Abstract
Background
Peri-operative intravenous administration of iron supplementation seems a good option to reduce allogeneic blood transfusion in major orthopaedic surgery. However, its efficacy in simultaneous bilateral total knee arthroplasty has not been studied.
Materials and methods
From December 2014 to May 2015, a total of 72 consecutive patients underwent simultaneous bilateral total knee arthroplasty and received peri-operative intravenous iron supplementation (iron isomaltoside 1000: 600 mg pre-operatively and 400 mg 1 week post-operatively) and intra-articular tranexamic acid (2 g in 20 mL saline at the end of surgery), and were managed with a restrictive transfusion trigger (haemoglobin <7 g/dL). Post-operatively, we observed patients closely for symptoms of anaemia and checked their haemoglobin levels on days 1, 6 and 13 after surgery.
Results
The mean baseline haemoglobin level was 13.1 g/dL. The levels remained above 7.0 g/dL on post-operative days 1, 6 and 13 (mean, 11.4 g/dL, 9.9 g/dL and 10.4 g/dL, respectively) in all but one patient who experienced melaena and required allogeneic blood transfusion.
Discussion
Intravenous iron supplementation combined with intra-articular administration of tranexamic acid seems to be an effective strategy for reducing the rate of allogeneic blood transfusion in patients undergoing simultaneous bilateral total knee arthroplasty managed with a restrictive transfusion trigger.
Keywords: intravenous iron supplement, tranexamic acid, transfusion, bilateral total knee arthroplasty
Introduction
The advantages of simultaneous bilateral total knee arthroplasty (BTKA) include lower costs and greater patients’ satisfaction1–4, lower infection and revision rates, shorter anaesthetic time and quicker recovery1. These advantages have led to the recommended use of simultaneous BTKA for young, healthy patients requiring replacement of both knee joints3. However, concerns about simultaneous BTKA include high mortality, risk of cardiac and pulmonary complications and high transfusion rate3,5.
With regards to transfusion, allogeneic blood transfusion (ABT) carries risks such as the transmission of blood-borne pathogens, induction of various immunomodulatory responses6 and development of peri-prosthetic joint infection7–9. With the aim of minimising recourse to ABT, various blood conservation strategies, including pre-operative autologous donation, use of erythropoietin, administration of antifibrinolytic agents and post-operative re-infusion drains, have been studied10–17. Trials of patients undergoing single TKA found that ABT is not needed with intra-articular or intravenous (IV) administration of the antifibrinolytic agent, tranexamic acid16,17. However, ABT can still be necessary in more than 10% of simultaneous BTKA, even when the patients are given tranexamic acid14,15.
IV iron supplementation is a good option to reduce the transfusion rate in patients with predicted blood loss during orthopaedic surgery18. Peri-operative IV iron supplements can reduce the blood transfusion rate after TKA18–22, whereas post-operative oral iron has been proven inefficacious in reducing blood transfusion or improving haemoglobin (Hb) levels23. However, the effect of IV iron supplementation in patients undergoing simultaneous BTKA has not been studied.
In the current study, we evaluated the results of IV iron supplementation and intra-articular administration of tranexamic acid in patients undergoing simultaneous BTKA.
Materials and methods
From December 2014 to May 2015, a total of 72 consecutive patients underwent simultaneous BTKA in our institution. All patients had severe osteoarthritis in both knees. For anaemic patients whose pre-operative Hb level was <11.0 g/dL, we recommended staged BTKA, rather than simultaneous BTKA, because extensive bleeding increases the risk of cardiac problems, surgical wound oxygen deprivation, delayed recovery and ABT. Anaemic patients were not, therefore, included in the current study. After obtaining the patient’s consent, simultaneous BTKA was conducted under spinal anaesthesia. Institutional review board approval was obtained (2015-0652-001) and all study protocols were approved.
Surgical procedures and topical tranexamic acid administration
After spinal anaesthesia and squeezing the lower extremities, a tourniquet was inflated to 350 mmHg. A 10–15 cm anterior midline incision was made, followed by a medial parapatellar approach. The cemented posterior-stabilised prostheses used in this study were Vanguard (Biomet, Warsaw, IN, USA), Emotion Pro (B. Braun Aesculap, Tuttlingen, Germany), Buechel-Pappas (Endotec, Orlando, FL, USA) and Oxinium (Smith & Nephew, Memphis, TN, USA). Each was implanted according to the surgical manual of its supplier. After the femoral medullary canal had been filled with autologous bone, implants were cemented and the capsule was repaired. After closing the capsule, 2 g tranexamic acid in 20 mL normal saline was administered intra-articularly, as previously described17. The tourniquet was deflated after suturing the skin and knee banding. The drain line was opened 6 h after deflation of the tourniquet and a low negative pressure was applied on the drainage bag. Post-operative pain was managed with an epidural patient-controlled analgesia pump for 2 days following surgery.
