Abstract
Diabetes mellitus (DM) is an important factor that contributes to the development of type I endometrial cancer (EC). Previous studies have demonstrated that metformin decreases mortality and risk of neoplasms in patients with DM. Since estrogen and estrogen receptor (ER) expression has been associated with the development of EC, the present study aimed to investigate the effects of metformin on cell proliferation and ER expression in EC cell lines that are sensitive to estrogen. The viability and proliferation of Ishikawa and HEC-1-A cells were measured following treatment with metformin and/or a 5′ AMP-activated protein kinase (AMPK) inhibitor (compound C) with or without treatment with estradiol (E2). In addition, the levels of ERα, ERβ, AMPK, ribosomal protein S6 kinase β-1 (p70S6K), myc proto-oncogene protein (c-myc) and proto-oncogene c-fos (c-fos) were measured following treatment. Metformin significantly decreased E2-stimulated cell proliferation; an effect that was rescued in the presence of compound C. Metformin treatment markedly increased the phosphorylation of AMPK while decreasing p70S6K phosphorylation, indicating that metformin exerts its effects through stimulation of AMPK and subsequent inhibition of the mammalian target of rapamycin (mTOR) signaling pathway. In addition, metformin significantly inhibited ERα expression while increasing ERβ expression, whereas treatment with compound C reversed these effects. Reverse transcription-quantitative polymerase chain reaction analysis demonstrated that c-fos and c-myc expression were attenuated by metformin, an effect that was rescued in the presence of compound C. Therefore, metformin regulates the expression of ERs, and inhibits estrogen-mediated proliferation of human EC cells through the activation of AMPK and subsequent inhibition of the mTOR signaling pathway.
Keywords: endometrial cancer, estrogen receptor, metformin, 5′ AMP-activated protein kinase
Introduction
Endometrial cancer (EC) is the fourth most common cancer in women worldwide and is the most common type of gynecological cancer (1). Patients with high estrogen levels are at increased risk of developing EC since estrogen exhibits growth-promoting properties in EC cells. Thus, estrogen serves as a tumor initiator, since it directly induces DNA mutations in tumor-suppressor genes and oncogenes (2). Upon binding to its receptor, estrogen triggers the transcription of a number of genes. There are two classes of estrogen receptor (ER), ERα and ERβ, which are encoded by the estrogen receptor 1 (ESR1) and ESR2 genes, bind to the same estrogen response elements (EREs) and regulate similar sets of genes (3). However, during the early stages of EC, the expression of ERα is increased compared with that of ERβ (4,5), which activates ERα upon estradiol (E2) binding. This stimulates the expression of estrogen target genes and leads to enhanced proliferation of the previously transformed cells, while causing additional errors in replication and potentially further DNA mutations (2).
Previous studies have demonstrated that EC is associated with a shift in the ratio of the two ER subtypes (4,6,7). In the classical model, estrogen regulates the downstream expression of genes by binding to ER and stimulating subsequent receptor dimerization and nuclear translocation (8–10). At the transcriptional level, estrogen regulates the expression of estrogen-responsive genes by binding to ER in the nucleus. In a number of target cells, ER regulates cell growth and differentiation by stimulating the transcription of proto-oncogene c-fos (c-fos), myc proto-oncogene protein (c-myc) and other proto-oncogenes (11).
Metformin, a biguanide compound, has been demonstrated to be effective in the treatment of polycystic ovarian syndrome, diabetes mellitus (DM) and insulin resistance (12). Metformin exhibits chemopreventive and anti-proliferative effects in a number of cancer types, including ovarian and breast cancer (13,14). In addition, application of metformin has been demonstrated to be significantly associated with a decrease in the incidence of cancer (15). Previous studies have reported that metformin inhibits cell proliferation and induces apoptosis in EC cell lines (16), in addition to inhibiting cell growth by non-insulin- and insulin-dependent mechanisms. Metformin regulates systemic insulin levels by increasing insulin receptor sensitivity and uptake (17). Metformin inhibits cell proliferation via liver kinase B1-mediated activation of 5′ AMP-activated protein kinase (AMPK) and reduction in mammalian target of rapamycin (mTOR) signaling (18). Activation of AMPK inhibits the mTOR signaling pathway, thereby regulating multiple signaling pathways involved in cell proliferation (19).
As previously reported, hypertension, DM and obesity are high risk factors for developing EC, with insulin resistance as a common pathophysiological basis (12). Estrogen and insulin have been demonstrated to be important risk factors leading to the development of EC (20). In non-diabetic patients with breast cancer, metformin has been demonstrated to decrease circulating estrogen levels (21). Markowska et al (22) demonstrated that the expression of ER was decreased in patients with EC and type 2 DM (DM2) receiving metformin compared with that in patients treated with insulin. However, the molecular mechanism underlying the effect of metformin on ER expression remains unclear. Metformin may inhibit the proliferation of human EC cells by regulating the expression of ER and estrogen-responsive genes, thereby altering the sensitivity of cells towards estrogen. To test this hypothesis, in the present study, the anti-neoplastic activity of metformin in EC, and the role of metformin in the expression of ER and estrogen-responsive genes were investigated in vitro. In addition, the underlying molecular mechanisms of the effects of metformin on EC cells were identified.
Materials and methods
Cell culture and reagents
The EC cell lines Ishikawa (differentiated) and HEC-1-A (poorly differentiated) were purchased from The Cell Bank of Type Culture Collection of Chinese Academy of Sciences (Shanghai, China). The cell lines were maintained in RPMI-1640 (Thermo Fisher Scientific, Inc., Waltham, MA, USA) and McCoy's 5A (Sigma-Aldrich; Merck KGaA, Darmstadt, Germany) medium containing 10% fetal bovine serum (FBS; Thermo Fisher Scientific, Inc.) in a humidified incubator with 5% CO2 at 37°C. The cells were passaged every 3–5 days. Metformin and estradiol (E2) were purchased from Sigma-Aldrich; Merck KGaA. Compound C (AMPK inhibitor) was purchased from Santa Cruz Biotechnology, Inc. (Dallas, TX, USA). Primers were purchased from Sangon Biotech Co., Ltd. (Shanghai, China). The 5′-bromo-2′-deoxyuridine (BrdU) cell proliferation ELISA assay was obtained from Roche Molecular Diagnostics (Branchburg, NJ, USA). A bicinchoninic acid protein assay kit was obtained from Pierce (Thermo Fisher Scientific, Inc.). The anti-ERα (cat. no. BS6424), anti-ERβ (cat. no. BS8465), anti-phosphorylated (p)-AMPK (cat. no. BS4457P), anti-AMPK (cat. no. BS4457), anti-phospho-S6K (cat. no. BS4440P) and anti-S6K (cat. no. BS4440) antibodies were purchased from Bioworld Technology, Inc. (St. Louis Park, MN, USA).
