Abstract
Objective:
To determine whether Epstein-Barr virus (EBV) or cytomegalovirus (CMV) seropositivity is associated with multiple sclerosis (MS) in blacks and Hispanics and to what extent measures of the hygiene hypothesis or breastfeeding could explain these findings. EBV and CMV have been associated with MS risk in whites, and the timing and frequency of both viruses vary by factors implicated in the hygiene hypothesis.
Methods:
Incident cases of MS or its precursor, clinically isolated syndrome (CIS), and matched controls (blacks, 111 cases/128 controls; Hispanics, 173/187; whites, 235/256) were recruited from the membership of Kaiser Permanente Southern California. Logistic regression models accounted for HLA-DRB1*1501 status, smoking, socioeconomic status, age, sex, genetic ancestry, and country of birth.
Results:
Epstein-Barr nuclear antigen-1 (EBNA-1) seropositivity was independently associated with an increased odds of MS/CIS in all 3 racial/ethnic groups (p < 0.001 for blacks and whites, p = 0.02 for Hispanics). In contrast, CMV seropositivity was associated with a lower risk of MS/CIS in Hispanics (p = 0.004) but not in blacks (p = 0.95) or whites (p = 0.96). Being born in a low/middle-income country was associated with a lower risk of MS in Hispanics (p = 0.02) but not after accounting for EBNA-1 seropositivity. Accounting for breastfeeding did not diminish the association between CMV and MS in Hispanics.
Conclusions:
The consistency of EBNA-1 seropositivity with MS across racial/ethnic groups and between studies points to a strong biological link between EBV infection and MS risk. The association between past CMV infection and MS risk supports the broader hygiene hypothesis, but the inconsistency of this association across racial/ethnic groups implies noncausal associations.
The hygiene hypothesis purports that the modern-day rise in allergic and autoimmune conditions is due to prevention or delay of common early-life infections. Evidence supporting a role for early-life hygiene as a risk factor for multiple sclerosis (MS) includes an increased risk of MS associated with infectious mononucleosis (IM),1–3 the clinical manifestation of Epstein-Barr virus (EBV) infection delayed into adolescent or adulthood; an extremely low risk of MS in EBV antibody–negative individuals4–8; and a decreased MS risk associated with higher sibling exposure early in life9 in whites. More recently, lower rates of cytomegalovirus (CMV) infection have also been linked to increased risk of MS,10,11 albeit inconsistently.5,12
The seroprevalence and timing of infection with EBV and CMV vary by factors implicated in the hygiene hypothesis and by race/ethnicity. Both are ubiquitous early-life infections in developing countries.13 However, in developed countries, EBV infection and particularly CMV infection are often delayed into adolescence and adulthood. While EBV seroprevalence remains ubiquitous in adults in developed countries, CMV seropositivity ranges from 51% to 82% in US adults, depending on race/ethnicity and socioeconomic factors.14 CMV seropositivity is higher at younger ages in Hispanic and black compared to white Americans and those residing in crowded living conditions.14 CMV can be transmitted through breastmilk; therefore, declining breastfeeding rates during most of the 20th century15 could also contribute to declines and delays in CMV infection. In addition, serum EBV antibody titers are higher in black compared to white American youths.16
We sought to use this natural variation in seroprevalence to determine the strength and consistency of association of EBV and CMV with MS risk in blacks, Hispanics, and whites and to examine to what extent such associations could be explained by factors implicated in the hygiene hypothesis or breastfeeding.
METHODS
Study population.
Participants in the MS Sunshine Study were recruited from the Kaiser Permanente Southern California (KPSC) membership between December 2011 and December 2014 via mailings and telephone. KPSC is a large health maintenance organization with >4 million members representative of the general population in Southern California.17 KPSC uses an integrated electronic health record (EHR) system that includes all inpatient and outpatient encounters, diagnostic tests, diagnoses, medications, and some demographic and behavioral characteristics. Data were collected from the EHR and, after informed consent, structured in-person interview, blood draw, and self-administered questionnaire (SAQ).
Standard protocol approvals, registrations, and patient consents.
The study protocol was approved by the KPSC institutional review board.
Case identification.
Incident cases with MS or clinically isolated syndrome (CIS) were identified with methods similar to those previously described.18,19 Briefly, we searched EHRs monthly for the first mention of ICD-9 diagnostic codes for MS/CIS. Diagnoses were confirmed by an MS specialist (A.L.-G.) according to diagnostic criteria/consensus definitions for MS20 or CIS.21,22 Eligibility required diagnosis of MS or CIS within the past 1.5 years or symptom onset within the past 3 years and age ≥18 years.
Control selection.
