Abstract
Here we review recent data and the evolving understanding of the role of red blood cell-derived microparticles (RMPs) in normal physiology and in disease progression. Microparticles (MPs) are small membrane vesicles derived from various parent cell types. MPs are produced in response to a variety of stimuli through several cytoskeletal and membrane phospholipid changes. MPs have been investigated as potential biomarkers for multiple disease processes and are thought to have biological effects, most notably in: promotion of coagulation, production and handling of reactive oxygen species, immune modulation, angiogenesis, and in apoptosis. Specifically, RMPs are produced normally during RBC maturation and their production is accelerated during processing and storage for transfusion. Several factors during RBC storage are known to trigger RMP production, including: increased intracellular calcium, increased potassium leakage, and energy failure with ATP depletion. Of note, RMP composition differs from that of intact RBCs, and the nature and composition of RMP components are affected by both storage duration and the character of storage solutions. Recognised RMP bioactivities include: promotion of coagulation, immune modulation, and promotion of endothelial adhesion, as well as influence upon vasoregulation via nitric oxide (NO) scavenging. Of particular relevance, RMPs are more avid NO scavengers than intact RBCs and this feature has been proposed as a mechanism for the impaired oxygen delivery homeostasis that has been observed following transfusion. Preliminary human studies demonstrate that circulating RMP abundance increases with RBC transfusion and is associated with altered plasma vasoactivity and abnormal vasoregulation. In summary, RMPs are submicron particles released from stored RBCs, with demonstrated vasoactive properties that appear to disturb oxygen delivery homeostasis. The clinical impact of RMPs in transfusion recipients is an area of continued investigation.
Keywords: erythrocytes, nitric oxide, blood transfusion
Microparticles
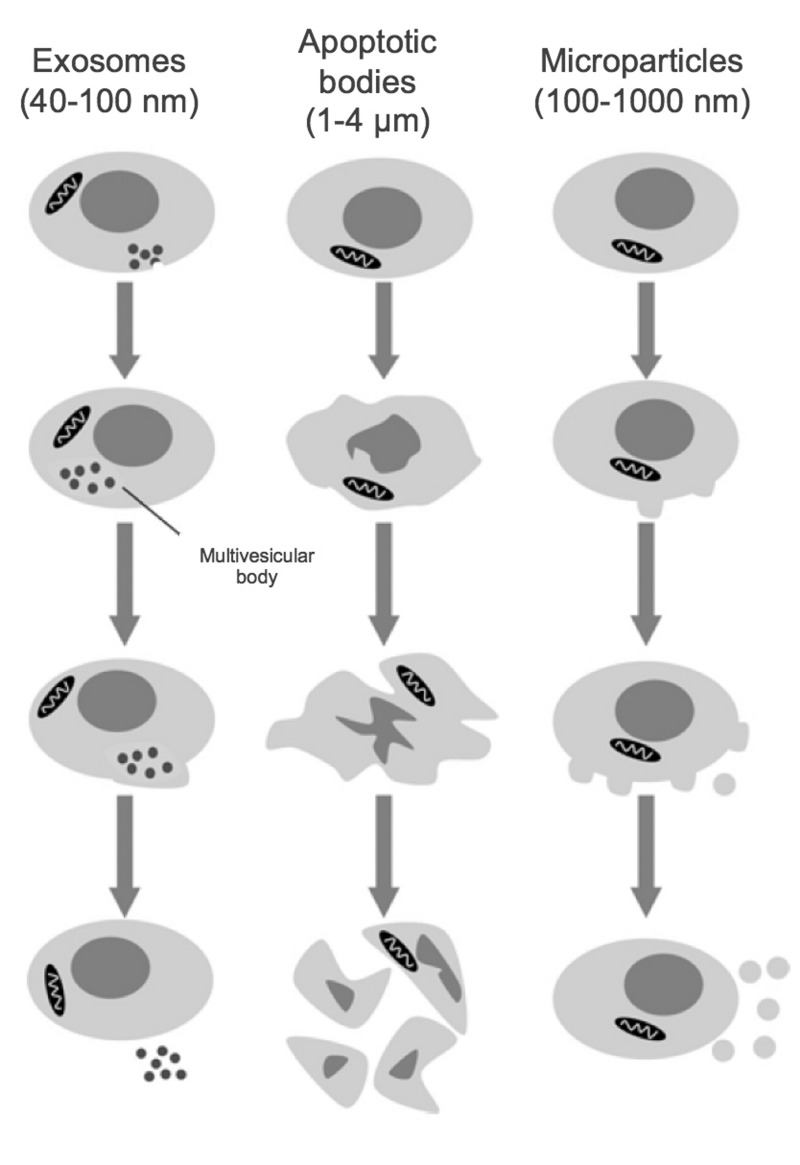
Release of small membrane vesicles (termed microparticles [MPs]) by activated and/or apoptotic cells was first described over 40 years ago1. MPs are formally defined as cell-derived vesicles that are 0.1–1.0 μm in size and are categorised by membrane proteins and cytosolic material specific to various parent cell populations2 (Figure 1). MPs are distinguished from exosomes and apoptotic bodies by size, composition and mechanism of formation3 (Figure 2). Exosomes are generally smaller (40–100 nm) and are formed by a multistep process that involves intracellular generation and subsequent extrusion of vesicles; apoptotic bodies are much larger (1–5 μm) and are formed by membrane shedding during apoptosis4.
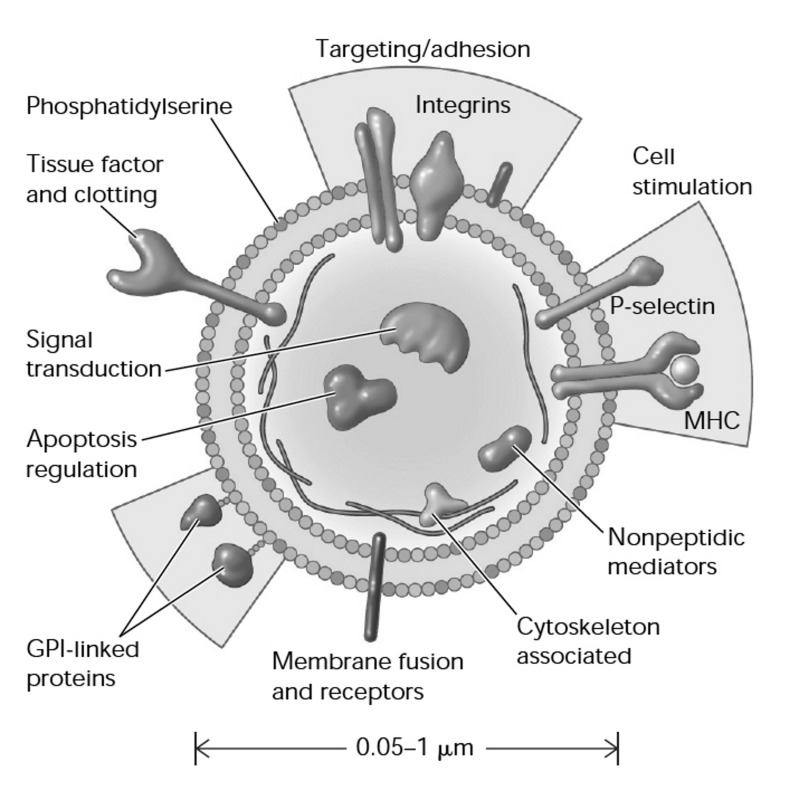
Figure 1.
Cellular microparticles: a mobile storage pool of bioactive effectors.
Membrane microparticles are shed from the plasma membrane of stimulated cells, harbouring cytoplasmic proteins as well as bioactive lipids implicated in a variety of fundamental processes. MHC: major histocompatibility complex; GPI: glycosylphosphatidylinositol. (Adapted with permission from Hugel et al., 2005113.)
Figure 2.
Size classes of extracellular vesicles.
Exosomes are formed through inward membrane budding, leading to formation of 40–100 nm intracellular vesicles which accumulate within multivesicular bodies that are subsequently released to the extracellular milieu. Apoptotic bodies may contain DNA and/or organelles and are formed during the late stages of apoptosis, after cell shrinkage. Microparticles are formed from the outward blebbing of membrane and released into the extracellular space. (Adapted with permission from Burger et al., 20133).
Microparticle-cell interaction
Microparticles serve as vehicles for inter-cellular exchange of biological material and information for which two principle mechanisms have been proposed: 1) MPs act similarly to circulating signaling modules, activating receptors on target cells by presenting organised clusters of membrane-associated bioactive molecules5; and/or 2) direct transfer of MP content, including proteins, bioactive lipids or RNA to recipient cell. These mechanisms result in phenotypic modification and reprogramming of cell functions6. As such, MP-based cell-cell communication enables a unique form of remote signalling from parental to target cells by presenting a complex arrangement of receptor ligands for membrane receptors that is paired with concentrated payloads of bioactive molecules and substrate for intracellular delivery7.
Microparticles as biomarkers of disease processes
Microparticle formation is enhanced under conditions of stress and injury and so MPs have been investigated as potential disease biomarkers. As such, it is important to recognise that circulating MP levels are determined by the balance between MP formation and clearance. MP levels, in particular platelet, leucocyte and endothelium-related MPs, are known to increase in states of vascular injury, pro-thrombotic and proinflammatory states. These include diabetes8, pulmonary hypertension9, chronic kidney disease10, preeclampsia11, atherosclerosis12, and heart failure13, amongst others.
