To the Editor:
Club cell secretory protein (CC16; encoding gene, SCGB1A1) is a homodimeric pneumoprotein that is produced mainly by club cells and other nonciliated epithelial cells in both proximal and distal airways (1). Higher airway expression and circulating levels of CC16 have been associated cross-sectionally with better lung function and lower prevalence and severity of chronic obstructive pulmonary disease (COPD) (2). Prospective studies have also shown that increased serum levels of CC16 at baseline are protective against subsequent development of COPD and accelerated FEV1 decline (3). In explaining these protective effects, growing evidence supports antiinflammatory and antioxidative properties of CC16 in the lungs, although results from animal models have been to some extent inconsistent. In line with direct protective effects, recombinant human CC16 has been shown to inhibit the cigarette smoke extract–induced release of IL-8 from bronchial epithelial cells isolated from patients with COPD (4). Thus, CC16 augmentation may be beneficial in the prevention and treatment of COPD.
Retinoic acid (RA)—an active metabolite of vitamin A—is known to play a key role in early lung development (5). These effects are largely dose specific (6). Studies on RA-induced alveolization in mice also indicate the importance of retinoic acid receptor (RAR) subtypes in mediating these effects of vitamin A, with decreased alveolar number selectively shown by RARγ null animals (7). In humans, despite conflicting results from reports that used estimates of vitamin A intake, epidemiological and clinical studies that actually measured circulating levels of vitamin A found consistently lower circulating levels of retinol and carotenoids in patients with COPD as compared with control subjects. In line with this scenario, baseline serum concentrations of β-carotene and retinol were inversely associated with respiratory symptoms in smokers from the ATBC (Alpha-Tocopherol, Beta-Carotene Cancer Prevention) Study (8), and vitamin A serum levels correlated significantly with FEV1 values among NHANES (National Health and Nutrition Examination Survey) III participants, particularly smokers (9). Of note, mice that were fed a purified diet containing reduced levels of vitamin A and were exposed to cigarette smoke for 3 months had increased susceptibility to lung emphysema, suggesting a potential causal link between vitamin A deficiency and smoking-related COPD (10).
Because of the effects of vitamin A on lung epithelial development, differentiation, and homeostasis, it is plausible that CC16—a major airway epithelial marker—may mediate some of the above associations. Yet, whether vitamin A affects CC16 production remains unknown. Here, we report that in vivo circulating levels of CC16 are up-regulated by vitamin A treatment and that in vitro CC16 expression in airway epithelial cells is increased by all-trans-retinoic acid (t-RA) acting mainly via RARα and RARγ.
In Vivo Studies
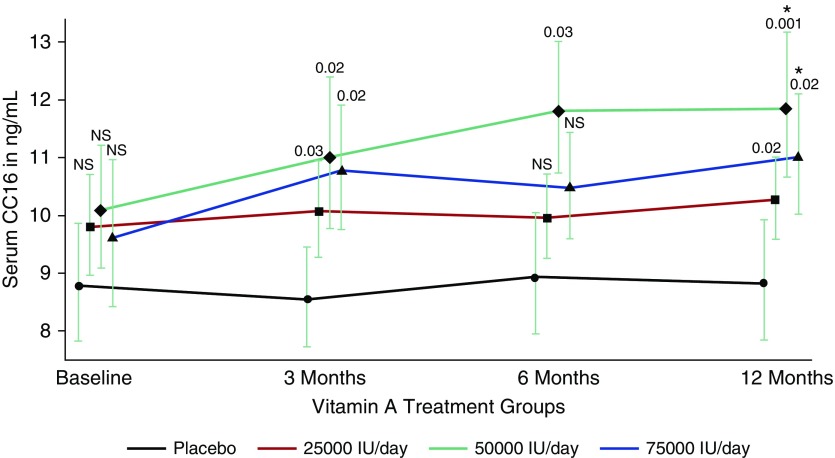
CC16 levels were measured with a commercially available ELISA kit (BioVendor, Asheville, NC) in serum samples collected from 71 subjects who participated in a placebo-controlled vitamin A trial for severely sun-damaged skin at baseline and at 3, 6, and 12 months of treatment (11). All participants had sun-damaged skin on their forearms, but they were otherwise required to be in general good health. Subjects were randomized to receive placebo or vitamin A (retinyl palmitate) at 25,000, 50,000, or 75,000 IU/d for 12 months. Overall, 65% of participants were male and 44% were never-smokers. Their mean (SD) age was 62 (7) years. Figure 1 