Intravenous iron supplementation
Peri- and post-operative IV iron supplementation was administered in the form of iron isomaltoside 1000 (Monofer®; Pharmacosmos, Holbaek, Denmark), which can be given safely as a high-dose infusion over a few minutes24. The cumulative IV iron dose was calculated using Ganzoni’s formula:
Consistent with a previous study on simultaneous BTKA14, we calculated the cumulative iron dose required. In calculating total iron dose, we assumed that: (i) Hb level decreased to 5~6 g/dL after simultaneous BTKA; (ii) the iron reserve did not need to be restored; (iii) the patients’ mean body mass index was 30 kg/m2 and (iv) their mean height was 150 cm. The estimated cumulative iron dose was 810~972 mg. Hence, each patient was given 1,000 mg of iron isomaltoside 1000 intravenously. Because the maximal daily dose of iron isomaltoside 1000 is 20 mg/kg and the current study included patients weighing under 50 kg, the total dose was administered in two separate doses. The day before surgery, 600 mg iron isomaltoside 1000 was given in 100 mL normal saline; the remaining 400 mg were administered in 100 mL normal saline 1 week later.
Post-operative protocol and evaluation
On post-operative day (POD) 1, mobilisation using a walker was permitted. On POD2, passive range of motion exercises were started using a continuous passive motion device once a day. The volume of fluids in the drains was checked daily and drains were removed on POD2–4 at the surgeon’s discretion. Prophylaxis of thromboembolism consisted of enoxaparin (Clexane; Sanofi-Aventis, Paris, France) 40 mg/day subcutaneously for the first 7 days after surgery, followed by oral rivaroxaban (Xarelto; Bayer, Leverkusen, Germany) 10 mg/day for the next 7 days.
Post-operative Hb levels were checked on POD1, POD6 and POD13. With close observation for symptoms of anaemia, all patients stayed in hospital for 2 weeks with continuous passive motion exercises and ambulation with a walker. ABT was administered if the patient’s Hb level decreased to <7.0 g/dL.
Statistical analyses
Alterations in Hb levels after surgery were evaluated by measures of the differences between Hb levels pre-operatively and on POD1, POD6 and POD 13 using Wilcoxon’s signed rank test. Stepwise multiple regression analysis was used to analyse factors affecting the post-operative changes in Hb level. Factors analysed included age, sex, medical history of hypertension, medication history of aspirin use, height, weight, body mass index, systolic blood pressure, diastolic blood pressure, heart rate, platelet count, prothrombin time, activated partial thromboplastin time and operating time. MANOVA testing was used to compare any differences among results associated with different surgeons or prostheses. All statistical analyses were performed using SPSS software version 12.0 (SPSS, Inc., Chicago, IL, USA) and differences were considered statistically significant for p values <0.05.
Results
The patients’ mean age was 69 years (range, 54–82 years), their mean height was 152 cm (range, 139–170 cm), their mean weight was 61 kg (range, 41–92 kg) and their mean body mass index was 26.5 kg/m2 (range, 17–35 kg/m2). The study cohort consisted of 68 female patients and four male patients (Table I). Preoperatively, the mean Hb level was 13.1 g/dL (range, 11.3–15.7 g/dL), mean platelet count was 260×109/L (range, 156–379×109/L), mean prothrombin time was 10.8 sec (range, 8.5–13.1 sec) and mean activated partial thromboplastin time was 30.2 sec (range, 22.4–46.5 sec). The mean operation time was 158 min (range, 130–200 min).
Table I.