BrdU assays for E2 and metformin
A BrdU ELISA kit was used to measure the effects of exposure of cells to estrogen (10−6 M) and/or metformin (5 mM) in the presence or absence of compound C (5 µM). The Ishikawa and HEC-1-A cell lines were plated on 96-well plates at a concentration of 8×103 cells/well. After 24 h, the cells were serum-starved for an additional 24 h and subsequently treated with E2 (10−6 M) in the presence or absence of metformin (5 mM) for 24 h at 37°C. To analyze the role of AMPK, cells were pre-treated with compound C (5 µM) for 24 h at 37°C prior to treatment with metformin and/or E2. The effect of metformin and estradiol was calculated as a percentage of the viability of control cells (cultured with medium without FBS) seeded in 96-well plates. Serum-free conditions were used for all the assays and tests. An immunoassay was performed to monitor the synthesis of DNA based on the incorporation of BrdU into the DNA as follows: Upon treatment with the aforementioned compounds, the cells were incubated with 10 µl/well BrdU labeling solution at 37°C (10 µM) for 24 h. Prior to incubation for 30 min at room temperature, the labeling medium was removed and 200 µl/well FixDenat (BioVision. Inc., Milpitas, CA, USA) was added. Subsequently, the cells were incubated for 30 min at room temperature and FixDenat solution was removed, and the cells were incubated with 100 µl/well anti-BrdU-peroxidase solution for 90 min at 37°C. Following the incubation, the antibody conjugate was removed and the cells were rinsed three times with 200 µl/well washing solution. The washing solution was then removed, 100 µl/well substrate solution was added and the cells were incubated at room temperature for 30 min. The absorbance of samples was measured at 490 nm. Each experiment was performed in triplicate and repeated three times to ensure the consistency of the results.
Reverse transcription-quantitative polymerase chain reaction (RT-qPCR)
Ishikawa and HEC-1-A cells were plated at a concentration of 105 cells/well in 6-well plates for 24 h at 37°C and subsequently treated with metformin (0, 1, 5 and 15 mM) in McCoy's 5tA or RPMI-1640 medium containing 5% FBS, respectively, for 24 h at 37°C. In addition, to assess the role of AMPK on ER expression, cells were treated with metformin (5 mM) with or without pre-treatment with compound C (5 µM) for 24 h. Total RNA was extracted from the cells using TRIzol reagent (Invitrogen; Thermo Fisher Scientific, Inc.) according to the manufacturer's protocol. The RNA samples were subjected to DNase I (TIANGEN Biotech Co., Ltd., Beijing, China) digestion to avoid possible genomic DNA contamination and were reverse transcribed with oligo-dT primers (Qiagen GmbH, Hilden, Germany) and Moloney murine leukemia virus reverse transcriptase (Promega Corporation, Madison, WI, USA). Reactions were performed using LightCycler 480 SYBR-Green I PCR Mastermix (Roche Diagnostics, Indianapolis, IN, USA) in a 20-µl reaction, containing 1 µl cDNA, 8.2 µl DNase/RNase-free deionized water, 10 µl SYBR-Green I Mastermix and 0.4 µl each primer (10 µM). Primer sequences are as follows: ERα forward, 5′-AGTGCCTTGTTGGATGCTG-3′ and reverse, 5′-TGCCAGGTTGGTCAGTAAGC-3′; ERβ forward, 5′-AGTCCCTGGTGTGAAGCAAG-3′ and reverse, 5′-TGAGCATCCCTCTTTGAACC-3′; c-myc forward, 5′-CCTCCACTCGGAAGGACTATC-3′ and reverse, 5′-TTCGCCTCTTGACATTCTCC-3′; c-fos forward, 5′-ACTACCACTCACCCGCAGAC-3′ and reverse, 5′-GGAATGAAGTTGGCACTGGA-3′; and GAPDH forward, 5′-CAGTCAGCCGCATCTTCTTTT-3′ and reverse, 5′-GTGACCAGGCGCCCAATAC-3′. The cycling conditions for PCR were as follows: 95°C for 30 sec, followed by 40 cycles (two steps) of 95°C for 5 sec and 60°C for 31 sec. PCR was performed in triplicate for each sample on an ABI 7500 Real Time PCR Instrument (Applied Biosystems; Thermo Fisher Scientific, Inc.) to detect the fluorescent signals. The accumulation of PCR product was determined as the increase in SYBR-Green fluorescence. The mRNA levels of ERα, ERβ, c-myc and c-fos were normalized to GAPDH. The relative mRNA levels were compared and expressed as the ratio to the control groups (23).
