Abstract
Objective:
To prospectively investigate the association between dietary sodium intake and multiple sclerosis (MS) risk.
Methods:
In this cohort study, we assessed dietary sodium intake by a validated food frequency questionnaire administered every 4 years to 80,920 nurses in the Nurses' Health Study (NHS) (1984–2002) and to 94,511 in the Nurses' Health Study II (NHSII) (1991–2007), and calibrated it using data from a validation study. There were 479 new MS cases during follow-up. We used Cox proportional hazards models to estimate hazard ratios (HR) and 95% confidence intervals (CI) for the effect of energy-adjusted dietary sodium on MS risk, adjusting also for age, latitude of residence at age 15, ancestry, body mass index at age 18, supplemental vitamin D intake, cigarette smoking, and total energy intake in each cohort. The results in both cohorts were pooled using fixed effects models.
Results:
Total dietary intake of sodium at baseline was not associated with MS risk (highest [medians: 3.2 g/d NHS; 3.5 g/d NHSII] vs lowest [medians: 2.5 g/d NHS; 2.8 g/d NHSII] quintile: HRpooled 0.98, 95% CI 0.74–1.30, p for trend = 0.75). Cumulative average sodium intake during follow-up was also not associated with MS risk (highest [medians: 3.3 g/d NHS; 3.4 g/d NHSII] vs lowest [medians: 2.7 g/d NHS; 2.8 g/d NHSII] quintile: HRpooled 1.02, 95% CI 0.76–1.37, p for trend = 0.76). Comparing more extreme sodium intake in deciles yielded similar results (p for trend = 0.95).
Conclusions:
Our findings suggest that higher dietary sodium intake does not increase the risk of developing MS.
Environmental factors like low vitamin D levels,1 infectious mononucleosis,2 smoking,3 and adiposity during adolescence4 have consistently been associated with multiple sclerosis (MS) risk, but it remains important to continue searching for novel, especially modifiable, risk factors.
The results of previous studies suggest that salt intake may be involved in the pathogenesis of MS and predict disease activity.5 High sodium concentrations in vitro were found to induce pathogenic Th17 cells, which play an important role in MS pathology.6,7 Furthermore, higher sodium intake has been related to increased disease activity in adult patients with MS8 and in experimental autoimmune encephalomyelitis (EAE),6,9 an MS animal model. The worldwide changes in dietary habits towards fast and processed food rich in sodium could thus have contributed to the increase in MS prevalence and incidence over the last decades, if sodium alters MS risk.10 However, recent findings in a pediatric population do not suggest an involvement of sodium in MS pathogenesis or exacerbation.11,12 Further, the association between dietary sodium and the risk of adult-onset MS has not yet been determined.
We therefore investigated prospectively whether total dietary sodium intake was associated with the risk of MS in 2 large US cohorts of women with an overall follow-up of 38 years, the Nurses' Health Study (NHS) and the Nurses' Health Study II (NHSII).
METHODS
Study design.
The ongoing NHS and NHSII are prospective cohort studies initiated in 1976 (NHS, 121,700 participants) and 1989 (NHSII, 116,671 participants).13 These are well-defined cohorts of registered nurses from different US states, 30–55 (NHS) and 25–42 (NHSII) years old at inclusion. They are followed over time with biennial self-administered postal questionnaires collecting information on different exposures, e.g., lifestyle, and health-related outcomes, with a high response rate (approximately 90%) in both cohorts in each questionnaire cycle.
Standard protocol approvals, registrations, and patient consents.
These cohort studies were approved by the institutional review board of Brigham and Women's Hospital.
Assessment of dietary sodium intake.
Diet was assessed in 80,920 women in NHS (in 1984, 1986, 1990, 1994, 1998, 2002) and 94,511 women in NHSII (in 1991, 1995, 1999, 2003, 2007) using a validated semiquantitative food frequency questionnaire (FFQ), described in detail elsewhere14–16 and sent out every 4 years as part of the main questionnaire. From 1984 in NHS and 1991 in NHSII, the FFQ remained similar, assessing the average portion size (e.g., 1 slice of bread, 2 slices of bacon, 1 8-oz glass of milk) and usual consumption (never or less than once per month, 1–3 per month, 1 per week, 2–4 per week, 5–6 per week, 1 per day, 2–3 per day, 4–5 per day, or 6+ per day) over the last year of about 130 food items covering the following categories: dairy products, fruits, vegetables, breads/cereals/starches, eggs/meats, beverages, sweets/baked goods/miscellaneous.
