Abstract
Taxol is the first-line chemotherapeutic agent for patients with castration-resistant prostate cancer. However, the mechanism of the sensitivity of prostate cancer cells to Taxol treatment remains to be elucidated. In the present study, it was found that paclitaxel induced more apoptosis and maspin expression in phosphatase and tensin homolog (PTEN)-positive 22Rv1 cells than PTEN-negative LNCaP cells. Knockdown of PTEN in 22Rv1 cells resulted in increased resistance to paclitaxel and impaired the induction of maspin expression by paclitaxel. Overexpression of PTEN sensitized LNCaP cells to paclitaxel treatment and increased maspin induction by paclitaxel. Furthermore, knocking down maspin abrogated PTEN-induced paclitaxel sensitivity in LNCaP cells. PTEN/maspin signaling may be important for regulating the susceptibility to paclitaxel in prostate cancer.
Keywords: paclitaxel, PTEN, maspin, drug sensitivity, prostate cancer cells
Introduction
Taxol, also termed paclitaxel, has been widely used in the treatment of several solid tumors, including prostate cancer (1–3). However, not all tumors are sensitive to paclitaxel treatment, and the mechanisms that distinguish resistant tumors from sensitive tumors are not well understood (4). Therefore, identifying the molecular characteristics associated with resistance or sensitivity to paclitaxel may help to determine the patients that are most likely to benefit from paclitaxel therapy.
Multiple mechanisms have been identified for paclitaxel-mediated chemotherapy of human cancers. For example, paclitaxel has been shown to induce apoptosis by binding to the tubulin protein of microtubules and inhibiting the depolymerization of microtubules (5). Several apoptotic signaling molecules, such as phosphatidyinositol-3 kinase (PI3K)/Akt, p53/p21 and c-Raf-1/Ras/B-cell lymphoma-2, have also been reported to be involved in apoptosis induction by paclitaxel (6,7). Previous studies have shown that Akt inactivation sensitized human ovarian cancer cells to cisplatin and paclitaxel (8–10). Phosphatase and tensin homolog (PTEN), an endogenous tumor suppressor, dephosphorylated the D3 position of phosphatidylinositol-3,4,5 triphosphate to negatively control PI3K activity, and thus inhibited a panel of cellular responses mediated by the PI3K/Akt pathway (11). Overexpression of PTEN in malignant cells also induced apoptosis and suppression of survival signaling (10). The potential role of PTEN in the clinical efficacy of prostate cancer therapy by paclitaxel and its underlying mechanism remains to be elucidated.
The present study aimed at determining the sensitivity of paclitaxel in PTEN-positive and PTEN-negative prostate cancer cells. Furthermore, the present study aimed at investigating the underlying molecular mechanism and function of PTEN in the regulation of mammary serine protease inhibitor or serpin (maspin) expression in paclitaxel sensitivity.
Materials and methods
Materials
Dharmacon PTEN siRNA, maspin siRNA and control siRNA were purchased from GE Healthcare Life Sciences (Little Chalfont, UK). Paclitaxel was purchased from Taihua Natural Plant Pharmaceutical Co. Ltd (Xi'an, China). Gibco RPMI-1640 medium and fetal bovine serum (FBS) were purchased from Thermo Fisher Scientific, Inc. (Waltham, MA, USA). All other chemicals used were of analytical grade and commercially available.
Cell culture and transfection
The prostate cancer LNCaP and 22Rv1 cell lines were purchased from American Type Culture Collection (Manassas, VA, USA). The cells were cultured in RPMI-1640 medium containing 10% FBS, 100 mg/ml streptomycin and 100 units/ml penicillin at 37°C in a humidified 5% CO2 atmosphere. The cells were transfected using Lipofectamine 2000 (Invitrogen, Thermo Fisher Scientific, Inc.), according to the manufacturer's instructions.
Cell cytotoxicity assay
The in vitro cytotoxicity of paclitaxel was evaluated by an MTT assay (12). Briefly, LNCaP cells and 22Rv1 cells were treated with different concentrations (0, 5, 10, 15 and 20 nM) of paclitaxel for 48 h or 10 nm paclitaxel for different time courses (0, 24, 48 and 72 h). The cells were washed with PBS and then 20 µl of 5 mg/ml MTT solution was added to the cells in each well. Plates were incubated for an additional 4 h at 37°C. The medium containing MTT was removed and 150 µl dimethyl sulfoxide was added to dissolve the formazan crystals. Absorbance was measured at 490 nm using a Labsystems iEMS microplate reader (Helsinki, Finland).