Western blot analysis
Cells were plated at a density of 2×105 cells/well in 6-well plates for 24 h. To determine the change in the expression of ERα and ERβ, the plated cell lines were treated with metformin (0, 5 and 15 mM) with or without compound C (5 µM) in RPMI-1640 or McCoy's 5A medium containing 5% FBS for 24 h. The p-AMPK/AMPK and p-p70S6K/p70S6K protein levels were detected to investigate the relevant signaling targets. Radioimmunoprecipitation assay buffer, containing 1% NP-40, 0.5% sodium deoxycholate and 0.1% SDS, was used for the preparation of cell lysates. Protein extracts (20 µg) were subjected to 10% SDS-PAGE, subsequently transferred to polyvinylidene fluoride membranes and blocked in 5% non-fat milk in 10 mM Tris, pH 7.5, 100 mM NaCl and 0.1% Tween-20 for 1 h at room temperature. The membranes were subsequently incubated with the aforementioned primary antibodies (dilution, 1:1,000; Bioworld Technology, Inc.) overnight at 4°C. The membrane was subsequently incubated with a secondary horseradish peroxidase-linked antibody (cat. no. 7074; dilution, 1:2,000; Cell Signaling Technology, Inc., Danvers, MA, USA) for 2 h following washing three times with PBS-Tween-20 for 5 min. Bands were visualized using enhanced chemiluminescence reagents (SuperSignal™ West Pico PLUS Chemiluminescent Substrate; cat. no. 34580), according to the manufacturer's protocol (Pierce; Thermo Fisher Scientific, Inc.). Membranes were subsequently stripped and re-probed using a primary antibody against β-actin (cat. no. AP0060; dilution, 1:1,000; Cell Signaling Technology, Inc.), pan-S6K (cat. no. BS4440; dilution, 1:1,000; Bioworld Technology, Inc.) or pan-AMPK (cat. no. BS4457; dilution, 1:1,000; Bioworld Technology, Inc.) to establish equal loading. The relative protein levels were normalized to β-actin and were expressed as the ratio to the non-treatment control groups. Densitometry with Quantity One® 1-D Analysis Software (version 4.6.9 for PC; Bio-Rad Laboratories, Inc., Hercules, CA, USA) was used for the quantification of protein bands, including β-actin.
Statistical analysis
Data are presented as the mean ± standard error of the mean. One-way analysis of variance was used for statistical analyses and was performed using SPSS software (version 13.0; SPSS, Inc., Chicago, IL, USA). The data between the two groups were compared using the least significant difference test. P<0.05 was considered to indicate a statistically significant difference.
Results
Compound C rescues the decrease in cell proliferation induced by metformin
A previous study from our group demonstrated that metformin significantly decreases the viability of Ishikawa and HEC-1-A cells in a time- and dose-dependent manner (24). Compound C has been identified as an adenosine triphosphate-competitive AMPK inhibitor (25). In the present study, treatment with metformin significantly decreased cell proliferation in HEC-1-A and Ishikawa cells compared with that in the control groups (P<0.05; Fig. 1), as measured using a BrdU assay. However, pre-treatment of Ishikawa and HEC-1-A cells with compoundC significantly rescued the decrease in cell proliferation induced by metformin (P<0.05; Fig. 1).
Figure 1.
Compound C rescues the anti-proliferative effect induced by metformin. Ishikawa and HEC-1-A cells were pre-treated with compound C for 24 h prior to incubation with metformin for 24 h. Proliferation was assessed using 5′-bromo-2′-deoxyuridine assays. Results represent the mean ± standard error of the mean of triplicate samples and are representative of three independent experiments. *P<0.05 compared with the control group. #P<0.05 compared with the Met group. Con, control; Met, 5 mM metformin; Met + Com, 5 mM metformin combined with pre-treatment with 5 µM compound C.
Metformin inhibits cell proliferation through the activation of AMPK
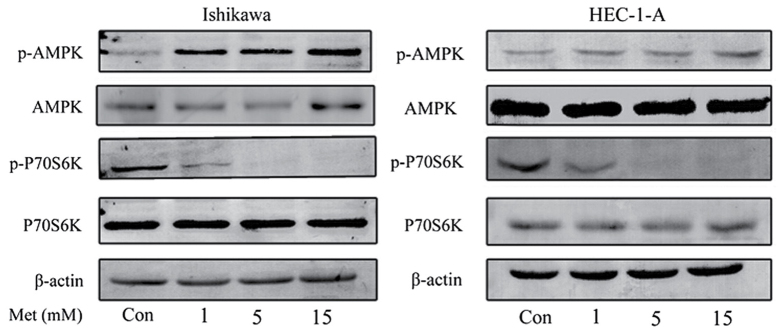
The effects of metformin on the mTOR signaling pathway were characterized in order to investigate the underlying molecular mechanisms of the anti-proliferative effects of metformin. Western blot analysis demonstrated that metformin induced the phosphorylation of AMPK in HEC-1-A and Ishikawa cells in a dose-dependent manner (Fig. 2). Previous studies have demonstrated that p70S6K is a downstream target of the mTOR signaling pathway (18,26). Metformin markedly inhibited the phosphorylation of p70S6K following 24 h of treatment; however, the effect of metformin on AMPK and p70S6K expression were not statistically significant. This suggests that metformin exhibits its anti-proliferative effect by activating AMPK and inhibiting the phosphorylation of p70S6K, which in turn results in the inhibition of the mTOR signaling pathway.
Figure 2.
Metformin activates AMPK and inhibits the mammalian target of rapamycin signaling pathway in the Ishikawa and HEC-1-A cell lines. Western blot analysis of p-AMPK, AMPK, p-p70S6K and p70S6K protein levels following treatment with the indicated concentrations of metformin for 24 h. Results are representative of three independent experiments. AMPK, 5′ AMP-activated protein kinase; p70S6K, ribosomal protein S6 kinase β-1; p, phosphorylated; Con, control; Met, metformin.
Inhibition of metformin attenuates the estrogen-mediated proliferation of EC cells
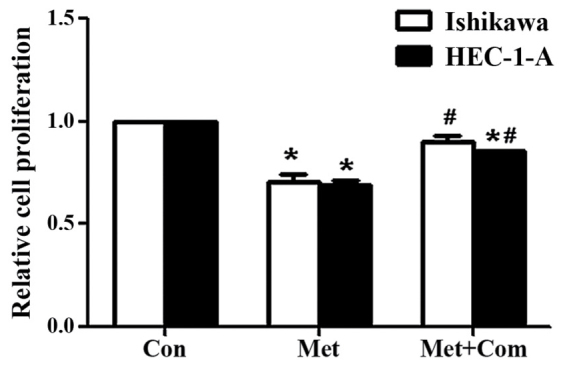
To establish the role of metformin in attenuating the estrogen-mediated proliferation of EC cells, Ishikawa and HEC-1-A cells were pre-treated with compound C for 24 h, and incubated with metformin and E2 for 24 h prior to performing a BrdU assay to assess cell proliferation. E2 significantly increased the proliferation of Ishikawa and HEC-1-A cells in comparison with that of the control group (P<0.05; Fig. 3). Co-treatment of cells with E2 and metformin significantly decreased cell proliferation compared with that of the E2 alone group (P<0.05). However, cell proliferation was significantly rescued when cells were pre-treated with compound C for 24 h (P<0.05, comparing the E2 + Met + Com group with the E2 + Met group).