Patients’ demographics.
| Characteristic | Mean |
|---|---|
| Age, years (min–max) | 69 (54–82) |
| Gender | M: 4, F: 68 |
| Medical history of hypertension (%) | 52 (72%) |
| History of aspirin use (%) | 20 (28%) |
| Height, cm (min–max) | 152 (139–170) |
| Weight, kg (min–max) | 61 (41–92) |
| Body mass index, kg/m2 (min–max) | 26.5 (17–35) |
| Systolic blood pressure, mmHg (min–max) | 126 (100–150) |
| Diastolic blood pressure, mmHg (min–max) | 77 (60–100) |
| Heart rate, beats per min (min–max) | 68 (40–84) |
| Pre-operative hemoglobin level, g/dL (min–max) | 13.1 (11.3–15.7) |
| Pre-operative platelet count, /μL (min–max) | 260,000 (156,000–379,000) |
| Pre-operative prothrombin time, sec (min–max) | 10.8 (8.5–13.1) |
| Pre-operative activated partial thromboplastin time, sec (min–max) | 30.2 (22.4–46.5) |
| Operation time, min (min–max) | 158 (130–200) |
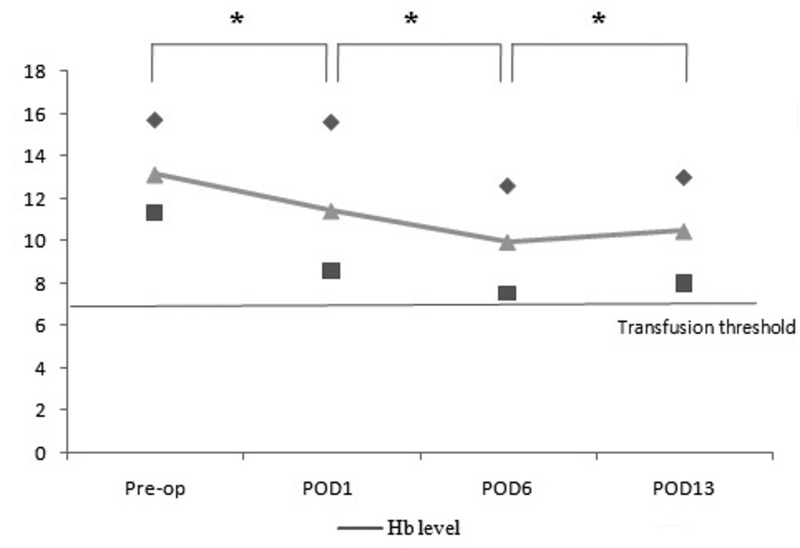
The mean total volume of post-operative drained blood was 862 mL (range, 215–1,955 mL). The volumes drained each day were 306 mL on POD1, 353 mL on POD2, 237 mL on POD3 for 46 patients and 172 mL on POD4 for 19 patients. Post-operatively, the mean Hb level decreased to 11.4 g/dL (range, 8.6–15.6 g/dL) on POD1 and further decreased on POD6 to 9.9 g/dL (range, 7.5–12.6 g/dL). Subsequently, the mean level recovered to 10.4 g/dL (range, 8.0–13.0 g/dL) (Figure 1). The mean Hb decrement was 1.7 g/dL (range, 0.1–3.1 g/dL) on POD1, 3.2 g/dL (range, 1.1–5.8 g/dL) on POD6, and 2.7 g/dL (range, −0.5–5.2 g/dL) on POD13. The differences were significant in Wilcoxon’s signed rank test. According to the multiple regression analysis, none of the variables studied had a statistically significant influence on postoperative Hb level or Hb decrement.
Figure 1.
Post-operative Hb levels.
Mean, ▲, and range: min, ■, and max, ◆. The Hb level was decreased on POD1 and POD6, and had begun to recover by POD13; *p-value <0.05 by Wilcoxon’s signed rank test. Hb: haemoglobin.
During close observation for 2 weeks, with the Hb transfusion threshold of <7.0 g/dL, only one patient received ABT on POD9 because of an unexpected decrease in Hb due to melaena. The overall transfusion rate was, therefore, 1.3% (1/72). Apart from this single case, none of the patients complained of dizziness or other symptoms of anaemia during the 2 weeks following simultaneous BTKA and none was recommended for or received transfusion. Among the 71 patients who were not transfused, three developed a Hb level <8 g/dL (7.5, 7.5 and 7.7 g/dL on POD6) with recovery to over 8 g/dL by POD13.