At least 1 control participant from the KPSC population, matched to the case on race/ethnicity, birthdate (within 2 years), sex, and KPSC facility (a surrogate measure for socioeconomic status), was identified from the EHR and recruited. The controls were assigned the same index date as their matched case (symptom onset date).
Data collection.
Self-identified race/ethnicity was obtained from the interview. White non-Hispanics were classified as white; any black race regardless of ethnicity was classified as black; and those who identified themselves as white and Hispanic were classified as Hispanics.
Covariates obtained from the in-person interview included history of IM, place of birth, income, and education. Details on number, birthdate, and cohabitation of siblings and breastfeeding (yes/no) were obtained from the SAQ. Age was defined as age at the index date. Infant sibling exposure was defined as cumulative infant-years of exposure to a younger sibling categorized as <1 vs ≥1 year before 6 years of age as previously described.9
Viral studies.
IgG antibodies to EBV viral capsid antigen (an indicator of remote EBV infection), Epstein-Barr nuclear antigen-1 (EBNA-1; an indicator of EBV latency), EBV early antigen (an indicator or recent EBV infection), and CMV were detected in serum with commercially available ELISAs (appendix e-1 at Neurology.org). The median time from diagnosis to serum measurement was relatively short (black cases 1 month, interquartile range 1–9 months; Hispanics 4 months, interquartile range = 0–12 months; whites 4 months, interquartile range = 0–10 months).
Genotyping.
DNA samples were genotyped for HLA-DRB1*15:01 status via tag single nucleotide polymorphism (rs3135388). Genetic ancestry was determined with STRUCTURE software version 2.3.119 using a genome-wide set of 67,547 linkage disequilibrium–pruned loci selected by PLINK 1.07.20 HLA-DRB1*15:03, an MS risk allele in blacks, was imputed (appendix e-1).
Statistical analysis.
The genotyping data were available on 1,159 (97.6%) of the 1,187 black, Hispanic, or white participants who had completed the study protocol by February 6, 2015. Sixty-nine participants were excluded because of missing data as follows: 4 missing serum viral titer values, 4 missing place of birth, 3 missing education status, 63 declined to answer income status, and 1 missing smoking status. The final analysis cohort included 1,090 (94.0%) participants: 239 blacks (111 cases/128 controls), 360 Hispanics (173 cases/187 controls), and 491 whites (235 cases/256 controls).
Multivariate logistic regression models were used to evaluate the associations of EBNA-1 and CMV seropositivity with MS by race/ethnicity group. The model was a priori defined and adjusted for factors that have been associated with higher EBNA-1 titers or CMV seropositivity in MS or the general population, including age, sex, education level (defined as college degree or higher vs less than college degree), annual household income (≥$65,000 or <$65,000, the median for California), HLA-DRB1*15:01 status,23 genetic ancestry, smoking (ever/never), and having been born in a low/middle-income country. In blacks, models were adjusted for carrying at least 1 copy of HLA-DRB1*15:01 or DRB1*15:03 risk allele.24 To avoid overfitting of models, birth country was included as a covariate only in Hispanics because very few whites and blacks were born in low/middle-income countries. The relationship of these variables to EBNA-1 and CMV seropositivity among controls was also evaluated to allow comparison with US population-based studies.14,16,25 Tests for multiplicative or additive interactions were also performed (appendix e-1).
Additional analyses were performed in the subgroup of participants (n = 869, 79.7%) who returned the SAQ for having been breastfed as an infant (yes/no) and infant sibling exposure because CMV can be transmitted through breastmilk and at least 1 infant-year contact has been associated with a reduced risk of IM and lower composite EBV titers.9
The Fisher exact test was used to compare frequencies of binary variables. The Wilcoxon rank-sum test was used to compare the medians of continuous variables between cases and controls stratified by race and between racial/ethnic groups. All analyses were conducted with SAS software version 9.3 (SAS Institute Inc, Cary, NC).
RESULTS
Participant characteristics.
Table 1 shows the demographic characteristics, prevalence of MS risk factors, EBV and CMV infection status, and selected factors that influence EBV and CMV seropositivity in cases and controls. Hispanic controls had a higher seroprevalence of CMV antibodies (p < 0.001) despite a younger age at index date than blacks and whites. Hispanics were more frequently born in a developing country (p < 0.001) and achieved lower education status than blacks or whites (p < 0.001). Blacks and Hispanics reported a lower annual household income than whites (p < 0.001). More black cases than controls carried at least 1 HLA-DRB1*1501 or 1503 risk allele (50.5% vs 31.3%, p = 0.0035), whereas Hispanics and whites rarely carried the HLA-DRB1*1503 allele.
Table 1.