The FFQ covers the main dietary sodium sources including the top 10 food items that contribute, according to the Centers for Disease Control and Prevention (CDC), to >40% of sodium eaten daily in the United States, i.e., breads/rolls, cold cuts/cured meats, pizza, poultry, soups, sandwiches, cheese, snacks, pasta, and meat dishes.17 A question on the habitual frequency of a salt shake added at the table during meals is also included, but not salt added during cooking. The FFQ takes also into account sodium sources other than the major source, salt (sodium chloride), including sodium glutamate, bicarbonate, citrate, nitrate, and benzoate.18 According to the CDC, 77% of the sodium in the average American diet comes from restaurant and processed food, while only 5% is added during cooking, and 6% at the table.19 The remaining sodium is a natural part of foods. On average, Americans consume >3,400 mg of sodium per day,19 which corresponds to about 1.5 teaspoons of salt and exceeds the daily limit of sodium intake of 2,300 mg recommended by the 2015 US dietary guidelines.20
The content of sodium in different food items was determined using the Harvard FFQ Nutrient Database, which is regularly updated and expanded for average nutrient and mineral content.21 Sodium intake from a specific food item was estimated by multiplying its sodium content by the reported frequency of intake, with frequency weighted such that 1 per day equaled 1. Total dietary sodium intake was calculated by summing sodium intake across the different foods. Subsequently, sodium intake was adjusted for energy intake using the residual method to isolate the mineral's contribution to risk independent of total caloric intake or food quantity consumed.22 The measurement of sodium by FFQ has been validated in the context of the Women's Lifestyle Validation Study (WLVS) conducted among participants in the NHS and NHSII to assess measurement error possible with self-reported dietary exposures.23,24 Using a slightly expanded version of the FFQ used in the original cohorts with in total 152 food items to include newer foods, the energy-adjusted random-error-corrected Spearman correlation coefficients with the sodium assessment by FFQ were 0.53 for sodium from 7-day dietary records,24 0.43 for sodium from 24-hour dietary recalls,24 and 0.48 for 24-hour urinary sodium (gold standard)23 (unpublished, under review). As FFQs systematically underestimate true sodium intake,25 we used sodium excretion measured in multiple 24-hour urine samples from women in WLVS to correct the sodium intake estimated by FFQ in our study for measurement error. The correction equation based on a linear regression with energy-adjusted sodium intake assessed by FFQ in WLVS as exposure and urinary sodium as outcome is (corrected sodium intake = 1,455.83 + [0.767 × uncorrected FFQ sodium intake]). Underreporting of true sodium intake in FFQs might vary according to other characteristics such as body mass index (BMI).25 However, the difference of the energy-adjusted sodium between the FFQ and biomarker measurement within WLVS was not associated with BMI (p = 0.35), age (p = 0.65), or smoking status (p = 0.32), suggesting that these factors are unrelated to reporting error in the study.
Case ascertainment.
We requested permission from women self-reporting an MS diagnosis on the biennial questionnaires to contact their treating neurologist and review their medical record. Since 2003, our study neurologist has been reviewing all medical records for confirmation. In this study, we defined the outcome as neurologist-confirmed probable or definite diagnosis of MS according to date of diagnosis (or date of first symptom in subanalyses). Cases of possible MS were not included. There were 130 new cases in NHS and 349 new cases in NHSII of probable or definite MS diagnosis during follow-up among women reporting dietary information.
Covariates.
Ancestry, reported in 1992 (NHS) and 1989 (NHSII), was categorized into southern European, Scandinavian, other Caucasian, or other. Women reported their state of residence at age 15 in 1992 (NHS) and 1993 (NHSII). We categorized the states into northern, middle, and southern tier, as previously described.26 Smoking status was updated biennially including information on number of cigarettes smoked per day, and categorized into never, <10, 10–24, and ≥25 pack-years. Women reported their height at baseline and their weight at age 18 in 1980 (NHS) and 1989 (NHSII). We calculated their BMI in kg/m2 from this information and categorized women as underweight, normal, overweight, or obese (<18.5, 18.5–<25.0, 25–<30, ≥30 kg/m2) according to cutoffs defined by the WHO. Supplemental vitamin D intake was categorized into none, <400, and ≥400 IU/d. Total energy intake was calculated from the FFQ.
Statistical analyses.
We estimated the association between total dietary sodium intake and MS risk during follow-up using Cox proportional hazards models. The energy-adjusted measurement error–corrected sodium was used as exposure in all analyses. Missing exposures were replaced with the last available value if the participant was not lost to follow-up. We reported (1) age- and total energy–adjusted and (2) multivariable-adjusted hazard ratios (HR) and 95% confidence interval (CI) at a significance level of 0.05. The women contributed person-years (time at risk) from return date of the baseline diet questionnaire (NHS: 1984, NHSII: 1991) to date of MS diagnosis, date of death, loss to or end of follow-up (NHS: June 2004, NHSII: June 2009), whichever occurred first. Women with daily caloric intake of below 500 or above 3,500 kcal and those who had returned only the baseline questionnaire were excluded from the analyses.
We assessed total dietary sodium intake at baseline continuously by incremental steps of 100 mg per day and categorically in quintiles and deciles, comparing those ranking in the top to those in the bottom category. Moreover, cumulative average intake of sodium during follow-up was assessed in quintiles and deciles, using the bottom category as reference. For the categorical analyses, we also reported the p value for linear trend across medians in each category. The multivariable models were adjusted for ancestry, latitude of residence at age 15, BMI at age 18, pack-years of smoking, supplemental vitamin D, and total energy intake. The latter 3 were updated biennially and introduced into the model as time-varying covariates. We conducted the analyses separately for each cohort and then pooled the results using fixed-effects models with the inverse of the variance of the risk estimates as the weight, and a Q statistic to assess heterogeneity.27 Mantel extension tests were performed to examine the trend across the pooled categories.
