Abstract
Introduction
Diabetic patients are associated with impaired peripheral microvascular function after cardiopulmonary bypass (CPB) and cardiac surgery. We hypothesized that upregulation of the inducible cyclooxygenase 2 (COX-2) contributes to altered microvascular reactivity of peripheral arterioles in diabetic patients undergoing CPB and cardiac surgery.
Methods
Skeletal muscle samples of non-diabetic (ND) and diabetic (DM) patients (n = 8/group) undergoing cardiac surgery were harvested before and after CPB. The protein expression/localization of COX-2 was assayed by Western blotting and immunohistochemistry. Peripheral arterioles were dissected from the harvested skeletal muscle tissue samples, the isolated arterioles (80–180μm) were cannulated and pressurized, and changes in diameter were measured with video microscopy. In-vitro relaxation responses of pre-contracted arterioles were examined in the presence of the endothelium-dependent vasodilator bradykinin (10−10 to 10−6M) and in the presence or absence of the selective COX-2 inhibitor NS398 (10−5M).
Results
The post-CPB protein levels of the inducible COX-2 were significantly increased compared with pre-CPB values in both the ND and DM groups (P<0.05), whereas, this increase was higher in DM than that of non-diabetics (P<0.05). In the DM arterioles, not the ND vessels, bradykinin-induced relaxation response was inhibited in the presence of the specific COX-2 inhibitor NS398 at baseline (P<0.05). After CPB, bradykinin-induced relaxation response of the ND and DM arterioles was inhibited in the presence of the specific COX-2 inhibitor NS398, but this effect was more pronounced in the diabetic patients (P<0.05).
Conclusion
Diabetes and CPB are associated with up-regulation in COX-2 expression/activation in human peripheral microvasculature. This alteration may lead to altered peripheral microvascular reactivity in diabetic patients undergoing cardiac surgery.
Keywords: Diabetes, COX-2, Microvasular Reactivity, Bradykinin, Skeletal muscle, CPB
Introduction
Patients with diabetes mellitus (DM) display a high risk of morbidity and mortality after cardiac surgery, especially in cases that require cardiopulmonary bypass (CPB).1–3 Diabetic patients are at risk for increased mortality, post-operative body weight, and length of hospital stay post-cardiac surgery.1,4,5 Recent studies have shown that diabetes is associated with increased vascular permeability and decreased vasomotor function,4,5 as well as decreased endothelium-dependent and independent relaxation response to vasodilators after cardiac surgery.6–9
The inducible isoform cyclooxygenase 2 (COX-2) is associated with the inflammatory response and is known to produce several mediators for coronary vasodilation and constriction.10–13 Unlike COX-1 which is present in tissues throughout the body, COX-2 expression has been shown to be upregulated to tissues experiencing inflammation or damage.10–15 Particularly relevant are tissues undergoing trauma due to surgery.10–15 We have previously shown increased protein and gene expression of COX-2 in the ischemic heart after cardioplegic arrest (CP) and cardiac surgery in non-diabetic patients.10,11 Recently, we have also found that diabetes induces COX-2 upregulation in ischemic myocardium and coronary arterioles in the setting of cardioplegic arrest and reperfusion.16 These findings suggest that upregulation of COX-2 expression may contribute to bradykinin-induced coronary arteriolar relaxation in diabetic patients undergoing cardiac surgery. However, it is not known whether diabetes and/or CPB may also affect COX-2 expression in the skeletal muscle. Therefore, we hypothesized that diabetes and/or CPB induces overexpression of the inducible COX-2 in the skeletal muscle tissue and peripheral vasculature, which may also contribute to bradykinin-induced peripheral microvascular relaxation, in the same manner as its effect on coronary microvasculature. Thus, the aim of this study is to measure COX-2 expression post-CPB in diabetic patients and analyze its effect on microvascular reactivity in vasodilation pathways involving bradykinin.