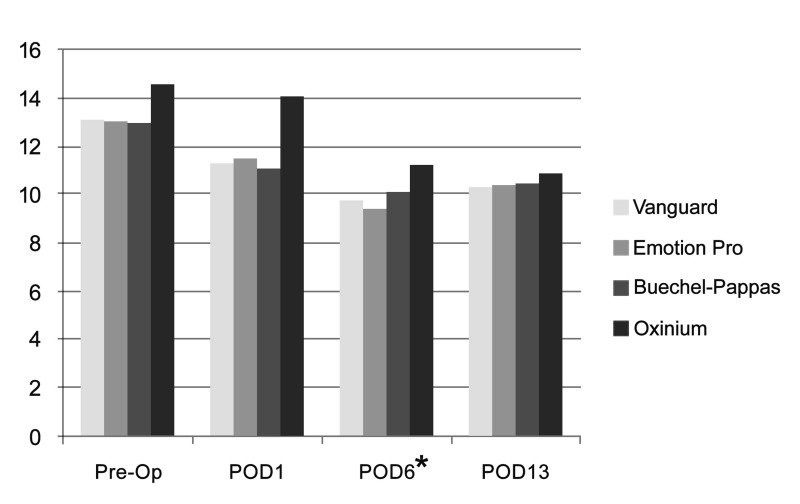
Six surgeons performed the operations using four commercial brands of prostheses. A Vanguard device was used in 24 patients, an Emotion Pro device in 17 patients, a Buechel-Pappas prosthesis in 19 patients and an Oxinium prosthesis in two. Because of the small number of patients and its complicating impact on statistical analysis, data regarding patients operated by one surgeon and those from patients in whom the Oximium prosthesis was implanted were excluded. With this exclusion, MANOVA testing revealed no statistical correlation between surgeons and Hb level or Hb decrement. As far as concerns the implanted devices, the Buechel-Pappas prosthesis was associated with a smaller decrement in Hb level on POD6 in comparison with the other prostheses. No difference was evident on POD13 (Figure 2).
Figure 2.
Hb level following surgery with the four prostheses.
The Buechel-Pappas system was associated with a smaller decrement in haemoglobin level on POD6, with no difference on POD13. *p-value <0.05 by the MANOVA test between the Vanguard, Emotion Pro and Buechel-Pappas prostheses. The Oxinium prosthesis was excluded from this analysis because of the small number of patients in whom it was used.
Discussion
The purpose of the current study was to evaluate IV iron supplementation with administration of intra-articular tranexamic acid in simultaneous BTKA as a new option to reduce ABT. Only one of 72 BTKA patients needed ABT due to unexpected melaena. All other patients had a Hb exceeding 7 g/dL with no symptoms of anaemia over the 2-week observation period. Thus, peri-operative IV iron supplementation with intra-articular tranexamic acid administration plus the use of a restrictive transfusion trigger seems to be a good strategy for avoiding ABT in patients undergoing simultaneous BTKA.
IV iron supplementation can promote faster recovery from surgical bleeding. Iron supplementation is commonly recommended for patients with iron deficiency anaemia who are waiting to undergo major orthopaedic surgery18,25,26. Peri-operative IV iron supplementation reportedly reduced Hb level decrement and transfusion rate in elderly hip fracture patients who had a pre-operative Hb concentration exceeding 12 g/dL27. In another study, peri-operative IV iron supplementation reduced the transfusion rate and hastened recovery from post-operative anaemia in TKA patients19,20. Hence, IV iron supplementation can also be recommended in major orthopaedic surgery without iron deficiency. We gave peri- and post-operative IV iron supplementation to patients undergoing simultaneous BTKA, who had a 2-fold higher predicted blood loss than those undergoing single TKA. No transfusion was required until POD6 and only one patient received ABT, for a reason other than surgical blood loss. Given that the transfusion rate for simultaneous BTKA in other studies has been reported to range between 7% and 24%4,5,14,15,28,29, it appears that IV iron supplementation can contribute to reduce transfusion rates.
Theussinger et al. reported a 2-week interval between the administration of IV iron supplements and an increment in Hb levels25 and, therefore, suggested administering IV iron 2–3 weeks before the scheduled surgical procedure. On the other hand, other studies reported that peri-operative or post-operative IV iron supplementation could also be effective at reducing the transfusion rate and hastening recovery19–22,27. We used peri- and post-operative IV iron supplementation and the patients did not require transfusion. Although we cannot clearly explain how IV iron exerts an effect within such a short time, it is conceivable that the administered iron quickly supplies the iron store needed to recover from acute bleeding and facilitates more rapid production of Hb in patients with on-going bleeding. Randomised controlled trials and laboratory studies are needed to determine the ideal time to start IV iron supplementation.