We repeated the baseline analyses in subgroups of never- and ever-smokers separately, as an association between sodium intake and another autoimmune disease has previously been reported only in smokers.28
Recently, α-linolenic acid (ALA), a polyunsaturated fatty acid, was found to be inversely associated with MS risk in the same cohorts.29 To assess whether this may confound the association, we adjusted the multivariable model of baseline sodium intake in quintiles in addition for ALA in quintiles determined from the diet questionnaires as described for sodium above.
In sensitivity analyses, we followed MS cases to date of onset instead of diagnosis. There were overall 289 new patients (59 in NHS, 230 in NHSII) diagnosed with probable or definite MS during follow-up who had information on onset date and diet.
RESULTS
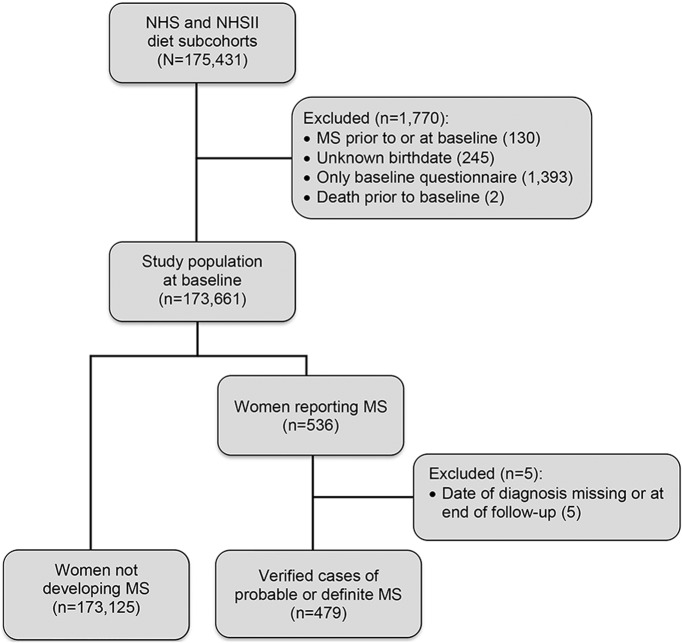
We followed 80,920 NHS and 94,511 NHSII participants over 3,096,959 person-years (figure 1). Women with higher sodium intake had on average a higher BMI at age 18 and a lower vitamin D supplementation at baseline (table 1). Women in the NHS with high sodium intake reported more pack-years of smoking and were more likely to have lived in the Northern tier at age 15. These trends were not seen in the NHSII. There were in total 479 incident MS cases during follow-up with a median age at diagnosis of 47 years; 69.5% had relapsing-remitting MS, 15.0% progressive MS (primary or secondary), and the remaining 15.4% had an unknown/other disease course.
Figure 1. Flowchart of the study population selection and sample size.
