Abstract
Introduction
Treatment for children with clinically aggressive, high-risk neuroblastoma remains challenging. Less than 50% of patients with high-risk neuroblastoma will survive long-term with current therapies, and survivors are at risk for serious treatment-related late toxicities. Here, we review new and evolving treatments that may ultimately improve outcome for children with high-risk neuroblastoma with decreased potential for late adverse events.
Areas covered
New strategies for treating high-risk neuroblastoma are reviewed including: radiotherapy, targeted cytotoxics, biologics, immunotherapy, and molecularly targeted agents. Recently completed and ongoing neuroblastoma clinical trials testing these novel treatments are highlighted. In addition, we discuss ongoing clinical trials designed to evaluate precision medicine approaches that target actionable somatic mutations and oncogenic cellular pathways.
Expert opinion
Advances in genomic medicine and molecular biology have led to the development of early phase studies testing biologically rational therapies targeting aberrantly activated cellular pathways. Because many of these drugs have a wider therapeutic index than standard chemotherapeutic agents, these treatments may be more effective and less toxic than current strategies. However, to effectively integrate these targeted strategies, robust predictive biomarkers must be developed that will identify patients who will benefit from these approaches and rapidly match treatments to patients at diagnosis.
Keywords: Neuroblastoma, immunotherapy, MIBG, molecularly targeted drugs, genomic trials
1. Introduction
Neuroblastoma, a common pediatric neoplasm of the sympathetic nervous system, is remarkable for its heterogeneous clinical behavior and varying response to treatment [1]. Subsets of patients are highly curable with surgery alone or with adjuvant chemotherapy [2, 3], while infants with localized tumors commonly achieve long-term survival without any treatment, including surgery [4]. Approximately 45% of patients have clinically aggressive, high-risk tumors that are relatively resistant to chemotherapy and radiation. Although treatment for this cohort remains challenging, increasingly intensive multi-modality treatments developed throughout the world that include myeloablative therapy and stem cell transplant have led to improved survival [5]. In addition, a seminal randomized Children's Oncology Group (COG) study demonstrated the efficacy of post-consolidation immunotherapy [6], leading to FDA approval of dinutuximab for high-risk patients. Most recently, 3-year overall survival rates of more than 70% were reported in a randomized COG trial testing further intensification of consolidation therapy with tandem cycles of high-dose therapy and autologous stem cell transplant [7]. While these results are promising, further follow-up will be needed to determine if this “more-is-better” approach will ultimately lead to improved long-term survival. Unfortunately, this aggressive treatment strategy confers an increased risk for organ dysfunction and other treatment-related late adverse events including second cancers [8]. Thus, it is clear that more effective strategies with a higher therapeutic index are desperately needed. This review will provide an overview of emerging and investigational therapies, many of which are based on a better understanding of the oncogenic drivers of this aggressive tumor. We will primarily focus on therapies that are currently being tested in early phase studies.
2. 131I-meta-iodobenzylguanidine (MIBG)
Although the anti-tumor activity of the radiopharmaceutical 131I-MIBG in refractory and relapsed neuroblastoma was first reported in the 1980's [9], it remains unclear if the addition of 131I-MIBG in treatment strategies for newly diagnosed neuroblastoma patients will lead to improved survival. Response rates in early phase studies of patients with relapsed and refractory disease have been promising, and in a large Phase II study of 164 patients, 37% achieved a partial or complete response with 131I-MIBG at a dose of 18 mCi/kg [10]. Efforts to further improve the rate of response have included rapid tandem infusion 131I-MIBG with autologous stem cell rescue and combination therapy with radiosensitizers or chemotherapy [11]. To directly test if response to 131I-MIBG is improved with radiosensitizers, The New Approaches to Neuroblastoma Therapy (NANT) consortium is currently conducting a randomized Phase II comparing single-agent 131I-MIBG to combination regimens with 131I-MIBG plus vincristine and irinotecan or vorinostat (NCT02035137, Table 1). The feasibility of incorporating 131I-MIBG into consolidation regimens with myeloablative chemotherapy and autologous stem cell transplant has also been demonstrated [12].
Table 1. Description of clinical trials currently available using 131I-MIBG and targeted cytotoxic therapy for patients with high-risk neuroblastoma.
| Trial Identifier | Study question | Sponsor | Study agent(s) | Phase | Disease eligibility | Age Range |
|---|---|---|---|---|---|---|
| NCT02035137 | Efficacy of single-agent 131I-MIBG compared to combination regimens with 131I-MIBG plus vincristine and irinotecan or vorinostat | NANT | 131I-MIBG, vincristine, irinotecan, vorinostat | 2 | Recurrent/ refractory neuroblastoma | 2 – 30 yrs. |
| NCT01175356 | Feasibility and tolerability of 12 mCi/kg, 15 mCi/kg, or 18 mCi/kg 131I-MIBG administered prior to myeloablative therapy with busulfan and melphalan | COG | 131I-MIBG | Pilot | New diagnosis neuroblastoma | 1 – 30 yrs. |
| NCT01962103 | Dose finding and efficacy of nab™-paclitaxel in recurrent/ refractory solid tumors | Celgene | nab™-paclitaxel | 1/2 | Recurrent/ refractory solid tumors | 6 mo. – 24 yrs. |
| NCT02013336 | Dose finding and tolerability of irinotecan sucrosofate liposome injection (MM-398) with cyclophosphamide in recurrent/ refractory solid tumors | Merrimack Pharmaceuticals/ SPOC | irinotecan sucrosofate (MM-398) | 1 | Recurrent/ refractory solid tumors | 1 – 20 yrs. |
| NCT02452554 | Efficacy of lorvotuzumab mertansine in recurrent/ refractory solid tumors | COG | lorvotuzumab mertansine (IMGN901) | 2 | Recurrent/ refractory solid tumors | 1 – 30 yrs. |
Abbreviations: COG; Children's Oncology Group, SPOC; South Plains Oncology Consortium, NANT: New Advances in Neuroblastoma
A small number of studies have tested 131I-MIBG during the induction or consolidation phase of treatment in newly diagnosed patients. Schmidt and colleagues retrospectively analyzed data from a German neuroblastoma trial in which 40 of 111 patients with residual disease following induction therapy were treated with 131I-MIBG [13]. Although significantly improved 3-year event-free survival and overall survival was observed in the cohort treated with 131I-MIBG, no difference in outcome was seen in a subgroup analysis of 66 patients who underwent high-dose chemotherapy with autologous stem cell transplantation. In a study conducted in the Netherlands, a response rate of 66% was observed following two cycles of 131I-MIBG administered to 41 newly diagnosed children prior to induction chemotherapy [14]. Recently, COG conducted a pilot study (NCT01175356, Table 1) testing the feasibility and tolerability of 12 mCi/kg, 15 mCi/kg, or 18 mCi/kg 131I-MIBG administered prior to myeloablative therapy with busulfan and melphalan in high-risk patients regardless of response to induction therapy. Significant toxicities were observed with the 18 mCi/Kg dose, although the stopping rules outlined in the protocol were not met. Based on these results, COG is now developing the first randomized Phase III study for newly diagnosed high-risk patients that will directly test if the addition of 131I-MIBG at a dose of 15 mCi/kg following cycle 3 of induction chemotherapy improves survival.
3. Targeted cytotoxic therapy
3.1. Nanoparticle Tumor Targeting Strategies
Protein nanoparticles were originally designed to address the toxicity of solvent-based formulations of hydrophobic anti-cancer agents. Additionally, traditional cytotoxic agents are being linked to antibodies which direct these agents specifically to tumor cells while sparing normal tissue. To reduce Cremophor® EL (CrEL) associated, dose-limiting hypersensitivity to paclitaxel, a 130-nanometer albumin-bound paclitaxel (nab™-paclitaxel) was first introduced and approved by the FDA in January 2005 for the treatment of relapsed metastatic breast cancer. The nab™ technology takes advantage of endogenous human albumin receptor mediated transcytosis and albumin binding proteins, such as secreted protein acidic and rich in cysteine (SPARC), to deliver higher doses of paclitaxel across the vascular endothelium and into tumors [15]. In preclinical studies, neuroblastoma xenografts were responsive to nab™-paclitaxel in a dose dependent fashion and nab™-paclitaxel produced significantly more favorable tumor/plasma ratios versus solvent-based drug, with enhanced delivery to tumor tissue, but lower systemic exposure [16]. These properties potentially allow for intratumoral dose intensification with decreased toxicity. A phase 2 trial of nab™-paclitaxel in neuroblastoma, rhabdomyosarcoma, and Ewing sarcoma is ongoing (NCT01962103, Table 1). Several other nanoparticle tumor targeting strategies are in development, including liposomal irinotecan (MM-398; Onivyde®; NCT02013336, Table 1) and a poly(lactide)-poly(ethylene glycol) (PLA-PEG)-based polymeric nanoparticle containing SN-38, the active metabolite of irinotecan [17].
3.2. Antibody-drug conjugates (ADC)
ADC is another unique avenue to direct cytotoxic agents to tumor. For neuroblastoma, lorvotuzumab mertansine (IMGN901) is the agent in class furthest along in the development pipeline. IMGN901 is a high-affinity humanized antibody recognizing CD56 (NCAM, neural cell adhesion molecule) conjugated to a derivative of maytansine, a highly potent microtubule inhibitor (DM1; mertansine) [18]. NCAM is almost ubiquitously expressed by neuroblastic tumors, although the particular isoform may differ depending on maturational status [19]. When IMGN901 binds CD56, it is internalized. The disulfide linker is cleaved within the cell to allow local concentrated release of mertansine and mitotic arrest, theoretically augmenting tumor kill while diminishing systemic side effects. A phase 2 study of IMGN901 in neuroblastoma and other CD56 expressing tumors through COG (NCT02452554, Table 1) was recently suspended due to drug availability. Recently, IMGN901 failed to show an improvement in survival in small-cell lung cancer patients and was attributed with increased infectious deaths [20]. Another potential ADC target being explored preclinically for neuroblastoma is the ALK receptor tyrosine kinase [21].
4. Immunotherapy
4.1. First Generation Anti-Neuroblastoma Antibodies
One of the most significant recent advances in the treatment of patients with high-risk neuroblastoma has been the development of therapies targeted to the cell surface GD2 disialoganglioside. Preclinical and early phase trials demonstrated that the chimeric antibody ch14.18 (dinutuximab) efficiently binds GD2 and culminated in the seminal COG ANBL0032 study which demonstrated that dinutuximab plus cytokines (GMS-CSF and IL-2) significantly improves EFS and OS [6]. In an effort to mitigate the pain and other toxicities that are seen when the antibody is infused over 8 to 20 hours, SIOPEN conducted a Phase II clinical trial testing a 10-day continuous infusion of Ch14.18 manufactured in Chinese hamster ovary (CHO) cells (ch14.18.CHO) in combination with sub-cutaneous (sc) IL-2 [22, 23]. Results of this Phase II study demonstrated clinically activity of the long-term delivery method of ch14.18/CHO and an improved toxicity profile. SIOPEN also recently conducted a randomized Phase III immunotherapy study to investigate the role of scIL-2 in the immune response, and no survival benefit of co-treatment with ch14.18/CHO and scIL-2 was observed [24] (NCT01704716, Table 2).
