Abstract
Red blood cells (RBCs) have historically been considered passive bystanders in thrombosis. However, clinical and epidemiological studies have associated quantitative and qualitative abnormalities in RBCs, including altered hematocrit, sickle cell disease, thalassemia, hemolytic anemias, and malaria, with both arterial and venous thrombosis. A growing body of mechanistic studies suggests that RBCs can promote thrombus formation and enhance thrombus stability. These findings suggest that RBCs may contribute to thrombosis pathophysiology and reveal potential strategies for therapeutically targeting RBCs to reduce thrombosis.
Introduction
Thrombosis is a leading cause of death and disability worldwide. Arterial thrombosis is typically associated with plaque rupture that triggers accumulation of platelets into platelet-rich (“white”) clots. Venous thromboembolism (deep vein thrombosis and/or pulmonary embolism, collectively VTE) is associated with endothelial dysfunction and blood stasis that result in the formation of fibrin- and red blood cell (RBC)-rich (“red”) clots. Interestingly, quantitative and qualitative abnormalities in RBCs have been associated with both arterial thrombosis and VTE. Although multiple mechanisms have been proposed (Figure 1), these remain largely theoretical with respect to understanding the role of RBCs in thrombosis. Herein, we briefly review clinical situations in which abnormal RBC quantity or quality has been associated with thrombosis and highlight mechanisms proposed to mediate these associations.
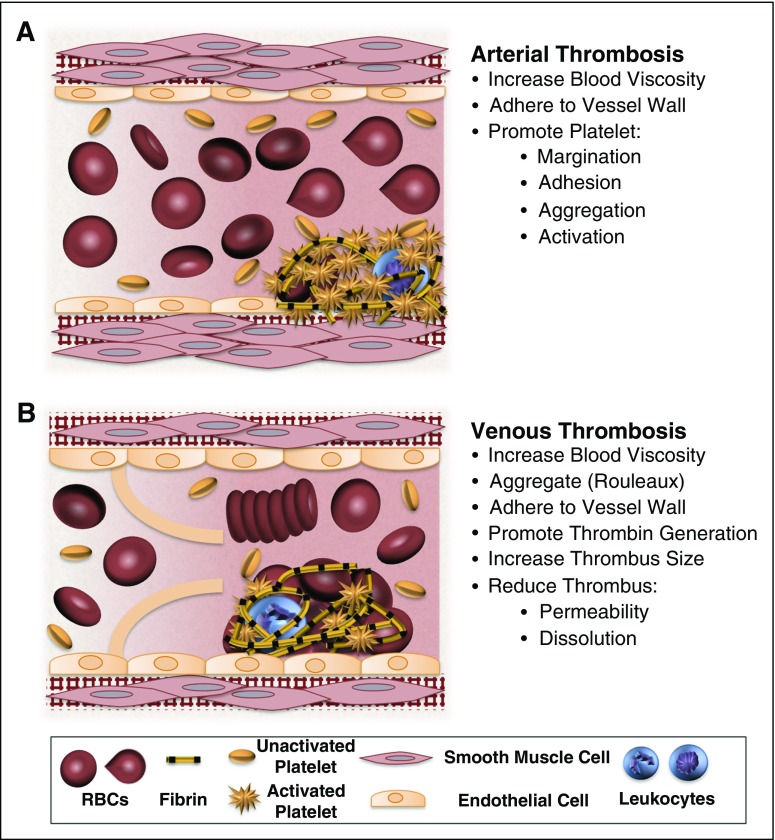
Figure 1.
Potential contributions of normal and abnormal RBCs to arterial and venous thrombosis/thromboembolism. (A) Arterial thrombi arise in vessels with high shear rates, which promotes the rapid formation of platelet-rich thrombi. During arterial thrombosis, RBCs promote platelet margination, increase platelet-thrombus interactions, and enhance platelet adhesion and activation. Although RBCs increase blood viscosity, this effect is lessened in arteries by high shear-induced shape change. (B) Venous thrombi form slowly in stasis or low flow (frequently in venous valve pockets) and are RBC and fibrin rich. In veins, RBC aggregation into stacked rouleaux structures increases blood viscosity. RBCs can also directly or indirectly adhere to the vessel wall and may contribute to thrombin generation within thrombi. Once incorporated into venous thrombi, RBCs increase thrombus size and reduce thrombus permeability and susceptibility to lysis. In disease states, abnormal RBCs and RBC-derived microvesicles may also adhere to the endothelium or extracellular matrix, activate platelets and other cells, and enhance local thrombin generation during thrombosis.
Clinical correlations between RBC abnormalities and thrombosis
Abnormal hematocrit
RBCs are the most abundant blood cell (4.2-6.1 × 109/mL in humans and 6.4-9.4 × 109/mL in mice). Consequently, RBCs comprise a large percentage of the blood volume. Reference intervals for hematocrit are higher in men than in women.