Figure 3.
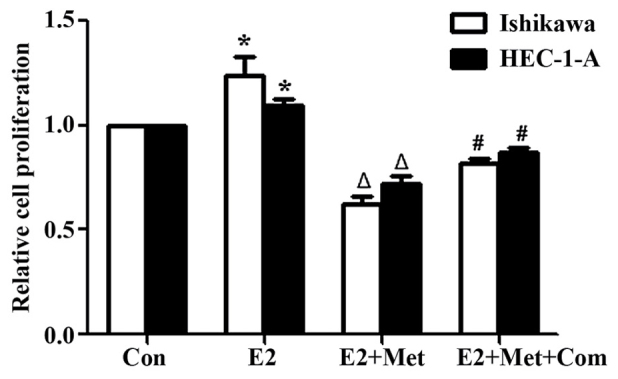
Compound C rescues the anti-proliferative effects induced by metformin following E2 stimulation. 5′-bromo-2′-deoxyuridine assay results in Ishikawa and HEC-1-A cells following treatment with E2, E2 and metformin or E2, metformin and pre-treatment with compound C for 24 h. Cells were incubated with metformin and/or E2 for 24 h with or without pre-treatment with compound C for 24 h. Results represent the mean ± standard error of the mean of triplicate samples and are representative of three independent experiments. *P<0.05, E2 group compared with control. ΔP<0.05, E2 + Met group compared with the E2 group. #P<0.05, E2 + Met + Com group compared with the E2 + Met group. Con, control; E2, 10−6 M 17β-estradiol; E2 + Met, 10−6 M 17β-estradiol combined with 5 mM metformin; E2+ Met + Com, 10−6 M 17β-estradiol combined with 5 mM metformin and pre-treatment with 5 µM compound C.
Regulation of ERα and ERβ expression levels in the Ishikawa and HEC-1-A cell lines
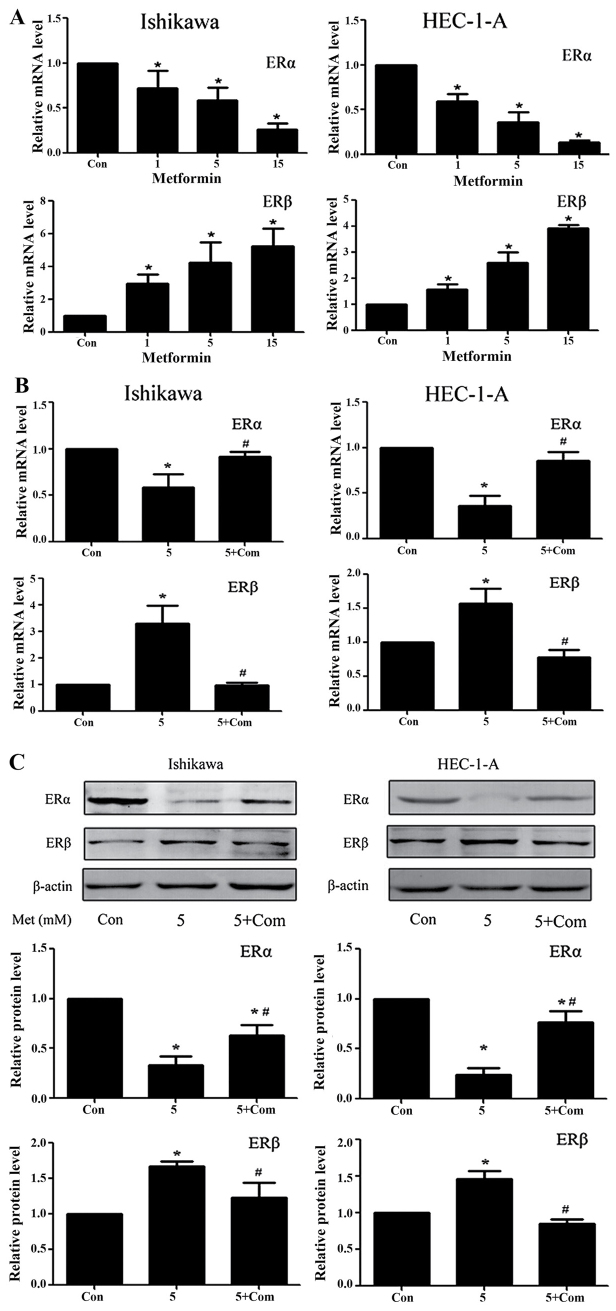
Previous studies from our group have demonstrated that metformin significantly downregulates ERα and upregulates ERβ protein levels in Ishikawa and HEC-1-A cells (24). RT-qPCR results from the present study demonstrated that, following treatment with metformin for 24 h, the ERα and ERβ mRNA levels in Ishikawa and HEC-1-A cells were significantly decreased and increased, respectively (P<0.05; Fig. 4A). Western blot analysis and RT-qPCR data from the present study suggest that metformin exhibits a regulatory effect on ER protein and mRNA levels. However, the regulatory effect of metformin on ER expression was rescued by pre-treatment with compound C at the mRNA and protein level (P<0.05; Fig. 4B and C).
Figure 4.