Cell apoptosis analysis
The cells were treated with different concentrations (0, 5, 10, 15 and 20 nM) of paclitaxel for 48 h or 10 nm paclitaxel for different time courses (0, 24, 48 and 72 h). Apoptosis was measured using an Annexin V/propidium iodide (PI) apoptosis detection kit according to the manufacturer's instructions (MultiSciences Biotech Co. Ltd, Zhejiang, China) and analyzed by a FACScan cytometer equipped with Cell Quest software (BD Biosciences, Franklin Lakes, NJ, USA).
Western blot analysis
Treated cells were harvested and lysed in RIPA lysis buffer [25 mM Tris-HCl (pH 7.6), 150 mM NaCl, 1% NP-40, 1% sodium deoxycholate, 0.1% SDS, 50 mM NaF, 1 mM Na3VO4 and complete protease inhibitor cocktail]. The insoluble materials were centrifuged at 10,000 × g at 4°C for 10 min and the supernatants were collected. The total proteins in supernatants were quantified by bicinchoninic acid protein assay kit (Beyotime Institute of Biotechnology, Haimen, China), according to the manufacturer's instructions. The proteins (10 µg) were separated on SDS-PAGE (10% gel) and transferred to polyvinylidene fluoride membranes. The membranes were blocked, incubated with primary antibodies (all diluted at 1:1,000 and incubated at 4°C overnight) including anti-PTEN (cat. no. 9556; Cell Signaling Technology, Inc., Danvers, MA, USA), anti-β-actin (cat. no. 3700; Cell Signaling Technology) and anti-maspin (cat. no. 271694; Santa Cruz Biotechnology, Inc., Dallas, TX, USA), washed and incubated with the goat anti-mouse immunoglobulin G conjugated to horseradish peroxidase (dilution, 1:5,000; cat. no. 2005; Santa Cruz Biotechnology, Inc.) at room temperature for 1 h. The protein bands were detected using enhanced chemiluminescence (Pierce; Thermo Fisher Scientific, Inc.). Expressions of these proteins were normalized to that of β-actin as a control and analyzed using Adobe Photoshop V7.01 software (Adobe Systems Inc., San Jose, CA, USA).
Reverse transcription-polymerase chain reaction (RT-PCR)
Total RNA from treated cells was isolated using TRIzol (Invitrogen; Thermo Fisher Scientific, Inc.), according to the manufacturer's protocol. The mRNA was reverse transcribed with Superscript II reverse transcriptase (Invitrogen; Thermo Fisher Scientific, Inc.), 1X transcription buffer containing 400 M dNTPs and 0.5 M oligo(dT)12-18 primer (Invitrogen; Thermo Fisher Scientific, Inc.). PCR reactions were carried out for 35 cycles (95°C, 30 sec; 58°C, 30 sec; 72°C, 30 sec) using primers specific for maspin (forward, 5′-CCCTATGCAAAGGAATTGGA-3′ and reverse, 5′-CAAAGTGGCCATCTGTGAGA-3′), GAPDH (forward, 5′-GAAGGTGAAGGTCGGAGTC-3′ and reverse, 5′-GAAGATGGTGATGGGATTTC-3′). The amplified products were separated on a 2% agarose gel containing ethidium bromide (0.5 µg/ml) and analyzed using Adobe Photoshop V7.01 software (Adobe Systems Inc.).
Statistical analysis
Experiments were performed with three replicates. Statistical analyses were performed using Student's t test. P<0.05 was considered to indicate a statistically significant difference.