Epidemiologic studies have detected associations between elevated hematocrit and arterial thrombosis.1-8 The largest of these found the risk of cardiovascular disease (CVD) is more than twofold greater in high- versus low-hematocrit groups.2 The British Regional Heart Study observed a 30% increase in major ischemic heart disease in individuals with high hematocrit, even after adjusting for age, physical activity, cholesterol, body mass index, and smoking.4 Although most studies have focused on risk in men,1-5 the Framingham Study detected correlations between hematocrit and CVD and CVD deaths in young women.7 This study also found a U-shaped risk relationship between hematocrit and stroke/transient ischemic attack in older women, with elevated risk at both low and high hematocrit.7 Analysis of men and women in the Thrombolysis in Myocardial Infarction trials revealed a J-shaped relationship between hemoglobin and 30-day cardiovascular mortality.6 Ischemic stroke risk is also increased in apparently healthy young children with iron-deficiency anemia.9 Although risk in anemic patients may reflect undiagnosed disease, comorbidities, or changes in other blood cells (eg, secondary thrombocytosis), these observations suggest multimodal effects of hematocrit on cardiovascular risk.
Fewer studies have examined associations between hematocrit and VTE. The largest of these reported a 1.5-fold increased risk of first VTE in patients with high versus low hematocrit; however, risk of unprovoked VTE was significantly elevated in men, but not women.10 Conversely, the Austrian Study on Recurrent Venous Thromboembolism found an association between elevated hematocrit and recurrent VTE in women, but not in men.11 Discordance between these studies may reflect the study populations or differing mechanisms underlying first vs recurrent VTE. Anemic patients receiving erythropoiesis-stimulating agents show increased VTE risk with increasing hemoglobin,12 associating even small increases in hematocrit with increased thrombosis risk.
Patients with erythrocytosis secondary to myeloproliferative neoplasia have increased risk of both arterial thrombosis and VTE,13,14 and ∼20% of polycythemia vera (PV) patients have thrombosis as the presenting symptom.14 Observations that packed cell volume correlates positively with vascular occlusive episodes led to the adoption of current clinical guidelines to maintain PV patients at a hematocrit <45%.15 Accordingly, the CYTO-PV trial showed therapeutic phlebotomy to maintain <45% hematocrit is associated with a thrombosis risk that is nearly fourfold lower than that of patients with hematocrits between 45% and 50%.16 Notably, however, differences in leukocyte counts between low- and high-hematocrit groups confounded assignment of an RBC-dependent pathologic mechanism,16 and the association between erythrocytosis and thrombosis in clonal erythrocytotic disease remains controversial.17,18 Moreover, since several polycythemic states are not associated with increased thrombosis (eg, emphysema), other factors and adaptive responses may modify thrombosis risk.
Abnormal RBC quality and function
Diseases involving qualitative RBC defects, including abnormal size, shape, and/or viscoelastic properties, are also associated with thrombosis. Sickle cell disease (SCD) patients experience vaso-occlusive crises, and ∼25% of SCD patients have clinically apparent strokes by age 45 years, ∼50% of which are ischemic.19,20 d-dimer is elevated in SCD patients at steady state and during crisis,21 suggesting these events involve a thrombotic component, although this is controversial. Interestingly, although isolated deep vein thrombosis is only weakly or not associated with SCD or sickle cell trait, studies consistently show that SCD/trait patients have two to fourfold increased risk of pulmonary embolism.22-26 These pulmonary thrombi may arise from in situ thrombus formation in the pulmonary vasculature rather than embolization from a distal site.22,23 Thrombosis risk is elevated in thalassemia patients, particularly following splenectomy.27 Similarly, in patients with hereditary spherocytosis (HS), risk of arterial thrombosis and VTE increases 7.2- and 3.3-fold, respectively, following splenectomy.28 In both cases, splenectomy may enhance thrombosis by decreasing clearance of abnormal RBCs.27,28 Thrombosis is also common in patients with paroxysmal nocturnal hemoglobinuria, a disorder marked by complement-mediated RBC hemolysis, and accounts for 40% to 67% of deaths.29 Thrombosis in these disorders is likely multifactorial in origin, involving both RBC dysfunction and effects on other blood cells and vasculature.