A restrictive transfusion trigger was recently introduced in the orthopaedic department of our hospital. The previous transfusion trigger level of Hb was <10 g/dL for the post-operative period or for ongoing bleeding. However, the complications of ABT prompted us to adopt a more restrictive transfusion trigger. Previous studies in other orthopaedic departments used either <8 g/dL as the level14,16,21 or the more restrictive value of <7 g/dL15,27. We used the latter. Three patients had a Hb concentration <8 g/dL at POD6. They had no symptoms and their Hb recovered to >8 g/dL after a week with only the scheduled IV iron supplement. This indicates that, with close observation, the more restrictive transfusion trigger level, <7 g/dL, can be used in the post-operative period.
Several studies have focused on the effect of tranexamic acid in TKA patients. Both IV and topical administration of tranexamic acid are effective in reducing blood loss and eliminating the need for transfusion in patients undergoing single TKA16,17,30,31. However, ABT is still required in some patients undergoing simultaneous BTKA, despite intra-articular or IV administration of tranexamic acid14,15,28,29. Hedge et al. reported that a mean of 0.23 and 0.63 units of ABT was required for simultaneous BTKA patients managed with intra-articular and IV tranexamic acid administration, respectively15. Karam et al. reported that IV tranexamic acid reduced blood loss in simultaneous BTKA, with the transfusion rate being decreased to 10%14. In the current study, only one patient required ABT and this was necessary because of melaena. Thus the transfusion rate was lower in this study than in previous studies, although there were differences between studies in transfusion trigger threshold, route or dosage of the tranexamic acid, pre-operative demographics, operating procedures or prostheses and the post-operative laboratory follow-up protocol. Further randomised controlled studies are needed to determine the real cause of the different transfusion rates.
Of the four prostheses used in the current study, the Buechel-Pappas prosthesis was associated with the least decrement in Hb level on POD6. Moon et al. reported that this prosthesis requires less intra-operative bone mass removal than that of other implants32. However, in our study the Hb level on POD13 was similar in patients given this prosthesis and the other prostheses. This means that the Buechel-Pappas prosthesis potentially causes less bleeding because of its design, such as less bone loss in the notch. However, the difference is small enough to be compensated for by the faster recovery within 2 weeks.
Our study has a few limitations. First, there was no control group and no long-term results. Second, there were no routine data in the period from POD2 to POD5, during which the Hb level may reach a nadir. Third, no clinical outcomes, such as pain or knee function, were considered.
Conclusions
IV iron supplementation and intra-articular administration of tranexamic acid, plus a restrictive transfusion trigger, seems to be an effective strategy for reducing the ABT rate in patients undergoing simultaneous BTKA.
Footnotes
Authorship contributions
DWS, SBH, JHP and BSK designed the study; KC and BSK collected and analysed data; DWS and BSK drafted the manuscript; DWS, SBH, JHP, KC, and BSK reviewed and revised the manuscript.
The Authors declare no conflicts of interests.
References
- 1.Fu D, Li G, Chen K, et al. Comparison of clinical outcome between simultaneous-bilateral and staged-bilateral total knee arthroplasty: a systematic review of retrospective studies. J Arthroplasty. 2013;28:1141–7. doi: 10.1016/j.arth.2012.09.023. [DOI] [PubMed] [Google Scholar]
- 2.Lane GJ, Hozack WJ, Shah S, et al. Simultaneous bilateral versus unilateral total knee arthroplasty. Outcomes analysis. Clin Orthop Relat Res. 1997:106–12. [PubMed] [Google Scholar]
