Abstract
Background
The aim of this study was to evaluate ex vivo and in vitro interference of a direct factor IIa inhibitor, dabigatran, on a prothrombinase-based assay to detect activated protein C resistance.
Materials and methods
An ex vivo study was performed in six heterozygous factor V Leiden carriers and 12 normal subjects without the factor V Leiden mutation who were treated with dabigatran. An in vitro study was also performed considering 12 plasma samples (six from normal subjects and six from heterozygous factor V Leiden carriers) spiked with dabigatran. The dabigatran concentration was evaluated using a diluted thrombin time assay, activated protein C resistance was evaluated using a prothrombinase-based assay.
Results
In both the ex vivo and in vitro studies dabigatran interfered significantly with activated protein C resistance ratios observed in normal subjects and in factor V Leiden heterozygous carriers.
Discussion
The results reported in this paper seem to confirm that dabigatran is able to interfere with the Penthafarm prothrombinase-based assay used to study activated protein C resistance, significantly increasing observed ratios. This effect appears to be present already at low concentrations of dabigatran (6 ng/mL) and affects both normal subjects and heterozygous carriers of factor V Leiden. In this group of patients, dabigatran, at concentrations in the therapeutic range (100–200 ng/mL), could markedly increase the activated protein C resistance ratio, bringing it up to within the reference range for normal subjects, thus potentially leading to misclassification of patients.
Keywords: APC resistance, dabigatran, factor V Leiden, prothrombinase-based assay
Introduction
Activated protein C (APC) is a natural anticoagulant that is capable of hydrolysing activated factor V (FVa) at three arginine sites (R306, R506, and R679). Factor V Leiden (FVL) is characterised by a single point mutation (G1691A) that changes the arginine in position 506 to a glutamine (R506Q); as a result of this mutation APC is less efficient at cleaving FVL. Thus FVL is “resistant” to APC, resulting in a hypercoagulable state1,2. Activated protein C resistance (APCr) is almost always caused by FVL. In clinical laboratories, the first approach to detecting the presence of FVL is usually a functional assay for APCr3.
The original APCr assay yields a ratio between a baseline activated partial thromboplastin time (aPTT) and the aPTT after purified exogenous APC has been added. APC-mediated cleavage of FVa prolongs the aPTT, and this prolongation is decreased in the presence of FVL. Both the sensitivity and specificity of these first-generation assays are unsatisfactory. This is in large part due to the assay not being accurate when the baseline aPTT is abnormal; conversely, low ratios that falsely suggest APCr are caused by a variety of conditions including acute phase reactions and pregnancy (due to elevated factor VIII and low protein S), and hormone replacement therapy or oral contraceptives (due to low protein S)3,4.
Second-generation APCr assays effectively eliminate the influence of abnormal factor levels by adding a step in which the patient’s plasma is diluted in FV-deficient (but otherwise normal) human plasma. Moreover, polybrene, a heparin neutraliser is added. These modifications increase the sensitivity and specificity of FVL detection. However, direct thrombin inhibitors and rivaroxaban can give falsely elevated APCr results3.
More recently, APCr assays have been developed that use Russell viper venom (RVV) from the snake Daboia russelli. The RVV time is analogous to the aPTT, except that the clotting cascade is initiated by the RVV factor X activator (RVV-X). This sidesteps the contact pathway and thereby eliminates influences of factor VIII, IX, XI and XII concentrations. There is no dilution into FV-deficient plasma, therefore low factor II or FV levels may interfere with these assays, as may low fibrinogen concentrations, direct thrombin inhibitors and direct factor Xa inhibitors. The reagent contains a heparin neutraliser as well as phospholipids intended to prevent any interference from lupus anticoagulants5.
In another APCr assay the role of factor Xa is replaced by noscarin, a FVa-dependent but phospholipid-independent prothrombin activator derived from the snake Notechis scutatus. Factor deficiencies or elevations are overcome by dilution into FV-deficient plasma. Moreover, phospholipid and calcium are not present in the reagent. Phospholipid independence should eliminate interference by lupus anticoagulants. FV is activated by RVV FV activator (RVV-V) in the presence or absence of exogenous APC. Another characteristic of this prothrombinase-based assay is that a ratio based on prothrombin time is used6.