MS = multiple sclerosis; NHS = Nurses' Health Study; NHSII = Nurses' Health Study II.
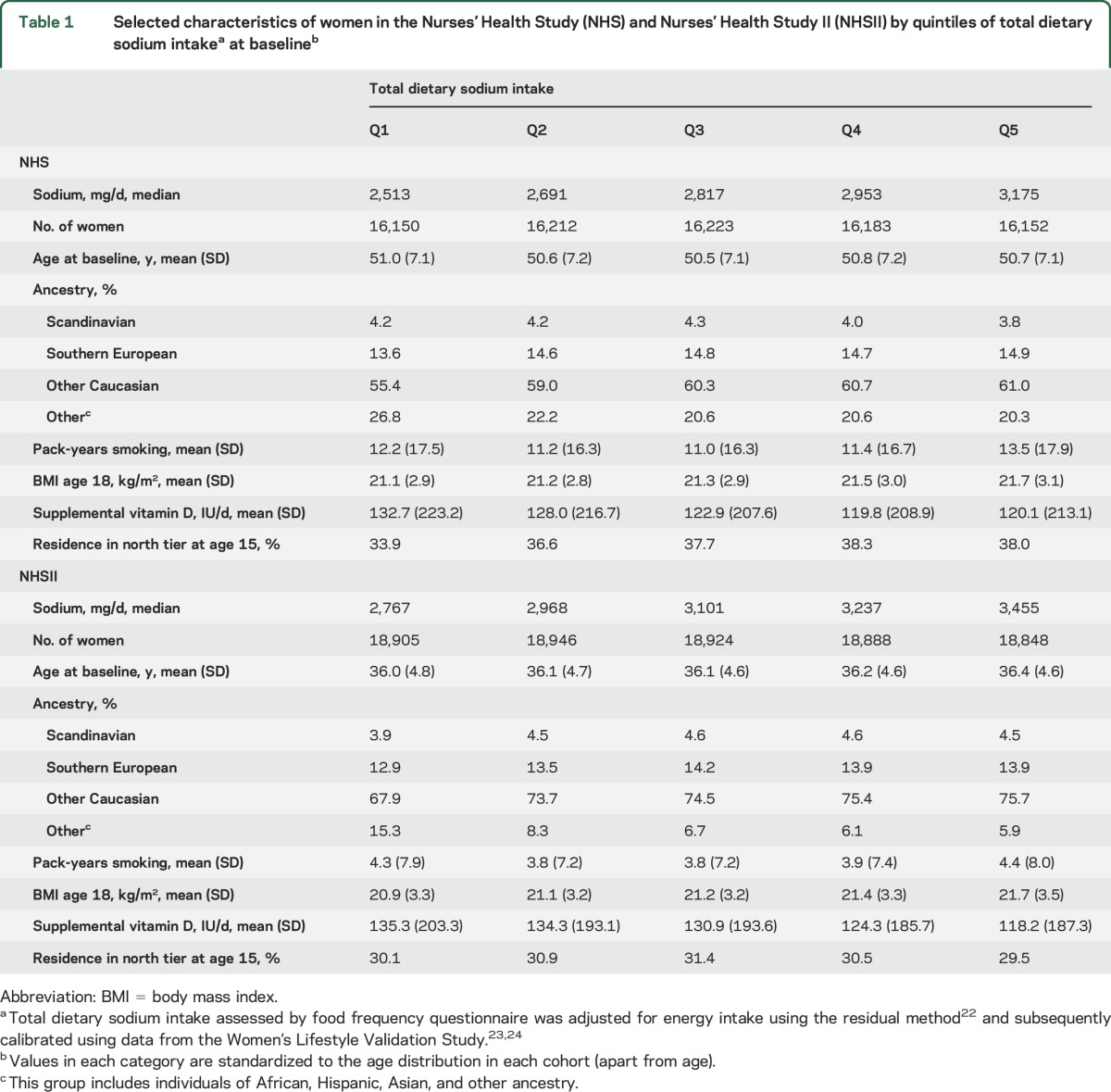
Table 1.
Selected characteristics of women in the Nurses' Health Study (NHS) and Nurses' Health Study II (NHSII) by quintiles of total dietary sodium intakea at baselineb
There was no association between sodium intake and the risk of developing MS (multivariable HRpooled for 100 mg per day increases in sodium = 1.00, 95% CI 0.96–1.03). In quintile analyses, there was also no association with MS risk comparing the top to the bottom quintile at baseline or cumulatively during follow-up, either in the age- and energy-only adjusted or in the multivariable models (table 2). Adjusting the multivariable baseline model additionally for ALA yielded similar results (top to bottom quintile of sodium intake: multivariable+ALA HRpooled 1.09, 95% CI 0.81–1.46, p for trend = 0.29); thus we did not include this covariate in the final model. More extreme comparisons led to similar results. We did not observe an association between deciles of total dietary sodium intake and MS risk, either at baseline (figure 2) (multivariable HRpooled 0.79, 95% CI 0.53–1.17, p for trend = 0.95) or for cumulative average intake (multivariable HRpooled 0.79, 95% CI 0.52–1.18, p for trend = 0.94), comparing women in the top to those in the bottom category (medians in highest vs lowest decile at baseline: 3.3 g/d [NHS] and 3.6 g/d [NHSII] vs 2.4 g/d [NHS] and 2.7 g/d [NHSII], cumulatively during follow-up: 3.5 g/d [NHS] and 3.5 g/d [NHSII] vs 2.6 g/d [NHS] and 2.7 g/d [NHSII]).
Table 2.