Selected characteristics at index date and CMV and EBV status of participants by race/ethnicity

A history of IM was reported less frequently by blacks and Hispanics compared to whites (table 1). However, a history of IM was consistently associated with a higher risk of MS/CIS among all 3 racial/ethnic groups independently of the effects of sex, age, smoking, HLA-DRB1*1501 carrier status, and genetic ancestry (blacks: odds ratio [OR] 4.43, 95% confidence interval [CI] 1.33–14.77, p = 0.015; whites: OR = 2.24, 95% CI = 1.32–3.79, p = 0.003; and Hispanics: OR = 3.66, 95% CI = 0.98–13.66, p = 0.053).
Association of CMV and EBNA-1 antibodies with MS within racial/ethnic groups.
Hispanic controls were more likely to be CMV seropositive and born in a low/middle- income country than cases (table 1). This association of CMV seropositivity with a lower risk of MS/CIS among Hispanics persisted after adjustment for HLA-DRB1*1501 carrier status, genetic ancestry, EBNA-1 positivity, and factors previously reported to be associated with CMV status, including country of birth, age, and socioeconomic status (figure, A). However, no association between CMV seropositivity and MS was detected in blacks or whites (table 1 and figure, A).
Figure. Association of MS with EBNA-1 and CMV seropositivity among blacks, Hispanics, and whites.
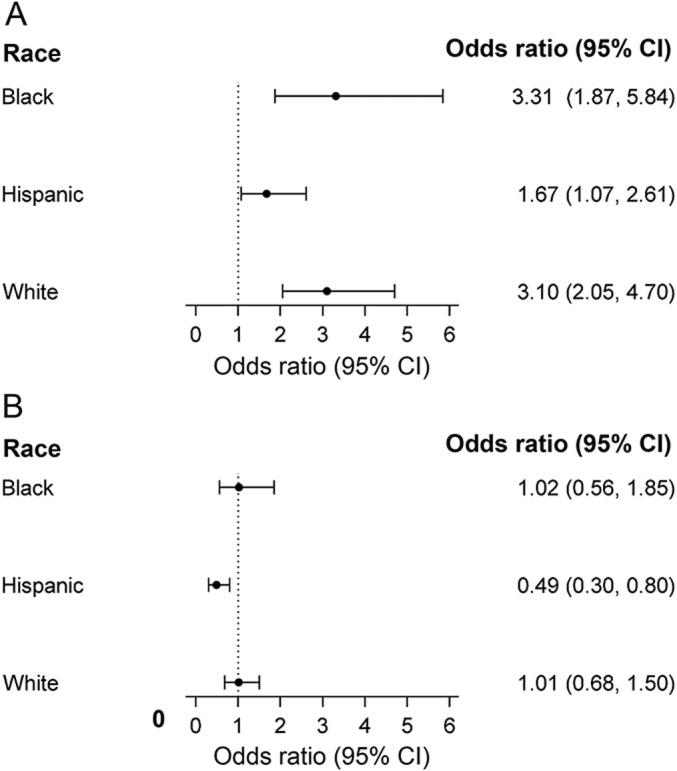
Depicted are the adjusted ORs and 95% CIs of the association between (A) EBNA-1 and (B) CMV seropositivity and MS/CIS among blacks (111 cases/128 controls), Hispanics (173 cases/187 controls), and whites (235 cases/256 controls) from the same model. ORs are adjusted for age, sex, education, household income, smoking, HLA-DRB1*15:01 (and HLA-DRB1*15:03 for blacks), genetic ancestry, and (A) CMV or (B) EBNA-1 seropositivity. Country of birth was also included in the model for Hispanics. CI = confidence interval; CIS = clinically isolated syndrome; CMV = cytomegalovirus; EBNA-1 = Epstein-Barr nuclear antigen-1; MS = multiple sclerosis; OR = odds ratio.
EBNA-1 titers and seroprevalence were consistently higher among cases than controls in all 3 racial/ethnic groups. Black and Hispanic controls also had higher EBNA-1 titers (p < 0.001) and frequency of seropositivity (p < 0.001) than white controls. The independent association of EBNA-1 seropositivity and higher risk of MS/CIS persisted in all 3 racial/ethnic groups after adjustment for HLA-DRB1*1501 carrier status, CMV positivity, socioeconomic status, and country of birth (figure, B). The frequency of EBV viral capsid antigen seropositivity differed between cases and controls in whites but not among blacks and Hispanics (table 1). EBV early antigen seropositivity was rare across all racial/ethnic groups and did not differ significantly by case/control status (table 1).
Interaction between EBNA-1 seropositivity and HLA-DRB1*15 on MS risk.
We detected an additive but not multiplicative interaction between EBNA-1 seropositivity and HLA-DRB1*1501/1503 carrier status in blacks (attributable proportion due to interaction 0.67, 95% CI = 0.39–0.95). No significant additive or multiplicative interactions were detected in whites or Hispanics (appendix e-1).