Results
PTEN induces differential sensitivity to paclitaxel treatment in prostate cancer cells
It has been shown that wild-type PTEN improves therapeutic efficacy in the treatment of human cancer (13). To determine whether PTEN would affect the sensitivity of prostate cancer cells to paclitaxel, the ability of paclitaxel to inhibit cell proliferation in PTEN-negative LNCaP cells and PTEN-positive 22Rv1 cells was first examined. As shown in Fig. 1A and B, paclitaxel inhibited growth of LNCaP and 22Rv1 cells in a concentration- and time-dependent manner. However, LNCaP cells revealed significantly increased resistance to paclitaxel treatment compared with 22Rv1 cells (P<0.01 at 20 nM treatment for 48 h, or 10 nM treatment for 72 h). The percentages of cell apoptosis induced by paclitaxel in LNCaP cells and 22Rv1 cells were further determined by Annexin V/PI staining and flow cytometry (Fig. 1C and D). As expected, paclitaxel induced apoptosis in a concentration- and time-dependent manner in LNCaP cells and 22Rv1 cells, and paclitaxel induced significantly increased apoptosis in 22Rv1 cells than LNCaP cells (P<0.01 at 20 nM treatment for 48 h, or 10 nM treatment for 72 h).
Figure 1.
Effect on the sensitivity to paclitaxel treatment in prostate cancer cells. (A) Effect of paclitaxel on the proliferation of 22Rv1 and LNCaP cells treated with different concentrations of paclitaxel for 48 h. (B) Effect of paclitaxel on the proliferation of 22Rv1 and LNCaP cells treated with 10 nM paclitaxel for different times. (C) Effects of paclitaxel on the cell apoptosis of 22Rv1 and LNCaP cells treated with different concentrations of paclitaxel for 48 h. (D) Effects of paclitaxel on the cell apoptosis of 22Rv1 and LNCaP cells treated with 10 nM paclitaxel for different times. Cell proliferation was determined by MTT assay and cell apoptosis was determined by Annexin V/propidium iodide staining. Results from three independent experiments were quantified. Error bars indicate standard deviation among three individual experiments. *P<0.05 and **P<0.01 compared with LNCaP cells at the corresponding paclitaxel concentration or treatment time.
Paclitaxel upregulates maspin expression in PTEN-positive prostate cancer cells
To explore the molecular mechanism involved in paclitaxel-induced sensitivity in prostate cancer cells, PTEN expression was determined by western blot analysis. As shown in Fig. 2A, exposure of 22Rv1 cells to paclitaxel resulted in a concentration- and time-dependent increase in the PTEN protein level. Notably, the increase of maspin protein level paralleled the elevated protein level of PTEN in 22Rv1 cells subsequent to paclitaxel treatment. However, the maspin protein level did not significantly change with paclitaxel treatment in PTEN-negative LNCaP cells (Fig. 2B). Furthermore, the induction of maspin mRNA level by paclitaxel in 22Rv1 cells and LNCaP cells was consistent with the change in maspin protein level (Fig. 2C). These data suggested that maspin induction by paclitaxel may occur in a PTEN-dependent manner.
Figure 2.
Effects of paclitaxel on PTEN and maspin expression in 22Rv1 and LNCaP cells. (A) PTEN protein expression after paclitaxel treatment in 22Rv1 cells. (B) Maspin protein expression after paclitaxel treatment in 22Rv1 and LNCaP cells. (C) Maspin mRNA expression level after paclitaxel treatment in 22Rv1 and LNCaP cells. 22Rv1 and LNCaP cells were treated with different concentrations of paclitaxel for 48 h. (A) PTEN and (B) maspin protein expression and (C) maspin mRNA levels were determined by western blot analysis and reverse transcription-polymerase chain reaction, respectively. The number underneath each band indicates the relative intensity of the corresponding band. PTEN, phosphatase and tensin homolog; IB, immunoblotting.
Knockdown of PTEN downregulates paclitaxel-induced maspin expression and apoptosis in 22RV1 cells
To determine whether PTEN-regulated maspin expression was involved in paclitaxel-induced apoptosis, PTEN was knocked down by PTEN-specific siRNA prior to paclitaxel treatment in 22Rv1 cells. As shown in Fig. 3A, knocking down PTEN increased resistance to paclitaxel treatment compared with cells transfected with non-specific siRNA. In addition, the increase in maspin protein and mRNA level subsequent to paclitaxel treatment was impaired when 22Rv1 cells were transfected with PTEN siRNA (Fig. 3B and C).
Figure 3.