Methods and materials
Skeletal Muscle Tissue Harvesting
Patients were identified among those undergoing cardiac surgery requiring cardiopulmonary bypass (CPB) at Rhode Island Hospital. Patients were divided into two groups based on their diabetic status: 1) non-diabetic (normal HgbA1C) and 2) diabetic (HgbA1C>8.5). Two samples of skeletal muscle were removed from the left interior chest wall of each patient. The first was taken before cannulation pre-CPB. The second was harvested after removal of the aortic cross clamp and weaning off of CPB. Both samples were then prepared for three experimental procedures: 1) in-vitro microvascular reactivity, 2) immunohistochemistry, 3) protein analysis western blotting. All procedures were approved by the Institutional Review Board of Rhode Island Hospital, and informed consent was obtained from all enrolled patients.
Microvascular reactivity
Arterioles (80–180 micrometer in diameter) were dissected from skeletal muscle samples before and after CPB. Arterioles were then stabilized in the organ chamber before being pre-treated and perfused with a thromboxane A-2 analogue U46619 (10−7M) (Sigma-Aldrich, St. Louis, MO) to 30–50% of the baseline diameter. The arterioles were then exposed to increasing concentrations of endothelium-dependent vasodilator bradykinin (Sigma-Aldrich) to measure the dose-dependent relaxation response. It has been well recognized that bradykinin induced vascular relaxation is endothelium-dependent.17–19 A sub-group of arterioles was also treated with a COX-2 inhibitor NS398 (10−5M) before the exposure to bradykinin for analysis of the effect of COX-2 on this vasodilation pathway.
Western blot
Protein samples were purified from the pre and post-CPB SKM tissue samples and prepared for immunoblotting. Membranes were incubated with individual polyclonal primary antibodies for COX-1 and COX-2, and then incubated with horseradish peroxidase-conjugated secondary antibodies. Peroxidase activity was visualized using a digital camera system and imaging bands were quantified using densitometry software.
Immunohistochemistry
After extraction, a portion of both pre- and post-CPB skeletal muscle samples were sectioned and parafinized for later use during microscopy techniques. Upon initiation of immunohistochemistry procedures, the sections were deparafinized in xylene, rehydrated using graded ethanol and PBS, and antigen-unmasked using sodium citrate. Slides were then washed with PBS and blocked with a 2% BSA and PBS solution at room temperature for 2 hours. After PBS wash, the slides were incubated overnight with anti-COX1, anti-COX2, and anti-mouse smooth muscle actin antibodies at 4°C. After PBS wash, the slides were incubated with the appropriate secondary antibody and mounted with fluorescent mounting medium. Slides were visualized using the Nikon epi-fluorescent microscope system. All images were taken at the same magnification and resolution, and later processed for optical density analysis.
Data analysis
All statistical analyses were performed with GraphPad Software (GraphPad Software, Inc, San Diego, CA). Data are expressed as the mean ± SD. For analysis of data from patient characteristics, protein expression and other imaging, Kruskal-Wallis tests with Dunn's multiple test were performed. For analysis of categorical data, Fisher’s exact test was employed. For analysis of microvessel data, two way repeated-measures ANOVA with a post hoc Bonferroni test was performed. P values less than 0.05 were considered significant.
Results
Patient characteristics are listed in the Table 1. The pre-operative hemoglobin A1C levels were 5.2 ± 0.3 in the ND patients and 9.0 ± 0.4 in the DM patients. All patients (n = 8/group) with preoperative hypertension were on antihypertensive medication [(aspirin and/or β-blocker, and/or calcium channel blocker, and/or statins and/or angiotensin-converting enzyme inhibitors (ACEI)].
Table 1.
