kit 2.0 (Thermo Fisher Scientific, Inc.) as previously before (6). Variants calling and annotations were performed using an Ion Reporter Server System (Thermo Fisher Scientific, Inc.), and buffy coat DNA was used as a control to detect variants in tumors by ‘AmpliSeq CHPv2 tumor-normal pair’ workflow.
Results
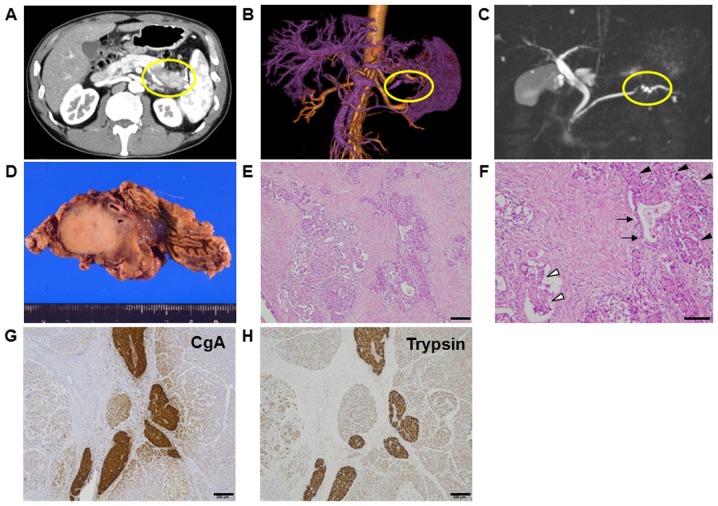
A 50-year-old male was referred to our hospital with intermittent symptoms of epigastralgia and back pain. He had no history of smoking or drinking, and underwent an appendectomy at 10 years of age. Physical examination revealed no anaemia or icteric findings. No tumor was palpable, but slight tenderness was felt at the epigastrium without rebound tenderness. Laboratory data showed slight elevation of amylase at 452 U/ml and lipase at 403 U/l, while serum tumor markers were not elevated: carcinoembryonic antigen (CEA) level at 1.0 ng/ml, carbohydrate antigen 19-9 (CA19-9) levels at 20.3 U/ml, Dupan-2 at <25 U/ml, and Span-1 at <11 U/ml. Abdominal computed tomography revealed a tumor measuring 3 cm in diameter within the body of the pancreas (Fig. 1A). Tumor enhancement from the splenic artery was indicated by magnetic resonance angiography (MRA) (Fig. 1B), and stenosis of the pancreatic duct was detected by magnetic resonance cholangiopancreatography (MRCP) (Fig. 1C). He was diagnosed with pancreatic cancer and underwent distal pancreatectomy with splenectomy. Gross findings showed a round, hard tumor with a serrated margin measuring 3 cm in diameter in the body of the pancreas, (Fig. 1D). The postoperative course was uneventful for 1 year.
Figure 1.
(A) Abdominal computed tomography revealing a tumor 3 cm in diameter in the body of the pancreas. (B) Tumor enhancement from the splenic artery was indicated by magnetic resonance angiography. (C) Stenosis of the pancreatic duct was observed by magnetic resonance cholangiopancreatography. (D) Gross findings showed a round, hard tumor with a serrated margin in the body of the pancreas. The tumor measured 3 cm in diameter and was growing within the pancreatic parenchyma. (E-H) Pathologic examination revealed a mixed pancreatic carcinoma. Representative image of haematoxylin and eosin staining with (E) low-power and (F) high-power magnification. Cellular morphology indicated tumor components of ductal carcinoma (arrow), acinar cell carcinomas (ACC; black arrow head), and neuroendocrine carcinomas (NEC; white arrow head) in (F). Immunohistochemical analysis showed positive staining for chromogranin A (CgA) as a marker of (G) NEC and trypsin as a marker of (H) ACC. Scale bars, 200 µm for (E, G and H); 100 µm for (F).