Effect of knocking down PTEN on cell apoptosis and maspin levels in 22Rv1 cells. (A) Paclitaxel induced apoptosis in 22Rv1 cells when PTEN was knocked down. (B) Paclitaxel induced change in maspin protein and mRNA levels in 22Rv1 cells when PTEN was knocked down. 22Rv1 cells were transfected with PTEN siRNA or control siRNA. At 48 h subsequent to transfection, cells were treated with 10 nM paclitaxel for 48 h. The cells were harvested and subjected to (A) apoptosis and (B) immunoblotting or RT-PCR analysis. (C and D) Densitometric analysis of maspin protein and mRNA level shown in (B), respectively. Results from three independent experiments were quantified. Error bars indicate the standard deviation among three individual experiments. *P<0.01. PTEN, phosphatase and tensin homolog; RT-PCR, reverse transcription-polymerase chain reaction; siRNA, small interfering RNA.
PTEN overexpression upregulates paclitaxel-induced maspin expression and apoptosis in LNCaP cells
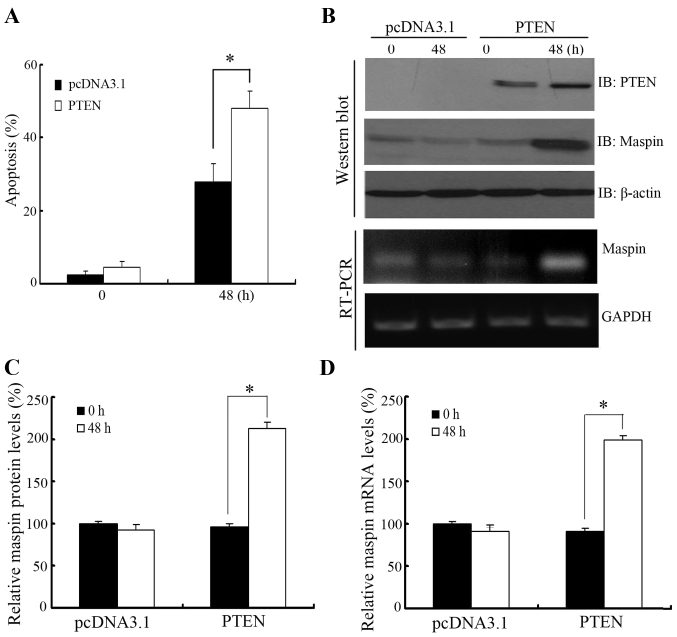
To further confirm the role of PTEN-regulated maspin expression in paclitaxel-induced apoptosis, PTEN was overexpressed in PTEN-negative LNCaP cells prior to paclitaxel treatment. As shown in Fig. 4A, LNCaP cells overexpressing PTEN showed more sensitivity to paclitaxel treatment compared with cells transfected with the empty vector. In addition, maspin protein and mRNA level were induced by overexpression of PTEN in LNCaP cells when treated with paclitaxel (Fig. 4B and C).
Figure 4.
Effect of PTEN overexpression on cell apoptosis and maspin levels in LNCaP cells. (A) Paclitaxel induced apoptosis in LNCaP cells when PTEN was overexpressed. (B) Paclitaxel induced change in maspin protein and mRNA levels when PTEN was overexpressed. LNCaP cells were transfected with PTEN. At 36 h after transfection, cells were treated with 10 nM paclitaxel for 48 h. The cells were harvested and subjected to (A) apoptosis and (B) immunoblotting or RT-PCR analysis. Densitometric analysis of the (C) maspin protein and (D) maspin mRNA levels shown in western blot analysis and RT-PCR, respectively, in (B). Results from three independent experiments were quantified. Error bars indicate the standard deviation among three individual experiments. *P<0.01. PTEN, phosphatase and tensin homolog; RT-PCR, reverse transcription-polymerase chain reaction.
Knockdown of maspin abrogates PTEN-induced paclitaxel sensitivity in LNCaP cells
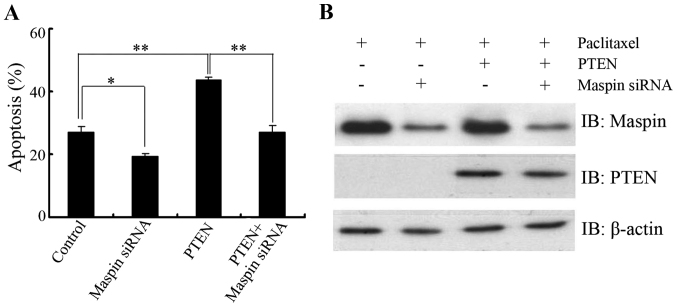
To clarify the biological significance of PTEN in the regulation of maspin in response to paclitaxel, LNCaP cells were transfected with PTEN in the presence or absence of maspin siRNA and then paclitaxel-induced apoptosis was determined. As expected, PTEN overexpression sensitized LNCaP cells to paclitaxel treatment. When the cells were cotransfected with maspin siRNA and PTEN, no significant difference in apoptosis was found compared with that of the control group (Fig. 5A and B), which further confirmed that the PTEN/maspin pathway may play a role in the regulation of paclitaxel sensitivity.