Table 2. Description of clinical trials currently available using immunotherapy for patients with high-risk neuroblastoma.
| Trial Identifier | Study question | Sponsor | Study agent(s) | Phase | Disease eligibility | Age Range |
|---|---|---|---|---|---|---|
| NCT01704716 | Compare efficacy of ch14.18/CHO with or without IL-2 in consolidation | SIOPEN | ch14.18/CHO, IL-2 | 3 | New diagnosis neuroblastoma | 1 mo. – 21 yrs. |
| NCT01767194 | Efficacy of dinutuximab plus irinotecan and temozolomide in first relapse | COG | dinutuximab, irinotecan, temozolomide | 2 | First recurrence/ progression neuroblastoma | All ages |
| NCT01757626 | Dose finding and efficacy of hu3F8 plus GM-CSF | Memorial Sloan Kettering Cancer Center | hu3F8, GM-CSF | 1/2 | Recurrent/ refractory neuroblastoma | ≥ 1 yr. |
| NCT01419834 | Dose finding and tolerability of hu3F8 alone | Memorial Sloan Kettering Cancer Center | hu3F8 | 1 | Recurrent/ refractory neuroblastoma | ≥ 1 yr. |
| NCT03033303 | Efficacy of hu3F8, GM-CSF, isotretinoin for consolidation of high-risk neuroblastoma in first remission | Memorial Sloan Kettering Cancer Center | hu3F8, GM-CSF, isotretinoin | 2 | Neuroblastoma in first remission | All ages |
| NCT01857934 | Efficacy of two courses of cyclophosphamide and topotecan combined with hu14.18K322A in induction chemotherapy | St. Jude | hu14.18K322A | 2 | New diagnosis neuroblastoma | ≤ 18 yrs. |
| NCT02573896 | Dose finding and tolerability of autologous NK cells, lenalidomide, and dinutuximab in recurrent/ refractory neuroblastoma | NANT | NK cells, lenalidomide, and dinutuximab | 1 | Recurrent/ refractory neuroblastoma | 1 mo. – 30 yrs. |
| NCT01576692 | Feasibility and tolerability of hu14.18K322A with allogeneic NK cells in recurrent/ refractory neuroblastoma | St. Jude | NK cells and hu14.18K322A | 1 | Recurrent/ refractory neuroblastoma | ≤ 21 yrs. |
| NCT02650648 | Feasibility and tolerability of hu3F8, cyclophosphamide, and allogenic NK cells in recurrent/ refractory neuroblastoma | Memorial Sloan Kettering Cancer Center | NK cells, hu3F8, and cyclophosphamide, | 1 | Recurrent/ refractory neuroblastoma | All ages |
| NCT02100891 | Efficacy of allogenic NK cells and haploidentical hematopoietic cell transplantation in recurrent/ refractory solid tumors | Medical College of Wisconsin | NK cells, stem-cell transplant | 2 | Recurrent/ refractory solid tumors | All ages |
| NCT02304458 | Dose finding and efficacy of nivolumab with or without ipilumimab in recurrent/ refractory solid tumors | COG | nivolumab, ipilumimab | 1/2 | Recurrent/ refractory solid tumors | 1 – 30 yrs. |
| NCT02332668 | Dose finding and efficacy of pembrolizumab in recurrent/ refractory solid tumors | Merck | pembrolizumab | 1/2 | Recurrent/ refractory solid tumors | 6 mo. – 17 yrs. |
| NCT00911560 | Dose finding and efficacy of a bivalent vaccine, OPT-821, and β-glucan for patients in neuroblastoma patients in remission > 6 months from end of treatment | Memorial Sloan Kettering Cancer Center | OPT-821, immunological adjuvant GD2L and GD3L, β-glucan | 1/2 | Neuroblastoma in remission | ≤ 21 yrs. |
| NCT02745756 | Feasibility and tolerability of a tumor lysate dendritic cell vaccine | H. Lee Moffitt Cancer Center and Research Institute | Dendritic cell vaccine | Pilot | All neuroblastoma patients undergoing autologous stem cell transplant and surgery | ≤ 21 yrs. |
Abbreviations: COG; Children's Oncology Group, SIOPEN; International Society of Pediatric Oncology Europe Neuroblastoma, NANT; New Advances in Neuroblastoma, IL-2; Interleuken-2, GM-CSF; Granulocyte macrophage colony-stimulating factor, NK; Natural killer
A randomized COG Phase II study has recently demonstrated the efficacy of combining chemotherapy (irinotecan and temozolomide) with dinutuximab for neuroblastoma patients in first relapse [25] (NCT01767194, Table 2). Specifically, nine of seventeen patients (52.9%) treated with dinutuximab and chemotherapy had a clinical response, and five (19.4%) achieved a complete remission. These encouraging results led an expanded enrollment on the antibody arm of this study in order to collect more data on the efficacy of this combination. In addition, a pilot study for newly diagnosed patient with high-risk neuroblastoma testing dinutuximab combined with induction chemotherapy is under development within the COG.
4.2. Second-generation GD2-targeted immunotherapy
A humanized version of the murine 3F8 (hu3F8) has been developed [26] and is being tested in multiple trials combined or not with sc GM-CSF in the relapsed setting (NCT01757626, NCT01419834, Table 2) or with sc GM-CSF and isotretinoin as consolidation therapy for patients in first remission (NCT03033303, Table 2). COG tested a fusion protein of humanized antibody (hu14.18) and IL-2 (hu14.18-IL2) (APN301) in a Phase II clinical trial and demonstrated a 21.7% response rate in relapsed patients without bulky disease [27]. A modified humanized antibody (hu14.18K322A) with a single amino acid substitution that blunts the complement activation thought to be responsible for neuropathic pain has shown a 19% response rate in patients with only MIBG detectable relapsed disease [28]. This antibody is now being evaluated as part of first line therapy (NCT01857934, Table 2) during induction.
4.3. Chimeric Antigen Receptor (CAR) T-cells
Based on the remarkable responses seen with CD19-CAR T cells in patients with acute lymphoblastic leukemia studies [29], CAR T-cells engineered to target neuroblastoma have been developed. Early trials have shown that it is feasible to engineer cells to target the neuroblastoma cell surface markers L1-CAM or GD2 [30]. Though these efforts were limited by the transiency of the engineered T-cells, they did demonstrate a tolerable safety profile [31]. Newer generations of CARs are thus being designed to improve efficacy while minimizing T-cell exhaustion.
4.4. Natural Killer (NK) cell therapy
Because NB cells do not express HLA class I, they can be a target of NK cell killing. Thus, administering NK cells in conjunction with anti-GD2 antibody or other immunostimulatory approaches may improve ADCC [32]. Multiple clinical trials are ongoing to test this hypothesis in relapsed neuroblastoma patients (NCT02573896, NCT01576692, NCT02650648, NCT02100891, Table 2).
4.5. Checkpoint inhibitors
In order to activate T-cells against tumor cells, both major histocompatibility complexes (MHC) and other co-stimulatory signals are required. Several signaling molecules, known as immune checkpoints, are capable of repressing T-cell activation by preventing co-stimulation. Thus, a patient's immune system could be induced to have antitumor activity by inhibiting these checkpoints. Agents targeting Cytotoxic T-lymphocyte antigen 4 (CTLA-4) became the first to demonstrate the potential of this approach [33]. Ipilumimab was the initial agent to target CTLA-4 and received FDA approval for metastatic melanoma in 2011 following successful phase 3 trials [34]. Subsequently, agents were developed against two other checkpoints, program death (PD-1) and its ligand (PD-L-1). Phase 3 trials in metastatic melanoma led to approval of the PD-1 inhibitors nivolumab and pembrolizumab for this indication [35, 36] and subsequent large trials in other tumor types have also been successful. The broad efficacy of these agents, which do not rely on disease specific targets, has led COG ADVL1412 is testing nivolumab with or without ipilumimab and (NCT02304458, Table 2) and ADVL1621 evaluating single agent pembrolizumab (NCT02332668, Table 2).
4.6. Tumor vaccines
Despite the intensive therapy given to patients with high-risk neuroblastoma, many patients in clinical remission clearly have minimal residual disease leading to late relapse [1], making vaccine approaches attractive in the post-consolidation setting. Multiple antigens have been explored as targets for these treatments including NY-ESO1 [37], GD2 [38], survivin [39], and MYCN [40]. However, efforts to use these approaches have had modest success, though new vaccines hold the potential for improved the immune response against neuroblastoma (NCT00911560, NCT02745756, Table 2).
5. Biologic agents
5.1. Fenretinide
Several agents are being explored for their immune-modulatory properties. The synthetic retinoid N-(4-hydroxyphenyl) retinamide (fenretinide) has been shown to have clinically relevant activity in neuroblastoma trials [41]. Unlike isotretinoin, fenretinide does not induce differentiation, but rather is selectively cytotoxic for cancer cells. The mechanism of action is complex, retinoic acid receptor and p53 independent, and includes the induction of de novo ceramide synthesis, generation of reactive oxygen species, and inhibition of angiogenesis [42]. Fenretinide also causes large, dose-dependent increases of intracellular of dihydroceramides, which are precursors of GD2. Recently it has been shown that fenretinide can induce GD2 target antigen expression, resulting in a significantly enhanced GD2-specific ADCC, as well as augmented complement-mediated, and NK-mediated killing suggesting that suggest that clinical activity of Dinutuximab would be boosted by fenretinide [43].
5.2. Lenalidomide
Lenalidomide is FDA approved in combination with dexamethasone for the treatment of multiple myeloma, is also being studies as an immune adjuvant. Although the mechanisms of lenalidomide are not completely understood, the overall effect in adult hematologic malignancy has been to increase anti-inflammatory cytokines, decrease pro-inflammatory cytokines, and induce proliferation, activation and survival of NK and cytotoxic T- lymphocytes, effectively enhancing both NK cell-mediated cytotoxicity and ADCC [44]. In children with refractory solid tumors and myelodysplastic syndrome, lenalidomide induces increases in plasma IL-2, IL-15 and GMCSF, to augment NK and T-cell mediated cytotoxicity, and to reduce the fraction of CD4+CD25+ regulatory T-cells thought to foster immune privilege [45]. In preclinical models, lenalidomide reversed the immunosuppressive effects of monocyte derived IL-6 and monocyte and neuroblastoma derived TGFβ1 on NK cell activity in vitro, and augmented the efficacy of ch14.18 immunotherapy in vivo [46]. A NANT phase I trial of lenalidomide in combination with dinutuximab and isotretinoin to evaluate the safety, preliminary activity and immunologic activity of this cassette in patients with recurrent/ refractory neuroblastoma (NCT01711554, Table 3) is nearing completion. Results of this study will provide clinically relevant information regarding the potential of replacing pharmacologic exogenous cytokines, particularly IL-2, during anti-GD2 immunotherapy which is perceived to be the most toxic component of the regimen, with lenalidomide.