RBC distribution width, a measure of the variability of circulating RBC volume, is positively correlated with risk of developing ischemic stroke30 and is seen in patients presenting with VTE.31 However, it is unclear whether this association indicates independent contributions of RBCs to thrombosis or a comorbidity that increases RBC variability and promotes thrombosis (eg, inflammation). RBC transfusion is also associated with increased incidence of arterial thrombosis and VTE in postoperative patients and hospitalized patients with cancer, and elevated risk persists in multivariable analysis.32-34 Interestingly, although RBC storage induces acquired RBC dysfunction, including membrane changes and release of RBC microvesicles (RBC-MVs),35 there appears to be no association between RBC storage time and thrombosis risk or mortality.34,36
Proposed mechanisms
Mechanisms mediating the association of quantitative and qualitative RBC defects with thrombosis have been difficult to identify because causation cannot be discerned from epidemiologic studies. Moreover, in vivo studies examining effects of RBCs on thrombosis can be difficult to interpret because RBC abnormalities typically occur in the context of comorbidities that produce complex phenotypes. For example, in humans, iron-deficiency anemia is associated with stroke in children,9 whereas iron administration shortens the bleeding time in iron-deficient patients and RBC transfusion shortens the bleeding time in thrombocytopenic individuals.37,38 Animal models of erythropoietin-induced erythrocytosis may reflect multiple, extrahematopoietic targets (eg, endothelial cells).39 Mouse models of JAK 2V617F-induced PV show increased FeCl3-induced mesenteric thrombosis but, paradoxically, bleeding after tail transection.40,41 Healthy mice infused with normal murine RBCs exhibit a shorter time to FeCl3-induced carotid artery occlusion and shorter tail bleeding times.42 These observations imply direct but complex relationships between hematocrit and coagulation. Studies employing in vitro, in silico, and in vivo model systems have identified biophysical and biochemical mechanisms by which RBCs may contribute to thrombus initiation, propagation, and resolution (Figure 1).
Blood rheology
Increased blood viscosity is an established risk factor for thrombosis.43 Due to their discoid shape, deformability, intrinsic viscoelastic properties, and fibrinogen-binding ability, RBCs are the primary determinants of blood viscosity.43-45 In general, elevated hematocrit is correlated with increased blood viscosity.46 In large vessels, viscosity increases exponentially with hematocrit,47 which may explain the increase in CVD risk observed with relatively small increases in hematocrit. In small vessels that permit only single-file flow of RBCs, viscosity increases linearly.47 Since blood behaves as a non-Newtonian fluid, the impact of RBCs on blood viscosity also depends on shear rate. At high (arterial) shear rates, transient deformation of RBCs reduces blood viscosity.48,49 Conversely, at low (venous) shear rates, slow movement of blood coupled with discoid RBC morphology allows electrostatic interactions to promote RBC aggregation into stacked “rouleaux” structures, which increase blood viscosity.49,50 Abnormal RBCs that cannot undergo shear-dependent morphologic changes can profoundly alter blood flow and viscosity.51 These effects may contribute to thrombosis in patients with SCD or HS.
Effects on the vessel wall
Intact RBCs can directly adhere to the endothelium or subendothelial matrix or bind through interactions with other blood proteins and/or cells, including neutrophils and platelets.52,53 Studies of sickled RBCs have implicated numerous receptors (eg, erythroid Lutheran blood group/basal cell adhesion molecule, integrin α4Bβ1, CD36, sulfate glycolipids, intercellular adhesion molecule-4, and phosphatidylserine) and ligands (eg, β3 integrins, thrombospondin, laminin, fibronectin, and fibrin[ogen]) in these adhesive interactions,54-58 which may particularly contribute to venous occlusion.56 Similarly, in cerebral malaria, infected RBCs exhibit enhanced binding to activated endothelium via interactions with platelet-decorated von Willebrand factor strings. This platelet CD36-dependent RBC adhesion may contribute to RBC sequestration and occlusion of the brain microvasculature.59 Hemoglobin released from hemolyzed RBCs can scavenge nitric oxide and inhibit endogenous mechanisms to prevent vasoconstriction, and it may enhance adhesive interactions with the vessel wall.60 In anemic patients, hypoxia-induced changes in gene expression may alter endothelial phenotype and increase thrombotic risk.61
Effects on platelets
RBCs have biophysical and biochemical effects on platelets. First, RBCs influence the physical location of platelets within vessels. In straight vessels with arterial shear rates, RBCs promote platelet margination, which can enrich the near-wall platelet concentration three to fivefold.62-64 In silico simulations suggest margination increases platelet–vessel wall interactions and enhances platelet deposition on thrombi by reducing the distance between flowing platelets and the thrombus and increasing the frequency and duration of these interactions.42,65 Accordingly, increasing hematocrit promotes platelet adhesion and accumulation on excised subendothelial matrices63 and von Willebrand factor– or collagen-coated surfaces.42,66 Second, hemoglobin and ADP released from hemolyzed or damaged RBCs biochemically enhance platelet activation and aggregation.60,67,68 Platelets isolated from RBC-transfused patients show slightly, but significantly, enhanced aggregation.69 Releaseates from platelets stimulated in the presence of RBCs enhance platelet P-selectin exposure and integrin αIIbβ3 activation,70 suggesting RBCs promote a prothrombotic feedback loop during platelet activation. Collectively, these results suggest elevated hematocrit increases the interaction and adhesion of platelets to thrombi.