Formation of microparticles
Microparticles arise from diverse cell types, including those related to vessels (endothelial cells, and vascular smooth muscle cells)14, blood (erythrocytes15, platelets and leucocytes), cardiomyocytes16 and podocytes3, as well as various cancers17 and progenitor cell populations18. MP formation occurs by outward blebbing and shedding of the plasma membrane19. This poorly understood process appears to involve two main steps: 1) initial cytoskeletal re-organisation that precedes outward membrane budding20 (actin filament rearrangements are essential to this process and are influenced by a number of intracellular enzymes including calpain21, rho kinase22 and transglutaminase23) (Figure 3); and 2) externalisation of phosphatidylserine (PS), a negatively charged aminophospholipid found almost exclusively on the plasma membrane inner leaflet in healthy cells24. PS “sidedness” is controlled by an ATP/calcium-dependent system involving 3 distinct enzymes: flippase, floppase and scramblase25. Of note, defective PS externalisation underlies Scott syndrome, a bleeding disorder associated with diminished platelet MP shedding26.
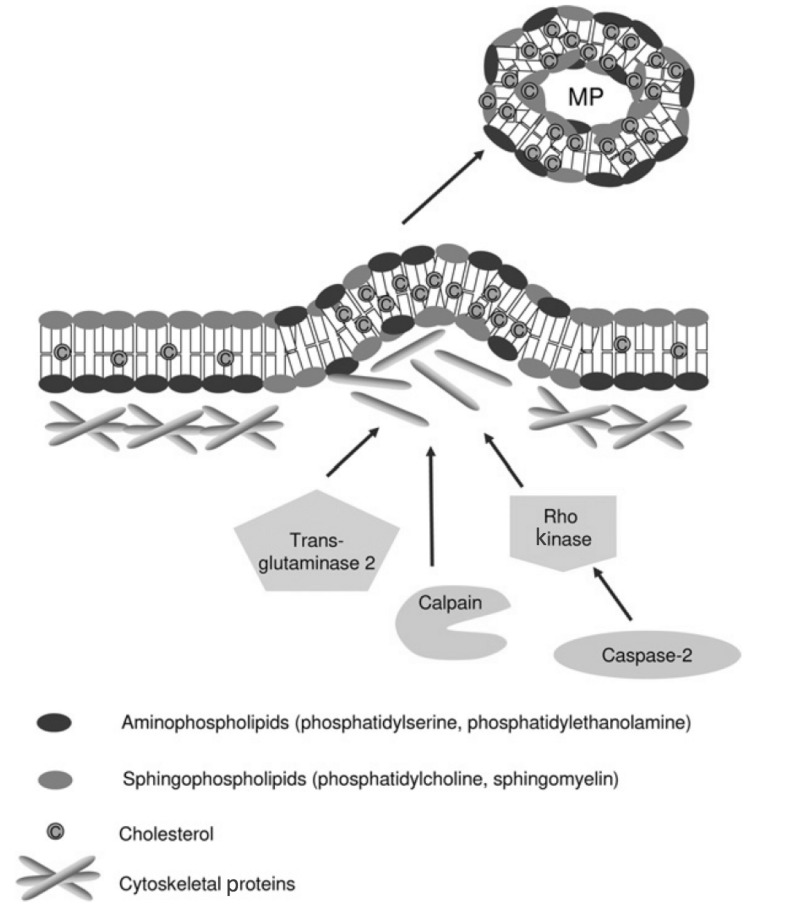
Figure 3.
Mechanisms proposed for cytoskeleton remodelling leading to microparticle (MP) formation.
Under normal conditions, aminophospholipids (phosphatidylserine and phosphatidylethanolamine) are found exclusively on the inner leaflet of the plasma membrane. During MP formation, membrane asymmetry is lost as aminophospholipids redistribute to the outer leaflet of the plasma membrane. Cytoskeletal re-organisation results in the outward blebbing of the plasma membrane and may be dependent upon actin polymerisation, caspase 2/Rho kinase, calpain and/or transglutaminase 2. Such processes may vary between different cell types. MP formation appears to occur selectively in lipid-rich microdomains (lipid rafts/caveolae) within the plasma membrane. (Adapted with permission from Burger et al., 20133). MP: microparticles.
Studies of cultured cells have identified several stimuli for MP formation, including: hormones, fatty acids, reactive oxygen species (e.g. hydrogen peroxide)27 and increased intracellular calcium levels28. Activation of several surface receptors has also been shown to drive MP production, such as by tumour necrosis factor (TNF)-α29 on monocytes, leucocytes and neutrophils, as well as by pro-inflammatory (lipopolysaccharide30, shiga toxin30, and cytokines31) and pro-coagulant ligands (thrombin32, collagen33, and norepinephrine34) on platelets and Toll-like receptor 4 on dendritic cells35 (Figure 4).
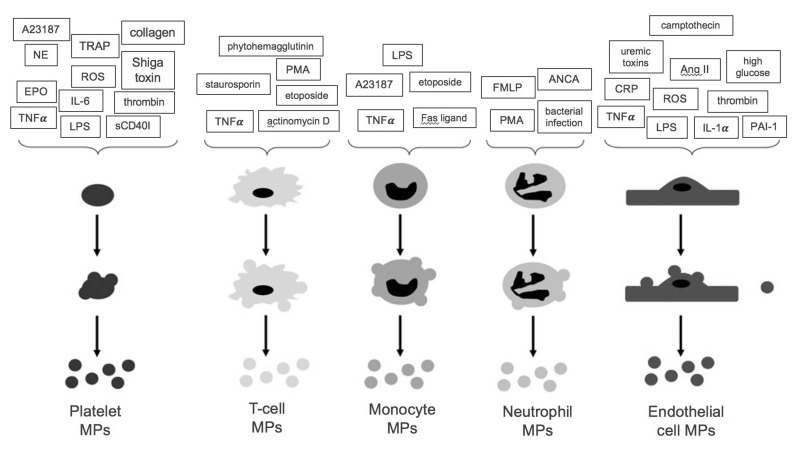
Figure 4.
Stimuli for microparticle (MP) formation from platelets, endothelial cells and leucocytes.
A summary of the stimuli which promote MP formation from platelets, endothelial cells and leucocytes. A23187: calcium ionophore A23187; TRAP: thrombin receptor activating peptide; NE: norepinephrine; ROS: reactive oxygen species; EPO: erythropoietin; IL-6: interleukin 6; TNFα: tumour necrosis factor α; LPS: lipopolysaccharide; sCD40I: soluble CD40 ligand; PMA: phorbol 12-myristate 13-acetate; FMLP: formyl-methionyl-leucyl-phenylalanine; ANCA: anti-neutrophil cytoplasmic antibodies; CRP: c-reactive protein; Ang II: angiotensin II; IL-1α: interleukin 1α; PAI-1: plasminogen activator inhibitor-1. (Adapted with permission from Burger et al., 20133).
Microparticle clearance
Microparticle elimination via the mononuclear phagocyte system (MPS) is believed to regulate circulating MP availability for target-cell fusion; however, less is known about this process than about MP formation3. Macrophages ingest co-cultured MPs and externalised PS is thought to signal scavenger receptors promoting MP endocytosis32. IgM expressed on the MP surface has also been shown to stimulate MP clearance by macrophages36.
Microparticle-mediated biological effects
Although cell activation enhances MP shedding, exocytosis is a constitutive process for the majority of cell types37. Depending on the stimulus, however, protein content of both “cytoplasm” and membrane for MPs derived from the same cell lineage can vary38. Moreover, the enzymes that govern plasma membrane remodelling during MP shedding can be selective, depending on the activating agonist and/or the microenvironments of parental cells39,40. Such tight regulation of MP production suggests that MPs may be important mediators of cell-cell communication. Of note, MPs are internalised by a variety of cells (macrophages and endothelial cells amongst others) in a dose-dependent manner, enabling “MP cargo” transfer between cells in a fashion that influences both functional and phenotypic characteristics of the target cells41.
The most well characterised processes influenced by MPs include the following items.
1) Coagulation
Coagulation is perhaps the most clearly established example of MP biology. Platelet-derived MPs have effects similar to activated platelets in initiation of thrombin generation and clot propagation, despite having at least 2-orders-of-magnitude difference in surface area42. Moreover, externalised phospholipids (mainly PS) create a negatively charged surface that anchors cationic domains of proteins involved in assembly of the multi-component complex involved in the thrombin burst43.
2) Oxidative stress
Microparticles of different derivation, produced under various stimuli, have been shown to affect the enzymatic systems controlling reactive oxygen species generation. Both endothelial and monocyte-derived MPs are known to increase superoxide44 and hydrogen peroxide production45, as well as to uncouple nitric oxide synthase (NOS)46. However, activated T-cell-related MPs have been shown to decrease reactive oxygen species production and to increase nitric oxide (NO) production47.
3) Inflammation
Pro-inflammatory stimuli provoke MP shedding; MPs may also directly contribute to the inflammatory response in an amplifying signalling loop (e.g. PMN-derived MPs promote endothelial IL-6 and monocyte chemotactic protein release)48. MPs are also thought to promote inter-cellular inflammatory cell interaction and adhesion; specifically, endothelial MPs increase expression of endothelial adhesion molecules and facilitate monocyte-endothelial cell interactions49, in addition to binding to monocytes and promoting transendothelial migration50.
4) Angiogenesis
Platelet-derived MPs have been implicated in regulation of angiogenesis. In rats, following myocardial ischemia, platelet MPs increase both post-ischaemic capillary density and proliferation51, and are reported to influence survival and tube formation of human umbilical vein endothelial cells52. This is not surprising, as platelets are known to contain at least 20 angiogenesis-regulating factors. Moreover, certain stimulated T-cell-related MPs have been shown to inhibit angiogenesis both in vivo and in vitro53.
5) Apoptosis
Endothelial and monocyte MPs appear to promote cellular senescence and apoptosis in circulating angiogenic and endothelial progenitor cells, respectively54,55. This process is thought to be dependent upon phagocytosis of MPs that contain high amounts of membrane arachidonic acid, leading to caspase activation and apoptosis initiation56.