shows the geometric means of circulating CC16 across the four treatment groups at baseline and at 3, 6, and 12 months of treatment. We found significant vitamin A treatment effects on serum CC16 as early as 3 months after initiation of treatment. By the completion of the trial all treatment groups had higher CC16 levels than the placebo group, with the 50,000-IU/d treatment having the highest levels. In addition, circulating CC16 levels increased significantly between baseline and the completion of the trial in both the 50,000- and 75,000-IU/d groups (P = 0.002 and 0.005, respectively). Similar trends for effects of vitamin A on CC16 levels were found in analyses stratified by smoking (data not shown), with CC16 levels at the end of the trial being significantly higher in the 50,000-IU/d group compared with the placebo group, both among the 31 never-smokers (P = 0.02) and among the 40 ever-smokers (P = 0.02). Thus, in these studies vitamin A treatment increased significantly the circulating levels of CC16 in vivo.
Figure 1.
Serum club cell secretory protein (CC16) levels at baseline, during, and after treatment in 71 subjects who participated in a placebo-controlled vitamin A trial for severely sun-damaged skin (11). CC16 levels (geometric mean ± SE) are shown for the placebo and the three treatment groups (vitamin A [retinyl palmitate] at 25,000, 50,000, or 75,000 IU/d) at baseline and at 3, 6, and 12 months of treatment. Number of subjects: Placebo (n = 20), 25,000 IU/d (n = 18), 50,000 IU/d (n = 15), and 75,000 IU/d (n = 18). P values (shown above error bars) refer to comparison with placebo group at each time point. Data were analyzed using random coefficients models to take into account intrasubject serial correlation. All analyses were adjusted for sex, age, and smoking status. *CC16 levels at 12 months significantly higher than baseline CC16 levels for treatment groups receiving 50,000 and 75,000 IU/d. NS = not significant.
In Vitro Studies
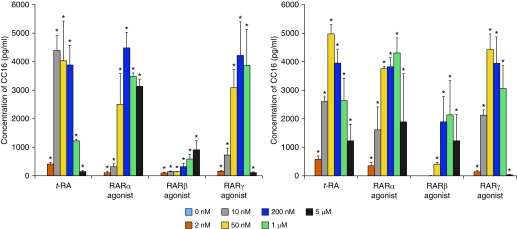
Because lung epithelium is the major contributor of serum CC16, we tested whether the effects of vitamin A on CC16 augmentation can be replicated in vitro in primary epithelial cell cultures. Because CC16 is expressed by epithelial cells from the bronchi to the bronchioles (1) and because of the technical barrier to isolate bronchiolar cells, we tested only bronchial epithelial cells in this study. Briefly, we cultured primary human bronchial epithelial cells from nine individuals with no lung disease (normal) and four patients with COPD (Global Initiative for Chronic Obstructive Lung Disease stage IV, receiving lung transplant). These cells were treated with various doses of t-RA, a stable metabolite that mediates physiological functions of vitamin A. As shown in Figure 2, we found that t-RA increased CC16 secretion in both normal and COPD cells by 72 hours after treatment with various doses, according to a bell-shaped curve. In addition, because t-RA binds with equal affinity to each retinoic acid receptor subtype (RARα, RARβ, and RARγ) (12), we tested whether any of the three subtypes was preferentially involved in the regulation of CC16. As also shown in Figure 2, by using agonists that are specific to RAR subtypes we found that both RARα-specific (AM580) and RARγ-specific (CD1530) agonists induced CC16 expression in a dose-dependent manner with comparable potency as t-RA, and that these responses were largely similar between normal and COPD cells. In contrast, the RARβ-specific agonist CD2314 induced CC16 expression with much less potency, although COPD cells tended to have stronger RARβ-specific responses than normal cells.
Figure 2.