Thrombin generation
A subset of normal, circulating RBCs, as well as RBC-MVs, have exposed phosphatidylserine on their outer membranes, and extracellular histones can increase RBC phosphatidylserine exposure.71 Although RBC phosphatidylserine can activate the contact pathway and support thrombin generation in vitro,72-76 the contribution of RBC- and RBC-MV–mediated thrombin generation to thrombosis in vivo is unknown. In a mouse model of arterial thrombosis, elevated hematocrit does not increase circulating thrombin–antithrombin complexes, a marker of coagulation activation.42 Moreover, RBC phosphatidylserine exposure does not predict thrombosis in a mouse model of HS,77 and ex vivo thrombin generation in whole blood from SCD patients is inversely correlated with RBC phosphatidylserine.78 However, both RBC phosphatidylserine exposure and RBC-MVs are elevated in SCD patients,74,78 and circulating prothrombin fragment 1.2, another marker of coagulation activation, is elevated in SCD patients and correlates with RBC phosphatidylserine.79 Thus, RBCs may augment thrombin generation in certain situations. RBCs may enhance procoagulant activity of lipopolysaccharide-treated monocytes80 and/or contribute to thrombin generation within RBC-rich venous thrombi.
Fibrinogen and fibrin
RBCs bind fibrinogen, and this interaction is a key mediator of erythrocyte sedimentation and blood viscosity.44,45 In addition, thrombus RBC content is strongly and positively associated with thrombus size.81 Findings from in vitro and in vivo models indicate RBCs are retained in clots via dual, but independent, contributions of fibrin network density and coagulation factor XIII(a)–mediated fibrin α-chain crosslinking.81,82 Once localized within clots, RBCs may impact thrombus structure and stability. In vitro, RBCs alter fibrin network organization and suppress plasmin generation.83-86 During platelet-mediated clot contraction, RBCs within thrombi are compressed into shapes (“polyhedrocytes”) that permit tight packing and reduce clot permeability.87 Thus, RBCs may delay access of thrombolytic enzymes to the clot and consequently prolong thrombus resolution.
Conclusions and future perspectives
Given differences in arterial thrombosis and VTE pathophysiology, as well as the diverse effects of RBCs on blood viscosity, cellular function, and thrombus formation, structure, and stability, RBCs likely contribute to arterial thrombosis and VTE in unique ways. Dissecting these contributions will require interdisciplinary methods that assess both biophysical and biochemical properties of RBCs and do so in complex experimental systems. Use of infusion-based in vivo models42,88 may enable the isolation and characterization of abnormal RBCs and their effects in complicated pathologies.
As our understanding of RBC contributions to thrombosis matures, so may the possibility of developing RBC-targeted antithrombotics. For example, blocking the interaction between RBCs and fibrin(ogen) or the endothelium may reduce VTE by preventing RBC aggregation and adhesion, respectively. Targeting the interaction between RBCs and platelets or effects of RBCs on platelet function may reduce arterial thrombosis risk; this premise is supported by the ability of platelet antagonists to reduce cardiovascular death and nonfatal myocardial infarction and stroke in PV patients.89 The discovery that factor XIII(a) mediates RBC retention in venous thrombi81 suggests that inhibiting factor XIII(a) may reduce thrombus size and accelerate VTE resolution. Reducing hematocrit through phlebotomy16 may reduce potential prothrombotic effects of RBCs in at-risk individuals. Basic and translational studies are needed to test these concepts.
Acknowledgments
The authors thank Nigel S. Key and Gordon D. O. Lowe for providing helpful comments.
The authors are supported by funding from the National Institutes of Health, National Heart, Lung, and Blood Institute (grant R01HL126974 [A.S.W.]) and a National Science Foundation Graduate Research Fellowship (DGE-1144081 [J.R.B.]).
Authorship
Contribution: J.R.B. and A.S.W. wrote the manuscript.
Conflict-of-interest: The authors declare no competing financial interests.
Correspondence: Alisa S. Wolberg, Department of Pathology and Laboratory Medicine, University of North Carolina at Chapel Hill, 819 Brinkhous-Bullitt Building, CB #7525, Chapel Hill, NC 27599-7525; e-mail: alisa_wolberg@med.unc.edu.