Red blood cell microparticles
Microparticle formation from red blood cells (RBCs) occurs during maturation and is accelerated during RBC storage57. RBC MPs are generally smaller than MPs of other origin, are more homogenous in size (approximately 0.15 μm in diameter), and are often accompanied by smaller vesicles, termed nanovesicles58. Of note, during their 120-day lifespan, RBCs lose approximately 20% of their volume through vesicle emission, increasing intraerythrocytic haemoglobin (Hb) concentration by approximately 14%. Metrics for production rates, circulating number and volume are presented in Table I59. It was originally thought that vesiculation served to rid RBCs (which lack lysosomes) of damaged or harmful components that may accumulate over time, such as denatured Hb, C5b-9 complement attack complexes or Band 3 neoantigens60. It has also been suggested that RMP shedding promotes recognition and clearance of senescent and/or damaged RBCs by removing integral self-marker membrane proteins (e.g. CD47)61.
Table I.
Estimated total circulating number, volume and rate of production of RMP, intact RBCs and their respective ratios in a healthy adult male (Willekens et al., 200859).
| Total circulating number | Volume per RMP/RBC | Rate of production | |||||||
|---|---|---|---|---|---|---|---|---|---|
|
|
|||||||||
| RMP | RBC | RMP | RBC | RMP | RBC | ||||
| Absolute number | 8.5×108 | 2.5×1013 | 0.065 μm3 | 88 μm3 | 5.8×108/sec | 1.4×106/sec | |||
| RMP:RBC ratio | 3.4×10−5:1 | 7.38×10−4:1 | 8,120:1 | ||||||
RMP: red blood cell-derived microparticles; RBC: red blood cell.
RMP production
Red blood cells spontaneously shed PS-positive MPs62; each RBC is estimated to generate approximately 230 vesicles during its lifespan63. Similar to other cell types, membrane phospholipid rearrangement is thought to be an integral step in RMP formation. The normal asymmetric distribution of the lipid bilayer is controlled by 3 different elements; flippase (ATP-dependent enzyme that promotes inward orientation of negatively charged lipids), floppase (responsible for maintaining outward orientation of phosphatidylcholine), and scramblase (facilitating bidirectional movement of all phospholipids)64. During RBC storage, ATP depletion and potassium leakage reduce flippase activity, while elevated intracellular calcium increases scramblase activity; consequently membrane asymmetry is lost, PS is exposed on the RBC surface, and vesicle shedding is promoted62. There is some controversy with regard to the RBC sub-population most responsible for RMP production. Some suggest that senescent RBCs are responsible for the majority of RMP production in vivo65, while others have shown that during storage younger RBC populations produce the majority of RMPs57. Of note, RMP composition/content may vary with specific storage conditions as well as with storage duration66. For example, RMPs generated in vitro by stimulation with Ca ionophores differ in size and cytoskeletal protein structure than RMPs produced during RBC storage58,67. RMPs isolated during storage seem to have less variation in size and shape than those isolated from circulation57. Moreover, hypotonic, alkaline storage solutions are associated with increased RMP production and with an RMP population that has significantly less cholesterol, phospholipids, as well as band 3 and protein 4.157. Leucoreduction appears to diminish RMP production by up to 40–50%, particularly under anaerobic conditions68. More generally, RBC storage is characterised by progressive depletion of energy resources and antioxidant defences, enabling accumulation of oxidative lesions, particularly involving the cytoskeleton and Band 369. Vesiculation may allow RBCs to eliminate such markers, as well as other harmful components that accumulate during storage70.
Triggers for RMP production
Little is known about the specific signalling pathways that regulate RBC vesiculation, both during RBC aging in vivo and during ex vivo storage. Furthermore, it is also important to recognise that unique changes may occur to stored RBCs in vivo following transfusion. Several triggers for RBC MP production have been identified, mostly pertaining to the changes RBCs undergo during storage. These include the following:
Increased cytosolic calcium (Ca2+) is perhaps the most well characterised trigger for activation of Ca2+-dependent proteases, leading to cytoskeletal damage and activation of Ca2+-dependent scramblase; both processes are integral to PS exposure and to MP shedding24.
ATP depletion impairs performance of the major ATP-dependent transporter proteins (flippase and floppase) responsible for maintaining cell membrane asymmetry; loss of asymmetry promotes MP budding and shedding62.
Increased potassium (K+) leakage has also been linked to disturbed erythrocytic membrane transporter activity, disturbing maintenance of membrane structure and is thought to lead to MP formation62.
Other cascades arising from energy failure in RBCs have been shown to increase RBC vesiculation and MP formation. These include G protein-coupled receptor signalling, the phosphoinositide 3-kinase (PI3K-Akt protein kinase B) pathway, the Jak-STAT (Janus kinase-signal transducer and activator of transcription) pathway, and the Raf-MEK (mitogen-activated protein kinase)-ERK (extracellular signal-regulated kinase) pathway25.
RMPs differ from intact RBCs
Proteomic analysis has demonstrated that RMPs contain many RBC proteins including carbonic anhydrase, peroxiredoxins and 14-3-3 proteins (regulators of a number of processes, such as modulation of protein kinase activity and signal transduction)71; however, RMPs are structurally and functionally different from intact RBCs (e.g. RMPs are not “just small RBCs”). As discussed earlier, the process of RMP shedding involves loss of normal RBC membrane asymmetry, and as a consequence there is a greater density of negatively charged phospholipids (e.g. PS) on the outer RMP membrane. RMP membranes are also enriched with specific proteins (Band 3 and Band 3 dimers) in comparison to parent RBCs60. Moreover, in comparison to intact RBCs, storage-related RMPs appear enriched with stomatin, relatively depleted in actin, and to have more stable glycophorin A71. Disruption of normal RBC cytoskeletal protein structure72 is also integral to RMP shedding73 and distinguishes daughter from parent cells. These structural characteristics, in addition to the discrepancy in size, result in differential “streaming characteristics” between RBCs and RMPs, with preferential RMP circulation in proximity to endothelial cells in the “cell-free” zone of the micro-circulation74. (It is to be noted that this feature has significant physiological implications; see below). Finally, RMPs encapsulate a significant amount of Hb75, which confers physiological characteristics closer to cell-free Hb than to intact RBCs, particularly with regard to interactions with nitric oxide.
Proposed RMP biological effects
There is increasing recognition of the biological effects of RMP, particularly in the context of transfusion. Proposed effects include the following items.
1) Promotion of coagulation by RMPs
It has been well established that negatively charged surfaces activate zymogen components of the coagulation cascade and it appears that RMPs act as pro-coagulant factors in this fashion. RMPs increase thrombin generation in the presence of low exogenous tissue factor and, in fact, are capable of initiating and propagating thrombin generation even in the absence of tissue factor76. There is some evidence that this pro-coagulant activity is dependent on Factor XII77. Some authors have suggested that tissue factor may be expressed on the RMP surface, contributing to their pro-coagulant effect78. Of note, RMP abundance is known to increase in certain pathological states, including sickle cell crises, which are associated with a hypercoagulable state79. Interestingly, RMPs also bind protein S, a co-factor for activated protein C, enhancing the degradation of Factors VIIIa and Va, inhibiting tenase and prothrombinase, and thus increasing clot breakdown80. As such, depending on context, balance amongst this and the other above mentioned effects may determine the RMP coagulation phenotype80.
2) Nitric oxide (NO) scavenging by RMPs
Nitric oxide (NO) is a vasodilator subserving physiological reflexes that maintain dynamic matching between regional blood flow and tissue respiration81. Of note, Hb reacts with NO in a diffusion-limited oxidation reaction that quenches NO bioactivity, disrupting vasoregulation and oxygen delivery homeostasis. Under normal conditions, this effect is limited by Hb compartmentalisation within RBCs82. Moreover, constraints upon Hb:NO interaction are substantially influenced by RBC size and membrane architecture83; however, reaction between de-compartmentalised, cell-free Hb and NO lacks such constraints84. Notably RMP:NO interactions more closely mirror those of cell-free Hb than that of intact RBCs, and as such, RMPs act as potent NO scavengers (RMP reaction with NO is approximately 1,000-fold faster than with RBC-encapsulated Hb85 and is only 2.5-3-fold slower than with cell-free Hb85). The potential impact of NO quenching by RMPs (following transfusion) dramatically increases with the increase in RMP abundance observed during storage. The degree by which RMPs influence NO bioavailability in vivo is dependent on several factors, most importantly, the degree to which RMPs enter the cell-free zone in the microcirculation (e.g. stream in proximity to endothelium)74.
3) Immune modulation by RMPs
Transfusion related immune modulation (TRIM) is a recognised, but poorly characterised, complication of transfusion. Given the known RMP increase with storage duration, a role for RMPs in TRIM pathobiology has been postulated86. RMPs influence the impact of antigen presenting cells (APC) and boost mitogen driven T-cell responses87. Specifically, RMPs amplify APC-based induction of pro-inflammatory cytokines and chemokines from peripheral blood mononuclear cells (PBMC) and promote PBMC survival87. Alternatively, RMPs may exert an immunosuppressive effect, by decreasing the release of various cytokines such as TNF-α, IL-8, or IL-1088. Of note, production of sickle cell-derived RMP (SS RMPs) is enhanced by inflammatory conditions. When engulfed by myeloid cells, SS RMPs promote pro-inflammatory cytokine secretion and endothelial cell adhesion, suggesting crosstalk between circulating inflammatory cells, and SS RMPs contributes to sickle cell disease pathogenesis89. In addition, RMPs have also been demonstrated to bear blood group antigens90; transfusion-related RMPs may, therefore, represent a significant immunogenic load59 and contribute to the severity of alloimmunisation in chronically transfused patients90.