Patient Characteristics
| Patient Characteristics | ND | DM | P values |
|---|---|---|---|
| Age (y)* | 68 ± 8 | 65 ± 7 | 0.62 |
| Male/Female (n) | 6/2 | 7/1 | 1.00 |
| HbA1c (%)* | 5.2 ± 0.3 | 9.0 ± 0.4 | 0.0001 |
| Patient Blood Glucose (mg/dL, pre-CP/CPB)* | 104 ± 12 | 206 ± 28 # | 0.0001 |
| Patient Blood Glucose (mg/dL, During-CP/CPB)* | 147 ± 15 | 186 ± 17 # | 0.001 |
| Pre-operative Insulin (n) | 0 | 6# | 0.47 |
| Intra-operative Insulin (n) | 1 | 8# | 0.001 |
| Obesity (BMI>30) | 4 | 6 | 0.61 |
| Hypercholestesterolemia (n) | 4 | 5 | 1.00 |
| Hypertension (n) | 6 | 7 | 1.00 |
| Current smokers | 0 | 0 | 1.00 |
| Atrial fibrillation (n) | 0 | 0 | 1.00 |
| Duration of CPB (min)* | 101 ± 9 | 109 ± 10 | 0.15 |
| Cross-clamp time (min)* | 86 ± 10 | 94 ± 11 | 0.16 |
| CABG only (n) | 6 | 5 | 1.00 |
| CABG plus AVR or MV replacement | 2 | 3 | 1.00 |
| Number of Graft | 2.63 ± 0.5 | 2.88 ± 0.7 | 0.35 |
| Oral Antidiabetic Medications | 0 | 5# | 0.03 |
| Aspirin | 7 | 8 | 1.00 |
vs. non-diabetics (ND); Mean ± SD; HbA1c = hemoglobin A1c
Microvessel Reactivity
Bradykinin induced dose-dependent relaxation response in both groups (Figure 1A–D). There was a significant decrease in relaxation response of the skeletal muscle arterioles to bradykinin in both groups post-CPB (P<0.05 vs. pre-CPB, Figure 1A and Figure 1B), and in the DM group pre-CPB (P<0.05 vs. ND), with the most significant decrease occurring in the DM group post-CPB (Figure 1A–D). There was no difference in the dose-dependent response to bradykinin between ND and DM patient groups at baseline (pre-CPB, Figure 1C). At baseline (pre-CPB), treatment of microvessels with the selective COX-2 inhibitor NS398 produced no effect on relaxation response of the pre-CPB vessels in the ND patient group (Figure 2). In contrast, treatment with the COX-2 inhibitor NS398 significantly diminished relaxation response to bradykinin in the pre-CPB DM microvessels (P<0.05 vs. bradykinin alone, Figure 4). After CPB, treatment with the COX-2 inhibitor NS398 significantly inhibited relaxation response to bradykinin in both of ND and DM microvessels (P<0.05 vs. bradykinin alone), but this effect was more pronounced in the DM group than that of ND. (P<0.05 vs. ND, Figure 2)
Fig. 1.
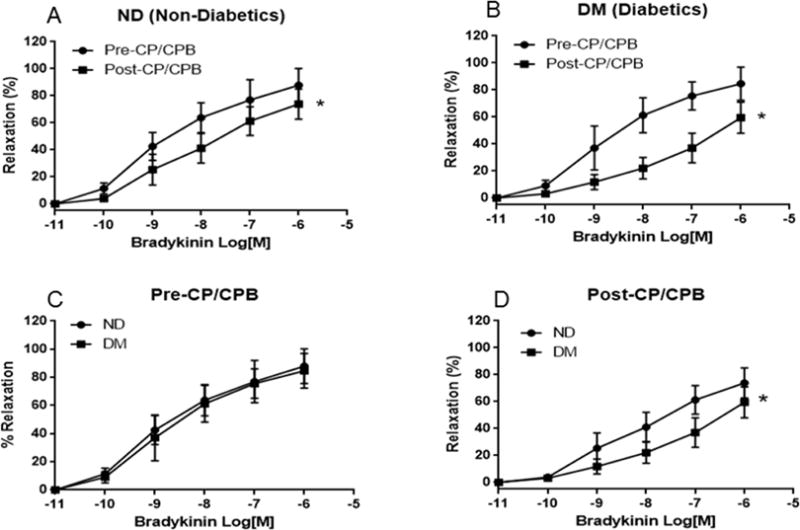
A. The relaxation responses of non-diabetic (ND), and diabetic (DM) arterioles to the dose-dependent, endothelium-dependent vasodilator bradykinin at baseline; Pre-CPB: before cardiopulmonary bypass; B. The relaxation response of ND, and DM peripheral arterioles to bradykinin after (post-) CPB; C. The relaxation response of non-diabetic (ND) peripheral arterioles to bradykinin between pre-CPB versus post-CPB; D. The relaxation response of DM peripheral arterioles to bradykinin between pre-CPB versus post-CPB; Data are means ± SD, n = 8/group, *P < 0.05 vs. ND; or pre-CPB,
Fig. 2.