Pooled hazard ratios (HRs) and 95% confidence interval (95% CI) of multiple sclerosis by quintiles of total dietary sodium intakea at baseline and cumulatively during follow-up in Nurses' Health Study (NHS, 1984–2004) and Nurses' Health Study II (NHSII, 1991–2009)
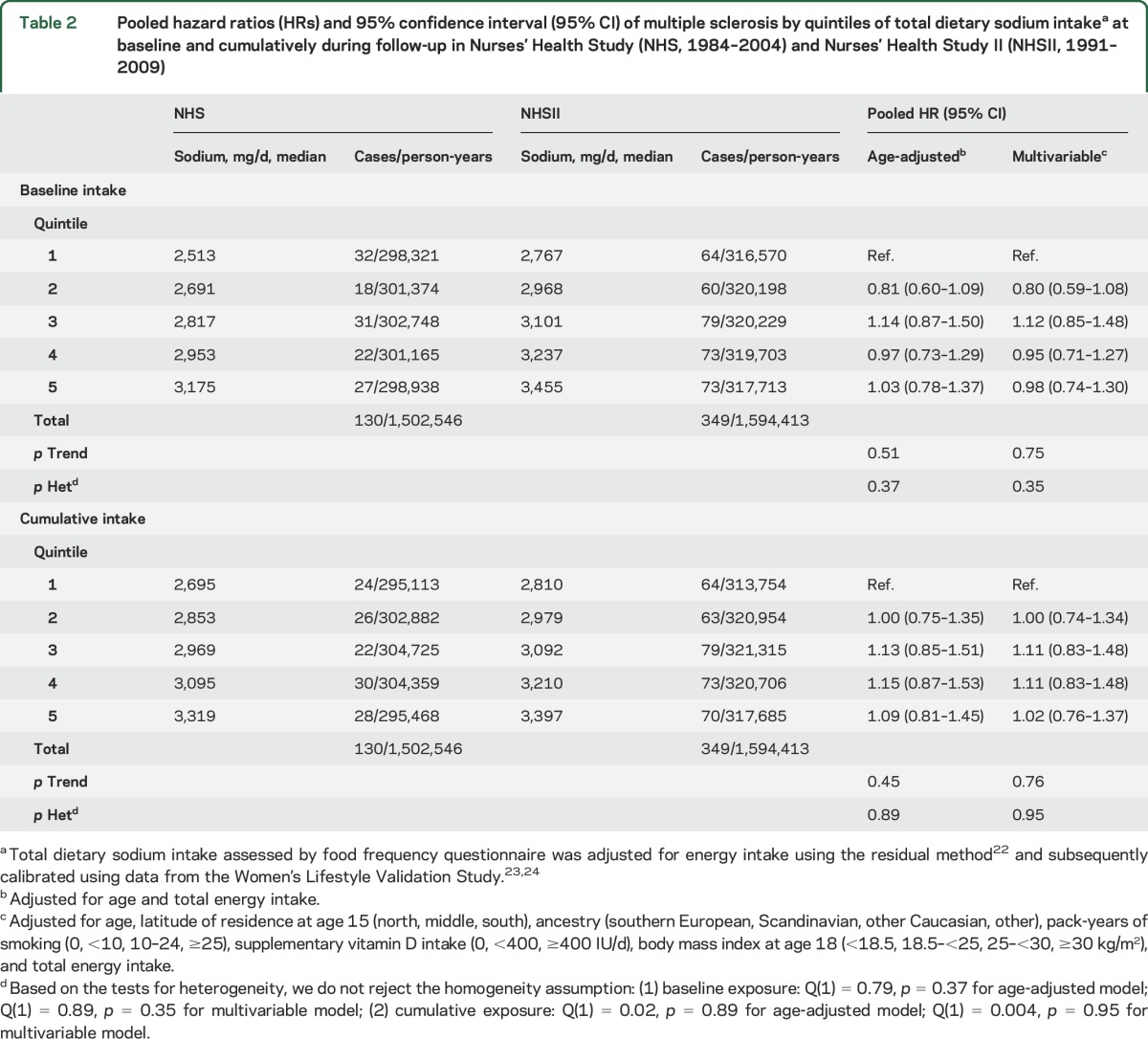
Figure 2. Total dietary sodium intake in deciles and multiple sclerosis risk in Nurses' Health Study (1984–2004) and Nurses' Health Study II (1991–2009).
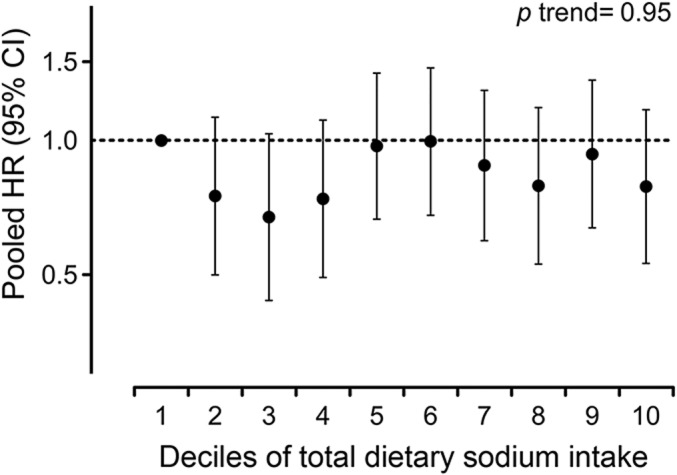
Total dietary sodium intake assessed by food frequency questionnaire at baseline was adjusted for energy intake using the residual method22 and subsequently calibrated using data from the Women's Lifestyle Validation Study.23,24 Pooled hazard ratios (HR) and 95% confidence intervals (CI) adjusted for age, latitude of residence at age 15 (north, middle, south), ancestry (southern European, Scandinavian, other Caucasian, other), pack-years of smoking (0, <10, 10–24, ≥25), supplementary vitamin D intake (0, <400, ≥400 IU/d), body mass index at age 18 (<18.5, 18.5–<25, 25–<30, ≥30 kg/m2), and total energy intake.