4) Promotion of endothelial adhesion by RMPs
As noted above, RMPs appear to play a significant role in the pathophysiology of sickle cell disease (SCD), during which RMP production is enhanced and promotes pro-inflammatory cytokine secretion79. SS RMPs have also been shown to enhance adhesion of intact RBCs to endothelial cells89. Of note, up to one-third of cell-free haeme in SCD may be carried by circulating RMPs91. Moreover, externalised membrane PS on SS RMPs retains haeme on the external RMP surface. Such haeme-laden SS RMPs transfer haeme directly to vascular endothelium, generating oxidative stress and endothelial apoptosis91. Such linkage of haemolysis to endothelial injury has been proposed as a trigger for SCD vaso-occlusive crises.
RMP clearance
Similar to other MP populations, RMPs are also likely to be cleared via the MPS, in particular by hepatic Kupffer cells92. Such removal appears to occur very rapidly and is thought to be mediated by PS-binding scavenger receptors and senescent cell antigen-specific autoantibodies92. Immunological analysis of RMPs elaborated by senescent RBCS demonstrates Band 3 clustering; this RMP subpopulation may, therefore, be cleared in a similar fashion to that for intact senescent RBCs59.
RMPs and RBC storage
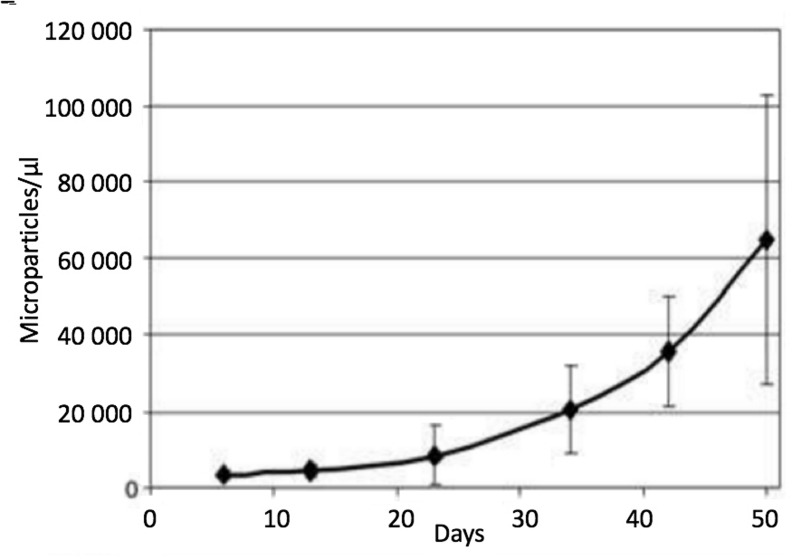
The RBC storage lesion is compromised by altered metabolism and biomechanics. In particular, energy failure (with impaired ATP and reduced equivalent production) is a common characteristic and is associated with a significant acidosis, decrease in 2,3-diphosphoglycerate levels, and failure of the membrane Na+/K+ ATPase pump with continuous potassium leakage93,94 (e.g. conditions known to promote RMP genesis95,96). Morphologically, stored RBCs slowly morph from smooth biconcave discs, to spiculated echinocytes, to dense spheroechinocytes. These changes are related to membrane loss consequent to RMP formation, as well as loss of cell volume regulation and diminished deformability57, thus greatly impacting post-transfusion survival and rheology97. In fact, RMP genesis appears to be directly proportional to storage duration, with a 20-fold increase after 50 days71 (Figure 5). The doubling time for RMP concentration during storage is estimated to be nine days (95% CI: 7.7–10.7 days)85. The specific reasons for such significant RMP production remain unknown although it has been suggested that storage activates a physiological process that serves (in vivo) as a means to prevent premature RBC clearance by shedding membrane proteins that would otherwise signal RBC senecence59,98.
Figure 5.
Microparticle (MP) count in red blood cell units during storage (without centrifugation).
Data are expressed as the mean±SD (n=7). At day 5: 3,371±1,188 MPs/μL were counted, whereas at day 50: MPs had approximately 20-fold (64,858±37,846 MPs/μL). MPs were stained with anti-human CD47. (Adapted with permission from Rubin et al.,71).
Physiological impact of storage-generated RMPs during RBC transfusion
Oxygen delivery homeostasis and vasoregulation
Tissue oxygen delivery is a function of blood O2 content and regional blood flow, with the latter being the principle determinant. The volume and distribution of regional blood flow is actively regulated to maintain dynamic coupling between O2 delivery and tissue respiration99. It is now commonly appreciated that RBCs act as both sensors and transducers in this physiology by regulating bioavailability of vasoactive effectors in plasma (and thereby, regulating resistance vessel caliber)81. This key physiological reflex is termed hypoxic vasodilation (HVD) and is primarily mediated by RBC-transported NO100–102. As such, by serving as HVD effector elements, RBCs function has a key role in maintaining O2 delivery homeostasis (Figure 6). This paracrine RBC function is governed by O2-linked transitions in Hb conformation which, because of differing reactions of deoxy- and oxy-Hb with NO, transduce regional pO2 gradients into NO bioactivity, thereby effecting vasodilation to resolve perfusion insufficiency (e.g. HVD)82. This physiology is disrupted when RBCs release Hb into plasma. Specifically, although Hb packaging in RBCs blunts NO consumption approximately 1,000-fold, once released, free Hb (and Hb containing RMP) readily inactivates NO, preventing facile NO-based traffic between RBCs and endothelium74,103.
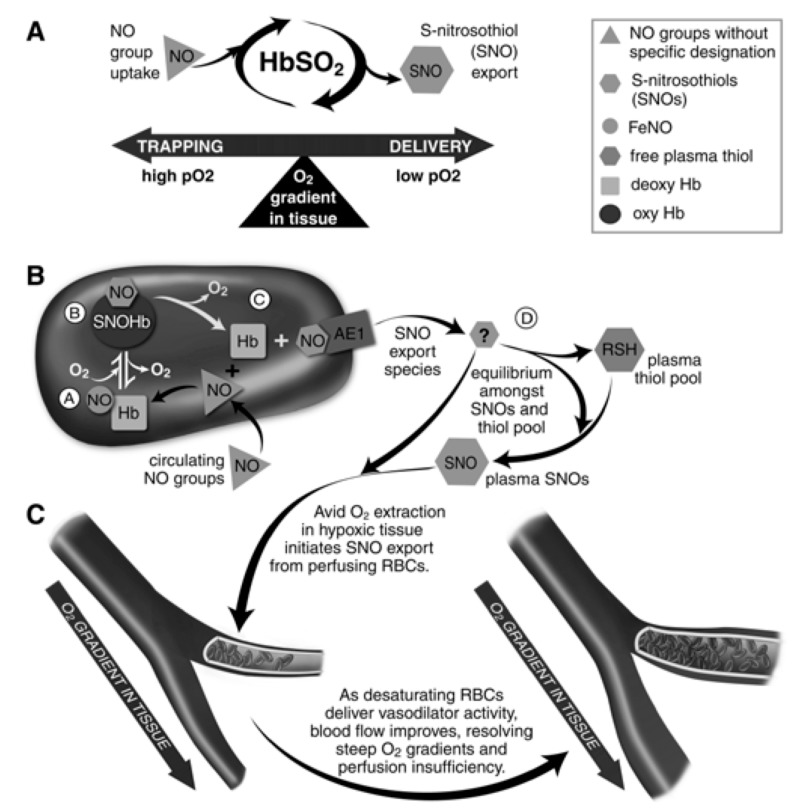
Figure 6.
Red blood cells (RBC) transduce regional O2 gradients in tissue to control nitric oxide (NO) bioactivity in plasma by trapping or delivering NO groups as a function of haemoglobin (Hb) O2 saturation (Hb SO2).
(A) Circulating NO groups are processed by Hb into the highly vasoactive (thiol-based) NO congener, S-nitrosothiol (SNO). By exporting SNOs as a function of Hb deoxygenation, RBCs precisely dispense vasodilator bioactivity in direct proportion to the lack of regional blood flow. (B) O2 delivery homeostasis requires biochemical coupling of vessel tone to environmental cues that match perfusion sufficiency to metabolic demand. Because oxygenated Hb (oxy Hb) and deoxygenated Hb (deoxy Hb) process NO differently, allosteric transitions in Hb conformation afford context-responsive (O2-coupled) control of NO bioavailability, thereby linking the sensor and effector arms of this system. Specifically, Hb conformation governs the equilibria among (A) deoxygenated Hb FeNO (NO sink), (B) oxygenated SNO-Hb (NO store), and (C) acceptor thiols including the membrane protein SNO-AE-1 (bioactive NO source). Direct SNO export from RBCs or S-transnitrosylation from RBCs to plasma thiols (D) yields vasoactive SNOs, which influence resistance vessel caliber and close this signalling loop. Thus, RBCs either trap (A) or export (D) NO groups to optimise blood flow. (C) NO processing in RBCs (A and B) couples vessel tone to tissue PO2; this system subserves hypoxic vasodilation in the arterial periphery and thereby calibrates blood flow to regional tissue hypoxia. (Adapted with permission from Doctor and Stamler, 201181. ©American Physiological Society). RSH: peptide or protein containing a thiol group.
Transfusion and vasoregulation
There is substantial evidence to suggest that RBC transfusion impairs HVD, although the mechanism has not been fully elucidated93,104,105. In addition to direct effects of released free Hb upon NO bioavailability in plasma, haemolysis may also impair endothelial NO production, possibly via release of arginase (which, through substrate depletion, constrains eNOS activity)85,106. Moreover, intact stored RBCs, with increasing storage duration, are 2–4 fold more avid NO scavengers than fresh RBCs107 and exhibit more pronounced inhibition of NO-mediated vasodilation. Furthermore, there is growing evidence to suggest stored RBC-derived free-Hb and Hb-rich RMPs dampens normal NO bioactivity in the microcirculation, leading to physiologically significant HVD impairment85,108–110. This latter observation is supported by in vivo data demonstrating RMP contributes to the initiation of vaso-occlusive crises in sickle cell disease91.