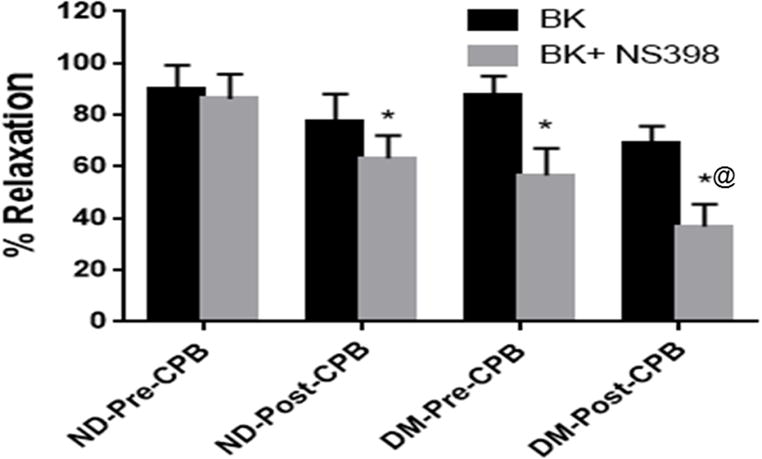
The relaxation response of pre-CPB arterioles to bradykinin (BK) in the presence and absence of the selective COX-2 inhibitor NS398 from ND and DM patients. Data are means ± SD. *P<0.05 vs. bradykinin, alone **P<0.001 vs. bradykinin alone, n = 6/group.
Fig. 4.
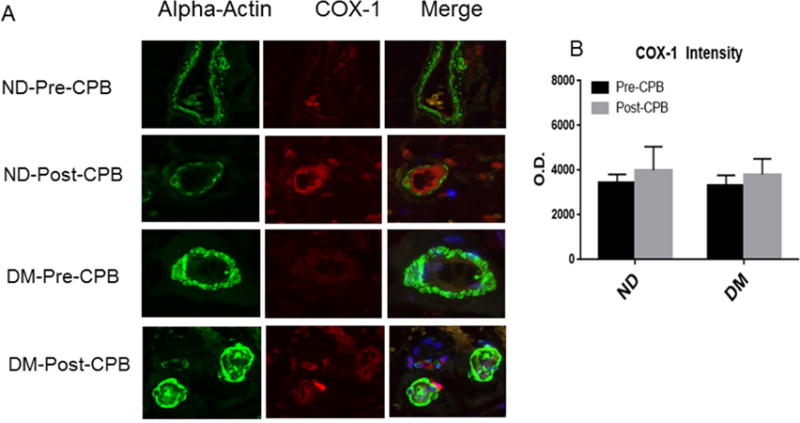
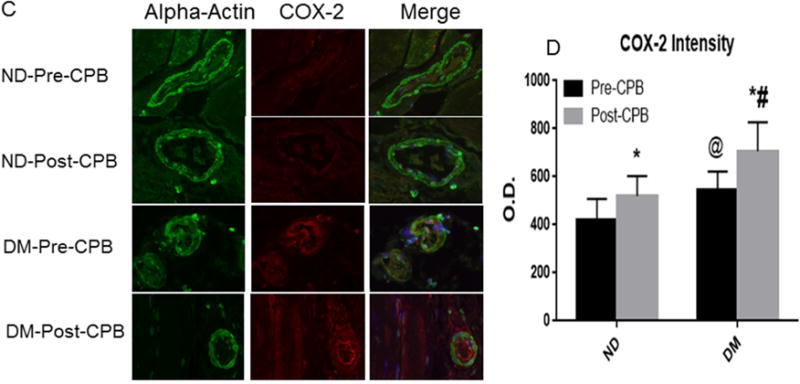
A, Immunofluorescence staining of COX-1 in paraffin-embedded human skeletal muscle tissue samples from ND and DM patients; Vessels were co-stained for cell nuclei (blue), smooth muscle α-actin (green) and COX-1 (red in endothelium and orange in smooth muscle). B, Densitometric analysis of signal intensities shows no significant differences in COX-1 distribution between the two groups or between pre- and post-CPB; C, Immuno-histochemical image of COX-2 in paraffin-embedded human skeletal muscle tissue samples from ND and DM patients; Vessels were co-stained for cell nuclei (blue), smooth muscle α-actin (green) and COX-2 (red in endothelium and orange in smooth muscle). D. Densitometric analysis of signal intensities shows differences in COX-2 distribution in the skeletal muscle arterioles between the two groups or between pre- and post-CPB; Data are means ± SEM, *p<0.05 vs. pre-CPB; @P<0.05 vs. ND at baseline, #P<0.05 vs. ND post-CPB; Data are means ± SD, n= 6/group.