Only few data concerning the interference that dabigatran may have on assays to detect APCr are available. Furthermore, most studies investigating this problem have been in vitro investigations and usually evaluated assays based on the aPTT ratio. The aim of this study was to evaluate ex vivo and in vitro interference of the direct factor IIa inhibitor dabigatran on a prothrombinase-based assay to detect APCr.
Materials and methods
Samples
Ex vivo study
We considered 18 subjects with non-valvular atrial fibrillation treated with dabigatran etexilate (150 mg twice daily) for thromboprophylaxis: 12 subjects were wild-type homozygotes for FVL and six were heterozygous for FVL. For each of these subjects at least four samples were available: a basal sample obtained before treatment, a pair of samples obtained after 1 month of treatment (the first before and the second 2 hours after taking the drug), and at least one more sample. The dabigatran concentration and APCr ratios were estimated in these samples. A total of 62 samples were taken from the normal subjects: 12 samples were obtained before drug administration (group O), in 15 samples the dabigatran concentration was below 100 ng/mL (group A), in 19 samples the dabigatran concentration was between 100 and 200 ng/mL (group B), and in 16 the concentration was over 200 ng/mL (group C). A total of 29 samples were taken from the FVL heterozygous carriers: six samples were obtained before drug administration (group O1), in seven samples the dabigatran concentration was below 100 ng/mL (group A1), in eight samples it was between 100 and 200 ng/mL (group B1), and in eight other samples the dabigatran concentration was over 200 ng/mL (group C1).
In vitro study
We prepared 12 plasma samples: six from normal subjects and six from FVL heterozygous patients. These samples were from subjects previously evaluated for FVL and stored at −80 °C. We made a solution containing 1 mg/mL of dabigatran7,8 and prepared six test-tubes for each plasma sample. No dabigatran was added to the first tube (D and D1 for normal subjects and FVL heterozygous carriers, respectively); the other tubes were spiked with dabigatran to obtain drug concentrations of 6 (E and E1), 12 (F and F1), 25 (G and G1), 50 (H and H1), and 100, ng/mL (I and I1). Each tube was tested in duplicate to evaluate the effective dabigatran concentration and APCr ratio.
Laboratory methods
Dabigatran concentration was determined using a diluted thrombin time assay supplied by Hyphen and a Sysmex Ca-7000 analyser (Dasit SpA, Milan, Italy)9. APCr was evaluated using a prothrombinase-based assay (Pefakit APC-R FVL; Pentapharm, Basel, Switzerland) and a Sysmex CA-7000 analyser10. The prothrombinase-based assay for detecting APCr evaluated in this study is a plasma-based functional clotting assay. It relies on a FVa elimination step and a FVa detection step. In accordance with the manufacturer’s instructions, in the first analytical step the patient’s plasma is mixed with normal human plasma that has been depleted of FV and a reagent that contains APC and a snake venom specifically activating the FV in the plasma sample (Russell viper venom-factor V [RVV-V] isolated from Daboia russelli venom). This step effectively eliminates the influence of abnormal coagulation factor levels; moreover, a heparin inhibitor (polybrene) is added to minimise interference from unfractionated or low-molecular weight heparin. During a first incubation period, FVa is inactivated by APC. The velocity of the inactivation of the FVa molecules depends on their binding kinetics to APC and, thus, is decelerated significantly in the case of a FVL mutation. Subsequently, a second reagent that contains a FV-dependent prothrombin activator (noscarin from the venom of Notechis scutatus scutatus) and EDTA are added. The prothrombin activator converts prothrombin to thrombin and, thus, induces clotting of the sample. Phospholipid and calcium are not present in the reagent. The clotting time is recorded. If the FVa molecules in the sample have been eliminated during the incubation step, the velocity of prothrombin activation by the FV-dependent noscarin is slow and, therefore, the clotting time is long. If the FVa elimination has been incomplete (e.g., because of a FVL mutation), the velocity of prothrombin activation is high and the clotting time is short. A second determination is performed under identical assay conditions, with the exception that no APC is added to the first reagent. Thus, the baseline clotting time is determined without the inactivation of the FVa molecules by APC. This determination is called the APC(−) measurement, as opposed to APC(+) for the test with the FVa elimination by APC. The ratio between the APC(+) and APC(−) results is calculated, so in this assay factor deficiencies or elevations are overcome by dilution into factor V-deficient plasma. The role of factor Xa is replaced by noscarin, a FVa-dependent but phospholipid-independent prothrombin activator11–14.