RMP impact upon vasoregulation
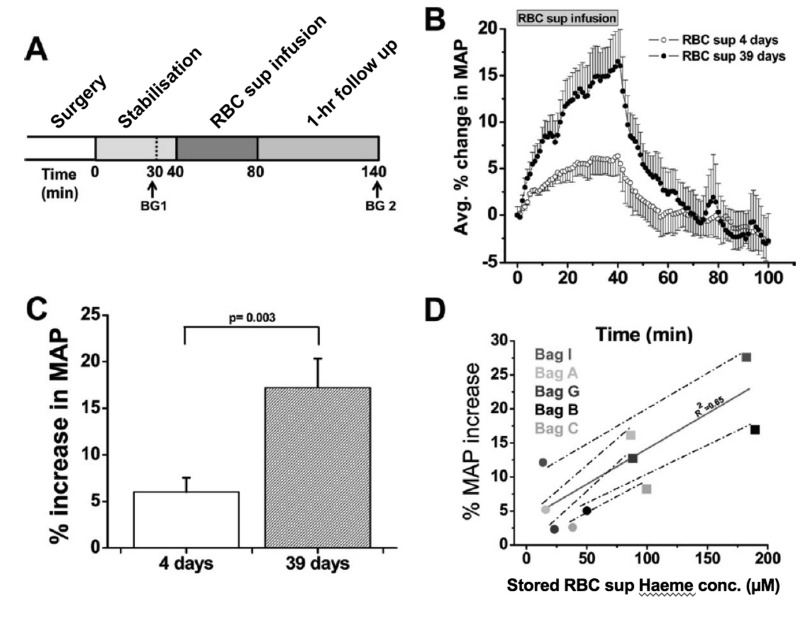
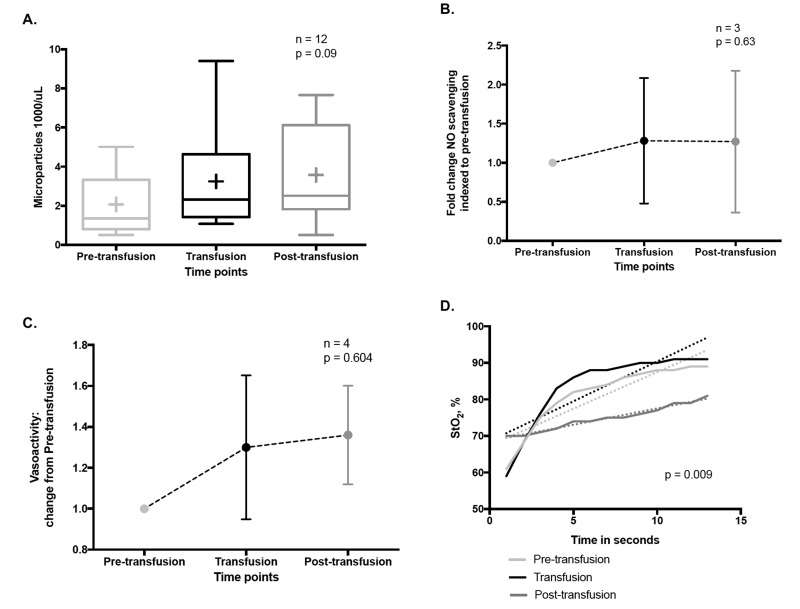
As discussed above, HVD is the principle physiological reflex that maintains dynamic coupling between regional oxygen delivery and tissue respiration, particularly during physiological stress. Evidence suggests that RMPs impair RBC-based HVD support of oxygen delivery homeostasis, by: 1) preferentially streaming in the cell-free zone of the microcirculation; and 2) acting as an NO sink. Specifically, animal studies demonstrate a significantly greater increase in mean arterial pressure (MAP) upon infusion of cell-free supernatants of longer stored RBC units (39 days) vs fresher units (4 days)85 (Figure 7). This increase correlates with the amount of extra-erythrocytic Hb (both free and in RMPs) in supernatants. Moreover, RMP half-life in this model was approximately 15–20 minutes, consistent with the time course for changes in blood pressure that occur with infusion of stored RBC supernatants85. This is particularly important since, unlike cell-free Hb, RMPs are not bound by haptoglobin and may, therefore, contribute significantly to this phenomenon85. Early results from the study of RMPs upon vasoregulation in critically-ill humans suggest an increase in circulating RMPs concurrent with transfusion, but not in MPs of other origin (Figure 8A). Moreover, these RMPs appear to increase NO scavenging (Figure 8B) and alter plasma vasoactivity (Figure 8C). In addition, in these subjects, a transfusion-associated increase in RMPs was associated with a significant increase in systemic vascular resistance and a corresponding decrease in cardiac output. Concurrently, tissue re-oxygenation following experimental vaso-occlusion (e.g. dynamic near infrared spectroscopy, a measure of HVD capacity111) was significantly dampened post transfusion as compared to pre- and intra-transfusion phases (Figure 8D)112.
Figure 7.
Vasoactivity of infused packed red cell supernatant/plasma.
(A) Experimental time line for packed red cell supernatant infusions. Rats were stabilised for 30 minutes (min) after surgery, and blood gasses were drawn as indicated (BG 1 and BG 2). Supernatant (1.6 mL) of packed red blood cells stored for either 4 or 39 days was infused for 40 min, after which the rats were followed for 1 hour (n=5). (B) Change in mean arterial pressure (MAP) over time after packed red blood cell (RBC) supernatant infusion and 60-min follow up. (C) Average percentage peak increase in MAP after infusion of packed RBC supernatants (RBC sup) (p=0.003). (D) Correlation (solid line) between packed red blood cell supernatant haeme concentration and percentage increase in MAP after 40-min infusion of packed red blood cell supernatant stored for either four days (black solid circle) or 39 days (black solid square; r2=0.65). Each data point was obtained from a separate rat infusion experiment, in a different rat (2 groups of n=5). All values are displayed as mean standard error of mean (SEM). Student t-test was used to compare the 2 groups of rats. Avg.: average; conc.: concentration. (Adapted with permission from Donadee et al., 201185).
Figure 8.
Pilot data of the vasoregulatory effects of transfusion related RMPs in humans.
(A) Circulating RMP abundance during RBC transfusion. Box and whisker plot of RMP concentration in plasma (flow cytometry, CD235a antibody) demonstrates an increasing trend during RBC transfusion (RM ANOVA). (B) NO scavenging relation to RBC transfusions. Using a validated NO consumption assay (Wang et al., 2004), we observe a ~1.3 fold increase in plasma NO scavenging following RBC transfusions (p=0.63, RM ANOVA). (C. Plasma vasoactivity is altered by RBC transfusions. Using a validated in vitro rabbit aortic ring array to assess vasoactivity, there was a 1.3 0.35 and 1.36 0.24 fold increase in plasma vasoconstrictive capacity during and after RBC transfusions compared to the pre-transfusion state, respectively. D. Change in hypoxic vasodilatory capacity in relation to RBC transfusion. A validated Dynamic Near Infrared Spectroscopy (NIRS) vascular occlusion test before, during and after RBC transfusion. Linear regression analysis showed a significantly slower rate of tissue resaturation post-transfusion as compared to the pre-and intra-transfusion states (p=0.0092). The rate of tissue resaturation is thought to correlate with capacity for capillary bed recruitment and hypoxic vasodilation (Said et al., 2016112).
Conclusions
Microparticles are submicron particles of various cell origins, the composition of which is dependent on parent cell identity, that form in response to a multitude of stimuli through cell membrane re-organisation, blebbing and shedding. MPs are thought to have several biological effects and may serve as vehicles for inter-cellular communication. RMPs form spontaneously during the RBC lifespan, with content and a cytoskeletal structure distinct from intact RBCs. RMP production accelerates during RBC storage due to associated biochemical changes: increased cytosolic calcium, ATP depletion, and increased potassium leakage. Moreover, RMP composition is affected by the trigger for their formation and by different storage conditions.
Proposed RMP biological effects include promotion of coagulation, immune modulation, and enhanced endothelial adhesion. Of particular importance, RMPs demonstrate significant NO trapping/consumption, disrupting regional matching between blood flow and tissue respiration that is essential to oxygen delivery homeostasis physiology. These effects have been demonstrated in animal models evaluating storage-related RMPs, which appear to provoke an increase in systemic vascular tone and blood pressure following infusion of cell-free RBC unit supernatants. This effect progresses with storage duration. Pilot data in transfused humans demonstrate similar findings, with an increase in circulating RMPs, in plasma NO scavenging, in systemic vascular resistance, and a reduction in tissue capacity for re-oxygenation following experimental vaso-occlusion.
Footnotes
The Authors declare no conflicts of interest.
Funding and support
Support for this manuscript was provided by the NIH grant R01GM113838.