Figure 5.
Role of PTEN-mediated maspin regulation in the sensitivity to paclitaxel treatment. LNCaP cells were transfected with PTEN in the presence or absence of maspin siRNA. After 48 h transfection, cells were treated with 10 nM paclitaxel. The cells were harvested after 48 h treatment and subjected to (A) apoptosis and (B) IB analysis. β-actin was used as a loading control. Results from three independent experiments were quantified. Error bars indicate the standard deviation of three individual experiments. *P<0.05, **P<0.01. PTEN, phosphatase and tensin homolog; siRNA, small interfering RNA; IB, immunoblotting.
Discussion
Prostate cancer is the most common cancer in men and the second leading cause of cancer-associated mortality in the western world (14). Androgen deprivation therapy (ADT) has become the standard treatment of patients with metastatic prostate cancer. Although the disease initially responds to ADT, tumors in the majority of patients eventually relapse and evolve into castration-resistant prostate cancer (CRPC) (15). Chemotherapy has demonstrated a benefit in improving the survival of patients with CRPC, and paclitaxel is a first-line chemotherapeutic agent in CRPC (1). However, the mechanism of apoptosis induction by paclitaxel remains to be elucidated.
The PTEN protein is a lipid phosphatase with putative tumor-suppressing abilities. Inactivating mutations or deletions of PTEN, which results in hyperactivation of the PI3K/Akt signaling pathway, are frequently observed in a high proportion of human cancers, including prostate cancer (16). PTEN deficiency is associated with a number of aggressive tumor cell phenotypes and poor prognosis of cancer patients (17,18). Previous studies have shown that cellular loss of functional PTEN leads to resistance or reduced sensitivity to chemotherapy and hormone therapy (19,20). To explore the potential role for PTEN in paclitaxel sensitivity in prostate cancer, the present study used two different prostate cancer cell lines, the PTEN-positive 22Rv1 cell line and the PTEN-negative LNCaP cell line, to assess paclitaxel sensitivity for apoptosis induction. The present data demonstrated that 22Rv1 cells were more sensitive to paclitaxel treatment compared with LNCaP cells, and paclitaxel caused PTEN overexpression in 22Rv1 cells, suggesting that paclitaxel may induce different amounts of apoptosis in prostate cancer cells due to their different PTEN status. To further confirm whether the presence of wild-type functional PTEN may benefit therapeutic efficacy in the treatment of prostate cancer, the effect of paclitaxel on the cells was determined when PTEN was knocked down in 22Rv1 cells or PTEN was overexpressed in LNCaP cells. As expected, knocking down PTEN in 22Rv1 cells induced resistance to paclitaxel treatment, while overexpression of PTEN sensitized LNCaP cells to paclitaxel treatment.
Maspin, also termed protease inhibitor 5, is characterized as a class II tumor suppressor based on its ability to inhibit tumor growth, metastasis and angiogenesis (21,22). Previous studies also showed that high expression of maspin was associated with response to chemotherapy in a number of human primary tumors (23,24). To elucidate the tumor-suppressive activity of maspin, certain proteins, including p53, have been identified to regulate maspin expression. The p53 protein binds directly to the p53-consensus-binding site present in the maspin promoter and induces maspin expression, which elucidates the role of p53 in cell growth, invasion and metastasis (25). In the present study, while investigating the mechanisms underlying PTEN-induced chemosensitivity, paclitaxel was found to upregulate maspin protein and mRNA levels in 22Rv1 cells. In addition, ectopic expression of PTEN was associated with elevated maspin expression in LNCaP cells and knockdown of PTEN caused reduced maspin induction in 22Rv1 cells when treated with paclitaxel. Furthermore, the proapoptotic effect of PTEN on paclitaxel-induced apoptosis can be abrogated by knocking down maspin. These data suggested that maspin may be involved in PTEN-induced chemosensitivity. Whether PTEN bound to the maspin promoter and regulated maspin expression requires additional investigation.