In the baseline analyses stratified by smoking status, there were 82 (NHS) and 159 (NHSII) ever-smoking and 48 (NHS) and 190 (NHSII) never-smoking new patients of definite or probable MS during follow-up. We did not observe an association between baseline sodium intake and MS risk when comparing women with highest to women with lowest intake according to quintiles in the subgroups separately (ever smokers: multivariable HRpooled 0.94, 95% CI 0.63–1.40, p for trend = 0.93; never smokers: multivariable HRpooled 1.06, 95% CI 0.71–1.59, p for trend = 0.50). This was also true for intake assessed continuously or in deciles (data not shown).
In sensitivity analyses, we used date of MS onset instead of diagnosis as outcome and the results were, similarly, null (data not shown).
DISCUSSION
In this large prospective study of women, we found that total dietary sodium intake was not associated with the risk of developing MS, suggesting that a decrease in sodium intake is unlikely to reduce MS risk.
Our results do not confirm the findings from experimental studies suggesting that increased salt consumption in humans could be an environmental MS risk factor.6,7,30 The inconsistency between our findings and those obtained in animal models could be explained by the sodium dose. Salt doses fed to mice displaying an accelerated onset and more severe EAE exceeded by far intakes observed in humans, even those with traditionally salt-rich diets.31,32 Further, although a marked in vitro Th17 cell induction was reported at salt concentrations meant to approximate interstitial sodium concentrations in humans,6,7 it is unclear whether these effects reproduce those observed in vivo, where sodium cations are buffered by glycosaminoglycans and interact with other molecules.33 Rather, our findings are consistent with those in the only other study assessing disease risk in humans, a case-control study in a pediatric population,11 in which no association was found between sodium intake and MS risk. It is possible that the effects on immune cells observed in experimental settings6,7 and in a small interventional study in healthy participants34 are insufficient to induce MS, but we cannot exclude that sodium could be involved in the development of other autoimmune diseases.28 As we did not investigate disease modification, we cannot evaluate whether sodium might worsen the disease course.5,8
There are several possible explanations for our findings. It could be that sodium does not play a role in MS. Experimental settings do not necessarily translate to the complex human biology and epidemiologic studies play a complementary role in producing evidence, especially when randomized clinical trials cannot be performed. It is also possible that there is an effect for more extreme sodium doses, which our study, though large, was not powered to investigate. Still, we could not detect any trend, or any indication of a potential dose–response effect. This suggests that a strong involvement of sodium in the MS pathogenesis is unlikely and such a finding would have implications for few individuals. Alternatively, sodium may manifest its harmful potential only in interaction with a certain genetic predisposition, as has been suggested in one study assessing effects on EAE course,35 or in interaction with other environmental factors, as has been reported for the risk of rheumatoid arthritis in smokers.28 However, we could not detect an association among ever-smokers in our study. Moreover, there could be residual negative confounding present from a still unidentified factor associated with salt intake and MS risk. Our findings could also be a chance finding, although this is unlikely because they are consistent for both baseline and cumulative exposure in 2 large cohorts and there was, as previously mentioned, no trend perceivable.
This study on the association between dietary sodium intake and risk of adult-onset MS has several strengths contributing to its validity and relevance. Information was collected prospectively, which is important for the assessment of dietary exposures, as retrospective studies on diet are especially prone to bias.22 Using a validated FFQ allows performing a study of this scale and still receiving reliable and valid information significantly correlated with the measurement from diet records. Repeated measurements allow for time-varying exposures and thus individual and secular behavioral changes over time to be considered. The regular update of the Harvard nutrient database contributed to minimizing the risk of exposure misclassification. As neurologists reviewed the medical records of every incident case, the probability of misclassification of onset and diagnosis was low in this study.
There are some limitations to this study. It includes only women and future studies need to verify whether the same observations can be made in men, who have a higher average sodium intake than women.36 An association only in men, however, could not be reconciled with a more pronounced increase in MS incidence in women over the last decades, which could at least partially explain the observed higher female-to-male ratio.37 Further, over 95% of women in this study are Caucasian, and we cannot conclude whether the lack of association between sodium intake and MS risk is generalizable to other ethnic groups. Moreover, we assessed sodium intake during adulthood, and it is possible that intake during childhood or adolescence may be the more relevant exposure.38 Further, even though the food sources contributing most to sodium intake are included in the study questionnaires, FFQs tend to underestimate true sodium intake.22 Individuals display a high day-to-day variability of sodium consumption and salt content in similar foods might be within a range of values. However, the gold standard to assess sodium intake, excretion in multiple 24-hour urine samples, is not practical for large, prospective, epidemiologic studies. Moreover, assessments by FFQ are reasonably correlated with those achieved by the gold standard according to the WLVS.23 By ranking individuals according to their exposure, we investigated their relative average sodium intake and compared extremes in a large population. We were thus not dependent on perfect accuracy in the estimation of absolute intake. In addition, based on data from this validation study, we could correct sodium intake reported by FFQ in our study for the systematic measurement error. Ideally, future studies should investigate the association between sodium intake and MS using the gold standard directly.39
This study provides evidence that increased dietary sodium intake does not increase MS risk, as previously suggested by experimental studies. Moderating dietary sodium intake is nevertheless favorable with regard to the prevention of cardiovascular disease.40
Supplementary Material
ACKNOWLEDGMENT
The authors thank Eilis O'Reilly, ScD, for input on data analyses.