References
- 1.Boulanger CM, Dignat-George F. Microparticles: an introduction. Arterioscler Thromb Vasc Biol. 2011;31:2–3. doi: 10.1161/ATVBAHA.110.220095. [DOI] [PubMed] [Google Scholar]
- 2.Morel O, Jesel L, Freyssinet JM, Toti F. Cellular mechanisms underlying the formation of circulating microparticles. Arterioscler Thromb Vasc Biol. 2011;31:15–26. doi: 10.1161/ATVBAHA.109.200956. [DOI] [PubMed] [Google Scholar]
- 3.Burger D, Schock S, Thompson CS, et al. Microparticles: biomarkers and beyond. Clin Sci. 2013;124:423–41. doi: 10.1042/CS20120309. [DOI] [PubMed] [Google Scholar]
- 4.Elmore S. Apoptosis: a review of programmed cell death. Toxicol Pathol. 2007;35:495–516. doi: 10.1080/01926230701320337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Del Conde I, Shrimpton CN, Thiagarajan P, Lopez JA. Tissue-factor-bearing microvesicles arise from lipid rafts and fuse with activated platelets to initiate coagulation. Blood. 2005;106:1604–11. doi: 10.1182/blood-2004-03-1095. [DOI] [PubMed] [Google Scholar]
- 6.Whale TA, Wilson HL, Tikoo SK, et al. Pivotal Advance: passively acquired membrane proteins alter the functional capacity of bovine polymorphonuclear cells. J Leukoc Biol. 2006;80:481–91. doi: 10.1189/jlb.0206078. [DOI] [PubMed] [Google Scholar]
- 7.Mause SF, Weber C. Microparticles: protagonists of a novel communication network for intercellular information exchange. Circ Res. 2010;107:1047–57. doi: 10.1161/CIRCRESAHA.110.226456. [DOI] [PubMed] [Google Scholar]
- 8.Feng B, Chen Y, Luo Y, et al. Circulating level of microparticles and their correlation with arterial elasticity and endothelium-dependent dilation in patients with type 2 diabetes mellitus. Atherosclerosis. 2010;208:264–9. doi: 10.1016/j.atherosclerosis.2009.06.037. [DOI] [PubMed] [Google Scholar]
- 9.Forest A, Pautas E, Ray P, et al. Circulating microparticles and procoagulant activity in elderly patients. J Gerontol A Biol Sci Med Sci. 2010;65:414–20. doi: 10.1093/gerona/glp187. [DOI] [PubMed] [Google Scholar]
- 10.Faure V, Dou L, Sabatier F, et al. Elevation of circulating endothelial microparticles in patients with chronic renal failure. J Thromb Haemost. 2006;4:566–73. doi: 10.1111/j.1538-7836.2005.01780.x. [DOI] [PubMed] [Google Scholar]
- 11.Gonzalez-Quintero VH, Jimenez JJ, Jy W, et al. Elevated plasma endothelial microparticles in preeclampsia. Am J Obstet Gynecol. 2003;189:589–93. doi: 10.1067/s0002-9378(03)00469-1. [DOI] [PubMed] [Google Scholar]
- 12.Bernal-Mizrachi L, Jy W, Jimenez JJ, et al. High levels of circulating endothelial microparticles in patients with acute coronary syndromes. Am Heart J. 2003;145:962–70. doi: 10.1016/S0002-8703(03)00103-0. [DOI] [PubMed] [Google Scholar]
- 13.Amabile N, Guerin AP, Tedgui A, et al. Predictive value of circulating endothelial microparticles for cardiovascular mortality in end-stage renal failure: a pilot study. Nephrol Dial Transplant. 2012;27:1873–80. doi: 10.1093/ndt/gfr573. [DOI] [PubMed] [Google Scholar]
- 14.Rautou PE, Vion AC, Amabile N, et al. Microparticles, vascular function, and atherothrombosis. Circ Res. 2011;109:593–606. doi: 10.1161/CIRCRESAHA.110.233163. [DOI] [PubMed] [Google Scholar]
- 15.Tissot JD, Rubin O, Canellini G. Analysis and clinical relevance of microparticles from red blood cells. Curr Opin Hematol. 2010;17:571–7. doi: 10.1097/moh.0b013e32833ec217. [DOI] [PubMed] [Google Scholar]
- 16.Antoniak S, Boltzen U, Eisenreich A, et al. Regulation of cardiomyocyte full-length tissue factor expression and microparticle release under inflammatory conditions in vitro. J Thromb Haemost. 2009;7:871–8. doi: 10.1111/j.1538-7836.2009.03323.x. [DOI] [PubMed] [Google Scholar]
- 17.Zahra S, Anderson JA, Stirling D, Ludlam CA. Microparticles, malignancy and thrombosis. Br J Haematol. 2011;152:688–700. doi: 10.1111/j.1365-2141.2010.08452.x. [DOI] [PubMed] [Google Scholar]
- 18.Chen TS, Lai RC, Lee MM, et al. Mesenchymal stem cell secretes microparticles enriched in pre-microRNAs. Nucleic Acids Res. 2010;38:215–24. doi: 10.1093/nar/gkp857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Dignat-George F, Boulanger CM. The many faces of endothelial microparticles. Arterioscler Thromb Vasc Biol. 2011;31:27–33. doi: 10.1161/ATVBAHA.110.218123. [DOI] [PubMed] [Google Scholar]
- 20.Cauwenberghs S, Feijge MA, Harper AG, et al. Shedding of procoagulant microparticles from unstimulated platelets by integrin-mediated destabilization of actin cytoskeleton. FEBS Lett. 2006;580:5313–20. doi: 10.1016/j.febslet.2006.08.082. [DOI] [PubMed] [Google Scholar]
- 21.Nolan S, Dixon R, Norman K, et al. Nitric oxide regulates neutrophil migration through microparticle formation. Am J Pathol. 2008;172:265–73. doi: 10.2353/ajpath.2008.070069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Sapet C, Simoncini S, Loriod B, et al. Thrombin-induced endothelial microparticle generation: identification of a novel pathway involving ROCK-II activation by caspase-2. Blood. 2006;108:1868–76. doi: 10.1182/blood-2006-04-014175. [DOI] [PubMed] [Google Scholar]
- 23.van den Akker J, van Weert A, Afink G, et al. Transglutaminase 2 is secreted from smooth muscle cells by transamidation-dependent microparticle formation. Amino Acids. 2012;42:961–73. doi: 10.1007/s00726-011-1010-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Bevers EM, Comfurius P, Dekkers DW, Zwaal RF. Lipid translocation across the plasma membrane of mammalian cells. Biochim Biophys Acta. 1999;1439:317–30. doi: 10.1016/s1388-1981(99)00110-9. [DOI] [PubMed] [Google Scholar]
- 25.Kostova EB, Beuger BM, Klei TR, et al. Identification of signalling cascades involved in red blood cell shrinkage and vesiculation. Biosci Rep. 2015;35:1–16. doi: 10.1042/BSR20150019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Leroyer AS, Ebrahimian TG, Cochain C, et al. Microparticles from ischemic muscle promotes postnatal vasculogenesis. Circulation. 2009;119:2808–17. doi: 10.1161/CIRCULATIONAHA.108.816710. [DOI] [PubMed] [Google Scholar]
- 27.Aoki N, Jin-no S, Nakagawa Y, et al. Identification and characterization of microvesicles secreted by 3T3-L1 adipocytes: redox- and hormone-dependent induction of milk fat globule-epidermal growth factor 8-associated microvesicles. Endocrinology. 2007;148:3850–62. doi: 10.1210/en.2006-1479. [DOI] [PubMed] [Google Scholar]
- 28.Fox JE, Austin CD, Reynolds CC, Steffen PK. Evidence that agonist-induced activation of calpain causes the shedding of procoagulant-containing microvesicles from the membrane of aggregating platelets. J Biol Chem. 1991;266:13289–95. [PubMed] [Google Scholar]
- 29.Eyre J, Burton JO, Saleem MA, et al. Monocyte- and endothelial-derived microparticles induce an inflammatory phenotype in human podocytes. Nephron Exp Nephrol. 2011;119:e58–66. doi: 10.1159/000329575. [DOI] [PubMed] [Google Scholar]
- 30.Stahl AL, Sartz L, Karpman D. Complement activation on platelet-leukocyte complexes and microparticles in enterohemorrhagic Escherichia coli-induced hemolytic uremic syndrome. Blood. 2011;117:5503–13. doi: 10.1182/blood-2010-09-309161. [DOI] [PubMed] [Google Scholar]
- 31.Nomura S, Nakamura T, Cone J, et al. Cytometric analysis of high shear-induced platelet microparticles and effect of cytokines on microparticle generation. Cytometry. 2000;40:173–81. [PubMed] [Google Scholar]
- 32.Terrisse AD, Puech N, Allart S, et al. Internalization of microparticles by endothelial cells promotes platelet/endothelial cell interaction under flow. J Thromb Haemost. 2010;8:2810–9. doi: 10.1111/j.1538-7836.2010.04088.x. [DOI] [PubMed] [Google Scholar]
- 33.Takano K, Asazuma N, Satoh K, et al. Collagen-induced generation of platelet-derived microparticles in whole blood is dependent on ADP released from red blood cells and calcium ions. Platelets. 2004;15:223–9. doi: 10.1080/09537100410001682797. [DOI] [PubMed] [Google Scholar]
- 34.Tschuor C, Asmis LM, Lenzlinger PM, et al. In vitro norepinephrine significantly activates isolated platelets from healthy volunteers and critically ill patients following severe traumatic brain injury. Crit Care. 2008;12:R80. doi: 10.1186/cc6931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Thery C, Ostrowski M, Segura E. Membrane vesicles as conveyors of immune responses. Nat Rev Immunol. 2009;9:581–93. doi: 10.1038/nri2567. [DOI] [PubMed] [Google Scholar]
- 36.Litvack ML, Post M, Palaniyar N. IgM promotes the clearance of small particles and apoptotic microparticles by macrophages. PLoS One. 2011;6:e17223. doi: 10.1371/journal.pone.0017223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Angelillo-Scherrer A. Leukocyte-derived microparticles in vascular homeostasis. Circ Res. 2012;110:356–69. doi: 10.1161/CIRCRESAHA.110.233403. [DOI] [PubMed] [Google Scholar]
- 38.Jimenez JJ, Jy W, Mauro LM, et al. Endothelial cells release phenotypically and quantitatively distinct microparticles in activation and apoptosis. Thromb Res. 2003;109:175–80. doi: 10.1016/s0049-3848(03)00064-1. [DOI] [PubMed] [Google Scholar]