Table 3. Description of clinical trials currently available using biologic agents for patients with high-risk neuroblastoma.
| Trial Identifier | Study question | Sponsor | Study agent(s) | Phase | Disease eligibility | Age Range |
|---|---|---|---|---|---|---|
| NCT01711554 | Dose finding and tolerability of lenalidomide, dinutuximab, with or without isotretinoin in recurrent/ refractory neuroblastoma | NANT | lenalidomide, dinutuximab, isotretinoin | 1 | Recurrent/ refractory neuroblastoma | ≤ 21 yrs. |
| NCT02308527 | Efficacy of bevacizumab and chemotherapy in recurrent/ refractory neuroblastoma | University of Birmingham | bevacizumab, temozolomide, irinotecan. and topotecan | 2 | Recurrent/ refractory neuroblastoma | 1 – 21 yrs. |
| NCT02298348 | Dose finding and tolerability of sorafenib, topotecan, and cyclophosphamide in recurrent/ refractory neuroblastoma | NANT | sorafenib, cyclophosphamide, and topotecan | 1 | Recurrent/ refractory neuroblastoma | ≤ 30 yrs. |
Abbreviations: NANT; New Advances in Neuroblastoma
5.3. Vascular Endothelial Growth Factor (VEGF) Inhibitors
Intriguingly, biologic agents can have multiple mechanisms by which they affect the tumor microenvironment. VEGF and multi-tyrosine kinases inhibitors (MTKI) were primarily designed to curtail endothelial survival, inhibit new vessel growth, and normalize tumor vasculature. In doing so, they can also modulate the immune response and the effects of pro-inflammatory cytokines. VEGF is known to inhibit immune infiltration of tumors, decrease the proliferation and activity of dendritic cells and alter the activity of T regulatory cells. Multiple studies are exploiting this connection by combining the VEGF inhibitor bevacizumab or MTKI in combination with immunotherapies [47]. The best studied MTKI in neuroblastoma is sorafenib which inhibits VEGF, platelet-derived growth factor β (PDGFβ), c-Kit, and RET receptor signaling, as well as B-Raf and C-Raf activation [48]. In subcutaneous and orthotopic xenographs, sorafenib inhibits tumor progression, reduces blood vessel density, decreases neuroblastoma proliferation, induces cell cycle arrest and diminishes signaling through the MAPK/ERK pathway (Figure 1) [49]. Furthermore, sorafenib has also been shown to inhibit the JAK2/ STAT3 pathway as well as activation of STAT3 by the pleiotropic, stromal derived cytokine IL-6, which is known to promote neuroblastoma proliferation, survival and drug resistance [50]. In pediatric embryonal tumors, the clinical activity of single agent sorafenib, as well as other VEGF blocking agents, has been disappointing [51, 52]. The BEACON trial studying temozolomide and irinotecan with and without bevacizumab (NCT02308527, Table 3) and NANT13-02 looking at the combination of cyclophosphamide, topotecan and sorafenib in refractory neuroblastoma (NCT02298348, Table 3) may provide further insight into the role of these agents which primarily target the tumor microenvironment.
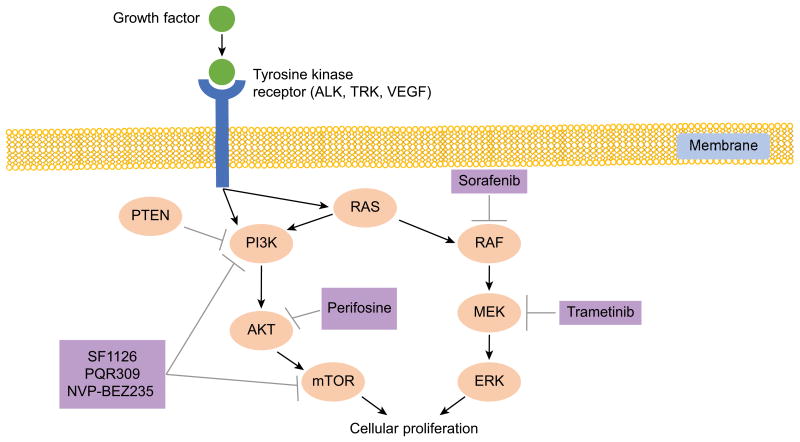
Figure 1.
PI3K and RAS are activated by multiple growth factors in neuroblastoma tumors. This activation leads to cellular proliferation and has been associated with relapsed disease and poor outcomes. Multiple drugs (purple) are in clinical development which inhibit one or more of the proteins in these pathways and may help improve outcomes for patients with high-risk neuroblastoma.
6. Molecularly targeted agents
6.1. ALK Inhibitors
Anaplastic lymphoma kinase (ALK), encoded by the ALK gene on chromosome 2p23.1, is a receptor tyrosine kinase belonging to the insulin receptor superfamily. It is normally expressed in the developing nervous system and is thought to play a role in neuronal differentiation [53]. Oncogenic ALK translocations have been described in multiple malignancies of adults. In neuroblastoma, ALK is also a tractable molecular target, although activating point mutations are more commonly detected than ALK translocations [54, 55].
6.1.1. Crizotinib
Crizotinib, a small molecule competitive inhibitor of ALK and MET kinase activity, has shown significant response rates in non-small cell lung cancer with ALK translocations, for which it is FDA approved [56]. A phase 1/2 trial of crizotinib (NCT00939770) was conducted in children, adolescents, and young adults with relapsed or refractory solid tumors or anaplastic large cell lymphoma, with a dedicated phase 2 cohort for individuals with neuroblastoma with ALK+ disease. In the phase 1 portion of the study, the recommended phase 2 dose (RP2D) was declared as 280 mg/m2 BID, almost twice the RP2D determined in adults. Therapy was overall well tolerated, and the most common toxicities during nausea and vomiting. Of the 11 patients with neuroblastoma and known ALK mutations, 1 patient had a CR and 2 had SD. Individuals with neuroblastoma who had a CR or SD received anywhere from 4-39 cycles of therapy, with some continuing on treatment at the time of publication of the phase 1 results [57]. The phase 2 portion of this study investigating response in those with neuroblastoma with ALK mutations or amplification has met accrual and may provide additional information on which patients may benefit from ALK inhibition. A COG phase 1 study of crizotinib with combination chemotherapy (cyclophosphamide/ topotecan or vincristine/ dexrazoxane/ doxorubicin) in children with relapsed or refractory solid tumors or anaplastic large cell lymphoma (NCT01606878, Table 4) is ongoing. Development plans for the next COG phase 3 study (ANBL1531) include integrating crizotinib in the upfront setting for individuals with ALK+ neuroblastoma.
Table 4. Description of clinical trials currently available using molecularly targeted agents for patients with high-risk neuroblastoma.
| Trial Identifier | Study question | Sponsor | Study agent(s) | Phase | Disease eligibility | Age Range |
|---|---|---|---|---|---|---|
| NCT01606878 | Dose finding and tolerability of crizotinib and chemotherapy in recurrent/ refractory solid tumors | COG | crizotinib | 1 | Recurrent/ refractory solid tumors | 1 – 21 yrs. |
| NCT01742286 | Dose finding and tolerability of ceritinib in recurrent/ refractory solid tumors | Novartis | ceritinib | 1 | Recurrent/ refractory solid tumors | 1 – 17 yrs. |
| NCT02650401 | Dose finding and tolerability of entrectonib in recurrent/ refractory solid tumors | Ignyta Inc. | entrectonib (RXDX-101) | 1/1b | Recurrent/ refractory solid tumors | 2 – 22 yrs. |
| NCT02124772 | Dose finding and efficacy of trametinib in recurrent/ refractory solid tumors | GlaxoSmithKline | trametinib | 1/2 | Recurrent/ refractory solid tumors | 1 mo. – 17 yrs. |
| NCT02337309 | Dose finding and tolerability of SF1126 in recurrent/ refractory solid tumors | NANT | SF1126 | 1 | Recurrent/ refractory neuroblastoma | 1 – 21 yrs. |
| NCT01587703 | Dose finding and efficacy of I-BET762 in NUT midline carcinoma or recurrent/ refractory solid tumors with MYCN-amplification | GlaxoSmithKline | I-BET762 (GSK525762) | 1/2 | NUT midline carcinoma or recurrent/ refractory MYCN-amplified solid tumors | ≥ 16 yrs. |
| NCT02030964 | Dose finding and tolerability of DFMO in combination with celecoxib, cyclophosphamide, and topotecan in recurrent/ refractory neuroblastoma | NANT | DFMO, celecoxib, cyclophosphamide, topotecan | 1 | Recurrent/ refractory neuroblastoma | 2 – 30 yrs. |
| NCT02808650 | Dose finding and tolerability of prexasertib in recurrent/ refractory solid tumors | COG | prexasertib (LY2606368) | 1 | Recurrent/ refractory solid tumors | 1 – 21 yrs. |
| NCT02095132 | Dose finding and efficacy of AZD1775 in recurrent/ refractory solid tumors | COG | AZD1775 | 1/2 | Recurrent/ refractory solid tumors | 1 – 21 yrs. |
| NCT01747876 | Dose finding and tolerability of ribociclib in recurrent/ refractory malignant rhabdoid tumors and neuroblastoma | Novartis | ribociclib | 1 | Recurrent/ refractory malignant rhabdoid tumors and neuroblastoma | 1 – 21 yrs. |
| NCT01971476 | Dose finding and tolerability of volasertib in recurrent/ refractory solid tumors | Boehringer-Ingelheim | volasertib (BI-6727) | 1 | Recurrent/ refractory solid tumors | 2 – 17 yrs. |
Abbreviations: COG; Children's Oncology Group, NANT; New Advances in Neuroblastoma
6.1.2. Lorlatinib
Pediatric phase 1 results of crizotinib showed modest responses in neuroblastoma with ALK mutations compared to ALK-rearranged malignancies, indicating differences in therapeutic targeting of the various ALK aberrations [58]. While crizotinib has shown antitumor activity in preclinical neuroblastoma models, cell lines and xenografts harboring F1174L-mutated ALK have shown reduced sensitivity. Crizotinib resistance to F1174L-mutatated ALK is thought to be, in part, secondary to increased ATP-binding affinity, which may be overcome by higher doses of crizotinib or higher-affinity inhibitors [59]. This has prompted investigation of the ALK/ROS1 inhibitor lorlatinib (PF-6463922), which has increased ALK-binding affinity to all variants and may, therefore, overcome the effects of primary resistance mutations. Indeed, lorlatinib was a more potent ALK-inhibitor than crizotinib across neuroblastoma mutations in vitro. Lorlatinib demonstrated significant antitumor activity in mouse xenograft models of neuroblastoma with and without primary crizotinib resistance and crizotinib naïve patient derived xenografts with F1174L and F1245C mutations. A NANT study with this agent is planned.