- 3.Vulcano E, Memtsoudis S, Della Valle AG. Bilateral total knee arthroplasty guidelines: are we there yet? J Knee Surg. 2013;26:273–9. doi: 10.1055/s-0032-1329721. [DOI] [PubMed] [Google Scholar]
- 4.Zhu M, Chen JY, Tan YR, et al. Effects of anesthetic technique on blood loss and complications after simultaneous bilateral total knee arthroplasty. Arch Orthop Trauma Surg. 2015;135:565–71. doi: 10.1007/s00402-015-2188-8. [DOI] [PubMed] [Google Scholar]
- 5.Bullock DP, Sporer SM, Shirreffs TG., Jr Comparison of simultaneous bilateral with unilateral total knee arthroplasty in terms of perioperative complications. J Bone Joint Surg Am. 2003;85-A:1981–6. doi: 10.2106/00004623-200310000-00018. [DOI] [PubMed] [Google Scholar]
- 6.Dellinger EP, Anaya DA. Infectious and immunologic consequences of blood transfusion. Crit Care. 2004;8( Suppl 2):S18–23. doi: 10.1186/cc2847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Pulido L, Ghanem E, Joshi A, et al. Periprosthetic joint infection: the incidence, timing, and predisposing factors. Clin Orthop Relat Res. 2008;466:1710–5. doi: 10.1007/s11999-008-0209-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Friedman R, Homering M, Holberg G, Berkowitz SD. Allogeneic blood transfusions and postoperative infections after total hip or knee arthroplasty. J Bone Joint Surg Am. 2014;96:272–8. doi: 10.2106/JBJS.L.01268. [DOI] [PubMed] [Google Scholar]
- 9.Newman ET, Watters TS, Lewis JS, et al. Impact of perioperative allogeneic and autologous blood transfusion on acute wound infection following total knee and total hip arthroplasty. J Bone Joint Surg Am. 2014;96:279–84. doi: 10.2106/JBJS.L.01041. [DOI] [PubMed] [Google Scholar]
- 10.Bezwada HP, Nazarian DG, Henry DH, Booth RE., Jr Preoperative use of recombinant human erythropoietin before total joint arthroplasty. J Bone Joint Surg Am. 2003;85-A:1795–800. doi: 10.2106/00004623-200309000-00020. [DOI] [PubMed] [Google Scholar]
- 11.Breakwell LM, Getty CJ, Dobson P. The efficacy of autologous blood transfusion in bilateral total knee arthroplasty. Knee. 2000;7:145–7. doi: 10.1016/s0968-0160(00)00032-6. [DOI] [PubMed] [Google Scholar]
- 12.Markar SR, Jones GG, Karthikesalingam A, et al. Transfusion drains versus suction drains in total knee replacement: meta-analysis. Knee Surg Sports Traumatol Arthrosc. 2012;20:1766–72. doi: 10.1007/s00167-011-1761-0. [DOI] [PubMed] [Google Scholar]
- 13.Sculco TP, Baldini A, Keating EM. Blood management in total joint arthroplasty. Instr Course Lect. 2005;54:51–66. [PubMed] [Google Scholar]
- 14.Karam JA, Bloomfield MR, DiIorio TM, et al. Evaluation of the efficacy and safety of tranexamic acid for reducing blood loss in bilateral total knee arthroplasty. J Arthroplasty. 2014;29:501–3. doi: 10.1016/j.arth.2013.08.005. [DOI] [PubMed] [Google Scholar]
- 15.Hegde C, Wasnik S, Kulkarni S, et al. Simultaneous bilateral computer assisted total knee arthroplasty: the effect of intravenous or intraarticular tranexamic acid. J Arthroplasty. 2013;28:1888–91. doi: 10.1016/j.arth.2013.03.018. [DOI] [PubMed] [Google Scholar]
- 16.Gomez-Barrena E, Ortega-Andreu M, Padilla-Eguiluz NG, et al. Topical intra-articular compared with intravenous tranexamic acid to reduce blood loss in primary total knee replacement: a double-blind, randomized, controlled, noninferiority clinical trial. J Bone Joint Surg Am. 2014;96:1937–44. doi: 10.2106/JBJS.N.00060. [DOI] [PubMed] [Google Scholar]
- 17.Ishida K, Tsumura N, Kitagawa A, et al. Intra-articular injection of tranexamic acid reduces not only blood loss but also knee joint swelling after total knee arthroplasty. Int Orthop. 2011;35:1639–45. doi: 10.1007/s00264-010-1205-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Muñoz M, García-Erce JA, Cuenca J, et al. On the role of iron therapy for reducing allogeneic blood transfusion in orthopaedic surgery. Blood Transfus. 2012;10:8–22. doi: 10.2450/2011.0061-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Cuenca J, García-Erce JA, Martinez F, et al. Perioperative intravenous iron, with or without erythropoietin, plus restrictive transfusion protocol reduce the need for allogeneic blood after knee replacement surgery. Transfusion. 2006;46:1112–9. doi: 10.1111/j.1537-2995.2006.00859.x. [DOI] [PubMed] [Google Scholar]