- 39.Peterson DB, Sander T, Kaul S, et al. Comparative proteomic analysis of PAI-1 and TNF-alpha-derived endothelial microparticles. Proteomics. 2008;8:2430–46. doi: 10.1002/pmic.200701029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Bernimoulin M, Waters EK, Foy M, et al. Differential stimulation of monocytic cells results in distinct populations of microparticles. J Thromb Haemost. 2009;7:1019–28. doi: 10.1111/j.1538-7836.2009.03434.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Diehl P, Fricke A, Sander L, et al. Microparticles: major transport vehicles for distinct microRNAs in circulation. Cardiovasc Res. 2012;93:633–44. doi: 10.1093/cvr/cvs007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Sinauridze EI, Kireev DA, Popenko NY, et al. Platelet microparticle membranes have 50- to 100-fold higher specific procoagulant activity than activated platelets. Thromb Haemost. 2007;97:425–34. [PubMed] [Google Scholar]
- 43.Owens AP, Mackman N. Microparticles in hemostasis and thrombosis. Circ Res. 2011;108:1284–97. doi: 10.1161/CIRCRESAHA.110.233056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Mastronardi ML, Mostefai HA, Soleti R, et al. Microparticles from apoptotic monocytes enhance nitrosative stress in human endothelial cells. Fundam Clin Pharmacol. 2011;25:653–60. doi: 10.1111/j.1472-8206.2010.00898.x. [DOI] [PubMed] [Google Scholar]
- 45.Burger D, Kwart DG, Montezano AC, et al. Microparticles induce cell cycle arrest through redox-sensitive processes in endothelial cells: implications in vascular senescence. J Am Heart Assoc. 2012;1:e001842. doi: 10.1161/JAHA.112.001842. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Essayagh S, Xuereb JM, Terrisse AD, et al. Microparticles from apoptotic monocytes induce transient platelet recruitment and tissue factor expression by cultured human vascular endothelial cells via a redox-sensitive mechanism. Thromb Haemost. 2007;98:831–7. [PubMed] [Google Scholar]
- 47.Agouni A, Mostefai HA, Porro C, et al. Sonic hedgehog carried by microparticles corrects endothelial injury through nitric oxide release. FASEB J. 2007;21:2735–41. doi: 10.1096/fj.07-8079com. [DOI] [PubMed] [Google Scholar]
- 48.Mesri M, Altieri DC. Leukocyte microparticles stimulate endothelial cell cytokine release and tissue factor induction in a JNK1 signaling pathway. J Biol Chem. 1999;274:23111–8. doi: 10.1074/jbc.274.33.23111. [DOI] [PubMed] [Google Scholar]
- 49.Burger D, Montezano AC, Nishigaki N, et al. Endothelial microparticle formation by angiotensin II is mediated via Ang II receptor type I/NADPH oxidase/Rho kinase pathways targeted to lipid rafts. Arterioscler Thromb Vasc Biol. 2011;31:1898–907. doi: 10.1161/ATVBAHA.110.222703. [DOI] [PubMed] [Google Scholar]
- 50.Jy W, Minagar A, Jimenez JJ, et al. Endothelial microparticles (EMP) bind and activate monocytes: elevated EMP-monocyte conjugates in multiple sclerosis. Front Biosci. 2004;9:3137–44. doi: 10.2741/1466. [DOI] [PubMed] [Google Scholar]
- 51.Brill A, Dashevsky O, Rivo J, et al. Platelet-derived microparticles induce angiogenesis and stimulate post-ischemic revascularization. Cardiovasc Res. 2005;67:30–8. doi: 10.1016/j.cardiores.2005.04.007. [DOI] [PubMed] [Google Scholar]
- 52.Kim HK, Song KS, Chung JH, et al. Platelet microparticles induce angiogenesis in vitro. Br J Haematol. 2004;124:376–84. doi: 10.1046/j.1365-2141.2003.04773.x. [DOI] [PubMed] [Google Scholar]
- 53.Yang C, Xiong W, Qiu Q, et al. Role of receptor-mediated endocytosis in the antiangiogenic effects of human T lymphoblastic cell-derived microparticles. Am J Physiol Regul Integr Comp Physiol. 2012;302:R941–9. doi: 10.1152/ajpregu.00527.2011. [DOI] [PubMed] [Google Scholar]
- 54.Huang PH, Huang SS, Chen YH, et al. Increased circulating CD31+/annexin V+ apoptotic microparticles and decreased circulating endothelial progenitor cell levels in hypertensive patients with microalbuminuria. J Hypertens. 2010;28:1655–65. doi: 10.1097/HJH.0b013e32833a4d0a. [DOI] [PubMed] [Google Scholar]
- 55.Distler JH, Akhmetshina A, Dees C, et al. Induction of apoptosis in circulating angiogenic cells by microparticles. Arthritis Rheum. 2011;63:2067–77. doi: 10.1002/art.30361. [DOI] [PubMed] [Google Scholar]
- 56.Huber LC, Jungel A, Distler JH, et al. The role of membrane lipids in the induction of macrophage apoptosis by microparticles. Apoptosis. 2007;12:363–74. doi: 10.1007/s10495-006-0622-7. [DOI] [PubMed] [Google Scholar]
- 57.Greenwalt TJ. The how and why of exocytic vesicles. Transfusion. 2006;46:143–52. doi: 10.1111/j.1537-2995.2006.00692.x. [DOI] [PubMed] [Google Scholar]
- 58.Allan D, Thomas P, Limbrick AR. The isolation and characterization of 60 nm vesicles (‘nanovesicles’) produced during ionophore A23187-induced budding of human erythrocytes. Biochem J. 1980;188:881–7. doi: 10.1042/bj1880881. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Willekens FL, Werre JM, Groenen-Dopp YA, et al. Erythrocyte vesiculation: a self-protective mechanism? Br J Haematol. 2008;141:549–56. doi: 10.1111/j.1365-2141.2008.07055.x. [DOI] [PubMed] [Google Scholar]
- 60.Bosman GJ, Werre JM, Willekens FL, Novotny VM. Erythrocyte ageing in vivo and in vitro: structural aspects and implications for transfusion. Transfus Med. 2008;18:335–47. doi: 10.1111/j.1365-3148.2008.00892.x. [DOI] [PubMed] [Google Scholar]
- 61.Stewart A, Urbaniak S, Turner M, Bessos H. The application of a new quantitative assay for the monitoring of integrin-associated protein CD47 on red blood cells during storage and comparison with the expression of CD47 and phosphatidylserine with flow cytometry. Transfusion. 2005;45:1496–503. doi: 10.1111/j.1537-2995.2005.00564.x. [DOI] [PubMed] [Google Scholar]
- 62.Burger P, Kostova E, Bloem E, et al. Potassium leakage primes stored erythrocytes for phosphatidylserine exposure and shedding of pro-coagulant vesicles. Br J Haematol. 2013;160:377–86. doi: 10.1111/bjh.12133. [DOI] [PubMed] [Google Scholar]
- 63.Bosch FH, Werre JM, Schipper L, et al. Determinants of red blood cell deformability in relation to cell age. Eur J Haematol. 1994;52:35–41. doi: 10.1111/j.1600-0609.1994.tb01282.x. [DOI] [PubMed] [Google Scholar]
- 64.Daleke DL. Regulation of transbilayer plasma membrane phospholipid asymmetry. J Lipid Res. 2003;44:233–42. doi: 10.1194/jlr.R200019-JLR200. [DOI] [PubMed] [Google Scholar]
- 65.Willekens FL, Roerdinkholder-Stoelwinder B, Groenen-Dopp YA, et al. Hemoglobin loss from erythrocytes in vivo results from spleen-facilitated vesiculation. Blood. 2003;101:747–51. doi: 10.1182/blood-2002-02-0500. [DOI] [PubMed] [Google Scholar]
- 66.Piccin A, Van Schilfgaarde M, Smith O. The importance of studying red blood cells microparticles. Blood Transfus. 2015;13:172–3. doi: 10.2450/2014.0276-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Salzer U, Hinterdorfer P, Hunger U, et al. Ca(++)-dependent vesicle release from erythrocytes involves stomatin-specific lipid rafts, synexin (annexin VII), and sorcin. Blood. 2002;99:2569–77. doi: 10.1182/blood.v99.7.2569. [DOI] [PubMed] [Google Scholar]
- 68.Jy W, Bidot C, Yoshida T, et al. Release of Microparticles During Blood Storage Is Influenced by Residual Platelets, Leukocytes and Oxygen Levels. Blood. 2012;120:3435. [Google Scholar]
- 69.Kriebardis AG, Antonelou MH, Stamoulis KE, et al. RBC-derived vesicles during storage: ultrastructur, protein composition, oxidation, and signaling components. Transfusion. 2008;48:1943–53. doi: 10.1111/j.1537-2995.2008.01794.x. [DOI] [PubMed] [Google Scholar]
- 70.Delobel J, Prudent M, Rubin O, et al. Subcellular fractionation of stored red blood cells reveals a compartment-based protein carbonylation evolution. J Proteomics. 2012;76:181–93. doi: 10.1016/j.jprot.2012.05.004. [DOI] [PubMed] [Google Scholar]
- 71.Rubin O, Crettaz D, Canellini G, et al. Microparticles in stored red blood cells: an approach using flow cytometry and proteomic tools. Vox Sang. 2008;95:288–97. doi: 10.1111/j.1423-0410.2008.01101.x. [DOI] [PubMed] [Google Scholar]
- 72.de Jong K, Beleznay Z, Ott P. Phospholipid asymmetry in red blood cells and spectrin-free vesicles during prolonged storage. Biochim Biophys Acta. 1996;1281:101–10. doi: 10.1016/0005-2736(96)00026-0. [DOI] [PubMed] [Google Scholar]
- 73.Rubin O, Canellini G, Delobel J, et al. Red blood cell microparticles: clinical relevance. Transfus Med Hemother. 2012;39:342–7. doi: 10.1159/000342228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Liu C, Zhao W, Christ GJ, et al. Nitric oxide scavenging by red cell microparticles. Free Radic Biol Med. 2013;65:1164–73. doi: 10.1016/j.freeradbiomed.2013.09.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Greenwalt TJ, McGuinness CG, Dumaswala UJ. Studies in red blood cell preservation: 4. Plasma vesicle hemoglobin exceeds free hemoglobin. Vox Sang. 1991;61:14–7. doi: 10.1111/j.1423-0410.1991.tb00920.x. [DOI] [PubMed] [Google Scholar]