In summary, the present study showed that PTEN was involved in paclitaxel sensitivity in prostate cancer cells. Mechanistically, it was demonstrated that PTEN-mediated paclitaxel sensitivity may be due to the induction of maspin expression. The PTEN/maspin signaling pathway may have an important role in regulating the susceptibility of prostate cancer to paclitaxel.
Acknowledgements
The present study was supported by the National Natural Science Foundation of China (grant no. 30801166).
References
- 1.Smith DC, Pienta KJ. Paclitaxel in the treatment of hormone-refractory prostate cancer. Semin Oncol. 1999;26(1 Suppl 2):S109–S111. [PubMed] [Google Scholar]
- 2.Tannock IF, de Wit R, Berry WR, Horti J, Pluzanska A, Chi KN, Oudard S, Théodore C, James ND, Turesson I, et al. Docetaxel plus prednisone or mitoxantrone plus prednisone for advanced prostate cancer. N Engl J Med. 2004;351:1502–1512. doi: 10.1056/NEJMoa040720. [DOI] [PubMed] [Google Scholar]
- 3.Petrylak DP, Tangen CM, Hussain MH, Lara PN, Jr, Jones JA, Taplin ME, Burch PA, Berry D, Moinpour C, Kohli M, et al. Docetaxel and estramustine compared with mitoxantrone and prednisone for advanced refractory prostate cancer. N Engl J Med. 2004;351:1513–1520. doi: 10.1056/NEJMoa041318. [DOI] [PubMed] [Google Scholar]
- 4.Nakayama S, Torikoshi Y, Takahashi T, Yoshida T, Sudo T, Matsushima T, Kawasaki Y, Katayama A, Gohda K, Hortobagyi GN, et al. Prediction of paclitaxel sensitivity by CDK1 and CDK2 activity in human breast cancer cells. Breast Cancer Res. 2009;11:R12. doi: 10.1186/bcr2231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Schiff PB, Fant J, Horwitz SB. Promotion of microtubule assembly in vitro by Taxol. Nature. 1979;277:665–667. doi: 10.1038/277665a0. [DOI] [PubMed] [Google Scholar]
- 6.Wang LG, Liu XM, Kreis W, Budman DR. The effect of antimicrotubule agents on signal transduction pathways of apoptosis: A review. Cancer Chemother Pharmacol. 1999;44:355–361. doi: 10.1007/s002800050989. [DOI] [PubMed] [Google Scholar]
- 7.Gan L, Wang J, Xu H, Yang X. Resistance to docetaxel-induced apoptosis in prostate cancer cells by p38/p53/p21 signaling. Prostate. 2011;71:1158–1166. doi: 10.1002/pros.21331. [DOI] [PubMed] [Google Scholar]
- 8.Kim SH, Juhnn YS, Song YS. Akt involvement in paclitaxel chemoresistance of human ovarian cancer cells. Ann N Y Acad Sci. 2007;1095:82–89. doi: 10.1196/annals.1397.012. [DOI] [PubMed] [Google Scholar]
- 9.Peng DJ, Wang J, Zhou JY, Wu GS. Role of the Akt/mTOR survival pathway in cisplatin resistance in ovarian cancer cells. Biochem Biophys Res Commun. 2010;394:600–605. doi: 10.1016/j.bbrc.2010.03.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Wu H, Cao Y, Weng D, Xing H, Song X, Zhou J, Xu G, Lu Y, Wang S, Ma D. Effect of tumor suppressor gene PTEN on the resistance to cisplatin in human ovarian cancer cell lines and related mechanisms. Cancer Lett. 2008;271:260–271. doi: 10.1016/j.canlet.2008.06.012. [DOI] [PubMed] [Google Scholar]
- 11.Ramaswamy S, Nakamura N, Vazquez F, Batt DB, Perera S, Roberts TM, Sellers WR. Regulation of G1 progression by the PTEN tumor suppressor protein is linked to inhibition of the phosphatidylinositol 3-kinase/Akt pathway. Proc Natl Acad Sci USA. 1999;96:2110–2115. doi: 10.1073/pnas.96.5.2110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Zhang R, Banika NL, Rayb SK. Differential sensitivity of human glioblastoma LN18 (PTEN-positive) and A172 (PTEN-negative) cells to Taxol for apoptosis. Brain Res. 2008;1239:216–225. doi: 10.1016/j.brainres.2008.08.075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Mosmann T. Rapid colorimetric assay for cellular growth and survival: Application to proliferation and cytoxicity assays. J Immunol Methods. 1983;65:55–63. doi: 10.1016/0022-1759(83)90303-4. [DOI] [PubMed] [Google Scholar]