Factors associated with CMV and EBNA-1 seropositivity among controls.
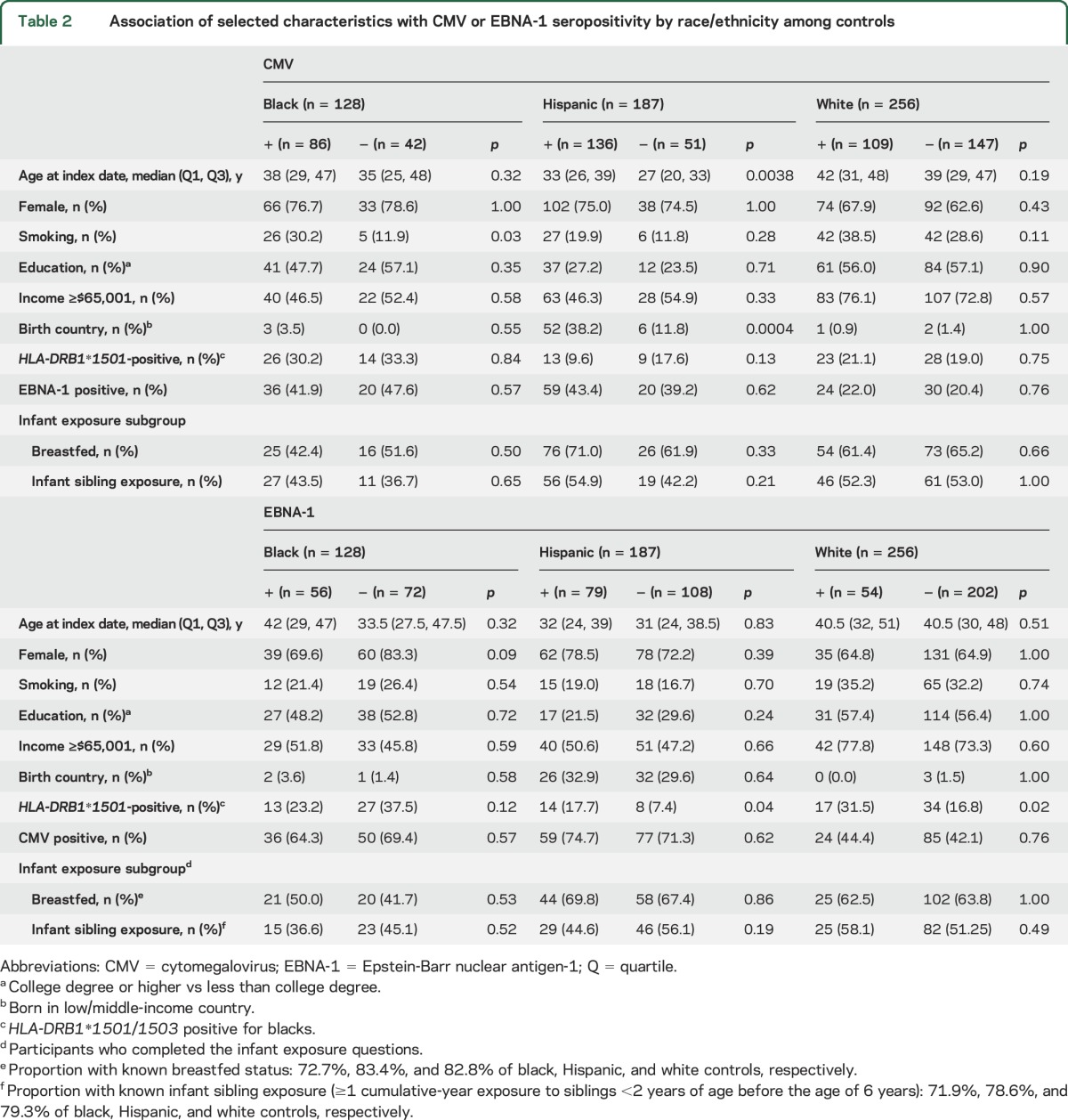
The associations among demographic and socioeconomic factors, country of birth, HLA-DRB1*1501 or DRB1*1501/1503 carrier status, and CMV or EBNA-1 seropositivity among controls are presented in table 2. Being born in a low/middle-income country and older age at index date were associated with CMV seropositivity in Hispanics. Hispanic and white controls who carried at least 1 copy of the HLA-DRB1*1501 risk allele were more likely to be seropositive for EBNA-1. Other factors showed no significant association with CMV or EBNA-1 seropositivity.
Table 2.
Association of selected characteristics with CMV or EBNA-1 seropositivity by race/ethnicity among controls
Breastfeeding, infant sibling exposure, CMV, EBV, and MS.