References
- 1.Burch GE, Depasquale NP. The hematocrit in patients with myocardial infarction. JAMA. 1962;180:62-63. [PubMed] [Google Scholar]
- 2.Sorlie PD, Garcia-Palmieri MR, Costas R Jr, Havlik RJ. Hematocrit and risk of coronary heart disease: the Puerto Rico Health Program. Am Heart J. 1981;101(4):456-461. [DOI] [PubMed] [Google Scholar]
- 3.Erikssen G, Thaulow E, Sandvik L, Stormorken H, Erikssen J. Haematocrit: a predictor of cardiovascular mortality? J Intern Med. 1993;234(5):493-499. [DOI] [PubMed] [Google Scholar]
- 4.Wannamethee G, Shaper AG, Whincup PH. Ischaemic heart disease: association with haematocrit in the British Regional Heart Study. J Epidemiol Community Health. 1994;48(2):112-118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Toss F, Nordström A, Nordström P. Association between hematocrit in late adolescence and subsequent myocardial infarction in Swedish men. Int J Cardiol. 2013;168(4):3588-3593. [DOI] [PubMed] [Google Scholar]
- 6.Sabatine MS, Morrow DA, Giugliano RP, et al. Association of hemoglobin levels with clinical outcomes in acute coronary syndromes. Circulation. 2005;111(16):2042-2049. [DOI] [PubMed] [Google Scholar]
- 7.Gagnon DR, Zhang TJ, Brand FN, Kannel WB. Hematocrit and the risk of cardiovascular disease--the Framingham study: a 34-year follow-up. Am Heart J. 1994;127(3):674-682. [DOI] [PubMed] [Google Scholar]
- 8.Danesh J, Collins R, Peto R, Lowe GD. Haematocrit, viscosity, erythrocyte sedimentation rate: meta-analyses of prospective studies of coronary heart disease. Eur Heart J. 2000;21(7):515-520. [DOI] [PubMed] [Google Scholar]
- 9.Maguire JL, deVeber G, Parkin PC. Association between iron-deficiency anemia and stroke in young children. Pediatrics. 2007;120(5):1053-1057. [DOI] [PubMed] [Google Scholar]
- 10.Braekkan SK, Mathiesen EB, Njølstad I, Wilsgaard T, Hansen JB. Hematocrit and risk of venous thromboembolism in a general population. The Tromso study. Haematologica. 2010;95(2):270-275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Eischer L, Tscholl V, Heinze G, Traby L, Kyrle PA, Eichinger S. Hematocrit and the risk of recurrent venous thrombosis: a prospective cohort study. PLoS One. 2012;7(6):e38705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Dicato M. Venous thromboembolic events and erythropoiesis-stimulating agents: an update. Oncologist. 2008;13(Suppl 3):11-15. [DOI] [PubMed] [Google Scholar]
- 13.Spivak JL. Polycythemia vera: myths, mechanisms, and management. Blood. 2002;100(13):4272-4290. [DOI] [PubMed] [Google Scholar]
- 14.Polycythemia vera: the natural history of 1213 patients followed for 20 years. Gruppo Italiano Studio Policitemia. Ann Intern Med. 1995;123(9):656-664. [DOI] [PubMed] [Google Scholar]
- 15.Pearson TC, Wetherley-Mein G. Vascular occlusive episodes and venous haematocrit in primary proliferative polycythaemia. Lancet. 1978;2(8102):1219-1222. [DOI] [PubMed] [Google Scholar]
- 16.Marchioli R, Finazzi G, Specchia G, et al. ; CYTO-PV Collaborative Group. Cardiovascular events and intensity of treatment in polycythemia vera. N Engl J Med. 2013;368(1):22-33. [DOI] [PubMed] [Google Scholar]
- 17.Di Nisio M, Barbui T, Di Gennaro L, et al. ; European Collaboration on Low-dose Aspirin in Polycythemia Vera (ECLAP) Investigators. The haematocrit and platelet target in polycythemia vera. Br J Haematol. 2007;136(2):249-259. [DOI] [PubMed] [Google Scholar]
- 18.Prchal JT, Gordeuk VR. Treatment target in polycythemia vera. N Engl J Med. 2013;368(16):1555-1556. [DOI] [PubMed] [Google Scholar]
- 19.Ohene-Frempong K, Weiner SJ, Sleeper LA, et al. Cerebrovascular accidents in sickle cell disease: rates and risk factors. Blood. 1998;91(1):288-294. [PubMed] [Google Scholar]
- 20.Verduzco LA, Nathan DG. Sickle cell disease and stroke. Blood. 2009;114(25):5117-5125. [DOI] [PubMed] [Google Scholar]
- 21.Devine DV, Kinney TR, Thomas PF, Rosse WF, Greenberg CS. Fragment D-dimer levels: an objective marker of vaso-occlusive crisis and other complications of sickle cell disease. Blood. 1986;68(1):317-319. [PubMed] [Google Scholar]
- 22.Stein PD, Beemath A, Meyers FA, Skaf E, Olson RE. Deep venous thrombosis and pulmonary embolism in hospitalized patients with sickle cell disease. Am J Med 2006;119(10):897. [DOI] [PubMed]
- 23.Novelli EM, Huynh C, Gladwin MT, Moore CG, Ragni MV. Pulmonary embolism in sickle cell disease: a case-control study. J Thromb Haemost. 2012;10(5):760-766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Austin H, Key NS, Benson JM, et al. Sickle cell trait and the risk of venous thromboembolism among blacks. Blood. 2007;110(3):908-912. [DOI] [PubMed] [Google Scholar]