- 14.Bilani N, Bahmad H, Abou-Kheir W. Prostate cancer and aspirin use: Synopsis of the proposed molecular mechanisms. Front Pharmacol. 2017;8:145. doi: 10.3389/fphar.2017.00145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Debes JD, Tindall DJ. Mechanisms of androgen refractory prostate cancer. N Engl J Med. 2004;351:1488–1490. doi: 10.1056/NEJMp048178. [DOI] [PubMed] [Google Scholar]
- 16.Cairns P, Okami K, Halachmi S, Halachmi N, Esteller M, Herman JG, Jen J, Isaacs WB, Bova GS, Sidransky D. Frequent inactivation of PTEN/MMAC1 in primary prostate cancer. Cancer Res. 1997;57:4997–5000. [PubMed] [Google Scholar]
- 17.Cully M, You H, Levine AJ, Mak TW. Beyond PTEN mutations: The PI3K pathways as an integrator of multiple inputs during tumorigenesis. Nat Rev Cancer. 2006;6:184–192. doi: 10.1038/nrc1819. [DOI] [PubMed] [Google Scholar]
- 18.Leslie NR, Downes CP. PTEN function: How normal cells control it and tumour cells lose it. Biochem J. 2004;382:1–11. doi: 10.1042/BJ20040825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Jiang Z, Pore N, Cerniglia GJ, Mick R, Georgescu MM, Bernhard EJ, Hahn SM, Gupta AK, Maity A. Phosphatase and tensin homologue deficiency in glioblastoma confers resistance to radiation and temozolomide that is reversed by the protease inhibitor nelfinavir. Cancer Res. 2007;67:4467–4473. doi: 10.1158/0008-5472.CAN-06-3398. [DOI] [PubMed] [Google Scholar]
- 20.Lee S, Choi EJ, Jin C, Kim DH. Activation of PI3K/Akt pathway by PTEN reduction and PIK3CA mRNA amplification contributes to cisplatin resistance in an ovarian cancer cell line. Gynecol Oncol. 2005;97:26–34. doi: 10.1016/j.ygyno.2004.11.051. [DOI] [PubMed] [Google Scholar]
- 21.Zou Z, Anisowicz A, Hendrix MJ, Thor A, Neveu M, Sheng S, Rafidi K, Seftor E, Sager R. Maspin, a serpin with tumor suppressing activity in human mammary epithelial cells. Science. 1994;263:526–529. doi: 10.1126/science.8290962. [DOI] [PubMed] [Google Scholar]
- 22.Zhang M, Volpert O, Shi YH, Bouck N. Maspin is an angiogenesis inhibitor. Nat Med. 2000;6:196–199. doi: 10.1038/72303. [DOI] [PubMed] [Google Scholar]
- 23.Marioni G, Koussis H, Gaio E, Giacomelli L, Bertolin A, D'Alessandro E, Scola A, Ottaviano G, de Filippis C, Jirillo A, et al. MASPIN's prognostic role in patients with advanced head and neck carcinoma treated with primary chemotherapy (carboplatin plus vinorelbine) and radiotherapy: Preliminary evidence. Acta Otolaryngol. 2009;129:786–792. doi: 10.1080/00016480802412789. [DOI] [PubMed] [Google Scholar]
- 24.Klasa-Mazurkiewicz D, Narkiewicz J, Milczek T, Lipińska B, Emerich J. Maspin overexpression correlates with positive response to primary chemotherapy in ovarian cancer patients. Gynecol Oncol. 2009;113:91–98. doi: 10.1016/j.ygyno.2008.12.038. [DOI] [PubMed] [Google Scholar]
- 25.Zou Z, Gao C, Nagaich AK, Connell T, Saito S, Moul JW, Seth P, Appella E, Srivastava S. p53 regulates the expression of the tumor suppressor gene maspin. J Biol Chem. 2000;275:6051–6054. doi: 10.1074/jbc.275.9.6051. [DOI] [PubMed] [Google Scholar]