To test whether associations of CMV or EBNA-1 antibodies and MS/CIS could be explained by other markers of the hygiene hypothesis, we examined the role of having been breastfed or exposed to infant siblings in the subgroup of participants for whom this information was available (70% of blacks, 79% of Hispanics, and 83% of whites for breastfeeding; 70% of blacks, 74% of Hispanics, and 81% of whites for infant sibling exposure). We did not find any association between infant sibling exposure or breastfeeding and CMV or EBNA-1 antibodies among controls (table 2). However, both factors showed some association with a reduced risk of MS/CIS.
Breastfeeding was associated with a reduced risk of MS in Hispanics (p = 0.03) and whites (p = 0.09; table 1). This finding was no longer statistically significant in either group after adjustment for EBNA-1 or CMV seropositivity, socioeconomic status, smoking, age, HLA-DRB1*1501, and genetic ancestry (adjusted OR = 0.59, 95% CI = 0.33–1.05, p = 0.073 in Hispanics; adjusted OR = 0.74, 95% CI = 0.46–1.20, p = 0.23 in whites).
Exposure to infant siblings before the age of 6 years was associated with a potentially reduced risk of MS in bivariable analyses in blacks and Hispanics (table 1), but this effect was no longer detectable after multivariable adjustment (p = 0.38 for Hispanics, p = 0.40 for blacks).
Even after simultaneously accounting for the possible confounding role of breastfeeding and infant sibling exposure, the significant protective association of CMV and MS in Hispanics (adjusted OR = 0.47, 95% CI = 0.26–0.84, p = 0.01) and significant association of EBNA-1 and increased risk of MS in all 3 racial/ethnic groups (p = 0.0018, p = 0.006, and p < 0.001 in blacks, Hispanics, and whites, respectively) remained in these subgroup analyses.
DISCUSSION
Studies like ours that include participants from multiple racial/ethnic groups (so-called transethnic or transancestral studies) can be a strong tool to test for potential biological associations between exposures and health outcomes. This is particularly important when the frequency of main exposures varies by racial/ethnic groups, as it does for EBNA-1 and CMV seroprevalence. If an exposure is truly biologically linked, one would expect to find consistent associations across racial/ethnic groups. If consistency is not demonstrated across racial/ethnic groups, it increases the likelihood that unmeasured confounders or mediators may better explain the association.
We found that serologic evidence of past EBV infection and a history of IM are consistently associated with an increased risk of MS/CIS in all 3 racial/ethnic groups. This consistency of EBNA-1 seropositivity and IM associated with MS across racial/ethnic groups in this study and between studies1–3,5–8 points to a strong biological link between EBV infection and MS risk. In contrast, CMV seropositivity was associated with a decreased risk of MS/CIS only in Hispanics. Although this association persisted after accounting for socioeconomic status, birth country, and other potential mediators of CMV infection, it should be interpreted cautiously. While the CMV findings support the broader hygiene hypothesis, the lack of association we found in blacks and whites and the inconsistent association between CMV and MS in previous studies5,12 imply a noncausal association.
EBV and CMV are ubiquitous early-life infections in low/middle-income countries, with seropositivity in most children by 3 years of age.26 Both are transmitted primarily by saliva and, after infection, remain latent with intermittent reactivation. While infection with EBV and CMV is usually asymptomatic, if exposure to EBV is delayed into adolescence or adulthood, it can be quite severe, manifesting as IM. Delay of CMV infection in females into adulthood can result in birth defects of their offspring from congenital CMV. The timing of these infections in the US varies by race/ethnicity.
CMV seropositivity is highest at younger ages in Hispanics (55% at 6–14 years, 59% at 15–29 years of age), followed by blacks (42% at 6–14 years, 64% at 15–29 years of age) compared to whites (41% at 6–14 years, 40.3% at 15–29 years of age).14 Why CMV seroprevalence is higher in very young Hispanic-Americans can be only partially explained by socioeconomic factors, household size, and country of birth.14 Another potential mediator of CMV infection that varies by cultural background is breastfeeding rates.
Our findings in blacks, whites, and Hispanics are consistent with previous studies examining the association of EBNA-1 seropositivity and IM with MS in whites.2,3,5–8 Before this study, only very limited data on EBV or CMV seropositivity and MS in nonwhites were available (n = 64 blacks, n = 26 Hispanics).27
We and others found that EBV seronegativity is rare among MS cases and controls5–8 and across all racial/ethnic groups.13 In addition, IM was strongly associated with MS risk across all groups, but the proportion of blacks and Hispanics with IM was lower than whites. This implies that delay of EBV infection into adolescence or adulthood may be the critical risk factor for MS. We, like others, have apparently EBV-seronegative cases, which warrants further investigation.