COX-1 and COX-2 Protein Expression
Immunoblot results show that there was some increase in COX-1 expression post-CPB in both ND and DM groups, however, this increase does not reach the level of statistical significance (Figure 3A, and Figure 3B). Immunoblot results indicate that COX-2 expression was significantly greater at baseline (pre-CPB) in the DM group than the ND group (P<0.05, Figure 3C and Figure 4D). COX-2 expression also increased significantly post-CPB in both patient groups and this affect was more pronounced in DM group than that of ND(P<0.05, Figure 3C and Figure3D).
Fig. 3.
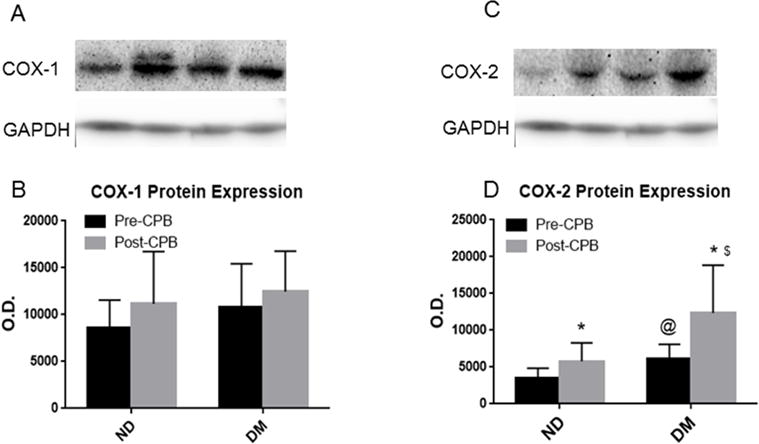
A, representative immunoblots of skeletal muscle tissue samples from non-ND, and DM patients, pre: pre-CPB, post-: post-CPB; B, Densitometric evaluation of immunoblot band intensity shows no significant differences in the levels of COX-1 between the two groups, or between pre- and post-CPB; C, representative immunoblots of skeletal muscle tissue samples from non-ND, and DM patients.; D, Densitometric evaluation of immunoblot band intensity shows significant differences in the levels of COX-2 between DM and ND groups, or between pre- and post-CPB, Data are means ± SD, n= 6/group, *P<0.05 vs. pre-CPB; @ P<0.05 vs. ND-pre-CPB, $P<0.05 vs. ND-post-CP/CPB.