Pathological findings
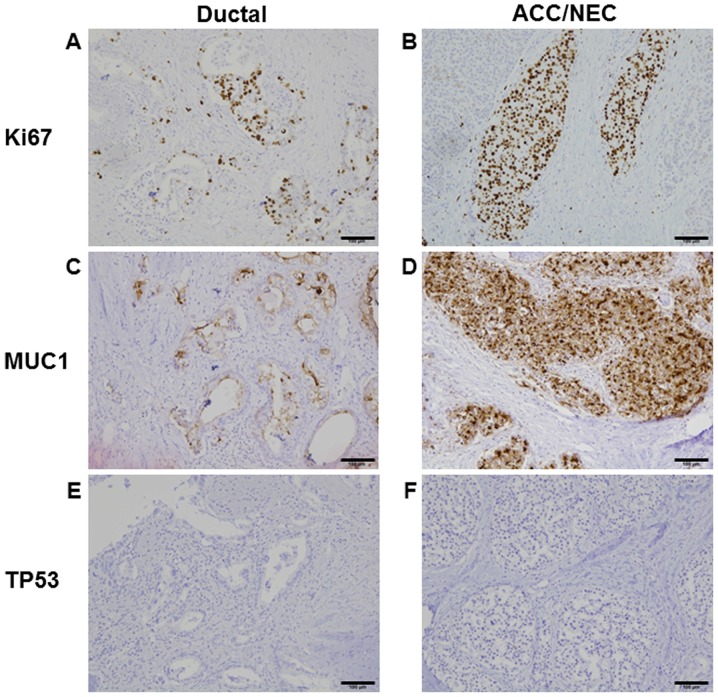
Pathological examinations showed that the tumor was histologically well to moderately differentiated adenocarcinoma. Eosinophilic cytoplasm was also observed in solid neoplasm, mixed with aforementioned ductal adenocarcinoma. This eosinophilic cytoplasm pattern resembled acinar cell and/or islet cell. Approximately 50% of each histological component was present in tumor (Fig. 1E and F). In the solid component, vascular invasion was observed. Lymph node metastasis from the ductal adenocarcinoma was observed in one lymph node (data not shown). Immunohistochemical analysis revealed positive expression of chromogranin A and trypsin as markers of NEC and ACC, respectively. These protein expressions were mostly overlapped in NEC and ACC, but not stained in ductal adenocarcinoma (Fig. 1G and H). Ki67 protein expression was 50% in ductal adenocarcinoma and 80% in NEC and ACC, suggesting Ki67 index was high in both components (Fig. 2A and B). Although MUC production was seen only in ductal adenocarcinoma (data not shown), MUC1 was positive in all tumor components (Fig. 2C and D). TP53 protein expression is negative in both ductal and ACC/NEC components (Fig. 2E and F). Mixed adenoneuroendocrine carcinoma (MANEC) was neglected because of the presence of ACC.
Figure 2.
Immunohistochemical analysis of ductal and acinar cell carcinoma (ACC)/neuroendocrine carcinoma (NEC) components. (A and B) Ki67 expression in (A) ductal and (B) ACC/NEC. (C and D) Mucin1 (MUC1) expression in (C) ductal and (D) ACC/NEC. (E and F) No TP53 expression in (E) ductal and (F) ACC/NEC components. Scale bars, 100 µm.
Genomic analysis
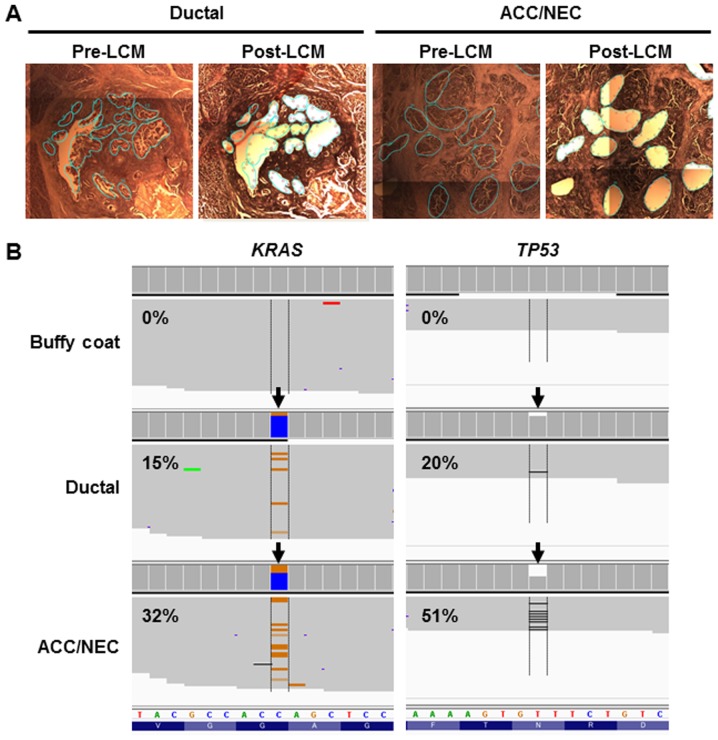
To examine whether genetic profiles were similar or distinct in ductal and ACC/NEC components, we performed targeted sequencing using AmpliSeq Cancer Hotspot Panel v2, which covers the hotspot regions of 50 cancer-related genes. We obtained the tumor cells from ductal or ACC/NEC components by laser-capture microdissection (Fig. 3A). Next-generation sequencing analysis yielded a sufficient number of mapped reads onto targeted regions, and the mean depth coverage was 2673x in the ductal tumor component and 4430x in the NEC/ACC mixed component (Table I). We identified the same two mutations (TP53 N210fs and KRAS G12R) in both ductal and acinar/neuroendocrine lesions (Fig. 3B and Table II). In accordance with this, TP53 protein expression was diminished (Fig. 2E and F), because the TP53 frameshift mutation was present in both neoplastic components. These results indicated that these distinct histological components derived from the same tumor clone and that the pancreatic ductal and ACC/NEC carcinomas developed from the same phylogeny in the present case.