A recent meta-analysis demonstrated an additive but not multiplicative interaction of HLA-DR*15:01 and EBV infection for MS risk.28 We were not able to detect this additive interaction with the main MS risk allele in whites or Hispanics. However, when we considered the 2 main risk alleles in blacks (HLA-DRB1*1501 and 1503), an additive interaction was detected. This finding supports a causal role of EBV infection in MS, at least in those carrying these major HLA risk alleles. These findings should be replicated in future racially/ethnically diverse cohorts.
In contrast, our CMV findings were inconsistent across racial/ethnic groups. Only 2 previous studies showed an inverse association between CMV seropositivity and MS: 1 large Swedish population-based incident case-control study11 and a small multiethnic US pediatric MS case-control study.10 Most previous studies found no association between CMV seropositivity and MS in their predominantly white, non-Hispanic populations.5,12 A possible explanation for the difference in our findings compared to those from the Swedish study is selection bias among controls in 1 or both studies because CMV seropositivity was higher among the Swedish controls (65%) than our white controls (58%) while seropositivity among cases was quite similar (57% and 58%, respectively).11 The Pediatric MS Study findings may be consistent with ours in that the protective association they found may have been driven by the inclusion of a significantly higher proportion of Hispanic cases than controls.10
To determine whether the protective association between CMV seropositivity and MS in Hispanics but not whites and blacks is better explained by other factors that increase seroprevalence, we examined the influence of being born in a low/middle-income country, higher infant sibling exposure, and lower socioeconomic status. These factors mark the hygiene hypothesis and were not considered in most previous studies. Rather than diminishing the associations between CMV or EBNA-1 and MS, accounting for these factors negated associations between birth country (Hispanics) or infant sibling exposure (Hispanics and blacks) and MS. This supports the idea that early infection with EBV explains much of the apparent protective effect of birth country reported herein and greater infant sibling exposure, as has been previously reported.9
Although we did not find an association between CMV seropositivity and breastfeeding, neither EBNA-1 nor CMV seropositivity could fully negate a possible protective effect of breastfeeding in Hispanics. The few previous studies examining breastfeeding and MS risk in Europeans29,30 and Mexicans31 found that breastfeeding, particularly if for >4 months, was associated with a lower risk of MS. One study found this protective association only in male participants.30 None of these studies accounted for EBV or CMV seropositivity.
The relationship between breastfeeding and the hygiene hypothesis is not straightforward. Breastfeeding can mark poor or superior hygiene, depending on the country and socioeconomic status. Breastfeeding is common among poor women in low/middle-income countries32 and better-educated women in developed countries.15 Breastfeeding can also result in vertical transmission of viruses such as HIV and CMV yet protect against common early-childhood infections.15 Thus, finding different effects of breastfeeding and MS across racial/ethnic groups might be expected. Whether breastfeeding is a modifiable risk factor for MS susceptibility, in particular independently of EBV, remains unclear and deserving of further study.
While we favor a noncausal association between CMV and MS, the biggest limitation of our study is the case-control design. Thus, we cannot exclude the possibility that it is earlier age at infection with CMV that is protective. Another big limitation is missing data for breastfeeding and infant sibling exposure because some participants did not complete the SAQ. In addition, reliance on self-report of IM and breastfeeding raises the issue of recall bias. Selection bias of controls is another potential limitation, although the seroprevalence for EBV and CMV is consistent with expected rates from multiethnic population-based studies.14,16,25
Strengths of this study include that it is the largest population-based multiethnic study of incident MS cases, all recruited from same geographic area over a relatively short period of time, thus minimizing the potential for selection bias, confounding by geography, or a birth cohort effect. In addition, factors were measured in the same way across racial/ethnic groups, allowing valid comparisons across groups.
This study highlights how multiethnic studies can improve our understanding of MS susceptibility. Taken together with previous studies, our findings point to a strong biological link between EBV infection and MS. Like previous studies, we cannot determine whether EBV infection is required to develop MS or whether the ability to detect EBNA-1 antibody titers is a reflection of altered cell-mediated immunity, the pathophysiologic hallmark of MS. In contrast, the inconsistency of association between MS and CMV seropositivity across racial/ethnic groups and between studies implies a noncausal association.