COX-1 and COX-2 Localization
Immuno-fluorescent staining demonstrated that COX-1 and COX-2 are localized in the skeletal muscle microvessels (Figure 4). There were no significant differences in COX-1 distribution in the skeletal muscle microvessels between ND and DM or between pre-and post-CPB.(Figure 4A and Figure 4B). At baseline, diabetes induced significant increases in COX-2 expression compared with ND group (Figure 4C and Figure 4D, P<0.05 vs pre-CPB-ND). The pre-CPB levels of COX-2 were significantly increased post-CPB in both groups (P<0.05 vs. pre-CPB), but this increase was greater in DM than ND groups. (Figure 4C and Figure 4D, P<0.05 vs. ND).
Discussion
CPB often causes peripheral arteriolar dilation (vasoplegia) and reduces vasomotor tone, manifested as systemic hypotension post-CPB. Clinically, anesthesiologists often administer the amount of norepinephrine or vasopressin necessary to maintain systemic blood pressure early after CPB. Consistent with the in-vivo observation, we have previously observed that the response of post-CPB microvessels (in-vitro, pre-dilated vessels) to vasodilators and vasopressors was significantly decreased.6,8,17,18 We have previously reported that CPB is associated with impairment of peripheral arteriolar endothelial function in patients after cardiac surgery.10,11,14 In particular, the relaxation responses of skeletal muscle microvessels to endothelium-dependent and independent vasodilators, ADP, substance P and SNP were significantly decreased after CPB and cardiac surgery.10,11,14 In the present study, we found that the relaxation response of the skeletal muscle arterioles to the endothelium-dependent vasodilator bradykinin was also impaired in patients after CPB and cardiac surgery. In this study, we observed that impaired relaxation response to bradykinin was more pronounced in the DM patients than the ND patients after CPB. These findings are consistent with our previous studies, suggesting diabetes causes endothelial dysfunction in human peripheral arterioles early after CPB and cardiac surgery.
Recently, our study demonstrates that COX-2 activation contributes to altered bradykinin-induced vasodilation of coronary arterioles.16 In the present study, we observed that bradykinin-induced vasodilation of the skeletal muscle arterioles was partially via COX-2 activation in diabetic patients, but not in non-diabetic patients at baseline. Importantly, after CPB, bradykinin-induced relaxation response was inhibited in the presence of the selective COX-2 inhibitor NS398 in both ND, and DM arterioles, whereas this effect was more pronounced in the DM group. These novel findings suggest that diabetes markedly enhances COX-2 activation in the peripheral microvasculature, indicating that COX-2 may play an important role in the vasodilation pathway involving bradykinin in the peripheral microcirculation early after CPB. Consistent with our previous study in the ischemic heart and coronary microvasculature,10,11,16 we found upregulation of COX-2 protein expression in the skeletal muscle and peripheral arterioles in the diabetic patients at baseline. In addition to the Western blot experiments, immune-histochemical staining also detected enhanced skeletal muscle and vascular localization of COX-2 in diabetic patients. The upregulation of COX-2 may contribute to bradykinin-induced vasodilation of peripheral arterioles in the diabetic patients.
We have previously found that cardioplegic arrest and reperfusion significantly upregulates COX-2 gene/protein expression of ischemic myocardium in patients undergoing cardiac surgery.10,11 In this study, we further observed that CPB caused more COX-2 protein expression/distribution in the skeletal muscle and peripheral arterioles in both ND and DM patients. This effect was more pronounced in diabetic patients. In contrast, CPB did not affect COX-1 protein expression/distribution in the skeletal muscle and peripheral arterioles in the patient with or without diabetes. These novel findings indicate that diabetes combined with CPB further up-regulates COX-2 protein expression and localization in the peripheral tissues and vessels. They also suggest that upregulated COX-2 plays an important role in the altered skeletal-muscle arteriolar reactivity in diabetic patients early after CPB.
However, the results of our study should be interpreted with several limitations. First, the sample size is still very small (8/group). Second, the use of different medications prior to cardiac surgery and the presence of varying comorbidities may have also affected the current results. Third, pre- and perioperative treatment with insulin might have influenced the outcomes of current studies. In conclusion, diabetes is associated with upregulation in COX-2 expression/distribution in human skeletal muscle and peripheral microvasculature early after CPB and cardiac surgery. These alterations may contribute to bradykinin-induced peripheral arteriolar relaxation in diabetic patients undergoing CP/CPB and cardiac surgery.