Metformin downregulates ERα and upregulates ERβ expression in Ishikawa and HEC-1-A cells in an AMPK-dependent manner. Reverse transcription-quantitative polymerase chain reaction analysis of ERα and ERβ expression in Ishikawa and HEC-1-A cells following treatment with (A) metformin (0, 1, 5 or 15 mM) or (B) 5 mM metformin pre-treatment with or without 5 µM compound C for 24 h. ERα and ERβ mRNA levels in each sample were calculated from a standard curve and normalized using GAPDH mRNA levels. Results represent the mean ± standard error of the mean of three independent experiments, each performed in triplicate (C) Western blot analysis of ERα and ERβ expression in Ishikawa and HEC-1-A cells following treatment with metformin (5 mM) and/or pre-treatment with compound C (5 µM) for 24 h. β-actin was used as a loading control. Results represent the mean ± standard error of the mean of three independent experiments. *P<0.05 compared with control. #P<0.05, 5+ Com group compared with the 5 mM metformin group. Con, control; 5, 5 mM metformin; 5+ Com, 5 mM metformin combined with pre-treatment with 5 µM compound C; Met, metformin; ER, estrogen receptor; AMPK, 5′ AMP-activated protein kinase.
Effect of metformin on c-fos and c-myc mRNA levels
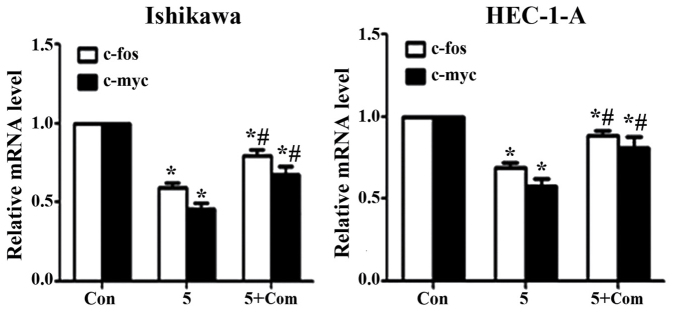
Treatment of Ishikawa and HEC-1-A cells with metformin significantly decreased the expression of c-fos and c-myc at the mRNA level (P<0.05; Fig. 5); however, the effect of metformin was reversed when cells were pre-treated with compound C prior to metformin addition (P<0.05).
Figure 5.
Compound C rescues the effects of metformin on c-fos and c-myc expression in Ishikawa and HEC-1-A cells. Reverse transcription-quantitative polymerase chain reaction analysis of c-fos and c-myc expression in Ishikawa and HEC-1-A cells following treatment with metformin (5 mM) for 24 h in the presence or absence of pre-treatment with compound C (5 µM) for 24 h. The c-fos and c-myc mRNA levels in each sample were calculated from a standard curve and normalized using GAPDH mRNA levels. Results represent the mean ± standard error of the mean of three independent experiments, each performed in triplicate. *P<0.05 compared with control. #P<0.05, 5+ Com group compared with the 5 mM metformin group. Con, control; 5, 5 mM metformin; 5+ Com, 5 mM metformin combined with pre-treatment with 5 µM compound C; c-fos, proto-oncogene c-fos; c-myc, myc proto-oncogene protein.
Discussion
A previous study from our group demonstrated the roles of metformin and estrogen in inhibiting and promoting the proliferation of EC cells (24). The results from the present study demonstrate the role of metformin in regulating ER expression; metformin significantly increased ERα while significantly decreasing ERβ expression at the mRNA and protein level in Ishikawa and HEC-1-A cells. In addition, metformin significantly inhibited the expression of c-fos and c-myc at the mRNA level. However, the molecular mechanisms underlying this regulation remain unclear.
Although its etiology remains unclear, previous studies have demonstrated the role of endocrine and genetic factors in its initiation and progression (27). According to a number of epidemiological studies, EC is associated with chronic exposure to high levels of estrogen (28). In addition, abnormalities in glucose and insulin levels are associated with EC (29).
A previous study demonstrated that metformin decreases the neoplastic proliferation of cells by modulating glucose metabolism, insulin sensitivity and intracellular signaling pathways (12). Data from the present study demonstrated that metformin inhibits the proliferation of EC cell lines; an effect that is reversible with pre-treatment with compound C, an AMPK inhibitor. Metformin treatment resulted in the activation of AMPK and its immediate downstream target, p70S6K. These results suggest that metformin exerts its effects through the mTOR signaling pathway and reveal a potential molecular mechanism for its antitumor effects on EC. These results are consistent with those from previous studies on ovarian, colon and prostate cancer cell lines (30,31).
It has previously been demonstrated that estrogen serves a critical role in the progression and development of EC (32,33). Previous studies have reported that women treated with metformin (1,500 mg/day) exhibit a significant reduction in E2 levels (−38%; P=0.02) and a borderline significant reduction in estrone (−10%; P=0.06) (21). Similar results were obtained in a randomized study in obese postmenopausal female patients with a history of polycystic ovary syndrome and/or insulin resistance (34); treatment with metformin (2,000 mg/day) resulted in a significant reduction in E2 levels (−27%). Additionally, metformin may decrease the concentration of estrogen in neoplastic tissue by locally inhibiting aromatase activity and suppressing the synthesis of the enzyme by interacting with its promoter, PII (35).
Estrogen has been demonstrated to promote the proliferation of EC cell lines. Results from the present study revealed that metformin attenuates the effect of E2 on the proliferation of EC cells. In addition, compound C rescued the anti-proliferative effect of metformin. Erdemoglu et al (36) demonstrated that metformin reduces estrogen-induced endometrial hyperplasia by inhibiting mTOR-mediated S6K1 activation, which acts as a potent regulator of protein synthesis and growth. Therefore, the metformin-induced attenuation of the effect of E2 on cell proliferation may be attributed to the activation of AMPK followed by the inhibition of the mTOR signaling pathway.
Estrogen-induced regulation of cell proliferation occurs via the ERα and ERβ isoforms. Estrogen and ERs serve critical roles in the initiation and development of EC (37). ERα is associated with estrogen-induced mitogenic signaling, whereas the function of ERβ is opposite to that of ERα (38). In the human mammary gland, E2 binds to ER isoforms, thereby regulating the proliferation and differentiation of cells (39). The E2-ERα complex functions as a regulator of the transcription of genes involved in the proliferation, differentiation and survival of cells. In various types of cancer, ERβ serves a critical role in inhibiting the ERα-mediated transcription and E2-induced proliferation of cells (38,40). Furthermore, in normal mammary epithelial cells, ERα and ERβ differentially regulate proliferation and apoptosis (41). The ERα/ERβ ratio has been reported to be a key element in modulating the activity of E2 in breast cancer cells (42). Markowska et al (22) reported a significant decrease in the expression of ER in female patients with DM2 and EC following administration of metformin compared with that in patients treated with insulin (P=0.004). However, the study did not demonstrate the effects of metformin on ER subtypes.