6.1.3. Ceritinib
Ceritinib is a second-generation small molecule ATP-competitive tyrosine inhibitor of ALK and IGF-1 [60]. Preclinical studies have shown significant antitumor activity against both crizotinib-sensitive and most crizotinib-resistant ALK-rearranged non-small cell lung cancer [61]. Enzymatic assay studies have shown that ceritinib is 20 times more potent against ALK than crizotinib. A phase 1 trial (+ expansion) of ceritinib in ALK-rearranged non-small cell lung cancer revealed high clinical activity, with an overall response rate of 56% (95% CI 45-67) was seen among patients who had received crizotinib previously [60]. As a result, ceritinib was FDA approved for the treatment of patients with ALK positive, metastatic non-small cell lung cancer (NSCLC) who have progressed on or are intolerant to crizotinib [62]. A pediatric phase 1 study of ceritinib in malignancies with ALK aberrations is ongoing (NCT01742286, Table 4).
6.2. TRK inhibitors
The TRK neurotrophin receptors play critical roles in development and maintenance of the central and peripheral nervous system. Activation of TRK family receptors (TrkA/NTRK1, TrkB/NTRK2, TrkC/NTRK3) is implicated in both pediatric and adult tumors. TrkB is co-expressed with its ligand, BDNF, and this autocrine pathway leads to invasion, metastasis, angiogenesis and drug resistance [63, 64]. Blocking TrkB with inhibitors leads to apoptosis and increased sensitivity to chemotherapy [64, 65]. Many pediatric and adult cancers have rearrangements or aberrant expression of TRK genes. However, mutations in or translocations involving TRK genes have not been found in neuroblastomas. Rather, autocrine activation of TrkB/BDNF pathway occurs in 50-60% of high-risk neuroblastoma, particularly in MYCN-amplified tumors [66]. Due to its frequency of activation, TRK has been proposed as a promising target for neuroblastoma.
6.2.1. Entrectinib
Entrectinib (RXDX-101) is a potent, small molecule inhibitor of the receptor tyrosine kinases TrkA/B/C, ROS1, and ALK. Entrectinib crosses the blood brain barrier, potentially enabling targeting of central nervous system (CNS) metastases and primary CNS tumors [67]. In vivo (SY5Y-TrkB xenografts), entrectinib demonstrates significant tumor growth inhibition and prolonged event free survival (EFS) when compared to vehicle alone. The addition of entrectinib to irinotecan and temozolomide enhanced tumor growth inhibition both in vitro and in vivo [68].
Phase 1 studies of entrectinib in adults with advanced or metastatic solid tumors harboring NTRK1/2/3, ROS1, or ALK molecular alterations have been completed and demonstrated a tolerable safety profile [69]. Phase 2 studies of entrectinib in adults are ongoing (NCT02097810, NCT02568267, NCT02587650). A phase 1/1b study of entrectinib is currently open in children with recurrent or refractory solid tumors and primary CNS tumors (NCT02650401, Table 4). Upon identification of the RP2D, one of the expansion cohorts in the phase 1b portion will open specifically for individuals with relapsed or refractory neuroblastoma to assess response.
6.2.2. Larotrectinib (LOXO-101)
Larotrectinib (LOXO-101), a selective small molecule inhibitor of TrkA/B/C is under clinical development for adult (NCT02122913, NCT02576431) and pediatric advanced solid tumors and brain tumors (NCT02122913). Once a RP2D is determined, expansion cohorts will open for individuals with alterations in one of the three NTRK genes or TRK proteins.
6.3. RAS-MAPK and MEK inhibitors
The RAS-MAPK pathway plays a role in cellular growth, survival, and differentiation and has been implicated in variety of malignancies (Figure 1) [70]. RAS-pathway related mutations are infrequent at diagnosis in neuroblastoma [71]. However, a recent report by Eleveld, et al. [72] showed an increased frequency of activating mutations not present at diagnosis in the RAS-MAPK pathway in relapsed neuroblastoma tumors. Specifically, mutations were identified in ALK, NF1, BRAF, PTPN11, FGFR1, KRAS, HRAS, and NRAS. Thus, inhibition of the RAS-MAPK pathway may serve as therapeutic target in the relapsed/refractory neuroblastoma population.
MAPK/ERK kinase (MEK) inhibitors have demonstrated anti-tumor effects in neuroblastoma in vitro [73]; binimetinib (MEK162) has shown tumor growth inhibition and increased survival in vivo [72]. Trametinib (MEK 1/2 inhibitor) was FDA approved in 2013 as an oral monotherapy for the treatment of adult patients with unresectable or metastatic melanoma containing BRAF V600E or V600K mutations [74], and in 2014 trametinib in combination with dabrafenib was FDA approved for the same indication [75]. A Phase I/IIa trial of trametinib alone or with dabrafenib for V600 mutations (NCT02124772, Table 4) is ongoing for pediatrics, and the study includes a dedicated expansion cohort (n=10) for individuals with relapsed or refractory neuroblastoma to begin to assess response.
6.4. PI3K/mTOR inhibitors
Aberrant activation of the PI3K/Akt/mTOR cell survival pathway has been demonstrated in neuroblastoma and is a focus of targeted therapy development (Figure 1). Chesler, et al. [76] showed that PI3K inhibition as a therapeutic strategy in neuroblastoma is, in part, based on its relationship with MYCN. The PI3K pathway appears to play a role in MYCN stabilization, and PI3K inhibition in MYCN-driven murine neuroblastoma leads to decreased levels of MYCN protein and tumor mass. MYCN mRNA levels are unchanged, suggesting that PI3K inhibition blocks MYCN at a post-transcriptional level. Thus, PI3K inhibition is of great interest, given the difficulty of directly blocking MYCN and other transcriptional factors [76, 77].
Chanthery, et al. [78] further showed that dual PI3K/mTOR inhibition with dactolisib (NVP-BEZ235) decreased levels of MYCN protein in tumor cells, with secondary paracrine blockade of angiogenesis in MYCN-driven neuroblastoma models. Elimination of MYCN protein by PI3K/mTOR inhibition, with compounds such as dactolisib, is closely associated with effective targeting of both mTORC1 and mTORC2 and suggests that mTOR kinase plays a significant role in the maintenance of MYCN stability [80]. The degree of MYCN-amplification and protein expression correlates with sensitivity to mTOR inhibition [78]. In addition, direct inhibition of AKT has been proposed as a therapeutic strategy in neuroblastoma. The AKT inhibitor perifosine (D21266) induced caspase-dependent apoptosis and decreased AKT phosphorylation in neuroblastoma cell lines and demonstrated tumor growth inhibition and/or tumor regression and longer survival in mouse xenograft models of neuroblastoma [79].
6.4.1. PI3K/mTOR inhibition
A phase 1 trial of SF1126, a pan-PI3K/mTOR inhibitor, is being conducted in children, adolescents, and young adults through the NANT consortium (NCT02337309, Table 4) using a 3+3 dose escalation design. SF1126 is a novel RGDS-conjugated LY294002 prodrug, which shows increased solubility and binds to integrins in the tumors compartment, improving delivery of drug to the tumor vasculature and tumor [80]. Upon determination of a RP2D, an expansion cohort of 10 patients with tumors demonstrating MYCN-amplification, MYCN over-expression, or MYC over-expression will be treated. COG is also planning a phase 1 trial using the PI3K/mTOR inhibitor PQR309 for patients with relapsed and refractory solid tumors.
6.4.2 Perifosine (AKT inhibitor)
A phase 1/1b study of the PI3K/AKT pathway inhibitor perifosine (NCT01049841) has recently been completed in children, adolescents, and young adults with advanced solid tumors, with a 1b expansion cohort for individuals with relapsed/ refractory neuroblastoma. Twenty-seven patients with neuroblastoma received perifosine. One of 24 patients had tumor with MYCN-amplification and none of the 21 tumor samples tested had ALK mutations. Therapy was overall well tolerated, without any dose limiting toxicities. Nine patients have had a progression free survival for a median of 54 months from study entry (range: 43-74 months) [81].
6.5. Epigenetic inhibitors
Targeting epigenetic modifications, or changes in the regulators of patterns of gene expression rather than the DNA sequence itself, continues to be growing area of anti-cancer therapy. Epigenetic pathways involving DNA methylation, histone modification, nucleosome remodeling, and non-coding RNAs are linked to oncogenesis, and several epigenetic inhibitors are under clinical development [82].
6.5.1. BET inhibitors
The bromodomain and extraterminal domain (BET) family (BRD2, BRD3, BRD4, and BRDT) plays a critical role in the activation of transcription. Bromodomains recognize and bind acetylated lysine residues on histone tails and regulate chromatin structure; thus, BET proteins are known as epigenetic “readers”. BET inhibitors have been found to block transcription of MYCN. BET inhibition with JQ1, I-BET726 (GSK1324726A), and OTX015 (now MK-8628) induced cell cycle arrest, apoptosis and down-regulation of MYCN expression and MYCN target genes in MYCN-amplified neuroblastoma cell lines [83, 84, 85]. MYCN-amplification was a strong predictive marker of sensitivity to JQ1 [83]. I-BET726 also induced growth inhibition and cytotoxicity in cell lines regardless of MYCN copy number or expression level, as well as direct suppression of BCL2 [84]. In various in vivo neuroblastoma models, treatment with JQ1, I-BET726, and OTX015 decreased tumor volume and prolonged overall survival [83, 84, 85]. Various BET inhibitors are under clinical investigation for adults. A phase 1 study of I-BET762 (GSK525762) is currently ongoing for individuals with NUT midline carcinoma and other cancers, including neuroblastoma and MYCN driven solid tumors, and includes individuals 16 years of age and older (NCT01587703, Table 4).