The F5 genotype was assessed using a Cepheid GeneXpert assay15 (Cepheid Italia Srl, Pero, MI, Italy).
Statistical analysis
We used MedCalc 8.0 (Medcalc Software, Ostend, Belgium) for the statistical computations. For comparisons of means we adopted a Student’s t-test: a p-value <0.05 was considered statistically significant. All results are reported as means±two standard deviations.
Results
Ex vivo study
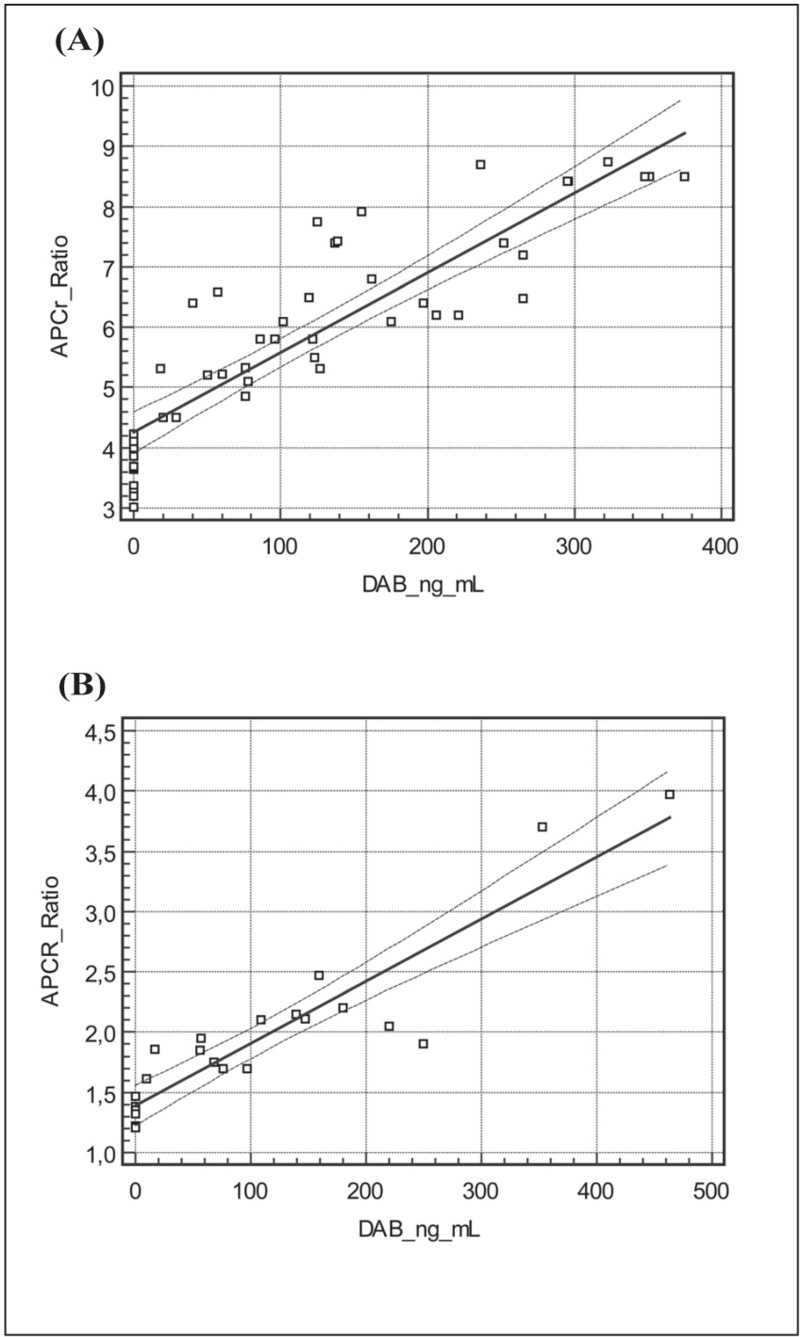
The results obtained in the FV wild-type homozygote patients (the so-called normal subjects) are reported in Table I. As shown in Figure 1A, there was a significant relationship between dabigatran concentration and APCr ratio (y=4.25+0.13x, r=0.88). The results obtained in FVL heterozygous patients are also reported in Table I. As shown in Figure 1B, there was a significant relationship between dabigatran concentration and APCr ratio also in this group (y=1.39+0.05x, r=0.92).
Table I.
Results from the ex vivo study.
| DAB concentration | APCr ratio | p | p | p | |
|---|---|---|---|---|---|
| O | 0 ng/mL | 3.69±0.41 | |||
| A | <100 ng/mL | 5.38±0.66 | O vs A p<0.01 | ||
| B | 100–200 ng/mL | 6.88±0.88 | O vs B p<0.005 | A vs B p<0.05 | |
| C | >200 ng/mL | 7.77±1.02 | O vs C p<0.001 | A vs C p<0.01 | B vs C P<0.05 |