Supplementary Material
GLOSSARY
- CI
confidence interval
- CIS
clinically isolated syndrome
- CMV
cytomegalovirus
- EBNA-1
Epstein-Barr nuclear antigen-1
- EBV
Epstein-Barr virus
- EHR
electronic health record
- ICD-9
International Classification of Diseases, ninth revision
- IM
infectious mononucleosis
- KPSC
Kaiser Permanente Southern California
- MS
multiple sclerosis
- OR
odds ratio
- SAQ
self-administered questionnaire
Footnotes
Supplemental data at Neurology.org
AUTHOR CONTRIBUTIONS
A.L.-G.'s contributions include study design, data collection, and drafting and revising the manuscript for content, including study concept and interpretation of data. She had full access to all of the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis. J.W. contributed to the analysis of the data, including statistical analysis, interpretation of data, and drafting and revising the manuscript for content. R.L. contributed to the study design, analysis of the data, including statistical analysis, interpretation of data, and revising the manuscript for content. J.S. contributed to the data collection, statistical analysis, and revision of the manuscript for content. E.G. contributed to the data collection and revision of the manuscript for content. L.A. contributed to revision of the manuscript for content. S.H. contributed to the data collection and revision of the manuscript for content. L.H.C. contributed to the analysis of the data, including statistical analysis, interpretation of data, and revising the manuscript for content. H.Q. contributed to the data analysis and revision of the manuscript for content. J.A.J. contributed to study design, interpretation of data, and revising the manuscript for content. L.F.B.’s contributions include study design and revising the manuscript for content. A.H.X. contributed to the study design, analysis of the data, including statistical analysis, interpretation of data, and revising the manuscript for content.
STUDY FUNDING
This research was supported by the National Institute of Neurologic Disorders and Stroke (NINDS; 1R01NS075308; principal investigator: Langer-Gould).
DISCLOSURE
A. Langer-Gould has been site principal investigator for 2 industry-sponsored phase 3 clinical trials (Biogen Idec, Hoffman-LaRoche). She receives grant support from the NIH, NINDS, Patient-Centered Outcomes Research Institute, and National MS Society. J. Wu reports no disclosures relevant to the manuscript. R. Lucas receives grant support from Cancer Australia and the National Health and Medical Research Council of Australia. J. Smith, E. Gonzales, L. Amezcua, S. Haraszti, L. Chen, H. Quach, J. James, L. Barcellos, and A. Xiang report no disclosures relevant to the manuscript. Go to Neurology.org for full disclosures.
REFERENCES
- 1.Almohmeed YH, Avenell A, Aucott L, Vickers MA. Systematic review and meta-analysis of the sero-epidemiological association between Epstein Barr virus and multiple sclerosis. PLoS One 2013;8:e61110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Belbasis L, Bellou V, Evangelou E, Ioannidis JP, Tzoulaki I. Environmental risk factors and multiple sclerosis: an umbrella review of systematic reviews and meta-analyses. Lancet Neurol 2015;14:263–273. [DOI] [PubMed] [Google Scholar]
- 3.Nielsen TR, Rostgaard K, Nielsen NM, et al. Multiple sclerosis after infectious mononucleosis. Arch Neurol 2007;64:72–75. [DOI] [PubMed] [Google Scholar]
- 4.Pakpoor J, Disanto G, Gerber JE, et al. The risk of developing multiple sclerosis in individuals seronegative for Epstein-Barr virus: a meta-analysis. Mult Scler 2013;19:162–166. [DOI] [PubMed] [Google Scholar]
- 5.Ascherio A, Munger KL, Lennette ET, et al. Epstein-Barr virus antibodies and risk of multiple sclerosis: a prospective study. JAMA 2001;286:3083–3088. [DOI] [PubMed] [Google Scholar]
- 6.Lucas RM, Ponsonby AL, Dear K, et al. Current and past Epstein-Barr virus infection in risk of initial CNS demyelination. Neurology 2011;77:371–379. [DOI] [PubMed] [Google Scholar]
- 7.DeLorenze GN, Munger KL, Lennette ET, Orentreich N, Vogelman JH, Ascherio A. Epstein-Barr virus and multiple sclerosis: evidence of association from a prospective study with long-term follow-up. Arch Neurol 2006;63:839–844. [DOI] [PubMed] [Google Scholar]
- 8.Sundstrom P, Juto P, Wadell G, et al. An altered immune response to Epstein-Barr virus in multiple sclerosis: a prospective study. Neurology 2004;62:2277–2282. [DOI] [PubMed] [Google Scholar]
- 9.Ponsonby AL, van der Mei I, Dwyer T, et al. Exposure to infant siblings during early life and risk of multiple sclerosis. JAMA 2005;293:463–469. [DOI] [PubMed] [Google Scholar]
