Abstract
Background
Mammalian cells contain three functional RAS proto-oncogenes, known as H-RAS, K-RAS, and N-RAS, which encode small GTP-binding proteins in terms of p21rass. RAS genes have been elucidated as major participants in the development and progression of cancer. A single nucleotide polymorphism (SNP) at H-RAS cDNA position 81 T→C (rs12628) has been found to be associated with the risk of many human cancers like gastrointestinal, oral, colon, bladder and thyroid carcinomas. Therefore, we hypothesized that this polymorphisms in H-RAS could influence susceptibility to chronic myeloid leukemia as well, and we conducted this study to test the hypothesis in Indian population.
Method
H-RAS polymorphism was studied in 100 chronic myeloid leukemia (CML) patients and 100 healthy controls by restriction fragmentation length polymorphism (RFLP-PCR). Associations between polymorphism and clinicopathological features of CML patients were investigated.
Results
In CML patients, the TT, TC and CC genotype frequency was 38%, 61% and 1% respectively, compared to 92%, 8% and 0% in healthy controls respectively. Compared to TT genotype, CT was significantly associated with increased risk of CML (odds ratio (OR): 8.4, P < 0.00001). There was a statistically significant correlation of H-RAS polymorphism with phases (P < 0.0003), molecular response (P < 0.0001), hematological response (P < 0.04) and thrombocytopenia (P < 0.003). However, there was no correlation of this polymorphism found with other clinical parameters.
Conclusion
H-RAS T81C polymorphism was found to be associated with CML risk and prognosis of CML. These results suggest that C heterozygosis may be considered a potential risk factor for CML development in the North Indian population.
Keywords: H-RAS T81C polymorphism, Restriction fragmentation length polymorphism, H-RAS, K-RAS, N-RAS, Chronic myeloid leukemia
Introduction
Chronic myeloid leukemia (CML) is a clonal myeloid stem cell disorder characterized by excessive accumulation of clonal myeloid cells in hematopoietic tissues. Clinically CML can be divided into three phases: the chronic phase (CP), the accelerated phase (AP), and the blast crisis (BC) [1, 2]. It occurs with an annual incidence of 1.0 - 1.5 per 100,000 persons [3]. CML occurs very rarely in children. More than 90% of CML patients are diagnosed when their disease is in a relatively early phase known as the CP [3]. CML is characterized by the presence of the Philadelphia (Ph) chromosome, a shortened 22 chromosome resulting from a (9;22) (q34;q11) reciprocal translocation that juxtaposes the c-abl oncogene 1 (ABL1) gene on chromosome 9 with the breakpoint cluster region (BCR) gene on chromosome 22 [4]. This reciprocal translocation gives rise to BCR-ABL fusion oncogene, resulting in fusion messenger RNA molecules (e1a2, b2a2, b3a2, and e19a2) of different lengths that are translated into different chimeric protein products (p190, p210 and p230 BCR-ABL respectively) that are characterized by constitutive activation of its tyrosine kinase activity [5]. H-RAS, K-RAS, and N-RAS are the three functional RAS proto-oncogenes, which encode small GTP-binding proteins. RAS genes have been characterized as major participants in the development and progression of a series of human tumors, such as gastrointestinal cancer, lung cancer, thyroid cancer, melanoma, and breast cancer [6]. H-RAS, N-RAS, and K-RAS proteins are widely expressed, with K-RAS expressing in almost all cell types. Members of the RAS gene family code for proteins of molecular weight 21,000 (p21); these proteins are membrane bound. They bind with GTP during their active state and GDP during their inactive state. The switch between their inactive and active forms, together with their ability to bind to target proteins, provides the mechanism for the cell differentiation, development and proliferation [7, 8]. Harvey-RAS (H-RAS) gene is located on chromosome 11 [9]. Oncogenic point mutations, namely the hotspot mutations in codons 12, 13 and 61, are frequently observed in many tumor types [10]. Besides the mutation hotspots, inherited polymorphisms in the H-RAS sequence were described [11, 12]. A single nucleotide polymorphism (SNP) at H-RAS cDNA position 81T/C (rs12628), in codon 27 of exon 1, was shown to be associated with the risk of human cancers [13]. H-RAS T/C polymorphism does not impair p21 protien structure and function as both CAT and CAC codons encode histidine (His27His). However, it was recently demonstrated that the C allele of this SNP could increase the risk of urinary bladder cancer, colon, and gastric cancer [14-16].