6.6. Modulation of MYCN
Polyamines are essential polycations that enhance transcription, translation and replication, as well as support MYCN functions. The ornithine decarboxylase gene ODC1, a direct target of MYCN, encodes the rate-limiting enzyme in polyamine synthesis, and thus inhibition of ODC1 mediates MYCN effects and serves as a therapeutic target in neuroblastoma. Alpha-difluoromethylornithine (DFMO), an irreversible ODC1 inhibitor, is FDA approved for the treatment of Trypanosomiasis and has been investigated in neuroblastoma for its role in polyamine depletion [86, 87]. In vivo, DFMO delays tumor initiation and enhances the antitumor activity of conventional cytotoxic chemotherapy [86]. DFMO in combination with celecoxib (an inducer of polyamine catabolism) alone or with conventional cytotoxic chemotherapy leads to tumor regression in vivo [87], possibly by altering the tumor microenvironment to support anti-tumor immunity. In a phase 1 trial of DFMO alone and in combination with oral etoposide conducted in patients with relapsed/refractory neuroblastoma, individuals tolerated 500-1500 mg/m2 of DFMO twice daily, without dose limiting toxicity [88]. A phase 1 Study of DFMO and celecoxib with cyclophosphamide/topotecan is ongoing (NCT02030964, Table 4).
In addition to ODC1, Aurora A kinase (AURKA) plays an important role in stabilizing the MYCN protein. In preclinical testing, the AURKA inhibitor alisertib (MLN8237) led to a complete response in three out of seven neuroblastoma xenografts [89]. A recent NANT Phase 1/2 study demonstrated that alisertib is well tolerated and an objective response was seen in 6 of 32 (18.8%) patients taking either the tablet or solution formulation [90]. Somewhat paradoxically, patients with MYCN-amplification had a trend towards lower response rates, necessitating improved biomarkers of activity in future trials of this agent.
6.7. Cell Cycle Checkpoint Kinase inhibitors
Loss of cell cycle regulatory control is a key step in tumorigenesis and is thus another promising therapeutic target. Numerous studies have shown in vitro and in vivo activity of agents targeting checkpoint kinases (CHK) 1/2/4/6, WEE1, and polo-like kinase 1 (PLK1) [91, 92]. Phase 1 testing in adults with refractory tumors has demonstrated tolerability and efficacy of the CHK1 inhibitor prexasertib (LY2606368), the WEE1 inhibitor AZD1775 and the PLK1 inhibitor volasertib (BI-6727) [93, 94, 95]. Additionally, the CDK4/6 inhibitor ribociclib recently received FDA approval after demonstrated improved survival in women with breast cancer. In pediatrics, phase 1 trials are ongoing for prexasertib (NCT02808650, Table 4), AZD1775 (NCT02095132, Table 4), ribociclib (NCT01747876, Table 4), and volasertib (NCT01971476, Table 4) for relapsed and refractory solid tumors.
6.8. Histone Deacetylase (HDAC) inhibitors
Decreased histone acetylation has long been a hallmark of cancer, altering chromatin structure and modifying transcriptional pathways [96]. HDAC inhibition leads to increased acetylation and a less malignant transcriptional profile. In neuroblastoma, vorinostat has shown the most potential in this category with a R2PD of 230mg/m2 [97]. Additionally, vorinostat is a sensitizer to 131I-MIBG and is tolerated at 180mg/m2 in combination with 18 mCi/kg 131I-MIBG [12]. This combination is currently being compared to 131I-MIBG alone or in combination with vincristine and irinotecan (NCT02035137, Table 1).
7. Genomic trials
In order to tackle the challenge of personalizing the use of the targeted agents described, several groups have evaluated genomic biomarkers to identify appropriate agents for patients with relapsed neuroblastoma [98, 99, 100, 101]. The goal of these trials was to determine the feasibility of using somatic and germline sequencing to recommend targeted treatment options. While feasibility was demonstrated with 31-50% of patients having a potentially targetable mutation, it is notable that only 3-19% received a drug specific to somatic aberrations with even fewer patients deriving clinical benefit from this approach. The NANT consortium recently activated a feasibility trial to sequence tumors from patients with relapsed neuroblastoma and provide informed treatment options (NCT02868268, Table 5). The important next question being addressed by these consortia is whether genomic approaches for drug selection provides a survival advantage compared to standard treatment options for relapsed and refractory patients. The Genomic Assessment Improves Novel Therapy (GAIN) consortium is currently accruing a total of 875 patients in an effort to demonstrate that genomic approaches will improve the survival of patients with relapsed solid tumors by recommending options to the family and provider through a molecular tumor board (NCT02520713, Table 5).
Table 5. Description of clinical trials currently available using genomic guided therapy for patients with high-risk neuroblastoma.
| Trial Identifier | Study question | Sponsor | Study agent(s) | Phase | Disease eligibility | Age Range |
|---|---|---|---|---|---|---|
| NCT02868268 | Identify targetable genomic alterations in ALK, MAPK pathway, metabolism, and immunologic mechanisms in recurrent/ refractory neuroblastoma | NANT | Recommendations made from genomic results | 2 | Recurrent/ refractory neuroblastoma | 1 – 30 yrs. |
| NCT02520713 | Identify targetable alterations using genome wide sequencing approaches in recurrent/ refractory solid tumors | Dana-Farber Cancer Institute | Recommendations made from genomic results | 2 | Recurrent/ refractory solid tumors | ≤ 30 yrs. |
| NCT02465060 | Identify targetable alterations using genomic approaches in recurrent/ refractory tumors | NCI | 24 unique treatment arms | 2 | Recurrent/ refractory solid tumors | ≥ 18 yrs. |
| NCT02693535 | Identify targetable alterations using genomic approaches in recurrent/ refractory tumors | ASCO | 15 unique treatment arms | 2 | Recurrent/ refractory solid tumors | ≥ 18 yrs. |
| NCT02780128 | Identify targetable genomic alterations in ALK, MAPK pathway, and p53 related mechanisms in recurrent/ refractory neuroblastoma | Children's Hospital of Philadelphia | 3 unique treatment arms | 2 | Recurrent/ refractory neuroblastoma | 1 – 21 yrs. |
Abbreviations: NANT; New Advances in Neuroblastoma, NCI; National Cancer Institute, ASCO: American Society of Clinical Oncology
One of the biggest barriers to precision medicine that emerged in feasibility studies was that many children did not receive a drug matched to their genomic profile because the patient could not obtain the recommended medication. To address this, groups have partnered with industry to design trials that have cassettes of readily available molecularly targeted agents and aim to identify patients for whom one of these drugs may be beneficial. The NCI-MATCH trial is accruing adult oncology patients regardless of tumor histology into one of 24 different treatment arms depending on identified somatic mutations (NCT02465060, Table 5). Plans are underway to allow children to enroll on this study through the NCI Pediatric MATCH which is likely to open for enrollment in 2017. The American Society of Clinical Oncology is also enrolling adults into the Targeted Agent and Profiling Utilization Registry (TAPUR) Study (NCT02693535, Table 5) which is determining the efficacy of using commercially available agents based on mutations detected in tumor profiling and will open the trial to children 12 years and older. Additionally, the NEPENTHE trial is accruing relapsed neuroblastoma patients into one of three treatment arms defined by ALK mutations, activations of the RAS-MAPK pathway, and those with wild-type p53. Patients in these three arms will be treated with ceritinib and ribociclib, trametinib, or HDM201 (an inhibitor of the HDM2 protein which inactivates p53), respectively (NCT02780128, Table 5). Thus, over the next several years, we will know if it is possible to identify a patient's individual tumor profile, treat them with a drug specific for those aberrations, and hopefully improve their prognosis.
8. Conclusion
The recent success of intensive induction chemotherapy and surgery, followed by tandem high-dose chemotherapy with autologous stem cell transplant and radiation and post-consolidation therapy with dinutuximab has improved outcomes for children with high-risk neuroblastoma. However, further gains in survival and a reduction in both short- and long-term side effects are needed. Advances in 131I-MIBG, immunotherapy, biologics, and molecularly targeted agents hold the promise of achieving these goals, but much work remains. Improved biomarkers will help guide new approaches for risk stratification and drug selection for patients with high-risk neuroblastoma.
9. Expert opinion
Advances in genomic medicine and molecular biology have led to a better understanding of the oncogenic drivers in neuroblastoma, and promising results have been observed in early phase studies testing therapies that target these aberrantly active cellular pathways. By implementing multiple therapeutic approaches including improved cytotoxic agents, immunotherapy, biologics, and molecularly targeted agents, advances are being made that have improved the outcomes for children diagnosed with high-risk neuroblastoma. Because many of these drugs have a wider therapeutic index and are more “precise” than standard chemotherapeutic agents, these treatments have the potential to be more effective and less toxic, leading to further improvement in survival for patients with newly diagnosed or relapsed neuroblastoma.
Currently, induction and consolidation therapy for patients with newly diagnosed, high-risk neuroblastoma consists primarily of large doses of traditional cytotoxic agents and radiation followed by immunotherapy. Patients are assigned to this treatment based on clinical and biologic variables identified decades ago. Unfortunately, up to 20% will not respond or will progress through induction therapy and more than 30% will relapse at later time points. To ultimately improve the survival of children with high-risk neuroblastoma, it will be critical to develop systems that will enable the rapid matching of effective treatments to patients in the diagnostic setting, thus decreasing the frequency of primary refractive disease. This will require the development of robust biomarkers that will distinguish patients who will respond to traditional treatment strategies from those who will not. For patients in the latter cohort, additional biomarkers will be needed to identify alternative treatment approaches that are most likely to be effective. Further, novel trial designs that will enable accurate assessment of the activity of these alternative regimens in small numbers of patients will be required.
DNA mutations serve as the most commonly used biomarkers for targeted agents at the present time, but are difficult to implement in neuroblastoma due to the paucity of these changes in this disease. However, DNA copy number changes and alterations in the transcriptome, epigenome, and proteome are all well described in neuroblastoma. Thus, a combination of “omic” measures are likely to be needed to develop robust predictive biomarkers for new agents, whether they be small molecules, biologics, or immunotherapeutics. For example, if an expression signature or genomic/epigenomic profile is prospectively validated to be predictive of response and outcome, it would open the way for the development of a molecular test that, ideally, would be rapidly implemented in the clinic and have clear therapeutic implications. A similar approach is currently planned for children with high-risk acute lymphoblastic leukemia wherein a low-density array expression panel will be used in the next phase 3 COG trial to identify those who would likely benefit from specific kinase inhibitors based on Philadelphia-like profiles.
Ultimately, emerging therapies for neuroblastoma are likely to continue to improve the survival rates for high-risk patients. With a deeper understanding of the underlying biology driving high-risk disease, we will continue to be able to develop additional precision medicine strategies that will hopefully be more effective and associated with less acute and long-term adverse effects. Intensification of therapy has improved outcomes for children with high-risk neuroblastoma thus far, but new, biomarker driven therapies will determine our future success.
Article highlights.
Long-term survival rates for patients with high-risk neuroblastoma remain at less than 50% despite intensive, multimodal therapy.