Figure 3.
Targeted next-generation sequencing of ductal and acinar cell carcinoma (ACC)/neuroendocrine carcinoma (NEC) tumors. (A) Representative image of microdissected specimen. Tumor cells were obtained from formalin-fixed and paraffin-embedded tissues using laser capture microdissection (LCM) from ductal and ACC/NEC tumors. Left image (Pre-LCM) is before microdissection; right image is after microdissection (Post-LCM). Cyan lines indicate the cutting area. (B) Representative image of sequencing read alignments visualized with IGV; the arrow indicates the single nucleotide variant in KRAS (orange line indicates nucleotide substitution, C>G) and deletion site in TP53 genes (black line indicates deletion site). The variant allelic fractions were denoted into the images.
Table I.
Sequencing reads and coverage data from next-generation sequencing.
| Sample name | Mapped reads | On target (%) | Mean depth | Uniformity (%) |
|---|---|---|---|---|
| Tumor ductal | 577,133 | 98.82 | 2,673 | 99.22 |
| Tumor ACC/NEC | 959,353 | 98.62 | 4,430 | 97.52 |
| Buffy coat | 888,135 | 96.13 | 4,045 | 98.31 |
ACC, acinar cell carcinoma; NEC, neuroendocrine carcinoma.
Table II.
Genetic alterations in ductal and ACC/NEC.
| Histology | Position | Reference | Variant | Gene | Mutation | Allelic fraction (%) |
|---|---|---|---|---|---|---|
| Ductal | chr12:25398280 | C | G | KRAS | G12R | 15 |
| Ductal | chr17:7578219 | GT | G | TP53 | N210fs | 20 |
| ACC/NEC | chr12:25398280 | C | G | KRAS | G12R | 32 |
| ACC/NEC | chr17:7578219 | GT | G | TP53 | N210fs | 51 |
ACC, acinar cell carcinoma; NEC, neuroendocrine carcinoma; chr, chromosome; fs, frameshift.
Discussion
The pancreas is composed of both exocrine and endocrine gland components: The exocrine part is formed from ductal and acinar cells, whereas the endocrine component is made up of endocrine cells. Most pancreatic tumors arise from a single cell type; therefore, ductal carcinomas and ACCs are considered to derive from the exocrine gland, and NECs from the endocrine gland. However, the existence of carcinomas with mixed differentiation complicates the situation. Additionally, few reports have defined the biological behaviour of mixed carcinomas. For example, pancreatic NEC and ACC appear to be distinct entities, but their pathological and morphological appearances can be similar. ACC has been reported to express neuroendocrine markers such as synaptophysin and chromogranin A, while the coexistence of ACC and NEC has also been reported. These observations suggest that the origin of the three types of pancreatic cancer (ductal, ACC, and NEC) is not clearly defined as either exocrine or endocrine glands.
Genetic profiles provide the direct evidence of tumor origins in the different histological components. While gene sequencing has demonstrated shared mutations in the different histologic components of mixed carcinomas (e.g., MANEC) of other digestive organs (7,8), it has not been proven in mixed acinar-ductal adenocarcinoma of the pancreas. Here, we present a case that showed the coexistence of ACC and NEC which were clearly seen together with ductal carcinoma. To investigate whether the different histological types derived from the same or a distinct tumor origin, we performed next-generation sequencing analysis and revealed genetic alterations in these distinct tumor histologies. We identified the same TP53 and KRAS mutations in both tumor samples from ductal carcinoma and mixed ACC/NEC carcinoma. Of note, there are the same genetic patterns between the two components, which were the distinct differentiation patterns. These results suggest that the same tumor clones are related to the development of pancreatic mixed acinar-neuroendocrine-ductal carcinoma. In line with this findings, previous studies also suggested the mixed neoplasm derived from same tumor origin in endocrine-exocrine tumors of the gut and MANEC of the gastrointestinal tract (7,8).