Therefore, in the present research, we hypothesize that the H-RAS T81C polymorphism may have an effect on the H-RAS activity, and ultimately may play a role in modulating the susceptibility to leukemia. In order to verify our hypothesis, a population-based case-control study was conducted to investigate the association between the H-RAS T81C genotypes and the risk of CML in Indian population.
Material and Methods
Sample collection
Peripheral blood samples, i.e. 5 mL venous blood samples, were collected in EDTA vials from 100 CML patients as well as from 100 healthy donors. Buffy coat was isolated and washed in red cells lysis buffer. All samples were stored at -80 °C until the RNA and genomic DNA was extracted. The diagnosis was confirmed by detecting t(9;22) or BCR/ABL fusion gene (p210bcr-abl) which is further categorized into b3a2 or b2a2 subtypes on the basis of the BCR breakpoint by reverse transcription polymerase chain reaction in the Molecular Oncology Lab, Department of Biochemistry.
Selection criteria of patients
Inclusion criteria
The study included newly diagnosed CML patients treated with imatinib mesylate with a dose of 400 to 800 mg/day. All three stages of cases were included: CP, AP, and BC.
Exclusion criteria
The exclusion criteria included chronic myelomonocytic leukemia (CMML) patients, and other myeloproliferative disorder patients. The patients’ follow-up was maintained regularly and samples were collected after every 6 months for imatinib response and mutation studies. The classic criteria used for imatinib mesylate responses in CML for hematologic and molecular responses are depicted in Tables 1 and 2.
Table 1. Hematologic Responses.
| Complete or major hematological response | Partial or minor hematological response | Lose or minimal hematological response |
|---|---|---|
| Platelet count >150 × 109/L WBC count < 10 × 109/L Basophils: < 5% Differential without immature granulocytes Absence of blasts and promyelocytes in peripheral blood Spleen: non-palpable spleen |
Platelet count < 450 × 109/L WBC count > 10 × 109/L Basophils: > 10% Presence of blasts and promyelocytes in peripheral blood Spleen: palpable spleen |
Platelet count < 450 × 109/L WBC count > 20 × 109/L Basophils: 15% Presence of blasts and promyelocytes in peripheral blood Spleen: palpable spleen |
Table 2. Molecular Response.
| Major molecular response | Minimal or no molecular response |
|---|---|
| It indicates non-quantifiable and non-detectable BCR-ABL gene transcript (BCR-ABL/ABL) ≤ 0.103* check every 3 months |
It indicates quantifiable and detectable BCR-ABL gene transcript (BCR-ABL/ABL) ≥ 0.103* check every 3 months |
*BCR-ABL to control gene ratio according to international scale (IS).
RNA isolation
Total RNA was isolated from mononuclear cells with guanidinium isothiocyanate (Trizol LSTM, Invitrogen), according to the protocol provided by the manufacturer. The presence of RNA was confirmed by running the product on 2% agarose gel.
cDNA synthesis
The concentration of RNA was measured spectrophotometrically. cDNA was then synthesized using M-MuLV reverse transcriptase and other reaction components (Fermentas Cat. No. K1622), according to the protocol provided by the manufacturer.
Multiplex RT-PCR for BCR-ABL
BCR-ABL transcripts were detected using allele-specific primers for p210 and p190 primer sequences, as already described [17] listed in Table 3. PCR was carried out in a total volume of 25 μL reaction mixture containing 1 U/μL Taq polymerase, 240 μM dNTP, 1.8 M MgCl2, and 0.6 μM of primers. A program was employed, under the following conditions: an initial denaturation step at 95 °C for 10 min, then followed by 40 cycles of denaturing at 94 °C for 40 s, primer annealing at 55 °C, extension at 72 °C for 45 s, and a final extension step at 72 °C for 5 min. The expected bands were as follows: 808 bp, normal BCR; 481 bp, e1a2; 385 bp, b3a2; 310 bp, b2a2.
Table 3. Sequence of Oligonucleotides Used in Multiplex RT-PCR for Detection of BCR-ABL Transcript as the Target Gene and BCR Transcripts as the Internal Control.
| BCR-ABL primers |
|---|
| C5e 5'-ATAGGATCCTTTGCAACCGGGTCTGAA-3' |
| B2B 5'-ACAGAATTCCGCTGACCATCAATAAG-3' |
| BCR-C 5'-ACCGCATGTTCCGGGACAAAAG-3' |
| CA3 5'-TGTTGACTGGCGTGATGTAGTTGCTTGG-3' |
DNA extraction
Genomic DNA from peripheral blood was extracted using genomic DNA extraction kit (Gene Aid Cat. No. GB 100). The quality and integrity of the DNA was determined by the A260/280 ratios.