The Children's Oncology Group is developing a Phase 3 clinical trial for patients with newly diagnosed high-risk neuroblastoma to evaluate the efficacy of the targeted radiopharmaceutical 131I-MIBG for patients with ALK- tumors and crizotinib for patients with ALK+ neuroblastoma.
Multiple agents including biologics, immunotherapies, and molecularly targeted compounds show promise in early phase trials.
Genomic driven trials have been developed to match patients with actionable tumor mutations with specific targeted agents.
Robust predictive biomarkers are needed to identify patients that will benefit most from novel therapies.
Acknowledgments
Funding: This research was supported in part by the Neuroblastoma Children's Cancer Society (SLC), the Children's Neuroblastoma Cancer Foundation (SLC), the Matthew Bittker Foundation (SLC), The Mark Staehely Foundation (SLC), a gift from Brittany and Michael MacRitchie (SLC), and NIH Grant Numbers K12CA139160 (MAA). The contents are solely the responsibility of the authors and do not necessarily represent the official views of the NIH.
Footnotes
Declaration of Interest: Susan Cohn owns Stock in Array BioPharma (I), Edwards Lifesciences (I), Gilead Sciences (I), LabCorp (I), United Therapeutics (I), Varian Medical Systems (I), Zogenix (I), AstraZeneca, Pfizer, Abbott Laboratories, Antares Pharmaceuticals, Array BioPharma, Auxilium Pharmaceuticals, Avanir Pharmaceuticals, Baxter International, BioReference Laboratories, DENTSPLY International, Dyax, Edwards Lifesciences, Gilead Sciences, Hansen Medical, Jazz Pharmaceuticals, LabCorp, MEDNAX Services, Novavax, OPKO Health, Smith & Nephew, Techne, United Therapeutics, Universal Health Services, Varian Medical Systems, Vermillion, WuXi AppTec, Zimmer Holdings And has received travel expenses from Novartis and United Therapeutics. The remaining authors have no relevant affiliations or financial involvement with any organization or entity with a financial interest in or financial conflict with the subject matter or materials discussed in the manuscript. This includes employment, consultancies, honoraria, stock ownership or options, expert testimony, grants or patents received or pending, or royalties.
References
Papers of special note have been highlighted as:
* of interest
** of considerable interest
- 1*.Matthay KK, Maris JM, Schleiermacher G, et al. Neuroblastoma. Nat Rev Dis Primers. 2016;2:16078. doi: 10.1038/nrdp.2016.78. Excellent review of the biology and clinical approach to neuroblastoma. [DOI] [PubMed] [Google Scholar]
- 2.Strother DR, London WB, Schmidt ML, et al. Outcome after surgery alone or with restricted use of chemotherapy for patients with low-risk neuroblastoma: results of Children's Oncology Group study P9641. J Clin Oncol. 2012;30:1842–8. doi: 10.1200/JCO.2011.37.9990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Baker DL, Schmidt ML, Cohn SL, et al. Outcome after reduced chemotherapy for intermediate-risk neuroblastoma. N Engl J Med. 2010;363:1313–23. doi: 10.1056/NEJMoa1001527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Nuchtern JG, London WB, Barnewolt CE, et al. A prospective study of expectant observation as primary therapy for neuroblastoma in young infants: a Children's Oncology Group study. Ann Surg. 2012;256:573–80. doi: 10.1097/SLA.0b013e31826cbbbd. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5*.Pinto NR, Applebaum MA, Volchenboum SL, et al. Advances in Risk Classification and Treatment Strategies for Neuroblastoma. J Clin Oncol. 2015;33:3008–17. doi: 10.1200/JCO.2014.59.4648. Excellent review of recent cooperative group clinical trials for neuroblastoma patients. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6**.Yu AL, Gilman AL, Ozkaynak MF, et al. Anti-GD2 antibody with GM-CSF, interleukin-2, and isotretinoin for neuroblastoma. N Engl J Med. 2010;363:1324–34. doi: 10.1056/NEJMoa0911123. Phase 3 trial demonstrating the survival benefit of dinutuximab with cytokines in newly diagnosed high-risk neuroblastoma. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7**.Park JR, Kreissman SG, London W, et al. A phase III randomized clinical trial (RCT) of tandem myeloablative autologous stem cell transplant (ASCT) using peripheral blood stem cell (PBSC) as consolidation therapy for high-risk neuroblastoma (HR-NB): A Children's Oncology Group (COG) study. J Clin Oncol. 2016;34 suppl abstr LBA3. Phase 3 trial demonstrating the survival benefit of trandem compared to single autologous stem cell transplant in newly diagnosed high-risk neuroblastoma. [Google Scholar]
- 8.Applebaum MA, Vaksman Z, Lee SM, et al. Neuroblastoma survivors are at increased risk for second malignancies: A report from the International Neuroblastoma Risk Group Project. Eur J Cancer. 2016;72:177–85. doi: 10.1016/j.ejca.2016.11.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Wilson JS, Gains JE, Moroz V, et al. A systematic review of 131I-meta iodobenzylguanidine molecular radiotherapy for neuroblastoma. Eur J Cancer. 2014;50:801–15. doi: 10.1016/j.ejca.2013.11.016. [DOI] [PubMed] [Google Scholar]
- 10*.Matthay KK, Yanik G, Messina J, et al. Phase II study on the effect of disease sites, age, and prior therapy on response to iodine-131-metaiodobenzylguanidine therapy in refractory neuroblastoma. J Clin Oncol. 2007;25:1054–60. doi: 10.1200/JCO.2006.09.3484. First large phase 2 study of 131I-MIBG in treatment of relapsed disease. [DOI] [PubMed] [Google Scholar]
- 11.Matthay KK, George RE, Yu AL. Promising therapeutic targets in neuroblastoma. Clin Cancer Res. 2012;18:2740–53. doi: 10.1158/1078-0432.CCR-11-1939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Parisi MT, Eslamy H, Park JR, et al. (131)I-Metaiodobenzylguanidine Theranostics in Neuroblastoma: Historical Perspectives; Practical Applications. Semin Nucl Med. 2016;46:184–202. doi: 10.1053/j.semnuclmed.2016.02.002. [DOI] [PubMed] [Google Scholar]
- 13.Schmidt M, Simon T, Hero B, et al. Is there a benefit of 131 I-MIBG therapy in the treatment of children with stage 4 neuroblastoma? A retrospective evaluation of The German Neuroblastoma Trial NB97 and implications for The German Neuroblastoma Trial NB2004. Nuklearmedizin. 2006;45:145–51. [PubMed] [Google Scholar]
- 14.de Kraker J, Hoefnagel KA, Verschuur AC, et al. Iodine-131-metaiodobenzylguanidine as initial induction therapy in stage 4 neuroblastoma patients over 1 year of age. Eur J Cancer. 2008;44:551–6. doi: 10.1016/j.ejca.2008.01.010. [DOI] [PubMed] [Google Scholar]
- 15.Desai N, Trieu V, Yao Z, et al. Increased antitumor activity, intratumor paclitaxel concentrations, and endothelial cell transport of cremophor-free, albumin-bound paclitaxel, ABI-007, compared with cremophor-based paclitaxel. Clin Cancer Res. 2006;12:1317–24. doi: 10.1158/1078-0432.CCR-05-1634. [DOI] [PubMed] [Google Scholar]
- 16.Houghton PJ, Kurmasheva RT, Kolb EA, et al. Initial testing (stage 1) of the tubulin binding agent nanoparticle albumin-bound (nab) paclitaxel (Abraxane((R))) by the Pediatric Preclinical Testing Program (PPTP) Pediatr Blood Cancer. 2015;62:1214–21. doi: 10.1002/pbc.25474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Iyer R, Croucher JL, Chorny M, et al. Nanoparticle delivery of an SN38 conjugate is more effective than irinotecan in a mouse model of neuroblastoma. Cancer Lett. 2015;360:205–12. doi: 10.1016/j.canlet.2015.02.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Chari RV, Martell BA, Gross JL, et al. Immunoconjugates containing novel maytansinoids: promising anticancer drugs. Cancer Res. 1992;52:127–31. [PubMed] [Google Scholar]
- 19.Winter C, Pawel B, Seiser E, et al. Neural cell adhesion molecule (NCAM) isoform expression is associated with neuroblastoma differentiation status. Pediatr Blood Cancer. 2008;51:10–6. doi: 10.1002/pbc.21475. [DOI] [PubMed] [Google Scholar]
- 20.Socinski MA, Kaye FJ, Spigel DR, et al. Phase 1/2 Study of the CD56-Targeting Antibody-Drug Conjugate Lorvotuzumab Mertansine (IMGN901) in Combination With Carboplatin/Etoposide in Small-Cell Lung Cancer Patients With Extensive-Stage Disease. Clin Lung Cancer. 2017;18:68–72. doi: 10.1016/j.cllc.2016.09.002. [DOI] [PubMed] [Google Scholar]
- 21.Sano R, Krytska K, Larmour C, et al. A novel antibody-drug conjugate directed to the ALK receptor tyrosine kinase demonstrates efficacy in models of neuroblastoma. Cancer Res. 2016;76(14 Suppl) doi: 10.1126/scitranslmed.aau9732. Abstract nr 2690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Siebert N, Eger C, Seidel D, et al. Pharmacokinetics and pharmacodynamics of ch14.18/CHO in relapsed/refractory high-risk neuroblastoma patients treated by long-term infusion in combination with IL-2. MAbs. 2016;8:604–16. doi: 10.1080/19420862.2015.1130196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Lode HN, Valteau Couanet D, Troschke-Meurer S, et al. Phase II clinical trial with long-term infusion of anti-GD2 antibody ch14.18/CHO in combination with interleukin-2 (IL2) in patients with high risk neuroblastoma. J Clin Oncol. 2016;34(suppl) abstr 10562. [Google Scholar]
- 24.Ladenstein R, Poetschger U, Gray J, et al. Toxicity and outcome of anti-GD2 antibody ch14.18/CHO in front-line, high-risk patients with neuroblastoma: Final results of the phase III immunotherapy randomisation (HR-NBL1/SIOPEN trial) J Clin Oncol. 2016;34 suppl abstr 10500. [Google Scholar]
- 25*.Mody R, Naranjo A, Van Ryn C, et al. ANBL1221: A Phase II Randomized Trial of Irinotecan and Temozolomide with Temsirolimus or Dinutuximab in Children with Refractory, Relapsed or Progressive Neuroblastoma: A Report From the Children's Oncology Group. J Clin Oncol. 2016;34 suppl abstr 10502. First trial to demonstrate activity of immunotherapy in combination with cytotoxic agents. [Google Scholar]