| DAB concentration | APCr ratio | ||||
| O1 | 0 ng/mL | 1.34±0.08 | |||
| A1 | <100 ng/mL | 1.79±0.11 | O1 vs A1 p<0.05 | ||
| B1 | 100–200 ng/mL | 2.23±0.14 | O1 vs B1 p<0.01 | A1 vs B1 p<0.05 | - |
| C1 | >200 ng/mL | 2.74±0.83 | O1 vs C1 p<0.001 | A1 vs C1 p<0.01 | B1 vs C1 p<0.05 |
APCr: activated protein C resistance; DAB: dabigatran etexilate; FV wild-type homozygous subjects: groups O, A, B and C. FV Leiden heterozygous patients: groups O1, A1, B1 and C1.
Figure 1.
Activated protein C resistance (APCr) ratios and dabigatran etexilate (DAB) concentrations in (A) FV wild-type subjects and (B) FV Leiden heterozygous patients: ex vivo results.
In vitro study
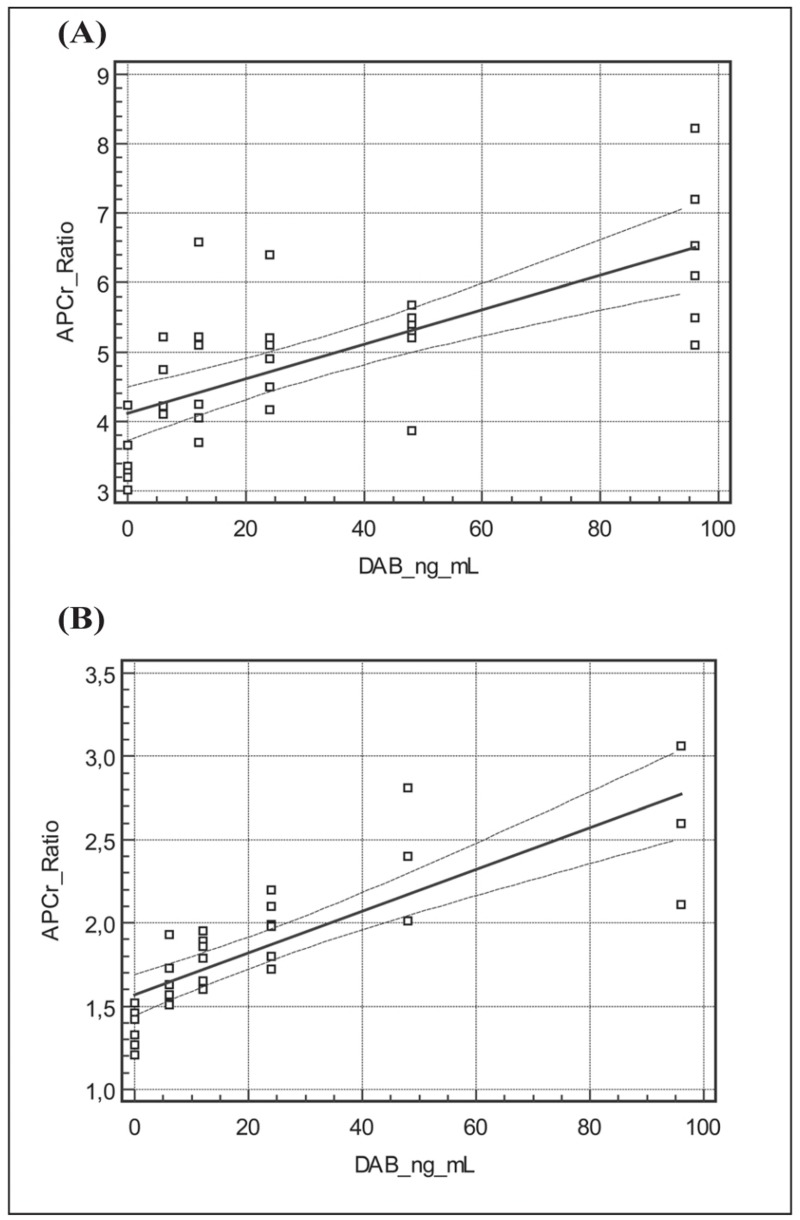
The results obtained in plasma samples from FV wild-type subjects are reported in Table II. As illustrated in Figure 2A, there was a significant relationship between dabigatran concentration and APCr ratio (y=4.11+0.25x, r=0.71). The corresponding results obtained in plasma samples from FVL heterozygous subjects are also reported in Table II. Likewise, Figure 2B shows that there was a significant relationship between dabigatran concentration and APCr ratio (y=1.57+0.12x, r=0.82) in plasma samples from FVL carriers.
Table II.
Results from the in vitro study.