- 25.Folsom AR, Tang W, Roetker NS, et al. Prospective study of sickle cell trait and venous thromboembolism incidence. J Thromb Haemost. 2015;13(1):2-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Manci EA, Culberson DE, Yang YM, et al. ; Investigators of the Cooperative Study of Sickle Cell Disease. Causes of death in sickle cell disease: an autopsy study. Br J Haematol. 2003;123(2):359-365. [DOI] [PubMed] [Google Scholar]
- 27.Cappellini MD, Robbiolo L, Bottasso BM, Coppola R, Fiorelli G, Mannucci AP. Venous thromboembolism and hypercoagulability in splenectomized patients with thalassaemia intermedia. Br J Haematol. 2000;111(2):467-473. [DOI] [PubMed] [Google Scholar]
- 28.Schilling RF, Gangnon RE, Traver MI. Delayed adverse vascular events after splenectomy in hereditary spherocytosis. J Thromb Haemost. 2008;6(8):1289-1295. [DOI] [PubMed] [Google Scholar]
- 29.Hill A, Kelly RJ, Hillmen P. Thrombosis in paroxysmal nocturnal hemoglobinuria. Blood. 2013;121(25):4985-4996, quiz 5105. [DOI] [PubMed] [Google Scholar]
- 30.Lappegård J, Ellingsen TS, Skjelbakken T, et al. Red cell distribution width is associated with future risk of incident stroke. The Tromsø Study. Thromb Haemost. 2016;115(1):126-134. [DOI] [PubMed] [Google Scholar]
- 31.Lippi G, Buonocore R, Cervellin G. Value of red blood cell distribution width on emergency department admission in patients with venous thrombosis. Am J Cardiol. 2016;117(4):670-675. [DOI] [PubMed] [Google Scholar]
- 32.Gangireddy C, Rectenwald JR, Upchurch GR, et al. Risk factors and clinical impact of postoperative symptomatic venous thromboembolism. J Vasc Surg. 2007;45(2):335-341, discussion 341-342. [DOI] [PubMed] [Google Scholar]
- 33.Khorana AA, Francis CW, Blumberg N, Culakova E, Refaai MA, Lyman GH. Blood transfusions, thrombosis, and mortality in hospitalized patients with cancer. Arch Intern Med. 2008;168(21):2377-2381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Kumar MA, Boland TA, Baiou M, et al. Red blood cell transfusion increases the risk of thrombotic events in patients with subarachnoid hemorrhage. Neurocrit Care. 2014;20(1):84-90. [DOI] [PubMed] [Google Scholar]
- 35.Larson MC, Karafin MS, Hillery CA, Hogg N. Phosphatidylethanolamine is progressively exposed in RBCs during storage. Transfus Med. 2017;27(2):136-141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Heddle NM, Cook RJ, Arnold DM, et al. Effect of short-term vs. long-term blood storage on mortality after transfusion. N Engl J Med. 2016;375(20):1937-1945. [DOI] [PubMed] [Google Scholar]
- 37.Duke WW. The relation of blood platelets to hemorrhagic disease. By W.W. Duke. JAMA. 1983;250(9):1201-1209. [PubMed] [Google Scholar]
- 38.Ho CH. Increase of red blood cells can shorten the bleeding time in patients with iron deficiency anemia. Blood. 1998;91(3):1094. [PubMed] [Google Scholar]
- 39.Broxmeyer HE. Erythropoietin: multiple targets, actions, and modifying influences for biological and clinical consideration. J Exp Med. 2013;210(2):205-208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Lamrani L, Lacout C, Ollivier V, et al. Hemostatic disorders in a JAK2V617F-driven mouse model of myeloproliferative neoplasm. Blood. 2014;124(7):1136-1145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Strassel C, Kubovcakova L, Mangin PH, et al. Haemorrhagic and thrombotic diatheses in mouse models with thrombocytosis. Thromb Haemost. 2015;113(2):414-425. [DOI] [PubMed] [Google Scholar]
- 42.Walton BL, Lehmann M, Skorczewski T, et al. Elevated hematocrit enhances platelet accumulation following vascular injury. Blood. 2017;129(18):2537-2546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Lowe GD, Lee AJ, Rumley A, Price JF, Fowkes FG. Blood viscosity and risk of cardiovascular events: the Edinburgh Artery Study. Br J Haematol. 1997;96(1):168-173. [DOI] [PubMed] [Google Scholar]
- 44.Holley L, Woodland N, Hung WT, Cordatos K, Reuben A. Influence of fibrinogen and haematocrit on erythrocyte sedimentation kinetics. Biorheology. 1999;36(4):287-297. [PubMed] [Google Scholar]
- 45.Rampling MW. The binding of fibrinogen and fibrinogen degradation products to the erythrocyte membrane and its relationship to haemorheology. Acta Biol Med Ger. 1981;40(4-5):373-378. [PubMed] [Google Scholar]
- 46.Kwaan HC, Wang J. Hyperviscosity in polycythemia vera and other red cell abnormalities. Semin Thromb Hemost. 2003;29(5):451-458. [DOI] [PubMed] [Google Scholar]
- 47.Pries AR, Neuhaus D, Gaehtgens P. Blood viscosity in tube flow: dependence on diameter and hematocrit. Am J Physiol. 1992;263(6 Pt 2):H1770-H1778. [DOI] [PubMed] [Google Scholar]