Genetic alterations in pancreatic cancer are different among the histological types. For instance, KRAS, TP53, SMAD4, and CDKN2A genes are most commonly mutated in pancreatic ductal cancer (9,10). In particular, KRAS mutations are observed about 90% of pancreatic ductal adenocarcinoma. Contrary, TP53 can be mutated in a subset of ACCs and, more frequently, in NECs (11,12). However, KRAS mutations are rarely observed in ACCs (13,14). Therefore, it is particularly noteworthy that a KRAS mutation was detected in the mixed ACC/NEC tumor component of the present case. To understand this, it is necessary to consider previously-reported step-wise pancreatic tumorigenesis models. The most common model is that pancreatic intraepithelial neoplasia is a neoplastic precursor of invasive pancreatic ductal adenocarcinoma (15). Another is that pancreatic cancer develops from acinar cells (16). In the genetic pancreatic cancer mouse model, KRAS activation induces the dedifferentiation of acinar cells, which transform and lose the expression of typical marker proteins; thus, acinar to ductal metaplasia (ADM) is a precancerous form of pancreatic ductal carcinoma (17–19), and acinar cell hyperplasia has been observed in the human pancreas (20). In this study, we identified TP53 and KRAS mutations that were shared by ductal and ACC/NEC tumors. This indicates that KRAS activation in acinar cells, as well as other genetic or epigenetic changes, triggers ADM and results in subsequent cell differentiation into ductal adenocarcinoma. To our knowledge, this is the first report of common mutational profiles in ductal and acinar carcinomas in a human pancreatic tumor, which may provide genetic evidence of pancreatic tumorigenesis involving ADM.
Acknowledgements
The authors would like to thank Hidetoshi Shigetomo, Yumi Kubota, and Ritsuko Yokouchi for their help. This study was supported by a Grant-in-Aid for Genome Research Project from Yamanashi Prefecture (to Y.H. and M.O.) and a grant from The YASUDA Medical Foundation (to Y.H.).
References
- 1.Hori M, Matsuda T, Shibata A, Katanoda K, Sobue T, Nishimoto H. Japan Cancer Surveillance Research Group: Cancer incidence and incidence rates in Japan in 2009: A study of 32 population-based cancer registries for the Monitoring of Cancer Incidence in Japan (MCIJ) project. Jpn J Clin Oncol. 2015;45:884–891. doi: 10.1093/jjco/hyv088. [DOI] [PubMed] [Google Scholar]
- 2.Longnecker DS. Pathologyof exocrine pancreatic neoplasms. https://www.uptodate.com/contents/pathology-of-exocrine-pancreatic-neoplasms. UpToDate. 2017 2017 Feb 06; Accessed. [Google Scholar]
- 3.Klimstra DS. Nonductal neoplasms of the pancreas. Mod Pathol. 2007;20(Suppl 1):S94–S112. doi: 10.1038/modpathol.3800686. [DOI] [PubMed] [Google Scholar]
- 4.Fukushima N, Hruban RH, Kato Y, et al. Ductal adenocarcinoma variants and mixed neoplasms of the pancreas. In: Bosman FT, Camerio F, Hruban RH, Thiise ND, editors. WHO classification of Tumors of the Digestive System. Lyon: IARC; 2010. [Google Scholar]
- 5.Hirotsu Y, Nakagomi H, Sakamoto I, Amemiya K, Mochizuki H, Omata M. Detection of BRCA1 and BRCA2 germline mutations in Japanese population using next-generation sequencing. Mol Genet Genomic Med. 2015;3:121–129. doi: 10.1002/mgg3.120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Hirotsu Y, Zheng TH, Amemiya K, Mochizuki H, Guleng B, Omata M. Targeted and exome sequencing identified somatic mutations in hepatocellular carcinoma. Hepatol Res. 2016;5:1145–1151. doi: 10.1111/hepr.12663. [DOI] [PubMed] [Google Scholar]
- 7.Furlan D, Cerutti R, Genasetti A, Pelosi G, Uccella S, La Rosa S, Capella C. Microallelotyping defines the monoclonal or the polyclonal origin of mixed and collision endocrine-exocrine tumors of the gut. Lab Invest. 2003;83:963–971. doi: 10.1097/01.LAB.0000079006.91414.BE. [DOI] [PubMed] [Google Scholar]