- 10.Waubant E, Mowry EM, Krupp L, et al. Common viruses associated with lower pediatric multiple sclerosis risk. Neurology 2011;76:1989–1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Sundqvist E, Bergstrom T, Daialhosein H, et al. Cytomegalovirus seropositivity is negatively associated with multiple sclerosis. Mult Scler 2014;20:165–173. [DOI] [PubMed] [Google Scholar]
- 12.Pakpoor J, Pakpoor J, Disanto G, Giovannoni G, Ramagopalan SV. Cytomegalovirus and multiple sclerosis risk. J Neurol 2013;260:1658–1660. [DOI] [PubMed] [Google Scholar]
- 13.Cherry JD, Harrison GJ, Kaplan SL, Hotez PJ, Steinbach WJ. Feigin and Cherry's Textbook of Pediatric Infectious Diseases. Philadelphia: Saunders/Elsevier; 2014. [Google Scholar]
- 14.Staras SA, Dollard SC, Radford KW, Flanders WD, Pass RF, Cannon MJ. Seroprevalence of cytomegalovirus infection in the United States, 1988–1994. Clin Infect Dis 2006;43:1143–1151. [DOI] [PubMed] [Google Scholar]
- 15.Hoddinott P, Tappin D, Wright C. Breast feeding. BMJ 2008;336:881–887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ford JL, Stowe RP. Racial-ethnic differences in Epstein-Barr virus antibody titers among U.S. children and adolescents. Ann Epidemiol 2013;23:275–280. [DOI] [PubMed] [Google Scholar]
- 17.Koebnick C, Langer Gould A, Gould MK, et al. Do the sociodemographic characteristics of members of a large, integrated health care system represent the population of interest? Permanente J 2012;16:37–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Langer-Gould A, Zhang JL, Chung J, Yeung Y, Waubant E, Yao J. Incidence of acquired CNS demyelinating syndromes in a multiethnic cohort of children. Neurology 2011;77:1143–1148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Langer-Gould A, Brara SM, Beaber BE, Zhang JL. Incidence of multiple sclerosis in multiple racial and ethnic groups. Neurology 2013;80:1734–1739. [DOI] [PubMed] [Google Scholar]
- 20.Polman CH, Reingold SC, Banwell B, et al. Diagnostic criteria for multiple sclerosis: 2010 revisions to the McDonald criteria. Ann Neurol 2011;69:292–302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Jacob A, Weinshenker BG. An approach to the diagnosis of acute transverse myelitis. Semin Neurol 2008;28:105–120. [DOI] [PubMed] [Google Scholar]
- 22.Transverse Myelitis Consortium Working Group. Proposed diagnostic criteria and nosology of acute transverse myelitis. Neurology 2002;59:499–505. [DOI] [PubMed] [Google Scholar]
- 23.Waubant E, Mowry EM, Krupp L, et al. Antibody response to common viruses and human leukocyte antigen-DRB1 in pediatric multiple sclerosis. Mult Scler 2013;19:891–895. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Oksenberg JR, Barcellos LF, Cree BA, et al. Mapping multiple sclerosis susceptibility to the HLA-DR locus in African Americans. Am J Hum Genet 2004;74:160–167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Dowd JB, Palermo T, Chyu L, Adam E, McDade TW. Race/ethnic and socioeconomic differences in stress and immune function in the National Longitudinal Study of Adolescent Health. Soc Sci Med 2014;115:49–55. [DOI] [PubMed] [Google Scholar]
- 26.Harrison GJ. Feigin and Cherry's Textbook of Pediatric Infectious Diseases, 7th ed. Philadelphia: Elsevier Saunders; 2014. [Google Scholar]
- 27.Munger KL, Levin LI, O'Reilly EJ, Falk KI, Ascherio A. Anti-Epstein-Barr virus antibodies as serological markers of multiple sclerosis: a prospective study among United States military personnel. Mult Scler 2011;17:1185–1193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Xiao D, Ye X, Zhang N, et al. A meta-analysis of interaction between Epstein-Barr virus and HLA-DRB1*1501 on risk of multiple sclerosis. Scientific Rep 2015;5:18083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Conradi S, Malzahn U, Paul F, et al. Breastfeeding is associated with lower risk for multiple sclerosis. Mult Scler 2013;19:553–558. [DOI] [PubMed] [Google Scholar]
- 30.Ragnedda G, Leoni S, Parpinel M, et al. Reduced duration of breastfeeding is associated with a higher risk of multiple sclerosis in both Italian and Norwegian adult males: the EnvIMS study. J Neurol 2015;262:1271–1277. [DOI] [PubMed] [Google Scholar]
- 31.Tarrats R, Ordonez G, Rios C, Sotelo J. Varicella, ephemeral breastfeeding and eczema as risk factors for multiple sclerosis in Mexicans. Acta Neurol Scand 2002;105:88–94. [DOI] [PubMed] [Google Scholar]
- 32.Barros FC, Victora CG, Scherpbier R, Gwatkin D. Socioeconomic inequities in the health and nutrition of children in low/middle income countries. Rev Saude Publica 2009;44:1–16. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