H-RAS T81C genotyping
H-RAS T81C polymorphism located in exon 1 was analyzed by RFLP-PCR using DRAIII enzyme. Restriction fragmentation length polymorphism (RFLP) assay was performed at cancer genetics laboratory, MAMC, New Delhi. A 200-bp segment was amplified by RFLP primers reported from elsewhere [14] represented in Table 4.
Table 4. Sequence of Oligonucleotides Used in Allele Specific PCR of H-RAS Gene.
| Gene | H-RAS |
|---|---|
| Forward primer | 5'-CTTGGCAGGTGGGGCAGGAGA-3' |
| Reverse primer | 5'-GGCACCTGGACGGCGGCGCTAG-3' |
The amplification was accomplished with a 25 μL reaction mixture containing 5 μL of 20 ng template DNA, 0.25 μL of 25 pmol each primer, 2.5 μL 10 mM dNTPs, 1.5 μL of 20 mM MgCl2, 0.3 μL of 5 U/μL Taq polymerase with 2.5 μL of 10× Taq buffer (FD1224, Fermantas). The amplification conditions were 10 min of initial denaturation at 95 °C, 40 cycles at 95 °C for 45 s, annealing temperature at 60 °C for 45 s and 72 °C for 45 s with a final 10 min extension step at 72 °C. Amplified PCR product (15 μL) was digested with 1 μL of 10 × digestion buffer containing 3 units fast digestion DraIII ((FD1224, Fermantas). After 15 min digestion at 37 °C, the fragments were separated on a 2% agarose gel stained with ethidium bromide. TT homozygote presented a single fragment of 200 bp, the CC homozygote with DraIII restriction site was cut into fragments of 145 bp and 55 bp, whereas the heterozygous CT genotype presented all the three fragments as shown in Figure 1.
Figure 1.
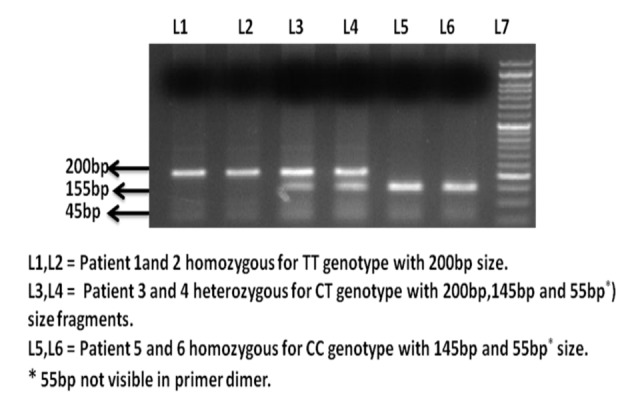
Ethidium bromide stained agarose gel electrophoresis image of H-RAS T81C polymorphism.
Statistical analysis
CML patients and controls were compared by statistical analysis performed using the SPSS 16.0 software package. Chi-square analysis and Fisher exact test were carried out to compare H-RAS T81C frequency with several clinical aspects, including sex, age, stage, BCR-ABL type, and thrombocytopenia. Hardy-Weinberg equilibrium was tested by a χ2 test to compare the observed genotype frequencies within the case-control groups. The associations between H-RAS T81C variant genotypes and risk of CML were estimated by computing the odds ratios (ORs) and their 95% confidence intervals (CIs) from both univariate and multivariate logistic regression analysis. P value of ≤ 0.05 was considered statistically significant.
Results
The study includes 100 CML patients and 100 healthy controls frequency matched with age and gender. A total of 65 males and 35 females already diagnosed with CML were included in the study. Two age groups were made, patients aged > 45 years included 36 cases, and above < 45 included 64 cases. The demographic characteristics of the study subjects, age and gender of patients, BCR-ABL type, thrombocytopenia, molecular response and hematological response are summarized in Table 5. Patients with a history of previous cancer were excluded. The Institutional Ethics Committees of Maulana Azad Medical College, New Delhi, India, approved the study and all patients provided written informed consent.