GLOSSARY
- ALA
α-linolenic acid
- BMI
body mass index
- CDC
Centers for Disease Control and Prevention
- CI
confidence interval
- EAE
experimental autoimmune encephalomyelitis
- FFQ
food frequency questionnaire
- HR
hazard ratio
- MS
multiple sclerosis
- NHS
Nurses' Health Study
- NHSII
Nurses' Health Study II
- WLVS
Women's Lifestyle Validation Study
Footnotes
Editorial, page 1314
AUTHOR CONTRIBUTIONS
Marianna Cortese contributed to data analysis and interpretation and to drafting and revising the manuscript and figures. Changzheng Yuan contributed to data analysis and interpretation and to revising the manuscript and figures. Tanuja Chitnis contributed to data acquisition and interpretation and to revising the manuscript and figures. Alberto Ascherio contributed to obtaining funding, study concept and design, data analysis and interpretation, and revising the manuscript and figures. Kassandra L. Munger contributed to obtaining funding, study concept and design, data analysis and interpretation, and drafting and revising the manuscript and figures.
STUDY FUNDING
This study was supported by a research grant from the National Multiple Sclerosis Society (PI: K.L. Munger) and by the US NIH (grants UM1 CA186107, UM1 CA176726).
DISCLOSURE
The authors report no disclosures relevant to the manuscript. Go to Neurology.org for full disclosures.
REFERENCES
- 1.Munger KL, Levin LI, Hollis BW, Howard NS, Ascherio A. Serum 25-hydroxyvitamin D levels and risk of multiple sclerosis. JAMA 2006;296:2832–2838. [DOI] [PubMed] [Google Scholar]
- 2.Levin LI, Munger KL, O'Reilly EJ, Falk KI, Ascherio A. Primary infection with the Epstein-Barr virus and risk of multiple sclerosis. Ann Neurol 2010;67:824–830. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Hernan MA, Olek MJ, Ascherio A. Cigarette smoking and incidence of multiple sclerosis. Am J Epidemiol 2001;154:69–74. [DOI] [PubMed] [Google Scholar]
- 4.Munger KL, Chitnis T, Ascherio A. Body size and risk of MS in two cohorts of US women. Neurology 2009;73:1543–1550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Hucke S, Wiendl H, Klotz L. Implications of dietary salt intake for multiple sclerosis pathogenesis. Mult Scler 2016;22:133–139. [DOI] [PubMed] [Google Scholar]
- 6.Kleinewietfeld M, Manzel A, Titze J, et al. Sodium chloride drives autoimmune disease by the induction of pathogenic TH17 cells. Nature 2013;496:518–522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Wu C, Yosef N, Thalhamer T, et al. Induction of pathogenic TH17 cells by inducible salt-sensing kinase SGK1. Nature 2013;496:513–517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Farez MF, Fiol MP, Gaitan MI, Quintana FJ, Correale J. Sodium intake is associated with increased disease activity in multiple sclerosis. J Neurol Neurosurg Psychiatry 2015;86:26–31. [DOI] [PubMed] [Google Scholar]
- 9.Hucke S, Eschborn M, Liebmann M, et al. Sodium chloride promotes pro-inflammatory macrophage polarization thereby aggravating CNS autoimmunity. J Autoimmun 2016;67:90–101. [DOI] [PubMed] [Google Scholar]
- 10.Jorg S, Grohme DA, Erzler M, et al. Environmental factors in autoimmune diseases and their role in multiple sclerosis. Cell Mol Life Sci 2016;73:4611–4622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.McDonald J, Graves J, Waldman A, et al. A case-control study of dietary salt intake in pediatric-onset multiple sclerosis. Mult Scler Relat Disord 2016;6:87–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Nourbakhsh B, Graves J, Casper TC, et al. Dietary salt intake and time to relapse in paediatric multiple sclerosis. J Neurol Neurosurg Psychiatry 2016;87:1350–1353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Bao Y, Bertoia ML, Lenart EB, et al. Origin, methods, and evolution of the three nurses' health studies. Am J Public Health 2016;106:1573–1581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Willett WC, Sampson L, Stampfer MJ, et al. Reproducibility and validity of a semiquantitative food frequency questionnaire. Am J Epidemiol 1985;122:51–65. [DOI] [PubMed] [Google Scholar]
- 15.Salvini S, Hunter DJ, Sampson L, et al. Food-based validation of a dietary questionnaire: the effects of week-to-week variation in food consumption. Int J Epidemiol 1989;18:858–867. [DOI] [PubMed] [Google Scholar]
- 16.Willett WC, Sampson L, Browne ML, et al. The use of a self-administered questionnaire to assess diet four years in the past. Am J Epidemiol 1988;127:188–199. [DOI] [PubMed] [Google Scholar]
- 17.US Centers for Disease Control and Prevention. Top 10 Sources of Sodium [online]. Available at: cdc.gov/salt/sources.htm. Accessed May 8, 2017. [Google Scholar]
- 18.US Food and Drug Administration. Food Facts: Sodium in Your Diet [online]. Available at: fda.gov/downloads/Food/IngredientsPackagingLabeling/UCM315471.pdf. Accessed May 8, 2017. [Google Scholar]
- 19.US Centers for Disease Control and Prevention. Sodium and Food Sources [online]. Available at: cdc.gov/salt/food.htm. Accessed May 8, 2017. [Google Scholar]
- 20.DeSalvo KB, Olson R, Casavale KO. Dietary guidelines for Americans. JAMA 2016;315:457–458. [DOI] [PubMed] [Google Scholar]
- 21.Harvard T.H. Chan School of Public Health Nutrition Department's Food Composition Tables [online]. Available at: regepi.bwh.harvard.edu/health/nutrition/repeatUser.html. Accessed May 8, 2017. [Google Scholar]
- 22.Willett W. Nutritional Epidemiology. 3rd ed. Oxford: Oxford University Press; 2013. [Google Scholar]
- 23.Yuan C, Spiegelman D, Rimm EB, et al. Validation of nutrient intakes assessed by questionnaire, 24-hour recalls, and diet records compared with urinary and plasma biomarkers: findings for women. Paper presented at the International Conference on Dietary Assessment Methods; September 1–3, 2015; Brisbane, Australia.