- 8.Scardoni M, Vittoria E, Volante M, Rusev B, Bersani S, Mafficini A, Gottardi M, Giandomenico V, Malleo G, Butturini G, et al. Mixed adenoneuroendocrine carcinomas of the gastrointestinal tract: Targeted next-generation sequencing suggests a monoclonal origin of the two components. Neuroendocrinology. 2014;100:310–316. doi: 10.1159/000369071. [DOI] [PubMed] [Google Scholar]
- 9.Waddell N, Pajic M, Patch AM, Chang DK, Kassahn KS, Bailey P, Johns AL, Miller D, Nones K, Quek K, et al. Whole genomes redefine the mutational landscape of pancreatic cancer. Nature. 2015;518:495–501. doi: 10.1038/nature14169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Biankin AV, Waddell N, Kassahn KS, Gingras MC, Muthuswamy LB, Johns AL, Miller DK, Wilson PJ, Patch AM, Wu J, et al. Pancreatic cancer genomes reveal aberrations in axon guidance pathway genes. Nature. 2012;491:399–405. doi: 10.1038/nature11547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.La Rosa S, Bernasconi B, Frattini M, Tibiletti MG, Molinari F, Furlan D, Sahnane N, Vanoli A, Albarello L, Zhang L, et al. TP53 alterations in pancreatic acinar cell carcinoma: New insights into the molecular pathology of this rare cancer. Virchows Arch. 2016;468:289–296. doi: 10.1007/s00428-015-1882-9. [DOI] [PubMed] [Google Scholar]
- 12.Yachida S, Vakiani E, White CM, Zhong Y, Saunders T, Morgan R, de Wilde RF, Maitra A, Hicks J, Demarzo AM, et al. Small cell and large cell neuroendocrine carcinomas of the pancreas are genetically similar and distinct from well-differentiated pancreatic neuroendocrine tumors. Am J Surg Pathol. 2012;36:173–184. doi: 10.1097/PAS.0b013e3182417d36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Jiao Y, Yonescu R, Offerhaus GJ, Klimstra DS, Maitra A, Eshleman JR, Herman JG, Poh W, Pelosof L, Wolfgang CL, et al. Whole-exome sequencing of pancreatic neoplasms with acinar differentiation. J Pathol. 2014;232:428–435. doi: 10.1002/path.4310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Chmielecki J, Hutchinson KE, Frampton GM, Chalmers ZR, Johnson A, Shi C, Elvin J, Ali SM, Ross JS, Basturk O, et al. Comprehensive genomic profiling of pancreatic acinar cell carcinomas identifies recurrent RAF fusions and frequent inactivation of DNA repair genes. Cancer Discov. 2014;4:1398–1405. doi: 10.1158/2159-8290.CD-14-0617. [DOI] [PubMed] [Google Scholar]
- 15.Tuveson DA, Neoptolemos JP. Understanding metastasis in pancreatic cancer: A call for new clinical approaches. Cell. 2012;148:21–23. doi: 10.1016/j.cell.2011.12.021. [DOI] [PubMed] [Google Scholar]
- 16.Schmid RM. Acinar-to-ductal metaplasia in pancreatic cancer development. J Clin Invest. 2002;109:1403–1404. doi: 10.1172/JCI0215889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Eser S, Reiff N, Messer M, Seidler B, Gottschalk K, Dobler M, Hieber M, Arbeiter A, Klein S, Kong B, et al. Selective requirement of PI3K/PDK1 signaling for Kras oncogene-driven pancreatic cell plasticity and cancer. Cancer Cell. 2013;23:406–420. doi: 10.1016/j.ccr.2013.01.023. [DOI] [PubMed] [Google Scholar]
- 18.Liou GY, Döppler H, DelGiorno KE, Zhang L, Leitges M, Crawford HC, Murphy MP, Storz P. Mutant KRas-induced mitochondrial oxidative stress in acinar cells upregulates EGFR signaling to drive formation of pancreatic precancerous lesions. Cell Rep. 2016;14:2325–2336. doi: 10.1016/j.celrep.2016.02.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Shi C, Hong SM, Lim P, Kamiyama H, Khan M, Anders RA, Goggins M, Hruban RH, Eshleman JR. KRAS2 mutations in human pancreatic acinar-ductal metaplastic lesions are limited to those with PanIN: Implications for the human pancreatic cancer cell of origin. Mol Cancer Res. 2009;7:230–236. doi: 10.1158/1541-7786.MCR-08-0206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Longnecker DS, Shinozuka H, Dekker A. Focal acinar cell dysplasia in human pancreas. Cancer. 1980;45:534–540. doi: 10.1002/1097-0142(19800201)45:3<534::AID-CNCR2820450320>3.0.CO;2-G. [DOI] [PubMed] [Google Scholar]