Table 5. Clinical-Pathological Parameters of CML Patients.
| No. | % | |
|---|---|---|
| Patients | 100 | 100% |
| controls | 100 | 100% |
| Males | 65 | 65% |
| Female | 35 | 35% |
| CP-CML | 50 | 50% |
| AP-CML | 25 | 25% |
| BC-CML | 25 | 25% |
| Imatinib | 100 | 100% |
| MMR | 52 | 52% |
| No MR | 48 | 48% |
| MHR | 50 | 50% |
| Minor HR | 10 | 10% |
| Loss HR | 40 | 40% |
| Age > 45 | 36 | 36% |
| Age < 45 | 64 | 64% |
| Thrombocytopenia | 50 | 50% |
| No thrombocytopenia | 50 | 50% |
H-RAS T81C genotyping frequency
We identified frequent T>C variation in codon 27 of exon 1 at cDNA position 81 of H-RAS, and the frequency of H-RAS genotypes for TT, CT and CC was 38%, 61% and 1% in patients compared to 92%, 8% and 0% in healthy controls. A statistically significant difference was observed between patients and healthy controls (P > 0.0001). Genotyping results are summarized in Table 6. The frequencies of T allele and C allele were 0.69 and 0.32 and allele frequencies were consistent with Hardy-Weinberg equilibrium. The frequency of C rare allele observed in CML was about 32.2% which was significantly higher than that in controls 4.1% (χ2 = 48.7, P < 0.00000001). However, there was a non-significant correlation found between H-RAS T81C polymorphism and the clinical features like age, gender, imatinib therapy and BCR-ABL transcripts.
Table 6. Association of H-RAS T81C Polymorphism With the Clinicopathological Features.
| Clinical features | No. | TT | CT | CC | P value |
|---|---|---|---|---|---|
| Patients | 100 | 38 | 61 | 1 | < 0.0001 |
| Controls | 100 | 92 | 8 | 0 | |
| Males | 65 | 25 | 39 | 1 | < 0.7 |
| Female | 35 | 13 | 22 | 0 | |
| CP-CML | 50 | 29 | 20 | 1 | < 0.0003 |
| AP-CML | 25 | 7 | 18 | 0 | |
| BC-CML | 25 | 2 | 23 | 0 | |
| A2b2 | 31 | 13 | 18 | 0 | < 0.9 |
| A2b3 | 67 | 24 | 42 | 1 | |
| A2b2/A2b3 | 2 | 1 | 1 | 0 | |
| Imatinib | 100 | 38 | 61 | 1 | |
| MMR | 52 | 31 | 21 | 0 | < 0.0001 |
| No MR | 48 | 7 | 40 | 1 | |
| MHR | 50 | 26 | 24 | 0 | < 0.04 |
| Minor HR | 10 | 3 | 7 | 0 | |
| Loss HR | 40 | 9 | 30 | 1 | |
| Age > 45 | 36 | 11 | 24 | 1 | < 0.2 |
| Age ≤ 45 | 64 | 27 | 37 | 0 | |
| Thrombocytopenia | 50 | 11 | 38 | 1 | < 0.003 |
| No thrombocytopenia | 50 | 27 | 23 | 0 |
H-RAS genotype frequency in CML phases
A statistically significant correlation was found between H-RAS genotypes in CML phases. The rare C allele considerably increased from chronic phase to advanced phase patients. The genotype frequencies for TT, CT and CC were 58%, 40% and 2% in CP, 28%,72% and 0% in AP and 8% ,92% and 0% in BC respectively (Fig. 2). The CT heterozygosity was a common feature of AP and BC phase CML compared to TT homozygote suggesting that H-RAS T81C polymorphism plays a significant role in the progression of CML.
Figure 2.
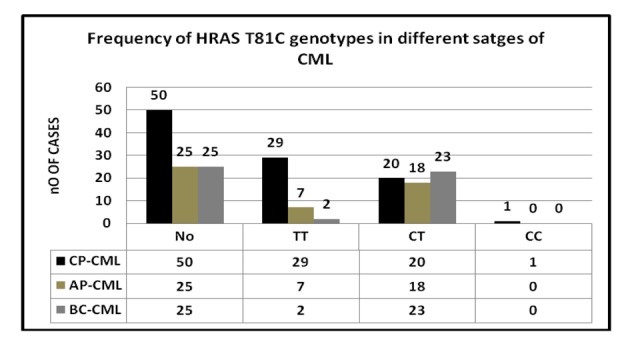
Association of H-RAS T81C with stages of CML.
Correlation of H-RAS polymorphism with molecular response
Among the 100 CML patients at the time of analysis, 52 displayed major MR, 48 minimal or no molecular responses. In MMR (major) group, 52.6%, 47.4% and 0% patients displayed TT, CT and CC genotypes compared to 17.5%, 83.3% and 0.2% genotypes in m HR (minor) respectively. A statistically significant difference was found between the two groups (P < 0.0001).