- 48.Lanotte L, Mauer J, Mendez S, et al. Red cells’ dynamic morphologies govern blood shear thinning under microcirculatory flow conditions. Proc Natl Acad Sci USA. 2016;113(47):13289-13294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Baskurt OK, Meiselman HJ. Iatrogenic hyperviscosity and thrombosis. Semin Thromb Hemost. 2012;38(8):854-864. [DOI] [PubMed] [Google Scholar]
- 50.Errill EW. Rheology of blood. Physiol Rev. 1969;49(4):863-888. [DOI] [PubMed] [Google Scholar]
- 51.Piety NZ, Reinhart WH, Pourreau PH, Abidi R, Shevkoplyas SS. Shape matters: the effect of red blood cell shape on perfusion of an artificial microvascular network. Transfusion. 2016;56(4):844-851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Wandersee NJ, Olson SC, Holzhauer SL, Hoffmann RG, Barker JE, Hillery CA. Increased erythrocyte adhesion in mice and humans with hereditary spherocytosis and hereditary elliptocytosis. Blood. 2004;103(2):710-716. [DOI] [PubMed] [Google Scholar]
- 53.Colin Y, Le Van Kim C, El Nemer W. Red cell adhesion in human diseases. Curr Opin Hematol. 2014;21(3):186-192. [DOI] [PubMed] [Google Scholar]
- 54.Wautier JL, Pintigny D, Wautier MP, et al. Fibrinogen, a modulator of erythrocyte adhesion to vascular endothelium. J Lab Clin Med. 1983;101(6):911-920. [PubMed] [Google Scholar]
- 55.Hillery CA, Du MC, Montgomery RR, Scott JP. Increased adhesion of erythrocytes to components of the extracellular matrix: isolation and characterization of a red blood cell lipid that binds thrombospondin and laminin. Blood. 1996;87(11):4879-4886. [PubMed] [Google Scholar]
- 56.Goel MS, Diamond SL. Adhesion of normal erythrocytes at depressed venous shear rates to activated neutrophils, activated platelets, and fibrin polymerized from plasma. Blood. 2002;100(10):3797-3803. [DOI] [PubMed] [Google Scholar]
- 57.Hermand P, Gane P, Huet M, et al. Red Cell ICAM-4 Is a Novel Ligand for Platelet-activated αIIbβ3 Integrin. J Biol Chem. 2003;278(7):4892-4898. [DOI] [PubMed] [Google Scholar]
- 58.Zennadi R, Hines PC, De Castro LM, Cartron JP, Parise LV, Telen MJ. Epinephrine acts through erythroid signaling pathways to activate sickle cell adhesion to endothelium via LW-alphavbeta3 interactions. Blood. 2004;104(12):3774-3781. [DOI] [PubMed] [Google Scholar]
- 59.Bridges DJ, Bunn J, van Mourik JA, et al. Rapid activation of endothelial cells enables Plasmodium falciparum adhesion to platelet-decorated von Willebrand factor strings. Blood. 2010;115(7):1472-1474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Rother RP, Bell L, Hillmen P, Gladwin MT. The clinical sequelae of intravascular hemolysis and extracellular plasma hemoglobin: a novel mechanism of human disease. JAMA. 2005;293(13):1653-1662. [DOI] [PubMed] [Google Scholar]
- 61.Sergueeva A, Miasnikova G, Shah BN, et al. Prospective study of thrombosis and thrombospondin-1 expression in Chuvash polycythemia. Haematologica. 2017;102(5):e166-e169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Turitto VT, Baumgartner HR. Platelet interaction with subendothelium in a perfusion system: physical role of red blood cells. Microvasc Res. 1975;9(3):335-344. [DOI] [PubMed] [Google Scholar]
- 63.Turitto VT, Weiss HJ. Red blood cells: their dual role in thrombus formation. Science. 1980;207(4430):541-543. [DOI] [PubMed] [Google Scholar]
- 64.Fogelson AL, Neeves KB. Fluid mechanics of blood clot formation. Annu Rev Fluid Mech. 2015;47:377-403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Wang W, Diacovo TG, Chen J, Freund JB, King MR. Simulation of platelet, thrombus and erythrocyte hydrodynamic interactions in a 3D arteriole with in vivo comparison. PLoS One. 2013;8(10):e76949. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Chen H, Angerer JI, Napoleone M, et al. Hematocrit and flow rate regulate the adhesion of platelets to von Willebrand factor. Biomicrofluidics. 2013;7(6):64113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Reimers RC, Sutera SP, Joist JH. Potentiation by red blood cells of shear-induced platelet aggregation: relative importance of chemical and physical mechanisms. Blood. 1984;64(6):1200-1206. [PubMed] [Google Scholar]
- 68.Santos MT, Valles J, Marcus AJ, et al. Enhancement of platelet reactivity and modulation of eicosanoid production by intact erythrocytes. A new approach to platelet activation and recruitment. J Clin Invest. 1991;87(2):571-580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Silvain J, Abtan J, Kerneis M, et al. Impact of red blood cell transfusion on platelet aggregation and inflammatory response in anemic coronary and noncoronary patients: the TRANSFUSION-2 study (impact of transfusion of red blood cell on platelet activation and aggregation studied with flow cytometry use and light transmission aggregometry). J Am Coll Cardiol. 2014;63(13):1289-1296. [DOI] [PubMed] [Google Scholar]