Acknowledgments
We would like to thank all nurses, physician assistants and perfusionists at cardiac-surgery-operation rooms in Lifespan Hospitals for collecting the tissue samples and data of patient characteristics. We would also like to thank the nurses and physician assistants at Division of Cardiac Surgery, Lifespan Hospitals for collecting patient consent forms.
Funding
This research project was supported by the National Heart, Lung, and Blood Institute HL-46716 (F.W.S), 1RO1HL127072 (J.F.), and supported in part by 1R01 HL136347-01(J.F.), NIGMS/NIH grant 1P20GM103652 (pilot project) (J.F.), AHA-Grant-in-Aid-15GRNT25710105 (J.F.), Undergraduate Research and Teaching Award (UTRA) of Brown University (K.A.).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Authors Contribution: Each of the authors listed below have made substantial contributions in each of these three areas: (1) conceiving and designing the study; or collecting the data; or analyzing and interpreting the data; (2) writing the manuscript or providing critical revisions that are important for the intellectual content; and (3) approving the final version of the manuscript. J.F.; K.A; S.M.P.; Y.H.L.; A.K.S.; A.E.; F.W. S:
Presented at the 12th Annual Academic Surgical Congress, Feb. 7–9, 2017 Las Vegas, NV
Disclosures
None
References
- 1.Carson JL, Scholz PM, Chen AY, Peterson ED, Gold J, Schneider SH. Diabetes mellitus increases short-term mortality and morbidity in patients undergoing coronary artery bypass graft surgery. J Am Coll Cardiol. 2002;40:418–423. doi: 10.1016/s0735-1097(02)01969-1. [DOI] [PubMed] [Google Scholar]
- 2.Woods SE, Smith JM, Sohail S, Sarah A, Engle A. The influence of type 2 diabetes mellitus in patients undergoing coronary artery bypass graft surgery: an 8-year prospective cohort study. Chest. 2004;126:1789–1795. doi: 10.1378/chest.126.6.1789. [DOI] [PubMed] [Google Scholar]
- 3.Halkos ME, Puskas JD, Lattouf OM, Kilgo P, Kerendi F. Elevated preoperative hemoglobin A1c level is predictive of adverse events after coronary artery bypass surgery. J Thorac Cardiovasc Surg. 2008;136:631–640. doi: 10.1016/j.jtcvs.2008.02.091. [DOI] [PubMed] [Google Scholar]
- 4.Emani S, Ramlawi B, Sodha NR, Li J, Bianchi C, Sellke FW. Increased vascular permeability after cardiopulmonary bypass in patients with diabetes is associated with increased expression of vascular growth factor and hepatocyte growth factor. J Thorac Cardiovasc Surg. 2009;138:185–91. doi: 10.1016/j.jtcvs.2008.12.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Feng J, Liu Y, Sabe AA, Sadek AA, Singh AK, Sodha NR, Sellke FW. Differential impairment of adherens-junction expression/phosphorylation after cardioplegia in diabetic versus non-diabetic patients. Eur J Cardiothoracic Surg. 2015 Jun 11; doi: 10.1093/ejcts/ezv202. pii: ezv20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Feng J, Liu YH, Chu LM, Singh AK, Dobrilovic N, Fingleton JG, Clements R, Bianchi C, Sellke FW. Changes in microvascular reactivity after cardiopulmonary bypass in patients with poorly controlled versus controlled diabetes. Circulation. 2012;126:S73–S80. doi: 10.1161/CIRCULATIONAHA.111.084590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Feng J, Chu LM, Dobrilovic N, Liu YH, Singh AK, Sellke FW. Decreased coronary microvascular reactivity after cardioplegic arrest in patients with uncontrolled diabetes mellitus. Surgery. 2012;152:282–289. doi: 10.1016/j.surg.2012.04.