Correlation of H-RAS T81C polymorphism with hematological response
Fifty CML patients had major HR, 10 minor HR and 40 minimal hematological responses respectively. The frequencies of H-RAS T81C genotypes TT, CT and CC were 52%, 48% and 0% in MHR, 30%, 70% and 0% in minor HR and 22.5%, 75% and 2.5% in loss HR group respectively. A significant correlation (P < 0.04) was reported between H-RAS polymorphism and hematological response in CML patients.
Correlation of H-RAS T81C polymorphism with thrombocytopenia
Higher percentage of heterozygosity was detected in patients with thrombocytopenia. The genotype frequencies were 22%, 76% and 2% in thromocytopenia group and 54%, 46% and 0% in no thrombocytopenia group for TT, CT and CC genotypes respectively. A statistically significant correlation was detected (P = 0.003).
H-RAS T81C polymorphism and CML risk
An unconditional logistic regression was used to estimate associations between the genotypes and risk of CML (Table 7). It was found that an increased risk of CML was associated with the C allele. Compared to the TT genotype, the OR of 18.4 (8.0 - 14.2) for the CT genotype, it was found that there was about more than 18-fold risk associated with heterozygosity, suggesting a possible dominant effect of this polymorphism in CML Indian population. Thus, carrying out OR calculation, it became possible to assess whether the occurrence of the polymorphic variants of H-RAS gene at codon 27 was in any way associated with the increased susceptibility to CML.
Table 7. Risk of CML Associated With the H-RAS Genotypes.
| Genotyping | Cases (n = 100) | Control (100) | OR* (95% CI) | P value |
|---|---|---|---|---|
| TT | 38 (38%) | 92(92%) | 1 | |
| TC | 61 (61%) | 8 (8%) | 18.4 (8.0-14.2) | < 0.0001 |
| CC | 1 (61%) | 0 (0%) | - | |
| Allele type | ||||
| T allele | 136(68.6%) | 184(95.8%) | 1 | |
| C allele | 62 (32.2%) | 8 (4.1%) | 10.4 (4.8-22.6) | < 0.0001 |
OR: odds ratio.
Discussion
RAS small GTPases are activated in many hematopoietic growth factor signaling and in hematological malignancies, but their role in hematopoiesis and leukemogenesis is not completely known. In addition to their normal cellular functions, RAS proteins play critical roles in tumorigenesis. Several molecular alterations in the RAS genes have been identified such as minisatellites and acquired mutations in various cancers. Mutated RAS genes are associated with approximately 30% of all human cancers, including both solid tumors and hematological malignancies [10].
In addition to the direct activation by mutations, RAS can also be functionally activated by other oncogenic mutations, such as the BCR/ABL fusion protein. Likewise, increased levels of RAS activation have been linked to CML resulting from the Brc/Abl translocation creating the Philadelphia chromosome [10]. Mutations in the RAS family of proto-oncogenes (H-RAS, N-RAS, and K-RAS) appear frequently in acute leukemias [18, 19]. Moreover, mutations in RAS genes may on occasion contribute to the transition from chronic-phase CML to the BC [20].
The role of H-RAS gene polymorphism in the pathogenesis of CML has not been reported. It has been suggested that H-RAS gene acts as enhancers of gene transcription. It was reported that polymorphic loci SNP 81T>C can indicate an increased risk of different types of human tumors, bladder, oral, gastrointestinal cancers, breast, lung, and skin [14, 16, 21-24]. H-RAS 81T-C polymorphism may induce aneuploidy through overexpression of the active p21 isoform of H-RAS in follicular thyroid tumors [25]. SNP 81T3C, although it does not alter the amino acid sequence of the p21 RAS protein, may affect the expression of the gene inducing overexpression [26]. It is conceivable that SNP 81T>C is linked to other polymorphic sites in functional intron regions of H-RAS.
We identified frequent polymorphism in the coding region of H-RAS in cDNA position 81T>C, which appeared to be a marker for the risk of CML development and progression. Our results show that heterozygote carriers of the variant C allele at the site of C/T polymorphism at codon 27 of the H-RAS gene have a decreased risk of CML. To confirm this, we screened cases and controls for T81C polymorphism. An unconditional logistic regression was performed to find the association of H-RAS polymorphism with CML; we found an 18-fold increased risk of CML in carriers of the heterozygous CT genotype in comparison to wild-type carriers (TT) (P > 0.0001, OR: 18.4). H-RAS 81 C allele has been shown to be a dominant genetic susceptibility factor for the development of many cancers in comparison to T allele; individuals harboring the homozygous and heterozygous 81C-genotype are at an increased risk of thyroid cancer, gastric cancer, colon cancer, bladder and oral cancer [14-16, 27-29].