| DAB concentration | APCr ratio | p | p | p | p | p | |
|---|---|---|---|---|---|---|---|
| D | 0 ng/mL | 3.45±0.44 | |||||
| E | 6 ng/mL | 4.42±0.47 | D vs E p<0.01 | ||||
| F | 12 ng/mL | 4.82±1.05 | D vs F p<0.005 | E vs F p<0.05 | |||
| G | 25 ng/mL | 5.05±0.77 | D vs G p<0.001 | E vs G p<0.01 | F vs G p<0.05 | ||
| H | 50 ng/mL | 5.16±0.66 | D vs H p<0.001 | G vs H p<0.01 | F vs H p<0.01 | G vs H p<0.05 | |
| I | 100 ng/mL | 6.44±1.15 | D vs I p<0.001 | E vs I p<0.001 | F vs I p<0.01 | G vs I p<0.01 | H vs I p<0.01 |
| DAB concentration | APCr ratio | ||||||
| D1 | 0 ng/mL | 1.33±0.12 | |||||
| E1 | 6 ng/mL | 1.66±0.15 | D1 vs E1 p<0.05 | ||||
| F1 | 12 ng/mL | 1.79±0.14 | D1 vs F1 p<0.01 | E1 vs F1 p<0.05 | |||
| G1 | 25 ng/mL | 1.97±0.18 | D1 vs G1 p<0.005 | E1 vs G1 p<0.01 | F1 vs G1 p<0.05 | ||
| H1 | 50 ng/mL | 2.41±0.40 | D1 vs H1 p<0.001 | E1 vs H1 p<0.01 | F1 vs H1 p<0.01 | G1 vs H1 p<0.05 | |
| I1 | 100 ng/mL | 2.59±0.48 | D1 vs I1 p<0.001 | E1 vs I1 p<0.01 | F1 vs I1 p<0.01 | G1 vs I1 p<0.01 | H1 vs I1 p<0.05 |
APCr: activated protein C resistance; DAB: dabigatran etexilate. Plasma samples from normal subjects (FV wild-type homozygotes): groups D, E, F, G, H and I. Plasma samples from heterozygous FVL carriers: groups D1, E1, F1, G1, H1 and I1.