- 26.Ahmed M, Goldgur Y, Hu J, et al. In silico driven redesign of a clinically relevant antibody for the treatment of GD2 positive tumors. PLoS One. 2013;8:e63359. doi: 10.1371/journal.pone.0063359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Shusterman S, London WB, Gillies SD, et al. Antitumor activity of hu14.18-IL2 in patients with relapsed/refractory neuroblastoma: a Children's Oncology Group (COG) phase II study. J Clin Oncol. 2010;28:4969–75. doi: 10.1200/JCO.2009.27.8861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Navid F, Sondel PM, Barfield R, et al. Phase I trial of a novel anti-GD2 monoclonal antibody, Hu14.18K322A, designed to decrease toxicity in children with refractory or recurrent neuroblastoma. J Clin Oncol. 2014;32:1445–52. doi: 10.1200/JCO.2013.50.4423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Lee DW, Kochenderfer JN, Stetler-Stevenson M, et al. T cells expressing CD19 chimeric antigen receptors for acute lymphoblastic leukaemia in children and young adults: a phase 1 dose-escalation trial. Lancet. 2015;385:517–28. doi: 10.1016/S0140-6736(14)61403-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Pule MA, Savoldo B, Myers GD, et al. Virus-specific T cells engineered to coexpress tumor-specific receptors: persistence and antitumor activity in individuals with neuroblastoma. Nat Med. 2008;14:1264–70. doi: 10.1038/nm.1882. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Louis CU, Savoldo B, Dotti G, et al. Antitumor activity and long-term fate of chimeric antigen receptor-positive T cells in patients with neuroblastoma. Blood. 2011;118:6050–6. doi: 10.1182/blood-2011-05-354449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Cheng M, Chen Y, Xiao W, et al. NK cell-based immunotherapy for malignant diseases. Cell Mol Immunol. 2013;10:230–52. doi: 10.1038/cmi.2013.10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Krummel MF, Allison JP. CD28 and CTLA-4 have opposing effects on the response of T cells to stimulation. J Exp Med. 1995;182:459–65. doi: 10.1084/jem.182.2.459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34*.Hodi FS, O'Day SJ, McDermott DF, et al. Improved survival with ipilimumab in patients with metastatic melanoma. N Engl J Med. 2010;363:711–23. doi: 10.1056/NEJMoa1003466. First paper to demonstrate clinical benefit of CTLA-4 inhibition. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Robert C, Schachter J, Long GV, et al. Pembrolizumab versus Ipilimumab in Advanced Melanoma. N Engl J Med. 2015;372:2521–32. doi: 10.1056/NEJMoa1503093. [DOI] [PubMed] [Google Scholar]
- 36.Larkin J, Chiarion-Sileni V, Gonzalez R, et al. Combined Nivolumab and Ipilimumab or Monotherapy in Untreated Melanoma. N Engl J Med. 2015;373:23–34. doi: 10.1056/NEJMoa1504030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Soling A, Schurr P, Berthold F. Expression and clinical relevance of NY-ESO-1, MAGE-1 and MAGE-3 in neuroblastoma. Anticancer Res. 1999;19:2205–9. [PubMed] [Google Scholar]
- 38.Fest S, Huebener N, Weixler S, et al. Characterization of GD2 peptide mimotope DNA vaccines effective against spontaneous neuroblastoma metastases. Cancer Res. 2006;66:10567–75. doi: 10.1158/0008-5472.CAN-06-1158. [DOI] [PubMed] [Google Scholar]
- 39.Fest S, Huebener N, Bleeke M, et al. Survivin minigene DNA vaccination is effective against neuroblastoma. Int J Cancer. 2009;125:104–14. doi: 10.1002/ijc.24291. [DOI] [PubMed] [Google Scholar]
- 40.Stermann A, Huebener N, Seidel D, et al. Targeting of MYCN by means of DNA vaccination is effective against neuroblastoma in mice. Cancer Immunol Immunother. 2015;64:1215–27. doi: 10.1007/s00262-015-1733-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Maurer BJ, Kang MH, Villablanca JG, et al. Phase I trial of fenretinide delivered orally in a novel organized lipid complex in patients with relapsed/refractory neuroblastoma: a report from the New Approaches to Neuroblastoma Therapy (NANT) consortium. Pediatr Blood Cancer. 2013;60:1801–8. doi: 10.1002/pbc.24643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Corazzari M, Lovat PE, Oliverio S, et al. Fenretinide: a p53-independent way to kill cancer cells. Biochem Biophys Res Commun. 2005;331:810–5. doi: 10.1016/j.bbrc.2005.03.184. [DOI] [PubMed] [Google Scholar]
- 43.Shibina A, Seidel D, Somanchi SS, et al. Fenretinide sensitizes multidrug-resistant human neuroblastoma cells to antibody-independent and ch14.18-mediated NK cell cytotoxicity. J Mol Med. 2013;91:459–72. doi: 10.1007/s00109-012-0958-0. [DOI] [PubMed] [Google Scholar]
- 44.Gribben JG, Fowler N, Morschhauser F. Mechanisms of Action of Lenalidomide in B-Cell Non-Hodgkin Lymphoma. J Clin Oncol. 2015;33:2803–11. doi: 10.1200/JCO.2014.59.5363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Berg SL, Cairo MS, Russell H, et al. Safety, Pharmacokinetics, and Immunomodulatory Effects of Lenalidomide in Children and Adolescents With Relapsed/Refractory Solid Tumors or Myelodysplastic Syndrome: A Children's Oncology Group Phase I Consortium Report. J Clin Oncol. 2011;29:316–23. doi: 10.1200/JCO.2010.30.8387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Xu Y, Sun J, Sheard MA, et al. Lenalidomide overcomes suppression of human natural killer cell anti-tumor functions by neuroblastoma microenvironment-associated IL-6 and TGFβ1. Cancer Immunol Immunother. 2013;62:1637–48. doi: 10.1007/s00262-013-1466-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Huang Y, Goel S, Duda DG, et al. Vascular Normalization as an Emerging Strategy to Enhance Cancer Immunotherapy. Cancer Res. 2013;73:2943–8. doi: 10.1158/0008-5472.CAN-12-4354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Wilhelm S, Carter C, Lynch M, et al. Discovery and development of sorafenib: a multikinase inhibitor for treating cancer. Nat Rev Drug Discov. 2006;5:835–44. doi: 10.1038/nrd2130. [DOI] [PubMed] [Google Scholar]
- 49.Kakodkar NC, Peddinti RR, Tian Y, et al. Sorafenib inhibits neuroblastoma cell proliferation and signaling, blocks angiogenesis, and impairs tumor growth. Pediatr Blood Cancer. 2012;59:642–7. doi: 10.1002/pbc.24004. [DOI] [PubMed] [Google Scholar]
- 50.Ara T, Song L, Shimada H, et al. Interleukin-6 in the bone marrow microenvironment promotes the growth and survival of neuroblastoma cells. Cancer Res. 2009;69:329–37. doi: 10.1158/0008-5472.CAN-08-0613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Glade Bender J, Yamashiro DJ, Fox E. Clinical development of VEGF signaling pathway inhibitors in childhood solid tumors. Oncologist. 2011;16:1614–25. doi: 10.1634/theoncologist.2011-0148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Kim A, Widemann BC, Krailo M, et al. Phase 2 trial of sorafenib in children and young adults with refractory solid tumors: A report from the Children's Oncology Group. Pediatr Blood Cancer. 2015;62:1562–6. doi: 10.1002/pbc.25548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Iwahara T, Fujimoto J, Wen D, et al. Molecular characterization of ALK, a receptor tyrosine kinase expressed specifically in the nervous system. Oncogene. 1997;14:439–49. doi: 10.1038/sj.onc.1200849. [DOI] [PubMed] [Google Scholar]
- 54**.George RE, Sanda T, Hanna M, et al. Activating mutations in ALK provide a therapeutic target in neuroblastoma. Nature. 2008;455:975–8. doi: 10.1038/nature07397. Seminal study demonstrating the potential for using ALK mutations as a molecular target in neuroblastoma. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55**.Chen Y, Takita J, Choi YL, et al. Oncogenic mutations of ALK kinase in neuroblastoma. Nature. 2008;455:971–4. doi: 10.1038/nature07399. Seminal study demonstrating the presence of recurrent ALK aberations in neuroblastoma. [DOI] [PubMed] [Google Scholar]
- 56.Malik SM, Maher VE, Bijwaard KE, et al. U.S. Food and Drug Administration approval: crizotinib for treatment of advanced or metastatic non-small cell lung cancer that is anaplastic lymphoma kinase positive. Clin Cancer Res. 2014;20:2029–34. doi: 10.1158/1078-0432.CCR-13-3077. [DOI] [PubMed] [Google Scholar]
- 57**.Mosse YP, Lim MS, Voss SD, et al. Safety and activity of crizotinib for paediatric patients with refractory solid tumours or anaplastic large-cell lymphoma: a Children's Oncology Group phase 1 consortium study. Lancet Oncol. 2013;14:472–80. doi: 10.1016/S1470-2045(13)70095-0. First clinical study to demonstrate activity of ALK inhibitors in relapsed and refractory neuroblastoma. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Bresler SC, Wood AC, Haglund EA, et al. Differential inhibitor sensitivity of anaplastic lymphoma kinase variants found in neuroblastoma. Sci Transl Med. 2011;3:108ra14. doi: 10.1126/scitranslmed.3002950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Infarinato NR, Park JH, Krytska K, et al. The ALK/ROS1 Inhibitor PF-06463922 Overcomes Primary Resistance to Crizotinib in ALK-Driven Neuroblastoma. Cancer Discov. 2016;6:96–107. doi: 10.1158/2159-8290.CD-15-1056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Shaw AT, Kim DW, Mehra R, et al. Ceritinib in ALK-rearranged non-small-cell lung cancer. N Engl J Med. 2014;370:1189–97. doi: 10.1056/NEJMoa1311107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Friboulet L, Li N, Katayama R, et al. The ALK inhibitor ceritinib overcomes crizotinib resistance in non-small cell lung cancer. Cancer Discov. 2014;4:662–73. doi: 10.1158/2159-8290.CD-13-0846. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Khozin S, Blumenthal GM, Zhang L, et al. FDA approval: ceritinib for the treatment of metastatic anaplastic lymphoma kinase-positive non-small cell lung cancer. Clin Cancer Res. 2015;21:2436–9. doi: 10.1158/1078-0432.CCR-14-3157. [DOI] [PubMed] [Google Scholar]