The frequency of T81C genotypes in different solid tumors is represented in Table 8 [14-16, 25, 29, 30] and also frequency of TT and CT genotype of T81C H-RAS in different cancers is shown Figures 3 and 4. Several molecular alternations in RAS gene have been identified such as minisatellites and mutations; however, research on the SNP in RAS gene was rare. This observation indicates that the distribution of H-RAS T81C polymorphism seems to be genetically different in various ethnics.
Table 8. Frequency of T81C Genotypes in Different Cancers.
| Cancer type | TT genotype | CT genotype | CC genotype | References |
|---|---|---|---|---|
| Rectal cancer | 75.2% | 24.7% | 0% | 16 |
| Colon cancer | 76.3% | 21.5% | 2.1% | 16 |
| Gastric cancer | 79.24% | 19.87% | 0.89% | 16 |
| Thyroid cancer | 37.6% | 44.7% | 17.7% | 29 |
| Urinary bladder cancer | 48.3% | 38.1% | 13.4% | 34 |
| Colon cancer | 60.5% | 36.5% | 3% | 35 |
| Oral cancer | 53.7% | 39.4% | 6% | 36 |
| Urinary bladder cancer | 34.1% | 48.5% | 17.4% | 37 |
Figure 3.
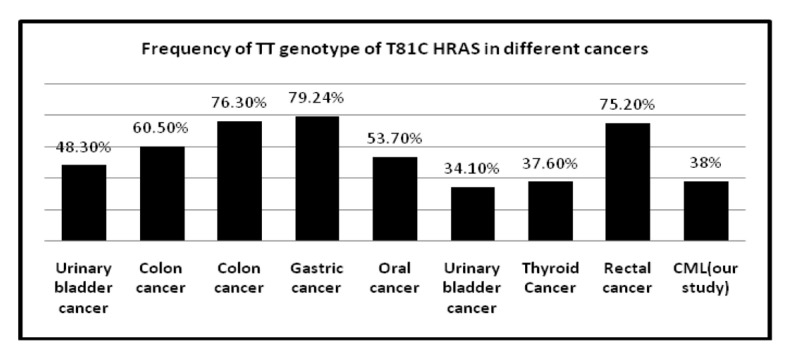
Frequency of TT genotype of T81C H-RAS in different cancers.
Figure 4.
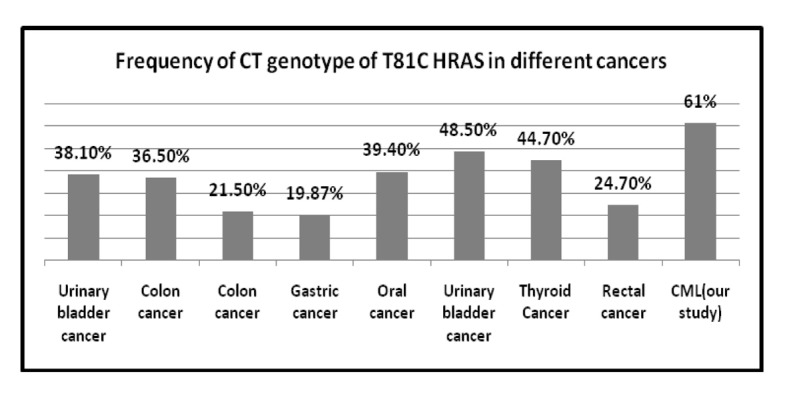
Frequency of CT genotype of T81C H-RAS in different cancers.
We found a strong association of H-RAS T81C polymorphism with the poor progression of CML. Compared to TT genotype, CT-genotype occurred more frequently in patients showing no molecular response and loss of hematogical response than good responders (P < 0.0001 and P < 0.003). Moreover, we found a statistically significant correlation of this polymorphism with thrombocytopenia with a higher of rare C allele in thrombocytoprenic group patients. We found a significant association of C allele occurrence in CML patients to the transition from CP CML to the BC (P < 0.0003).
Conclusion
In conclusion, a frequent genetic polymorphism in the H-RAS proto-oncogene appears to be epidemiologically relevant for CML risk and progression. Its possible polymorphic loci in H-RAS could be a promising subject of additional studies.