Figure 2.
Activated protein C resistance (APCr) ratios and dabigatran etexilate (DAB) concentrations in (A) FV wild-type subjects and (B) FV Leiden heterozygous patients: in vitro results.
Discussion
Dabigatran etexilate is an oral anticoagulant drug with a direct inhibitory action against factor IIa. Because of its action as a direct thrombin inhibitor, dabigatran is known to interfere with essentially all clot-based coagulation assays. Among routine coagulation assays, the most sensitive to the presence of dabigatran was found to be the thrombin time, which is prolonged even at low dabigatran concentrations. Relatively low levels of dabigatran (75 ng/mL) are consistent with prolongation of the aPTT, whereas relatively high levels of dabigatran (200 ng/mL) are needed to prolong the prothrombin time assay7,16,17.
Only few data concerning dabigatran’s influence on the assay for detecting APCr were available. Furthermore, most studies investigating this problem were in vitro investigations and usually evaluated assays based on the aPTT ratio. For example, Adcock et al.7 and Lindahl et al.17 observed, in two in vitro studies performed using aPTT-based functional assays, that dabigatran was able to increase the APCr ratio. However, only homozygous wild-type FV subjects were included in those studies. There are no studies evaluating the effect of inhibitors of factor IIa on the determination of APCr ratio performed using prothrombinase-based functional assays. In a previous paper we reported, in three FVL heterozygous carriers that, using a prothrombinase-based assay, APCr ratios observed during dabigatran treatment were significantly higher than values observed in samples obtained before treatment12.
In this study, both ex vivo and in vitro investigations were performed in order to evaluate the effect of dabigatran on a prothrombinase-based assay for the detection of APCr. In the ex vivo study 91 samples obtained from 18 subjects were considered: 29 samples were taken from six FVL heterozygous carriers and 62 samples were from 12 normal (FV wild-type homozygous) subjects. These samples were divided into four groups (O, A, B and C for normal subjects; O1, A1, B1 and C1 for FVL carriers) according to their dabigatran concentration: 0 ng/mL (groups O and O1), <100 ng/mL (groups A and A1), between 100 and 200 ng/mL (groups B and B1), and >200 ng/mL (groups C and C1).
In the ex vivo study, we observed a clear interference of dabigatran on APCr ratios among normal subjects. As indicated in Table I, the APCr ratios detected in the four groups of samples classified according to plasma dabigatran concentration (O, A, B and C) differed from each other in a statistically significant manner in all cases. As further confirmation of the ability of dabigatran to interfere with the study of APCr we constructed a regression line relating dabigatran plasma concentration and APCr ratios; as shown in Figure 1A we observed a satisfactory correlation with r=0.88.
In vitro, the mean APCr ratio observed in group D was 3.45±0.44, a value similar to that in group O (both groups studied without the presence of dabigatran). In group E (dabigatran concentration 6 ng/mL) the mean APCr ratio was significantly higher (p<0.05) than that in group O. Thus, even at concentrations as low as 6 ng/mL, much lower than the recommended therapeutic levels, dabigatran was able to interfere with the assay, significantly increasing the APCr ratios. As indicated in Table II, the APCr ratios determined in six plasma samples spiked with various concentrations of dabigatran, ranging from 0 to 100 ng/mL (D, E, F, G, H and I), differed from each other, in all cases in a statistically significant manner. As further confirmation of the ability of dabigatran to interfere with the study of APCr we constructed a regression line relating dabigatran plasma concentration and APCr ratios; as shown in Figure 2A, we observed a satisfactory correlation with r=0.71.