Dose-dependent club cell secretory protein (CC16) production in primary human bronchial epithelial cell cultures from normal individuals with no respiratory diseases (left) and from patients with COPD (GOLD stage IV; right) under immersed conditions in response to various doses of t-RA or RAR-specific agonist treatment. RAR-specific agonists included AM580 for RARα, CD2314 for RARβ, and CD1530 for RARγ. Secreted CC16 was measured in the culture medium. Values represent means ± SEM 72 hours after treatment. Number of subjects: Normal (n = 9), patients with COPD (n = 4). *P < 0.01 compared with untreated. COPD = chronic obstructive pulmonary disease; GOLD = Global Initiative for Chronic Obstructive Lung Disease; RAR = retinoic acid receptor; t-RA = all-trans-retinoic acid.
In summary, our studies indicate that retinoids increase CC16 secretion in human bronchial epithelial cells from both normal individuals and patients with COPD; that these effects are mediated mainly through RARα and RARγ; and that, in vivo, vitamin A treatment results in a significant increase in circulating CC16 levels in individuals with no COPD. Whether these results simply support the importance of vitamin A dietary intake or may also have pharmacological implications in the early and preclinical stages of COPD remains to be determined. In this context, two considerations are noteworthy. First, previous large randomized cancer prevention trials (13, 14) have reported an increased risk for lung cancer among participants receiving β-carotene supplementation. Thus, any intervention aimed at using carotenoids to increase CC16 production would first need to address and minimize the potential effects of these agents on lung cancer risk, particularly among smokers. Second, two previous clinical trials (15, 16) that tested oral γ-selective retinoid agonists in patients with moderate to severe COPD did not find significant effects in the advanced stages of disease. The rationale for these trials was largely based on the postulated effects of retinoids on alveologenesis. However, if the main mechanism of action of retinoids is related to up-regulation of CC16 production, their effects would be expected to be strongest at the early and preclinical stages of COPD, when the irreversible airway remodeling and parenchymal destruction have not been established and a sufficient number of CC16-producing cells are still present in the airways of patients. Further studies are warranted to evaluate these scenarios.
Acknowledgments
Acknowledgment
The authors are grateful to Amber Spangenberg, B.S., and Marilyn Halonen, Ph.D., for completion of the assays for the in vivo studies.
Footnotes
Supported by award AI113526 from the National Institute of Allergy, Immunology and Infectious Disease, clinical innovative award 123055-CIA from the Flight Attendant Medical Research Institute, biomedical investigator award BIG-3064 from the Arizona Biomedical Research Commission, awards HL107188 and HL095021 from the NHLBI, and awards CA02374 and CA027502 from the National Cancer Institute, National Institutes of Health.
Originally Published in Press as DOI: 10.1164/rccm.201608-1611LE on February 23, 2017
Author disclosures are available with the text of this letter at www.atsjournals.org.
References
- 1.Singh G, Katyal SK. Secretory proteins of Clara cells and type II cells. In: Parent RA, editor. Comparative biology of the normal lung. Boca Raton, FL: CRC Press; 1992. pp. 93–108. [Google Scholar]