The data from the present study demonstrate that metformin reduces the expression of ERα while promoting the expression of ERβ, which may be associated with the activation of AMPK. Therefore, the regulation of ER subtype expression following metformin treatment may affect cell proliferation and influence the prognosis of patients with EC. However, further studies are required to validate this hypothesis.
Studies on breast cancer cells (43) and rat uterus (44) have demonstrated estrogen-induced induction of c-fos, c-myc and N-myc proto-oncogene protein. In mammalian uterus, estrogen induces the expression of c-fos (45), c-myc (46) and c-jun (47). In addition, metformin acts as a chemopreventive agent by downregulating the expression of c-myc to restrict the initiation and transformation of prostatic neoplasia (48). According to Zhang et al (17), metformin treatment attenuates the estrogen-dependent proliferation of c-myc and c-fos expression in the endometrium of obese rats compared with that in untreated controls. The findings from the present study indicate that metformin treatment results in reduced expression of c-myc and c-fos in vitro. Metformin treatment activated the AMPK signaling pathway and concomitantly downregulated the expression of c-myc and c-fos. It has previously been demonstrated that metformin reduces the expression of c-myc in breast tumors (49) and prostatic neoplasia (48) by activating AMPK. According to Dang (50), c-myc and c-fos oncogenes are critical in the tumorigenesis of a number of human cancers.
In conclusion, the results from the present study indicate that metformin inhibits the proliferation of EC cells through the activation of AMPK and subsequent inhibition of the mTOR signaling pathway. Further studies are required to identify the role of metformin as chemopreventive agent in populations with a high risk of cancer.
Glossary
Abbreviations
- Met
metformin
- EC
endometrial cancer
- ER
estrogen receptor
- E2
estradiol
- AMPK
5′ AMP-activated protein kinase
- Com
compound C
- Con
control
References
- 1.Fader AN, Arriba LN, Frasure HE, von Gruenigen VE. Endometrial cancer and obesity: Epidemiology, biomarkers, prevention and survivorship. Gynecol Oncol. 2009;114:121–127. doi: 10.1016/j.ygyno.2009.03.039. [DOI] [PubMed] [Google Scholar]
- 2.Rižner T. Estrogen biosynthesis, phase I and phase II metabolism, and action in endometrial cancer. Mol Cell Endocrinol. 2013;381:124–139. doi: 10.1016/j.mce.2013.07.026. [DOI] [PubMed] [Google Scholar]
- 3.Klinge CM. Estrogen receptor interaction with estrogen response elements. Nucleic Acids Res. 2001;29:2905–2919. doi: 10.1093/nar/29.14.2905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Sakaguchi H, Fujimoto J, Aoki I, Toyoki H, Khatun S, Tamaya T. Expression of oestrogen receptor alpha and beta in uterine endometrial and ovarian cancers. Eur J Cancer. 2002;38(Suppl 6):S74–S75. doi: 10.1016/S0959-8049(02)00296-4. [DOI] [PubMed] [Google Scholar]
- 5.Utsunomiya H, Suzuki T, Harada N, Ito K, Matsuzaki S, Konno R, Sato S, Yajima A, Sasano H. Analysis of estrogen receptor alpha and beta in endometrial carcinomas: Correlation with ER beta and clinicopathologic findings in 45 cases. Int J Gynecol Pathol. 2000;19:335–341. doi: 10.1097/00004347-200010000-00007. [DOI] [PubMed] [Google Scholar]
- 6.Hu K, Zhong G, He F. Expression of estrogen receptors ERalpha and ERbeta in endometrial hyperplasia and adenocarcinoma. Int J Gynecol Cancer. 2005;15:537–541. doi: 10.1111/j.1525-1438.2005.15321.x. [DOI] [PubMed] [Google Scholar]
- 7.Saegusa M, Okayasu I. Changes in expression of estrogen receptors alpha and beta in relation to progesterone receptor and pS2 status in normal and malignant endometrium. Jpn J Cancer Res. 2000;91:510–518. doi: 10.1111/j.1349-7006.2000.tb00975.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Bolton JL, Thatcher GR. Potential mechanisms of estrogen quinone carcinogenesis. Chem Res Toxicol. 2008;21:93–101. doi: 10.1021/tx700191p. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Hammes SR, Levin ER. Minireview: Recent advances in extranuclear steroid receptor actions. Endocrinology. 2011;152:4489–4495. doi: 10.1210/en.2011-1470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Prossnitz ER, Barton M. The G-protein-coupled estrogen receptor GPER in health and disease. Nat Rev Endocrinol. 2011;7:715–726. doi: 10.1038/nrendo.2011.122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Yamashita S, Takayanagi A, Shimizu N. Temporal and cell-type specific expression of c-fos and c-jun protooncogenes in the mouse uterus after estrogen stimulation. Endocrinology. 1996;137:5468–5475. doi: 10.1210/endo.137.12.8940373. [DOI] [PubMed] [Google Scholar]
- 12.Bjørge T, Lukanova A, Jonsson H, Tretli S, Ulmer H, Manjer J, Stocks T, Selmer R, Nagel G, Almquist M, et al. Metabolic syndrome and breast cancer in the me-can (metabolic syndrome and cancer) project. Cancer Epidemiol Biomarkers Prev. 2010;19:1737–1745. doi: 10.1158/1055-9965.EPI-10-0230. [DOI] [PubMed] [Google Scholar]