- 63.Acheson A, Conover JC, Fandl JP, et al. A BDNF autocrine loop in adult sensory neurons prevents cell death. Nature. 1995;374:450–3. doi: 10.1038/374450a0. [see comments] [DOI] [PubMed] [Google Scholar]
- 64.Ho R, Eggert A, Hishiki T, et al. Resistance to chemotherapy mediated by TrkB in neuroblastomas. Cancer Res. 2002;62:6462–6. [PubMed] [Google Scholar]
- 65.Evans AE, Kisselbach KD, Liu X, et al. Effect of CEP-751 (KT-6587) on neuroblastoma xenografts expressing TrkB. Medical & Pediatric Oncology. 2001;36:181–4. doi: 10.1002/1096-911X(20010101)36:1<181::AID-MPO1043>3.0.CO;2-Q. [DOI] [PubMed] [Google Scholar]
- 66.Brodeur GM, Minturn JE, Ho R, et al. Trk receptor expression and inhibition in neuroblastomas. Clin Cancer Res. 2009;15:3244–50. doi: 10.1158/1078-0432.CCR-08-1815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Ardini E, Menichincheri M, Banfi P, et al. Entrectinib, a Pan-TRK, ROS1, and ALK Inhibitor with Activity in Multiple Molecularly Defined Cancer Indications. Mol Cancer Ther. 2016;15:628–39. doi: 10.1158/1535-7163.MCT-15-0758. [DOI] [PubMed] [Google Scholar]
- 68.Iyer R, Wehrmann L, Golden RL, et al. Entrectinib is a potent inhibitor of Trk-driven neuroblastomas in a xenograft mouse model. Cancer Lett. 2016;372:179–86. doi: 10.1016/j.canlet.2016.01.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.De Braud F, Niger M, Damian S, et al. Alka-372-001: First-in-human, phase I study of entrectinib – an oral pan-trk, ROS1, and ALK inhibitor – in patients with advanced solid tumors with relevant molecular alterations. J Clin Oncol. 2015;33 [Google Scholar]
- 70.Dhillon AS, Hagan S, Rath O, et al. MAP kinase signalling pathways in cancer. Oncogene. 2007;26:3279–90. doi: 10.1038/sj.onc.1210421. [DOI] [PubMed] [Google Scholar]
- 71*.Pugh TJ, Morozova O, Attiyeh EF, et al. The genetic landscape of high-risk neuroblastoma. Nat Genet. 2013;45:279–84. doi: 10.1038/ng.2529. Described the genomic landscape of high-risk neuroblastoma. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72*.Eleveld TF, Oldridge DA, Bernard V, et al. Relapsed neuroblastomas show frequent RAS-MAPK pathway mutations. Nat Genet. 2015;47:864–71. doi: 10.1038/ng.3333. First study to demonstrate the genetic landscape in relapsed neuroblastoma. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Tanaka T, Higashi M, Kimura K, et al. MEK inhibitors as a novel therapy for neuroblastoma: Their in vitro effects and predicting their efficacy. J Pediatr Surg. 2016;51:2074–9. doi: 10.1016/j.jpedsurg.2016.09.043. [DOI] [PubMed] [Google Scholar]
- 74.Wright CJ, McCormack PL. Trametinib: first global approval. Drugs. 2013;73:1245–54. doi: 10.1007/s40265-013-0096-1. [DOI] [PubMed] [Google Scholar]
- 75.Menzies AM, Long GV. Dabrafenib and trametinib, alone and in combination for BRAF-mutant metastatic melanoma. Clin Cancer Res. 2014;20:2035–43. doi: 10.1158/1078-0432.CCR-13-2054. [DOI] [PubMed] [Google Scholar]
- 76.Chesler L, Schlieve C, Goldenberg DD, et al. Inhibition of phosphatidylinositol 3-kinase destabilizes Mycn protein and blocks malignant progression in neuroblastoma. Cancer Res. 2006;66:8139–46. doi: 10.1158/0008-5472.CAN-05-2769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Chanthery YH, Gustafson WC, Itsara M, et al. Paracrine signaling through MYCN enhances tumor-vascular interactions in neuroblastoma. Sci Transl Med. 2012;4:115ra3. doi: 10.1126/scitranslmed.3002977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Vaughan L, Clarke PA, Barker K, et al. Inhibition of mTOR-kinase destabilizes MYCN and is a potential therapy for MYCN-dependent tumors. Oncotarget. 2016 doi: 10.18632/oncotarget.10544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Li Z, Tan F, Liewehr DJ, et al. In vitro and in vivo inhibition of neuroblastoma tumor cell growth by AKT inhibitor perifosine. J Natl Cancer Inst. 2010;102:758–70. doi: 10.1093/jnci/djq125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Garlich JR, De P, Dey N, et al. A vascular targeted pan phosphoinositide 3-kinase inhibitor prodrug, SF1126, with antitumor and antiangiogenic activity. Cancer Res. 2008;68:206–15. doi: 10.1158/0008-5472.CAN-07-0669. [DOI] [PubMed] [Google Scholar]
- 81.Kushner BH, Cheung NV, Modak S, et al. A phase I/Ib trial targeting the Pi3k/Akt pathway using perifosine: Long-term progression-free survival of patients with resistant neuroblastoma. Int J Cancer. 2017;140:480–4. doi: 10.1002/ijc.30440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Dawson MA, Kouzarides T. Cancer epigenetics: from mechanism to therapy. Cell. 2012;150:12–27. doi: 10.1016/j.cell.2012.06.013. [DOI] [PubMed] [Google Scholar]
- 83.Puissant A, Frumm SM, Alexe G, et al. Targeting MYCN in neuroblastoma by BET bromodomain inhibition. Cancer Discov. 2013;3:308–23. doi: 10.1158/2159-8290.CD-12-0418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Wyce A, Ganji G, Smitheman KN, et al. BET inhibition silences expression of MYCN and BCL2 and induces cytotoxicity in neuroblastoma tumor models. PLoS One. 2013;8:e72967. doi: 10.1371/journal.pone.0072967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Henssen A, Althoff K, Odersky A, et al. Targeting MYCN-Driven Transcription By BET-Bromodomain Inhibition. Clin Cancer Res. 2016;22:2470–81. doi: 10.1158/1078-0432.CCR-15-1449. [DOI] [PubMed] [Google Scholar]
- 86.Hogarty MD, Norris MD, Davis K, et al. ODC1 is a critical determinant of MYCN oncogenesis and a therapeutic target in neuroblastoma. Cancer Res. 2008;68:9735–45. doi: 10.1158/0008-5472.CAN-07-6866. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Evageliou NF, Haber M, Vu A, et al. Polyamine Antagonist Therapies Inhibit Neuroblastoma Initiation and Progression. Clin Cancer Res. 2016;22:4391–404. doi: 10.1158/1078-0432.CCR-15-2539. [DOI] [PubMed] [Google Scholar]
- 88.Saulnier Sholler GL, Gerner EW, Bergendahl G, et al. A Phase I Trial of DFMO Targeting Polyamine Addiction in Patients with Relapsed/Refractory Neuroblastoma. PLoS One. 2015;10:e0127246. doi: 10.1371/journal.pone.0127246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Maris JM, Morton CL, Gorlick R, et al. Initial testing of the aurora kinase A inhibitor MLN8237 by the Pediatric Preclinical Testing Program (PPTP) Pediatr Blood Cancer. 2010;55:26–34. doi: 10.1002/pbc.22430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.DuBois SG, Marachelian A, Fox E, et al. Phase II study of alisertib, irinotecan, and temozolomide in children with relapsed and refractory neuroblastoma: A report from the New Approaches to Neuroblastoma Therapy (NANT) consortium. J Clin Oncol. 2016;34 doi: 10.1200/JCO.2015.65.4889. suppl abstr 10556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Rader J, Russell MR, Hart LS, et al. Dual CDK4/CDK6 inhibition induces cell-cycle arrest and senescence in neuroblastoma. Clin Cancer Res. 2013;19:6173–82. doi: 10.1158/1078-0432.CCR-13-1675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Ackermann S, Goeser F, Schulte JH, et al. Polo-like kinase 1 is a therapeutic target in high-risk neuroblastoma. Clin Cancer Res. 2011;17:731–41. doi: 10.1158/1078-0432.CCR-10-1129. [DOI] [PubMed] [Google Scholar]
- 93.Hong D, Infante J, Janku F, et al. Phase I Study of LY2606368, a Checkpoint Kinase 1 Inhibitor, in Patients With Advanced Cancer. J Clin Oncol. 2016;34:1764–71. doi: 10.1200/JCO.2015.64.5788. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Do K, Wilsker D, Ji J, et al. Phase I Study of Single-Agent AZD1775 (MK-1775), a Wee1 Kinase Inhibitor, in Patients With Refractory Solid Tumors. J Clin Oncol. 2015;33:3409–15. doi: 10.1200/JCO.2014.60.4009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.de Braud F, Cascinu S, Spitaleri G, et al. A phase I, dose-escalation study of volasertib combined with nintedanib in advanced solid tumors. Ann Oncol. 2015;26:2341–6. doi: 10.1093/annonc/mdv354. [DOI] [PubMed] [Google Scholar]
- 96.Johnstone RW. Histone-deacetylase inhibitors: novel drugs for the treatment of cancer. Nat Rev Drug Discov. 2002;1:287–99. doi: 10.1038/nrd772. [DOI] [PubMed] [Google Scholar]
- 97.Fouladi M, Park JR, Stewart CF, et al. Pediatric phase I trial and pharmacokinetic study of vorinostat: a Children's Oncology Group phase I consortium report. J Clin Oncol. 2010;28:3623–9. doi: 10.1200/JCO.2009.25.9119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98**.Mody RJ, Wu YM, Lonigro RJ, et al. Integrative Clinical Sequencing in the Management of Refractory or Relapsed Cancer in Youth. JAMA. 2015;314:913–25. doi: 10.1001/jama.2015.10080. First study to demonstrate that pediatric tumors can be sequenced clinically to identify potential therapeutic targets. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Harris MH, DuBois SG, Glade Bender JL, et al. Multicenter Feasibility Study of Tumor Molecular Profiling to Inform Therapeutic Decisions in Advanced Pediatric Solid Tumors: The Individualized Cancer Therapy (iCat) Study. JAMA Oncol. 2016 doi: 10.1001/jamaoncol.2015.5689. [DOI] [PubMed] [Google Scholar]
- 100.Worst BC, van Tilburg CM, Balasubramanian GP, et al. Next-generation personalised medicine for high-risk paediatric cancer patients - The INFORM pilot study. Eur J Cancer. 2016;65:91–101. doi: 10.1016/j.ejca.2016.06.009. [DOI] [PubMed] [Google Scholar]
- 101.Parsons DW, Roy A, Yang Y, et al. Diagnostic Yield of Clinical Tumor and Germline Whole-Exome Sequencing for Children With Solid Tumors. JAMA Oncol. 2016 doi: 10.1001/jamaoncol.2015.5699. [DOI] [PMC free article] [PubMed] [Google Scholar]