- 2.Laucho-Contreras ME, Polverino F, Gupta K, Taylor KL, Kelly E, Pinto-Plata V, Divo M, Ashfaq N, Petersen H, Stripp B, et al. Protective role for club cell secretory protein-16 (CC16) in the development of COPD. Eur Respir J. 2015;45:1544–1556. doi: 10.1183/09031936.00134214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Guerra S, Halonen M, Vasquez MM, Spangenberg A, Stern DA, Morgan WJ, Wright AL, Lavi I, Tarès L, Carsin AE, et al. Relation between circulating CC16 concentrations, lung function, and development of chronic obstructive pulmonary disease across the lifespan: a prospective study. Lancet Respir Med. 2015;3:613–620. doi: 10.1016/S2213-2600(15)00196-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Gamez AS, Gras D, Petit A, Knabe L, Molinari N, Vachier I, Chanez P, Bourdin A. Supplementing defect in club cell secretory protein attenuates airway inflammation in COPD. Chest. 2015;147:1467–1476. doi: 10.1378/chest.14-1174. [DOI] [PubMed] [Google Scholar]
- 5.Shi W, Chen F, Cardoso WV. Mechanisms of lung development: contribution to adult lung disease and relevance to chronic obstructive pulmonary disease. Proc Am Thorac Soc. 2009;6:558–563. doi: 10.1513/pats.200905-031RM. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Ochiai A. Differentiation of Clara cell (distal type) antigen in human fetal bronchial epithelial cell line (HFBE) Exp Toxicol Pathol. 1992;44:223–234. doi: 10.1016/S0940-2993(11)80232-0. [DOI] [PubMed] [Google Scholar]
- 7.McGowan S, Jackson SK, Jenkins-Moore M, Dai HH, Chambon P, Snyder JM. Mice bearing deletions of retinoic acid receptors demonstrate reduced lung elastin and alveolar numbers. Am J Respir Cell Mol Biol. 2000;23:162–167. doi: 10.1165/ajrcmb.23.2.3904. [DOI] [PubMed] [Google Scholar]
- 8.Rautalahti M, Virtamo J, Haukka J, Heinonen OP, Sundvall J, Albanes D, Huttunen JK. The effect of α-tocopherol and β-carotene supplementation on COPD symptoms. Am J Respir Crit Care Med. 1997;156:1447–1452. doi: 10.1164/ajrccm.156.5.96-11048. [DOI] [PubMed] [Google Scholar]
- 9.McKeever TM, Lewis SA, Smit HA, Burney P, Cassano PA, Britton J. A multivariate analysis of serum nutrient levels and lung function. Respir Res. 2008;9:67. doi: 10.1186/1465-9921-9-67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.van Eijl S, Mortaz E, Versluis C, Nijkamp FP, Folkerts G, Bloksma N. A low vitamin A status increases the susceptibility to cigarette smoke–induced lung emphysema in C57BL/6J mice. J Physiol Pharmacol. 2011;62:175–182. [PubMed] [Google Scholar]
- 11.Alberts D, Ranger-Moore J, Einspahr J, Saboda K, Bozzo P, Liu Y, Xu XC, Lotan R, Warneke J, Salasche S, et al. Safety and efficacy of dose-intensive oral vitamin A in subjects with sun-damaged skin. Clin Cancer Res. 2004;10:1875–1880. doi: 10.1158/1078-0432.ccr-03-0188. [DOI] [PubMed] [Google Scholar]
- 12.Apfel C, Bauer F, Crettaz M, Forni L, Kamber M, Kaufmann F, LeMotte P, Pirson W, Klaus M. A retinoic acid receptor α antagonist selectively counteracts retinoic acid effects. Proc Natl Acad Sci USA. 1992;89:7129–7133. doi: 10.1073/pnas.89.15.7129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Omenn GS, Goodman GE, Thornquist MD, Balmes J, Cullen MR, Glass A, Keogh JP, Meyskens FL, Valanis B, Williams JH, et al. Effects of a combination of β carotene and vitamin A on lung cancer and cardiovascular disease. N Engl J Med. 1996;334:1150–1155. doi: 10.1056/NEJM199605023341802. [DOI] [PubMed] [Google Scholar]
- 14.Alpha-Tocopherol Beta Carotene Cancer Prevention Study Group. The effect of vitamin E and beta carotene on the incidence of lung cancer and other cancers in male smokers. N Engl J Med. 1994;330:1029–1035. doi: 10.1056/NEJM199404143301501. [DOI] [PubMed] [Google Scholar]
- 15.Jones PW, Rames AD. Tesra (Treatment of Emphysema with a Selective Retinoid Agonist) study results [abstract] Am J Respir Crit Care Med. 2011;183:A6418. [Google Scholar]
- 16.Stolk J, Stockley RA, Stoel BC, Cooper BG, Piitulainen E, Seersholm N, Chapman KR, Burdon JG, Decramer M, Abboud RT, et al. Randomised controlled trial for emphysema with a selective agonist of the γ-type retinoic acid receptor. Eur Respir J. 2012;40:306–312. doi: 10.1183/09031936.00161911. [DOI] [PubMed] [Google Scholar]