- 13.Del Barco S, Vazquez-Martin A, Cufi S, Oliveras-Ferraros C, Bosch-Barrera J, Joven J, Martin-Castillo B, Menendez JA. Metformin: Multi-faceted protection against cancer. Oncotarget. 2011;2:896–917. doi: 10.18632/oncotarget.387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Dowling RJ, Goodwin PJ, Stambolic V. Understanding the benefit of metformin use in cancer treatment. BMC Med. 2011;9:33. doi: 10.1186/1741-7015-9-33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Rocha GZ, Dias MM, Ropelle ER, Osório-Costa F, Rossato FA, Vercesi AE, Saad MJ, Carvalheira JB. Metformin amplifies chemotherapy-induced AMPK activation and antitumoral growth. Clin Cancer Res. 2011;17:3993–4005. doi: 10.1158/1078-0432.CCR-10-2243. [DOI] [PubMed] [Google Scholar]
- 16.Cantrell LA, Zhou C, Mendivil A, Malloy KM, Gehrig PA, Bae-Jump VL. Metformin is a potent inhibitor of endometrial cancer cell proliferation-implications for a novel treatment strategy. Gynecol Oncol. 2010;116:92–98. doi: 10.1016/j.ygyno.2009.09.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Zhang Q, Celestino J, Schmandt R, McCampbell AS, Urbauer DL, Meyer LA, Burzawa JK, Huang M, Yates MS, Iglesias D, et al. Chemopreventive effects of metformin on obesity-associated endometrial proliferation. Am J Obstet Gynecol. 2013;209:24.e1–24.e12. doi: 10.1016/j.ajog.2013.03.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Dowling RJ, Zakikhani M, Fantus IG, Pollak M, Sonenberg N. Metformin inhibits mammalian target of rapamycin-dependent translation initiation in breast cancer cells. Cancer Res. 2007;67:10804–10812. doi: 10.1158/0008-5472.CAN-07-2310. [DOI] [PubMed] [Google Scholar]
- 19.Viollet B, Guigas B, Garcia Sanz N, Leclerc J, Foretz M, Andreelli F. Cellular and molecular mechanisms of metformin: An overview. Clin Sci (Lond) 2012;122:253–270. doi: 10.1042/CS20110386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Esteva FJ, Moulder SL, Gonzalez-Angulo AM, Ensor J, Murray JL, Green MC, Koenig KB, Lee MH, Hortobagyi GN, Yeung SC. Phase I trial of exemestane in combination with metformin and rosiglitazone in nondiabetic obese postmenopausal women with hormone receptor-positive metastatic breast cancer. Cancer Chemother Pharmacol. 2013;71:63–72. doi: 10.1007/s00280-012-1977-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Campagnoli C, Berrino F, Venturelli E, Abbà C, Biglia N, Brucato T, Cogliati P, Danese S, Donadio M, Zito G, Pasanisi P. Metformin decreases circulating androgen and estrogen levels in nondiabetic women with breast cancer. Clin Breast Cancer. 2013;13:433–438. doi: 10.1016/j.clbc.2013.08.012. [DOI] [PubMed] [Google Scholar]
- 22.Markowska A, Pawałowska M, Filas V, Korski K, Gryboś M, Sajdak S, Olejek A, Bednarek W, Spiewankiewicz B, Lubin J, Markowska J. Does Metformin affect ER, PR, IGF-1R, β-catenin and PAX-2 expression in women with diabetes mellitus and endometrial cancer? Diabetol Metab Syndr. 2013;5:76. doi: 10.1186/1758-5996-5-76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Xie Y, Wang YL, Yu L, Hu Q, Ji L, Zhang Y, Liao QP. Metformin promotes progesterone receptor expression via inhibition of mammalian target of rapamycin (mTOR) in endometrial cancer cells. J Steroid Biochem Mol Biol. 2011;126:113–120. doi: 10.1016/j.jsbmb.2010.12.006. [DOI] [PubMed] [Google Scholar]
- 24.Zhang J, Zhang B, Yin Z, Chen F, Liu T, Xu H, Liu Y, Zhou X. Effects of metformin on the estrogen-induced proliferation and the expression of ER in human endometrial cancer cells. Zhonghua Fu Chan Ke Za Zhi. 2014;49:932–937. (In Chinese) [PubMed] [Google Scholar]
- 25.Emerling BM, Viollet B, Tormos KV, Chandel NS. Compound C inhibits hypoxic activation of HIF-1 independent of AMPK. FEBS Lett. 2007;581:5727–5731. doi: 10.1016/j.febslet.2007.11.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Jefferies HB, Fumagalli S, Dennis PB, Reinhard C, Pearson RB, Thomas G. Rapamycin suppresses 5′TOP mRNA translation through inhibition of p70s6k. EMBO J. 1997;16:3693–3704. doi: 10.1093/emboj/16.12.3693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Ellenson LH, Wu TC. Focus on endometrial and cervical cancer. Cancer Cell. 2004;5:533–538. doi: 10.1016/j.ccr.2004.05.029. [DOI] [PubMed] [Google Scholar]
- 28.Farnell YZ, Ing NH. The effects of estradiol and selective estrogen receptor modulators on gene expression and messenger RNA stability in immortalized sheep endometrial stromal cells and human endometrial adenocarcinoma cells. J Steroid Biochem Mol Biol. 2003;84:453–461. doi: 10.1016/S0960-0760(03)00076-1. [DOI] [PubMed] [Google Scholar]
- 29.Soliman PT, Wu D, Tortolero-Luna G, Schmeler KM, Slomovitz BM, Bray MS, Gershenson DM, Lu KH. Association between adiponectin, insulin resistance, and endometrial cancer. Cancer. 2006;106:2376–2381. doi: 10.1002/cncr.21866. [DOI] [PubMed] [Google Scholar]
- 30.Zakikhani M, Dowling RJ, Sonenberg N, Pollak MN. The effects of adiponectin and metformin on prostate and colon neoplasia involve activation of AMP-activated protein kinase. Cancer Prev Res (Phila) 2008;1:369–375. doi: 10.1158/1940-6207.CAPR-08-0081. [DOI] [PubMed] [Google Scholar]