Acknowledgments
The authors are grateful to all the patients who formed the study group.
Competing Interests
The authors declare that they have no competing interests.
References
- 1.Savage DG, Szydlo RM, Goldman JM. Clinical features at diagnosis in 430 patients with chronic myeloid leukaemia seen at a referral centre over a 16-year period. Br J Haematol. 1997;96(1):111–116. doi: 10.1046/j.1365-2141.1997.d01-1982.x. [DOI] [PubMed] [Google Scholar]
- 2.Spiers AS. The clinical features of chronic granulocytic leukaemia. Clin Haematol. 1977;6(1):77–95. [PubMed] [Google Scholar]
- 3.Perrotti D, Jamieson C, Goldman J, Skorski T. Chronic myeloid leukemia: mechanisms of blastic transformation. J Clin Invest. 2010;120(7):2254–2264. doi: 10.1172/JCI41246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Chasseriau J, Rivet J, Bilan F, Chomel JC, Guilhot F, Bourmeyster N, Kitzis A. Characterization of the different BCR-ABL transcripts with a single multiplex RT-PCR. J Mol Diagn. 2004;6(4):343–347. doi: 10.1016/S1525-1578(10)60530-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Nowell PC, Hungerford DA. A minute chromosome in human chronic granulocytic leukemia. Science. 1960;132:1497–1499. [Google Scholar]
- 6.Barbacid M. ras genes. Annu Rev Biochem. 1987;56:779–827. doi: 10.1146/annurev.bi.56.070187.004023. [DOI] [PubMed] [Google Scholar]
- 7.Bos JL. The ras gene family and human carcinogenesis. Mutat Res. 1988;195(3):255–271. doi: 10.1016/0165-1110(88)90004-8. [DOI] [PubMed] [Google Scholar]
- 8.Malumbres M, Barbacid M. RAS oncogenes: the first 30 years. Nat Rev Cancer. 2003;3(6):459–465. doi: 10.1038/nrc1097. [DOI] [PubMed] [Google Scholar]
- 9.Junien C, McBride OW. Report of the committee on the genetic constitution of chromosome 11. Cytogenet Cell Genet. 1989;51(1-4):226–258. doi: 10.1159/000132793. [DOI] [PubMed] [Google Scholar]
- 10.Bos JL. ras oncogenes in human cancer: a review. Cancer Res. 1989;49(17):4682–4689. [PubMed] [Google Scholar]
- 11.Furth ME, Aldrich TH, Cordon-Cardo C. Expression of ras proto-oncogene proteins in normal human tissues. Oncogene. 1987;1(1):47–58. [PubMed] [Google Scholar]
- 12.Pethe V, Shekhar PV. Estrogen inducibility of c-Ha-ras transcription in breast cancer cells. Identification of functional estrogen-responsive transcriptional regulatory elements in exon 1/intron 1 of the c-Ha-ras gene. J Biol Chem. 1999;274(43):30969–30978. doi: 10.1074/jbc.274.43.30969. [DOI] [PubMed] [Google Scholar]
- 13.Taparowsky E, Suard Y, Fasano O, Shimizu K, Goldfarb M, Wigler M. Activation of the T24 bladder carcinoma transforming gene is linked to a single amino acid change. Nature. 1982;300(5894):762–765. doi: 10.1038/300762a0. [DOI] [PubMed] [Google Scholar]
- 14.Johne A, Roots I, Brockmoller J. A single nucleotide polymorphism in the human H-ras proto-oncogene determines the risk of urinary bladder cancer. Cancer Epidemiol Biomarkers Prev. 2003;12(1):68–70. [PubMed] [Google Scholar]
- 15.Catela Ivkovic T, Loncar B, Spaventi R, Kapitanovic S. Association of H-ras polymorphisms and susceptibility to sporadic colon cancer. Int J Oncol. 2009;35(5):1169–1173. doi: 10.3892/ijo_00000433. [DOI] [PubMed] [Google Scholar]
- 16.Zhang Y, Jin M, Liu B, Ma X, Yao K, Li Q, Chen K. Association between H-RAS T81C genetic polymorphism and gastrointestinal cancer risk: a population based case-control study in China. BMC Cancer. 2008;8:256. doi: 10.1186/1471-2407-8-256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Rosaline H, Majdan R, Wan Z. et al. One-step multiplex RT-PCR for detection of BCR/ABL gene in Malay patients with chronic myeloid leukaemia. Asia Pacific Journal of Molecular Biology and Biotechnology. 2008;16(2):41–44. [Google Scholar]