Among FVL heterozygous patients we observed, ex vivo, a clear influence of dabigatran on APCr ratios. As indicated in Table I, APCr ratios in the four groups of samples classified according to plasma dabigatran concentration (O1, A1, B1 and C1) were all statistically significantly different from each other and the regression line relating dabigatran plasma concentration and APCr ratio (Figure 1B) showed a satisfactory correlation (r=0.92), thus further confirming the ability of dabigatran to interfere with APCr assays.
In vitro, the mean APCr ratio in group D1 was 1.33±0.12, a value similar to that obtained in group O1 (both groups studied in the absence of dabigatran). In group E1 (dabigatran concentration 6 ng/mL) the mean APCr ratio was significantly higher (p<0.05) than that in group O. Thus, in FVL heterozygous patients, just as in normal subjects, at concentrations as low as6 ng/mL, much lower than recommended therapeutic levels, dabigatran interfered with the APCr assay, significantly increasing the ratios. As indicated in Table II, APCr ratios detected in the six plasma samples spiked with various amounts of dabigatran (range of concentrations from 0 to 100 ng/mL; D1, E1, F1, G1, H1 and I1) were all statistically significantly different from each other. Once again the ability of dabigatran to interfere with the APCr assay was confirmed by the regression line relating the drug’s plasma concentration and APCr ratio, which had a satisfactory correlation of r=0.82 (Figure 2B).
Conclusions
The results reported in this paper seem to confirm that dabigatran is able to interfere with the Penthafarm prothrombinase-based assay for the study of APCr, significantly increasing the ratios. This effect appears to be present already at low concentrations of dabigatran (6 ng/mL) and affects both normal subjects and heterozygous carriers of FVL. In this latter group of patients, at concentrations of dabigatran in the therapeutic range (100–200 ng/mL), there could be marked increases in the APCr ratio, bring it up to within the reference range for normal subjects, thus potentially leading to misclassification of patients with APCr.
Footnotes
Ethics
The study protocol was approved by the competent ethical committee. Each patient signed consent to drug administration and enrolment in this study.
Authorship contributions
GG designed the study, coordinated the group of authors and prepared the manuscript. SV collected data and performed the statistical analysis. LV and RV enrolled patients and performed the follow-up. PC and FG performed the laboratory assays. RV revised the manuscript.
Disclousure of conflicts of interest
GG, SV, LV, FG and RV are employers of Italian National Health Service. PC is an employee of DASIT SpA.
References
- 1.Van Cott EM, Khor B, Zehnder JL. Factor V Leiden. Am J Hematol. 2016;91:46–9. doi: 10.1002/ajh.24222. [DOI] [PubMed] [Google Scholar]
- 2.Saemundsson Y, Sveinsdottir SV, Svantesson H, et al. Homozygous factor V Leiden and double heterozygosity for factor V Leiden and prothrombin mutation. J Thromb Thrombolysis. 2013;36:324–31. doi: 10.1007/s11239-012-0824-5. [DOI] [PubMed] [Google Scholar]
- 3.Kadauke S, Khor B, Van Cott EM. Activated protein C resistance testing for factor V Leiden. Am J Hematol. 2014;89:1147–50. doi: 10.1002/ajh.23867. [DOI] [PubMed] [Google Scholar]