- 31.Gotlieb WH, Saumet J, Beauchamp MC, Gu J, Lau S, Pollak MN, Bruchim I. In vitro metformin anti-neoplastic activity in epithelial ovarian cancer. Gynecol Oncol. 2008;110:246–250. doi: 10.1016/j.ygyno.2008.04.008. [DOI] [PubMed] [Google Scholar]
- 32.Terry KL, Missmer SA. Epidemiology of ovarian and endometrial cancers. Pathol Epidemiol Cancer. 2017 doi: 10.1007/978-3-319-35153-7_13. [DOI] [Google Scholar]
- 33.Zhao H, Jiang Y, Liu Y, Yun C, Li L. Endogenous estrogen metabolites as biomarkers for endometrial cancer via a novel method of liquid chromatography-mass spectrometry with hollow fiber liquid-phase microextraction. Horm Metab Res. 2015;47:158–164. doi: 10.1055/s-0034-1371865. [DOI] [PubMed] [Google Scholar]
- 34.Patel SM, Iqbal N, Kaul S, Ratcliffe SJ, Rickels MR, Reilly MP, Scattergood T, Basu A, Fuller C, Cappola AR. The effects of metformin and leuprolide acetate on insulin resistance and testosterone levels in non-diabetic postmenopausal women: A randomized, placebo-controlled trial. Fertil Steril. 2010;94:2161–2166. doi: 10.1016/j.fertnstert.2010.01.064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Brown KA, Hunger NI, Docanto M, Simpson ER. Metformin inhibits aromatase expression in human breast adipose stromal cells via stimulation of AMP-activated protein kinase. Breast Cancer Res Treat. 2010;123:591–596. doi: 10.1007/s10549-010-0834-y. [DOI] [PubMed] [Google Scholar]
- 36.Erdemoglu E, Güney M, Giray SG, Take G, Mungan T. Effects of metformin on mammalian target of rapamycin in a mouse model of endometrial hyperplasia. Eur J Obstet Gynecol Reprod Biol. 2009;145:195–199. doi: 10.1016/j.ejogrb.2009.04.034. [DOI] [PubMed] [Google Scholar]
- 37.Burns KA, Korach KS. Estrogen receptors and human disease: An update. Arch Toxicol. 2012;86:1491–1504. doi: 10.1007/s00204-012-0868-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Ström A, Hartman J, Foster JS, Kietz S, Wimalasena J, Gustafsson JA. Estrogen receptor beta inhibits 17beta-estradiol-stimulated proliferation of the breast cancer cell line T47D. Proc Natl Acad Sci USA. 2004;101:1566–1571. doi: 10.1073/pnas.0308319100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Helguero LA, Faulds MH, Gustafsson JA, Haldosén LA. Estrogen receptors alfa (ERalpha) and beta (ERbeta) differentially regulate proliferation and apoptosis of the normal murine mammary epithelial cell line HC11. Oncogene. 2005;24:6605–6616. doi: 10.1038/sj.onc.1208807. [DOI] [PubMed] [Google Scholar]
- 40.Paruthiyil S, Parmar H, Kerekatte V, Cunha GR, Firestone GL, Leitman DC. Estrogen receptor beta inhibits human breast cancer cell proliferation and tumor formation by causing a G2 cell cycle arrest. Cancer Res. 2004;64:423–428. doi: 10.1158/0008-5472.CAN-03-2446. [DOI] [PubMed] [Google Scholar]
- 41.Grober OM, Mutarelli M, Giurato G, Ravo M, Cicatiello L, De Filippo MR, Ferraro L, Nassa G, Papa MF, Paris O, et al. Global analysis of estrogen receptor beta binding to breast cancer cell genome reveals an extensive interplay with estrogen receptor alpha for target gene regulation. BMC Genomics. 2011;12:36. doi: 10.1186/1471-2164-12-36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Matthews J, Gustafsson JA. Estrogen signaling: A subtle balance between ER alpha and ER beta. Mol Interv. 2003;3:281–292. doi: 10.1124/mi.3.5.281. [DOI] [PubMed] [Google Scholar]
- 43.Ali SH, O'Donnell AL, Balu D, Pohl MB, Seyler MJ, Mohamed S, Mousa S, Dandona P. Estrogen receptor-alpha in the inhibition of cancer growth and angiogenesis. Cancer Res. 2000;60:7094–7098. [PubMed] [Google Scholar]
- 44.Ali SH, O'Donnell AL, Balu D, Pohl MB, Seyler MJ, Mohamed S, Mousa S, Dandona P. High levels of oestrogen receptor-alpha in tumorigenesis: Inhibition of cell growth and angiogenic factors. Cell Prolif. 2001;34:223–231. doi: 10.1046/j.0960-7722.2001.00207.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Loose-Mitchell DS, Chiappetta C, Stancel GM. Estrogen regulation of c-fos messenger ribonucleic acid. Mol Endocrinol. 1988;2:946–951. doi: 10.1210/mend-2-10-946. [DOI] [PubMed] [Google Scholar]
- 46.Murphy LJ, Murphy LC, Friesen HG. Estrogen induction of N-myc and c-myc proto-oncogene expression in the rat uterus. Endocrinology. 1987;120:1882–1888. doi: 10.1210/endo-120-5-1882. [DOI] [PubMed] [Google Scholar]
- 47.Weisz A, Cicatiello L, Persico E, Scalona M, Bresciani F. Estrogen stimulates transcription of c-jun protooncogene. Mol Endocrinol. 1990;4:1041–1050. doi: 10.1210/mend-4-7-1041. [DOI] [PubMed] [Google Scholar]
- 48.Akinyeke T, Matsumura S, Wang X, Wu Y, Schalfer ED, Saxena A, Yan W, Logan SK, Li X. Metformin targets c-MYC oncogene to prevent prostate cancer. Carcinogenesis. 2013;34:2823–2832. doi: 10.1093/carcin/bgt307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Blandino G, Valerio M, Cioce M, Mori F, Casadei L, Pulito C, Sacconi A, Biagioni F, Cortese G, Galanti S, et al. Metformin elicits anticancer effects through the sequential modulation of DICER and c-MYC. Nat Commun. 2012;3:865. doi: 10.1038/ncomms1859. [DOI] [PubMed] [Google Scholar]
- 50.Dang CV. MYC on the path to cancer. Cell. 2012;149:22–35. doi: 10.1016/j.cell.2012.03.003. [DOI] [PMC free article] [PubMed] [Google Scholar]