- 18.Zou X, Calame K. Signaling pathways activated by oncogenic forms of Abl tyrosine kinase. J Biol Chem. 1999;274(26):18141–18144. doi: 10.1074/jbc.274.26.18141. [DOI] [PubMed] [Google Scholar]
- 19.Bos JL, Verlaan-de Vries M, van der Eb AJ, Janssen JW, Delwel R, Lowenberg B, Colly LP. Mutations in N-ras predominate in acute myeloid leukemia. Blood. 1987;69(4):1237–1241. [PubMed] [Google Scholar]
- 20.Bos JL, Toksoz D, Marshall CJ, Verlaan-de Vries M, Veeneman GH, van der Eb AJ, van Boom JH. et al. Amino-acid substitutions at codon 13 of the N-ras oncogene in human acute myeloid leukaemia. Nature. 1985;315(6022):726–730. doi: 10.1038/315726a0. [DOI] [PubMed] [Google Scholar]
- 21.Liu E, Hjelle B, Bishop JM. Transforming genes in chronic myelogenous leukemia. Proc Natl Acad Sci U S A. 1988;85(6):1952–1956. doi: 10.1073/pnas.85.6.1952. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Sathyan KM, Nalinakumari KR, Abraham T, Kannan S. Influence of single nucleotide polymorphisms in H-Ras and cyclin D1 genes on oral cancer susceptibility. Oral Oncol. 2006;42(6):607–613. doi: 10.1016/j.oraloncology.2005.10.019. [DOI] [PubMed] [Google Scholar]
- 23.Kotsinas A, Gorgoulis VG, Zacharatos P, Mariatos G, Kokotas S, Liloglou T, Ikonomopoulos J. et al. Additional characterization of a hexanucleotide polymorphic site in the first intron of human H-ras gene: comparative study of its alterations in non-small cell lung carcinomas and sporadic invasive breast carcinomas. Cancer Genet Cytogenet. 2001;126(2):147–154. doi: 10.1016/S0165-4608(00)00407-6. [DOI] [PubMed] [Google Scholar]
- 24.Papadakis EN, Dokianakis DN, Spandidos DA. Genetic analysis of H-ras intron-1 polymorphic and variable tandem repeat regions in human breast cancer. Int J Biol Markers. 2003;18(3):195–199. doi: 10.1177/172460080301800307. [DOI] [PubMed] [Google Scholar]
- 25.Castro P, Soares P, Gusmao L, Seruca R, Sobrinho-Simoes M. H-RAS 81 polymorphism is significantly associated with aneuploidy in follicular tumors of the thyroid. Oncogene. 2006;25(33):4620–4627. doi: 10.1038/sj.onc.1209491. [DOI] [PubMed] [Google Scholar]
- 26.Kreimer-Erlacher H, Seidl H, Back B, Kerl H, Wolf P. High mutation frequency at Ha-ras exons 1-4 in squamous cell carcinomas from PUVA-treated psoriasis patients. Photochem Photobiol. 2001;74(2):323–330. doi: 10.1562/0031-8655(2001)074<0323:HMFAHR>2.0.CO;2. [DOI] [PubMed] [Google Scholar]
- 27.Yoo J, Park SY, Robinson RA, Kang SJ, Ahn WS, Kang CS. ras Gene mutations and expression of Ras signal transduction mediators in gastric adenocarcinomas. Arch Pathol Lab Med. 2002;126(9):1096–1100. doi: 10.5858/2002-126-1096-RGMAEO. [DOI] [PubMed] [Google Scholar]
- 28.Murugan AK, Hong NT, Cuc TT, Hung NC, Munirajan AK, Ikeda MA, Tsuchida N. Detection of two novel mutations and relatively high incidence of H-RAS mutations in Vietnamese oral cancer. Oral Oncol. 2009;45(10):e161–166. doi: 10.1016/j.oraloncology.2009.05.638. [DOI] [PubMed] [Google Scholar]
- 29.Sathyan KM, Nalinakumari KR, Kannan S. H-Ras 81T -> C Polymorphism Associated with Oral Cancer Risk. Indian J Med Res. 2005;85(2) [Google Scholar]
- 30.Magdalena T, Edyta B, Marek R. et al. Polymorphic variants of H-RA S proto oncogene and their possible role in bladder cancer etiology. Central European Journal of Urology. 2012;65(2) doi: 10.5173/ceju.2012.02.art6. [DOI] [PMC free article] [PubMed] [Google Scholar]