- 76.Rubin O, Delobel J, Prudent M, et al. Red blood cell-derived microparticles isolated from blood units initiate and propagate thrombin generation. Transfusion. 2013;53:1744–54. doi: 10.1111/trf.12008. [DOI] [PubMed] [Google Scholar]
- 77.Van Der Meijden PE, Van Schilfgaarde M, Van Oerle R, et al. Platelet- and erythrocyte-derived microparticles trigger thrombin generation via factor XIIa. J Thromb Haemost. 2012;10:1355–62. doi: 10.1111/j.1538-7836.2012.04758.x. [DOI] [PubMed] [Google Scholar]
- 78.Biro E, Sturk-Maquelin KN, Vogel GM, et al. Human cell-derived microparticles promote thrombus formation in vivo in a tissue factor-dependent manner. J Thromb Haemost. 2003;1:2561–8. doi: 10.1046/j.1538-7836.2003.00456.x. [DOI] [PubMed] [Google Scholar]
- 79.van Beers EJ, Schaap MC, Berckmans RJ, et al. Circulating erythrocyte-derived microparticles are associated with coagulation activation in sickle cell disease. Haematologica. 2009;94:1513–9. doi: 10.3324/haematol.2009.008938. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Koshiar RL, Somajo S, Norstrom E, Dahlback B. Erythrocyte-derived microparticles supporting activated protein C-mediated regulation of blood coagulation. PLoS One. 2014;9:e104200. doi: 10.1371/journal.pone.0104200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Doctor A, Stamler JS. Nitric oxide transport in blood: a third gas in the respiratory cycle. Compr Physiol. 2011;1:541–68. doi: 10.1002/cphy.c090009. [DOI] [PubMed] [Google Scholar]
- 82.Singel DJ, Stamler JS. Chemical physiology of blood flow regulation by red blood cells: the role of nitric oxide and S-nitrosohemoglobin. Annu Rev Physiol. 2005;67:99–145. doi: 10.1146/annurev.physiol.67.060603.090918. [DOI] [PubMed] [Google Scholar]
- 83.Huang KT, Han TH, Hyduke DR, et al. Modulation of nitric oxide bioavailability by erythrocytes. Proc Natl Acad Sci USA. 2001;98:11771–6. doi: 10.1073/pnas.201276698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Lancaster JR., Jr Simulation of the diffusion and reaction of endogenously produced nitric oxide. Proc Natl Acad Sci USA. 1994;91:8137–41. doi: 10.1073/pnas.91.17.8137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Donadee C, Raat NJ, Kanias T, et al. Nitric oxide scavenging by red blood cell microparticles and cell-free hemoglobin as a mechanism for the red cell storage lesion. Circulation. 2011;124:465–76. doi: 10.1161/CIRCULATIONAHA.110.008698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Muszynski JA, Spinella PC, Cholette JM, et al. Transfusion-related immunomodulation: review of the literature and implications for pediatric critical illness. Transfusion. 2017;57:195–206. doi: 10.1111/trf.13855. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Danesh A, Inglis HC, Jackman RP, et al. Exosomes from red blood cell units bind to monocytes and induce proinflammatory cytokines, boosting T-cell responses in vitro. Blood. 2014;123:687–96. doi: 10.1182/blood-2013-10-530469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Sadallah S, Eken C, Schifferli JA. Erythrocyte-derived ectosomes have immunosuppressive properties. J Leukoc Biol. 2008;84:1316–25. doi: 10.1189/jlb.0108013. [DOI] [PubMed] [Google Scholar]
- 89.Awojoodu AO, Keegan PM, Lane AR, et al. Acid sphingomyelinase is activated in sickle cell erythrocytes and contributes to inflammatory microparticle generation in SCD. Blood. 2014;124:1941–50. doi: 10.1182/blood-2014-01-543652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Canellini G, Rubin O, Delobel J, et al. Red blood cell microparticles and blood group antigens: an analysis by flow cytometry. Blood Transfus. 2012;10:s39–45. doi: 10.2450/2012.007S. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Camus SM, De Moraes JA, Bonnin P, et al. Circulating cell membrane microparticles transfer heme to endothelial cells and trigger vasoocclusions in sickle cell disease. Blood. 2015;125:3805–14. doi: 10.1182/blood-2014-07-589283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Willekens FL, Werre JM, Kruijt JK, et al. Liver Kupffer cells rapidly remove red blood cell-derived vesicles from the circulation by scavenger receptors. Blood. 2005;105:2141–5. doi: 10.1182/blood-2004-04-1578. [DOI] [PubMed] [Google Scholar]
- 93.Bennett-Guerrero E, Veldman TH, Doctor A, et al. Evolution of adverse changes in stored RBCs. Proc Natl Acad Sci USA. 2007;104:17063–8. doi: 10.1073/pnas.0708160104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.D’Alessandro A, Kriebardis AG, Rinalducci S, et al. An update on red blood cell storage lesions, as gleaned through biochemistry and omics technologies. Transfusion. 2015;55:205–19. doi: 10.1111/trf.12804. [DOI] [PubMed] [Google Scholar]
- 95.Karon BS, Hoyer JD, Stubbs JR, Thomas DD. Changes in Band 3 oligomeric state precede cell membrane phospholipid loss during blood bank storage of red blood cells. Transfusion. 2009;49:1435–42. doi: 10.1111/j.1537-2995.2009.02133.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Bosman GJ, Lasonder E, Luten M, et al. The proteome of red cell membranes and vesicles during storage in blood bank conditions. Transfusion. 2008;48:827–35. doi: 10.1111/j.1537-2995.2007.01630.x. [DOI] [PubMed] [Google Scholar]
- 97.Relevy H, Koshkaryev A, Manny N, et al. Blood banking-induced alteration of red blood cell flow properties. Transfusion. 2008;48:136–46. doi: 10.1111/j.1537-2995.2007.01491.x. [DOI] [PubMed] [Google Scholar]
- 98.Solheim BG, Flesland O, Seghatchian J, Brosstad F. Clinical implications of red blood cell and platelet storage lesions: an overview. Transfus Apher Sci. 2004;31:185–9. doi: 10.1016/j.transci.2004.09.004. [DOI] [PubMed] [Google Scholar]
- 99.Kulandavelu S, Balkan W, Hare JM. Regulation of oxygen delivery to the body via hypoxic vasodilation. Proc Natl Acad Sci USA. 2015;112:6254–5. doi: 10.1073/pnas.1506523112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Doctor A, Platt R, Sheram ML, et al. Hemoglobin conformation couples erythrocyte S-nitrosothiol content to O2 gradients. Proc Natl Acad Sci USA. 2005;102:5709–14. doi: 10.1073/pnas.0407490102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Gonzalez-Alonso J, Richardson RS, Saltin B. Exercising skeletal muscle blood flow in humans responds to reduction in arterial oxyhaemoglobin, but not to altered free oxygen. J Physiol. 2001;530:331–41. doi: 10.1111/j.1469-7793.2001.0331l.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.McMahon TJ, Moon RE, Luschinger BP, et al. Nitric oxide in the human respiratory cycle. Nat Med. 2002;8:711–7. doi: 10.1038/nm718. [DOI] [PubMed] [Google Scholar]
- 103.Vaughn MW, Huang KT, Kuo L, Liao JC. Erythrocytes possess an intrinsic barrier to nitric oxide consumption. J Biol Chem. 2000;275:2342–8. doi: 10.1074/jbc.275.4.2342. [DOI] [PubMed] [Google Scholar]
- 104.Reynolds JD, Hess DT, Stamler JS. The transfusion problem: role of aberrant S-nitrosylation. Transfusion. 2011;51:852–8. doi: 10.1111/j.1537-2995.2011.03097.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Bonaventura J. Clinical implications of the loss of vasoactive nitric oxide during red blood cell storage. Proc Natl Acad Sci USA. 2007;104:19165–6. doi: 10.1073/pnas.0708871105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Alexander JT, El-Ali AM, Newman JL, et al. Red blood cells stored for increasing periods produce progressive impairments in nitric oxide-mediated vasodilation. Transfusion. 2013;53:2619–28. doi: 10.1111/trf.12111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Stapley R, Owusu BY, Brandon A, et al. Erythrocyte storage increases rates of NO and nitrite scavenging: implications for transfusion-related toxicity. Biochem J. 2012;446:499–508. doi: 10.1042/BJ20120675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Roback JD. Vascular effects of the red blood cell storage lesion. Hematology Am Soc Hematol Educ Program. 2011;2011:475–9. doi: 10.1182/asheducation-2011.1.475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Chen K, Pittman RN, Popel AS. Nitric oxide in the vasculature: where does it come from and where does it go? A quantitative perspective. Antioxid Redox Signal. 2008;10:1185–98. doi: 10.1089/ars.2007.1959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Kim-Shapiro DB, Lee J, Gladwin MT. Storage lesion: role of red blood cell breakdown. Transfusion. 2011;51:844–51. doi: 10.1111/j.1537-2995.2011.03100.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Lipcsey M, Woinarski NC, Bellomo R. Near infrared spectroscopy (NIRS) of the thenar eminence in anesthesia and intensive care. Ann Intensive Care. 2012;2:11. doi: 10.1186/2110-5820-2-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Said AS, Gazit A, Jackups R, et al. Transfusion related vasoactivity: relationship to circulating RBC Microparticles. Toronto: WFPICC; 2016. [Google Scholar]
- 113.Hugel B, Martinez MC, Kunzelmann C, et al. Membrane microparticles: two sides of the coin. Physiology. 2005;20:22–7. doi: 10.1152/physiol.00029.2004. [DOI] [PubMed] [Google Scholar]