- 24.Yuan C, Spiegelman D, Rimm EB, et al. Validity of a dietary questionnaire assessed by comparison with multiple weighed dietary records or 24-hour recalls. Am J Epidemiol 2017;185:570–584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Freedman LS, Commins JM, Moler JE, et al. Pooled results from 5 validation studies of dietary self-report instruments using recovery biomarkers for potassium and sodium intake. Am J Epidemiol 2015;181:473–487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Hernan MA, Olek MJ, Ascherio A. Geographic variation of MS incidence in two prospective studies of US women. Neurology 1999;53:1711–1718. [DOI] [PubMed] [Google Scholar]
- 27.DerSimonian R, Laird N. Meta-analysis in clinical trials. Control Clin Trials 1986;7:177–188. [DOI] [PubMed] [Google Scholar]
- 28.Sundstrom B, Johansson I, Rantapaa-Dahlqvist S. Interaction between dietary sodium and smoking increases the risk for rheumatoid arthritis: results from a nested case-control study. Rheumatology 2015;54:487–493. [DOI] [PubMed] [Google Scholar]
- 29.Bjornevik K, Chitnis T, Ascherio A, Munger KL. Polyunsaturated fatty acids and the risk of multiple sclerosis. Mult Scler Epub 2017 Jan 1. [DOI] [PMC free article] [PubMed]
- 30.Hernandez AL, Kitz A, Wu C, et al. Sodium chloride inhibits the suppressive function of FOXP3+ regulatory T cells. J Clin Invest 2015;125:4212–4222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Trieu K, Neal B, Hawkes C, et al. Salt reduction initiatives around the world: a systematic review of progress towards the global target. PLoS One 2015;10:e0130247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Brown IJ, Tzoulaki I, Candeias V, Elliott P. Salt intakes around the world: implications for public health. Int J Epidemiol 2009;38:791–813. [DOI] [PubMed] [Google Scholar]
- 33.Nijst P, Verbrugge FH, Grieten L, et al. The pathophysiological role of interstitial sodium in heart failure. J Am Coll Cardiol 2015;65:378–388. [DOI] [PubMed] [Google Scholar]
- 34.Yi B, Titze J, Rykova M, et al. Effects of dietary salt levels on monocytic cells and immune responses in healthy human subjects: a longitudinal study. Transl Res 2015;166:103–110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Krementsov DN, Case LK, Hickey WF, Teuscher C. Exacerbation of autoimmune neuroinflammation by dietary sodium is genetically controlled and sex specific. FASEB J 2015;29:3446–3457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Bailey RL, Parker EA, Rhodes DG, et al. Estimating sodium and potassium intakes and their ratio in the American diet: data from the 2011–2012 NHANES. J Nutr Epub 2016 Mar 9. [DOI] [PMC free article] [PubMed]
- 37.Koch-Henriksen N, Sorensen PS. The changing demographic pattern of multiple sclerosis epidemiology. Lancet Neurol 2010;9:520–532. [DOI] [PubMed] [Google Scholar]
- 38.Handel AE, Giovannoni G, Ebers GC, Ramagopalan SV. Environmental factors and their timing in adult-onset multiple sclerosis. Nat Rev Neurol 2010;6:156–166. [DOI] [PubMed] [Google Scholar]
- 39.Dyer A, Elliott P, Chee D, Stamler J. Urinary biochemical markers of dietary intake in the INTERSALT study. Am J Clin Nutr 1997;65:1246S–1253S. [DOI] [PubMed] [Google Scholar]
- 40.Aburto NJ, Ziolkovska A, Hooper L, Elliott P, Cappuccio FP, Meerpohl JJ. Effect of lower sodium intake on health: systematic review and meta-analyses. BMJ 2013;346:f1326. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.