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Feng J, Liu YH, Khabbaz K, Hagberg R, Robich MP, Clements RT, Bianchi C, Sellke FW. Decreased contractile response to endothelin-1 of peripheral microvasculature from diabetic patients. Surgery. 2011;149:247–252. doi: 10.1016/j.surg.2010.07.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Liu YH, Xie A, Singh AK, Ehsan A, Choudhary G, Dudley S, Sellke FW, Feng J. Inactivation of endothelial SKCa/IKCa contributes to coronary arteriolar dysfunction in diabetic patients. Journal of American Heart Association. 4(8) doi: 10.1161/JAHA.115.002062. online 2015 Aug 24. pii: e002062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Metais C, Li JY, Li J, Simons M, Sellke FW. Serotonin-induced coronary contraction increases after blood cardioplegia-reperfusion: Role of COX-2 expression. Circulation. 1999;100:II-328–II334. doi: 10.1161/01.cir.100.suppl_2.ii-328. [DOI] [PubMed] [Google Scholar]
- 11.Metais C, Bianchi C, Li JY, Li J, Simons M, Sellke FW. Serotonin-induced human coronary microvascular contraction during acute myocardial ischemia is blocked by COX-2 inhibition. Basic Res Cardiol. 2001;96:59–67. doi: 10.1007/s003950170078. [DOI] [PubMed] [Google Scholar]
- 12.Szerafin T, Erdei N, Fülöp T, Pasztor ET, Edes I, Koller A, Bagi Z. Increased cyclooxygenase-2 expression and prostaglandin-mediated dilation in coronary arterioles of patients with diabetes mellitus. Circ Res. 2006 Sep 1;99(5):e12–7. doi: 10.1161/01.RES.0000241051.83067.62. [DOI] [PubMed] [Google Scholar]
- 13.Vane JR, Botting RM. The mechanism of action of aspirin. Thromb Res. 2003 Jun 15;110(5–6):255–258. doi: 10.1016/s0049-3848(03)00379-7. [DOI] [PubMed] [Google Scholar]
- 14.Przygodzki T, Talar M, Przygodzka P, Watala C. Inhibition of cyclooxygenase-2 causes a decrease in coronary flow in diabetic mice. The possible role of PGE2 and dysfunctional vasodilation mediated by prostacyclin receptor. J Physiol Biochem. 2015 Sep;71(3):351–358. doi: 10.1007/s13105-015-0415-y. [DOI] [PubMed] [Google Scholar]
- 15.Warner TD, Mitchell JA, Vane JR. Cyclo-oxygenase-2 inhibitors and cardiovascular events. Lancet. 2002 Nov 23;360(9346):1700–1701. doi: 10.1016/S0140-6736(02)11635-7. [DOI] [PubMed] [Google Scholar]
- 16.Feng J, Anderson K, Mitchell H, Singh AK, Ehsan A, Liu Y, Sellke FW. Diabetic upregulation of COX-2 contributes to altered coronary reactivity after cardiac surgery. Ann Thorac Surgery. 2017 doi: 10.1016/j.athoracsur.2016.11.025. In press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Feng J, Liu YH, Singh AK, Dobrilovic N, Feng WC, Chu LM, Robich MP, Khabbaz KR, Sellke FW. Impaired contractile response of human peripheral arterioles to thromboxane A-2 after cardiopulmonary bypass. Surgery. 2011;150:263–271. doi: 10.1016/j.surg.2011.06.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Feng J, Chu LM, Robich M, Clements RT, Khabbaz K, Hagberg R, Liu YH, Osipov RM, Sellke FW. Endothelin-1 induced contraction and signaling in the human skeletal muscle microcirculation. Circulation. 2010;122:S150–S155. doi: 10.1161/CIRCULATIONAHA.109.928226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Feng J, Liu Y, Singh AK, Ehsan A, Sellke N, Liang J, Sellke FW. Effects of diabetes and cardiopulmonary bypass on adherens-junction-protein expression of human peripheral tissue. Surgery. 2017 Mar;161(3):823–829. doi: 10.1016/j.surg.2016.08.057. [DOI] [PMC free article] [PubMed] [Google Scholar]