- 4.de Ronde H, Bertina RM. Laboratory diagnosis of APC-resistance: a critical evaluation of the test and the development of diagnostic criteria. Thromb Haemost. 1994;72:880–6. [PubMed] [Google Scholar]
- 5.Quehenberger P, Handler S, Mannhalter C, et al. The factor V (Leiden) test: evaluation of an assay based on dilute Russell viper venom time for the detection of the factor V Leiden mutation. Thromb Res. 1999;96:125–33. doi: 10.1016/s0049-3848(99)00090-0. [DOI] [PubMed] [Google Scholar]
- 6.Wilmer M, Stocker C, Bühler B, et al. Improved distinction of factor V wild-type and factor V Leiden using a novel prothrombin-based activated protein C resistance assay. Am J Clin Pathol. 2004;122:836–42. doi: 10.1309/T8AV-VH7Q-WGL0-QTF5. [DOI] [PubMed] [Google Scholar]
- 7.Adcock DM, Gosselin R, Kitchen S, et al. The effect of dabigatran on select specialty coagulation assays. Am J Clin Pathol. 2013;139:102–9. doi: 10.1309/AJCPY6G6ZITVKPVH. [DOI] [PubMed] [Google Scholar]
- 8.Douxfils J, Mullier F, Robert S, et al. Impact of dabigatran on a large panel of routine or specific coagulation assays. Laboratory recommendations for monitoring of dabigatran etexilate. Thromb Haemost. 2012;107:985–97. doi: 10.1160/TH11-11-0804. [DOI] [PubMed] [Google Scholar]
- 9.Avecilla S, Ferrell C, Chandler WL, et al. Plasma-diluted thrombin time to measure dabigatran concentrations during dabigatran etexilate therapy. Am J Clin Pathol. 2012;137:572–4. doi: 10.1309/AJCPAU7OQM0SRPZQ. [DOI] [PubMed] [Google Scholar]
- 10.Gessoni G, Valverde S. Clinical evaluation of a functional prothrombin time-based assay for identification of factor V Leiden carriers in a group of Italian patients with venous thrombosis. Blood Coagul Fibrinolysis. 2007;18:603–10. doi: 10.1097/MBC.0b013e3282891e2f. [DOI] [PubMed] [Google Scholar]
- 11.Tsutsumi Y, Shimono J, Ohhigashi H, et al. Analysis of the influence of dabigatran etexilate on coagulation factors and inhibitors. Int J Lab Hematol. 2015;37:225–30. doi: 10.1111/ijlh.12270. [DOI] [PubMed] [Google Scholar]
- 12.Gessoni G, Valverde S, Gessoni F, et al. The effect of dabigatran and rivaroxaban on a prothrombinase-based assay for activated protein C resistance: a preliminary study in subjects heterozygous for factor V Leiden. Blood Transfus. 2015;13:666–8. doi: 10.2450/2015.0224-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Herskovits A, Lemire S, Longtine J, et al. Comparison of Russell viper venom-based and activated partial thromboplastin time-based screening assays for resistance to activated protein C. Am J Clin Pathol. 2008;130:796–804. doi: 10.1309/AJCP7YBJ6URTVCWP. [DOI] [PubMed] [Google Scholar]
- 14.Gessoni G, Valverde S, Manoni F. Comparison between two functional assays to detect factor V Leiden. Int J Lab Hematol. 2010;32:188–9. doi: 10.1111/j.1751-553X.2009.01144.x. [DOI] [PubMed] [Google Scholar]
- 15.Gessoni G, Valverde S, Manoni F. Evaluation of the GeneXpert assay in the detection of factor V Leiden and prothrombin 20210 in stored, previously classified samples. Clin Chim Acta. 2012;413:814–6. doi: 10.1016/j.cca.2012.01.016. [DOI] [PubMed] [Google Scholar]
- 16.Tripodi A. The laboratory and the direct oral anticoagulants. Blood. 2011;121:4032–5. doi: 10.1182/blood-2012-12-453076. [DOI] [PubMed] [Google Scholar]
- 17.Lindahl TL, Baghaei F, Blixter IF, et al. Effects of the oral, direct thrombin inhibitor dabigatran etexilate on five common coagulation assays. Thromb Haemost. 2011;105:371–8. doi: 10.1160/TH10-06-0342. [DOI] [PubMed] [Google Scholar]