- 70.Vallés J, Santos MT, Aznar J, et al. Platelet-erythrocyte interactions enhance αIIbβ3 integrin receptor activation and P-selectin expression during platelet recruitment: down-regulation by aspirin ex vivo. Blood. 2002;99(11):3978-3984. [DOI] [PubMed] [Google Scholar]
- 71.Semeraro F, Ammollo CT, Esmon NL, Esmon CT. Histones induce phosphatidylserine exposure and a procoagulant phenotype in human red blood cells. J Thromb Haemost. 2014;12(10):1697-1702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Kuypers FA, Lewis RA, Hua M, et al. Detection of altered membrane phospholipid asymmetry in subpopulations of human red blood cells using fluorescently labeled annexin V. Blood. 1996;87(3):1179-1187. [PubMed] [Google Scholar]
- 73.Peyrou V, Lormeau JC, Hérault JP, Gaich C, Pfliegger AM, Herbert JM. Contribution of erythrocytes to thrombin generation in whole blood. Thromb Haemost. 1999;81(3):400-406. [PubMed] [Google Scholar]
- 74.van Beers EJ, Schaap MC, Berckmans RJ, et al. ; CURAMA study group. Circulating erythrocyte-derived microparticles are associated with coagulation activation in sickle cell disease. Haematologica. 2009;94(11):1513-1519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Whelihan MF, Zachary V, Orfeo T, Mann KG. Prothrombin activation in blood coagulation: the erythrocyte contribution to thrombin generation. Blood. 2012;120(18):3837-3845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Van Der Meijden PE, Van Schilfgaarde M, Van Oerle R, Renné T, ten Cate H, Spronk HM. Platelet- and erythrocyte-derived microparticles trigger thrombin generation via factor XIIa. J Thromb Haemost. 2012;10(7):1355-1362. [DOI] [PubMed] [Google Scholar]
- 77.Wandersee NJ, Tait JF, Barker JE. Erythroid phosphatidyl serine exposure is not predictive of thrombotic risk in mice with hemolytic anemia. Blood Cells Mol Dis. 2000;26(1):75-83. [DOI] [PubMed] [Google Scholar]
- 78.Whelihan MF, Lim MY, Mooberry MJ, et al. Thrombin generation and cell-dependent hypercoagulability in sickle cell disease. J Thromb Haemost. 2016;14(10):1941-1952. [DOI] [PubMed] [Google Scholar]
- 79.Setty BN, Rao AK, Stuart MJ. Thrombophilia in sickle cell disease: the red cell connection. Blood. 2001;98(12):3228-3233. [DOI] [PubMed] [Google Scholar]
- 80.Østerud B, Unruh D, Olsen JO, Kirchhofer D, Owens AP III, Bogdanov VY. Procoagulant and proinflammatory effects of red blood cells on lipopolysaccharide-stimulated monocytes. J Thromb Haemost. 2015;13(9):1676-1682. [DOI] [PubMed] [Google Scholar]
- 81.Aleman MM, Byrnes JR, Wang J-G, et al. Factor XIII activity mediates red blood cell retention in venous thrombi. J Clin Invest. 2014;124(8):3590-3600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Byrnes JR, Duval C, Wang Y, et al. Factor XIIIa-dependent retention of red blood cells in clots is mediated by fibrin α-chain crosslinking. Blood. 2015;126(16):1940-1948. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Carr ME Jr, Hardin CL. Fibrin has larger pores when formed in the presence of erythrocytes. Am J Physiol. 1987;253(5 Pt 2):H1069-H1073. [DOI] [PubMed] [Google Scholar]
- 84.van Gelder JM, Nair CH, Dhall DP. The significance of red cell surface area to the permeability of fibrin network. Biorheology. 1994;31(3):259-275. [DOI] [PubMed] [Google Scholar]
- 85.Gersh KC, Nagaswami C, Weisel JW. Fibrin network structure and clot mechanical properties are altered by incorporation of erythrocytes. Thromb Haemost. 2009;102(6):1169-1175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Wohner N, Sótonyi P, Machovich R, et al. Lytic resistance of fibrin containing red blood cells. Arterioscler Thromb Vasc Biol. 2011;31(10):2306-2313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Cines DB, Lebedeva T, Nagaswami C, et al. Clot contraction: compression of erythrocytes into tightly packed polyhedra and redistribution of platelets and fibrin. Blood. 2014;123(10):1596-1603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Gilson CR, Kraus TS, Hod EA, et al. A novel mouse model of red blood cell storage and posttransfusion in vivo survival. Transfusion. 2009;49(8):1546-1553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Landolfi R, Marchioli R, Kutti J, et al. ; European Collaboration on Low-Dose Aspirin in Polycythemia Vera Investigators. Efficacy and safety of low-dose aspirin in polycythemia vera. N Engl J Med. 2004;350(2):114-124. [DOI] [PubMed] [Google Scholar]