- 20.García-Erce JA, Cuenca J, Martinez F, et al. Perioperative intravenous iron preserves iron stores and may hasten the recovery from post-operative anaemia after knee replacement surgery. Transfus Med. 2006;16:335–41. doi: 10.1111/j.1365-3148.2006.00682.x. [DOI] [PubMed] [Google Scholar]
- 21.Muñoz M, Naveira E, Seara J, Cordero J. Effects of postoperative intravenous iron on transfusion requirements after lower limb arthroplasty. Br J Anaesth. 2012;108:532–4. doi: 10.1093/bja/aes012. [DOI] [PubMed] [Google Scholar]
- 22.Beris P, Muñoz M, García-Erce JA, et al. Perioperative anaemia management: consensus statement on the role of intravenous iron. Br J Anaesth. 2008;100:599–604. doi: 10.1093/bja/aen054. [DOI] [PubMed] [Google Scholar]
- 23.Mundy GM, Birtwistle SJ, Power RA. The effect of iron supplementation on the level of haemoglobin after lower limb arthroplasty. J Bone Joint Surg Br. 2005;87:213–7. doi: 10.1302/0301-620x.87b2.15122. [DOI] [PubMed] [Google Scholar]
- 24.Jahn MR, Andreasen HB, Futterer S, et al. A comparative study of the physicochemical properties of iron isomaltoside 1000 (Monofer), a new intravenous iron preparation and its clinical implications. Eur J Pharm Biopharm. 2011;78:480–91. doi: 10.1016/j.ejpb.2011.03.016. [DOI] [PubMed] [Google Scholar]
- 25.Theusinger OM, Leyvraz PF, Schanz U, et al. Treatment of iron deficiency anaemia in orthopedic surgery with intravenous iron: efficacy and limits: a prospective study. Anesthesiology. 2007;107:923–7. doi: 10.1097/01.anes.0000291441.10704.82. [DOI] [PubMed] [Google Scholar]
- 26.Na HS, Shin SY, Hwang JY, et al. Effects of intravenous iron combined with low-dose recombinant human erythropoietin on transfusion requirements in iron-deficient patients undergoing bilateral total knee replacement arthroplasty. Transfusion. 2011;51:118–24. doi: 10.1111/j.1537-2995.2010.02783.x. [DOI] [PubMed] [Google Scholar]
- 27.Serrano-Trenas JA, Ugalde PF, Cabello LM, et al. Role of perioperative intravenous iron therapy in elderly hip fracture patients: a single-center randomized controlled trial. Transfusion. 2011;51:97–104. doi: 10.1111/j.1537-2995.2010.02769.x. [DOI] [PubMed] [Google Scholar]
- 28.Kim TK, Chang CB, Kang YG, et al. Clinical value of tranexamic acid in unilateral and simultaneous bilateral TKAs under a contemporary blood-saving protocol: a randomized controlled trial. Knee Surg Sports Traumatol Arthrosc. 2014;22:1870–8. doi: 10.1007/s00167-013-2492-1. [DOI] [PubMed] [Google Scholar]
- 29.MacGillivray RG, Tarabichi SB, Hawari MF, Raoof NT. Tranexamic acid to reduce blood loss after bilateral total knee arthroplasty: a prospective, randomized double blind study. J Arthroplasty. 2011;26:24–8. doi: 10.1016/j.arth.2009.11.013. [DOI] [PubMed] [Google Scholar]
- 30.Zhao-Yu C, Yan G, Wei C, et al. Reduced blood loss after intra-articular tranexamic acid injection during total knee arthroplasty: a meta-analysis of the literature. Knee Surg Sports Traumatol Arthrosc. 2014;22:3181–90. doi: 10.1007/s00167-013-2814-3. [DOI] [PubMed] [Google Scholar]
- 31.Yang ZG, Chen WP, Wu LD. Effectiveness and safety of tranexamic acid in reducing blood loss in total knee arthroplasty: a meta-analysis. J Bone Joint Surg Am. 2012;94:1153–9. doi: 10.2106/JBJS.K.00873. [DOI] [PubMed] [Google Scholar]
- 32.Moon KH, Hong SH, Hong TH. Total knee replacement arthroplasty with Buechel and Pappas knee: minimum 2-year follow-up. Clin Orthop Surg. 2015;7:62–8. doi: 10.4055/cios.2015.7.1.62. [DOI] [PMC free article] [PubMed] [Google Scholar]