Abstract
Rationale: Prior sepsis studies evaluating antibiotic timing have shown mixed results.
Objectives: To evaluate the association between antibiotic timing and mortality among patients with sepsis receiving antibiotics within 6 hours of emergency department registration.
Methods: Retrospective study of 35,000 randomly selected inpatients with sepsis treated at 21 emergency departments between 2010 and 2013 in Northern California. The primary exposure was antibiotics given within 6 hours of emergency department registration. The primary outcome was adjusted in-hospital mortality. We used detailed physiologic data to quantify severity of illness within 1 hour of registration and logistic regression to estimate the odds of hospital mortality based on antibiotic timing and patient factors.
Measurements and Main Results: The median time to antibiotic administration was 2.1 hours (interquartile range, 1.4–3.1 h). The adjusted odds ratio for hospital mortality based on each hour of delay in antibiotics after registration was 1.09 (95% confidence interval [CI], 1.05–1.13) for each elapsed hour between registration and antibiotic administration. The increase in absolute mortality associated with an hour’s delay in antibiotic administration was 0.3% (95% CI, 0.01–0.6%; P = 0.04) for sepsis, 0.4% (95% CI, 0.1–0.8%; P = 0.02) for severe sepsis, and 1.8% (95% CI, 0.8–3.0%; P = 0.001) for shock.
Conclusions: In a large, contemporary, and multicenter sample of patients with sepsis in the emergency department, hourly delays in antibiotic administration were associated with increased odds of hospital mortality even among patients who received antibiotics within 6 hours. The odds increased within each sepsis severity strata, and the increased odds of mortality were greatest in septic shock.
Keywords: sepsis, septic shock, antibacterial agents
At a Glance Commentary
Scientific Knowledge on the Subject
Prior work evaluating antibiotic timing in sepsis has shown mixed results and focused on more severely ill patients, often including patients with long delays in antibiotic administration. This has resulted in clinical equipoise regarding timing thresholds for antibiotic administration in sepsis.
What This Study Adds to the Field
We evaluated 35,000 patients treated within a contemporary multicenter sepsis quality improvement program using granular data including vital signs, laboratory values, and severity of illness indices. Although increased time to antibiotics after emergency department presentation was associated with increased mortality in all sepsis severity groups, the increase in the odds of mortality was greatest in septic shock.
It is widely accepted and biologically plausible that giving antibiotics as early as possible to patients with sepsis should improve their outcomes (1, 2). This has motivated international guidelines and quality benchmarks in sepsis care (1, 3, 4). It is further motivating several planned clinical trials of administering antibiotics to suspected patients with sepsis even in prehospital settings and before full hospital evaluation (5–7).
However, the desire to shorten the time to antibiotic administration may also incur potential harms and costs (8–10). Such harms might arise from a greater proportion of patients receiving antibiotics unnecessarily because less time is available for clinicians to evaluate alternate etiologies for the patient’s presentation (9). Unnecessary antibiotics can result in adverse patient-specific and community-level consequences (11, 12). Within resource-constrained settings like the emergency department (ED), the focus on antibiotic timing could also result in decreased attention to, and investment in, other time-sensitive patient needs (13). Prior efforts to mandate and report antibiotic timing in pneumonia were challenged for several reasons, including antibiotic overuse, and subsequently withdrawn (8–10).
In the absence of a randomized clinical trial to evaluate the benefits of early antibiotic administration, the current evidence remains mixed (14–32). Although no one disputes the need for prompt antibiotic therapy in patients with sepsis, additional study is necessary. Specifically, the availability of granular data from the electronic medical record now permits asking whether administering antibiotics within 1 hour provides more benefit than antibiotics given at 2 or 3 hours. Differences in outcomes related to decision-making in these very early intervals of care could have an important impact on clinical practice, care guidelines, and reporting metrics. We sought to examine data drawn from a multicenter setting to quantify the association between antibiotic timing and mortality among patients with sepsis of all severity levels. Some of the results of these studies have been previously reported in the form of an abstract at the American Thoracic Society International Conference in 2016 (33).
Methods
This study was approved by the Kaiser Permanente Northern California Institutional Review Board.
Subjects
We conducted a retrospective study of patients with sepsis aged greater than or equal to 18 years hospitalized through the ED at the 21 hospitals in the Kaiser Permanente Northern California integrated healthcare delivery system between July 1, 2010, and December 31, 2013. We based our sepsis definitions on prior international consensus definitions of sepsis because they were in clinical use during the period in which this study was conducted (34). We included patients with sepsis based on previously described methods including the presence of inpatient International Classification of Disease Clinical Modification ninth edition diagnosis codes of 038 and subtypes 995.91, 995.92, and/or 785.52 (35–37); and their receipt of antibiotics (i.e., antibacterial agents) within 6 hours of ED registration time. We randomly selected 5,000 patients hospitalized in 2010 and 10,000 patients hospitalized in each year between 2011 and 2013; we selected fewer cases from late 2010 because a regional sepsis quality improvement program was completing implementation.
Hospitalization Data
We linked patients with sepsis with corresponding electronic databases based on methods described in prior studies using electronic medical record flowsheet, laboratory, diagnosis, and treatment data (38–41), incorporating composite comorbid disease burden (Comorbidity Point Score 2) and acute severity of illness (Laboratory Acute Physiology Score 2 [LAPS2]) scores. We determined predicted hospital mortality with an automated hospital risk prediction model that demonstrated good discrimination in this population (C statistic, 0.80). We assessed intensive care unit admission from the ED using bed history records and determined patients’ resuscitation care order status at hospital admission as “full code” versus “not full code” (42). We ascertained hospital mortality from inpatient records (38–41).
ED Data
To minimize confounding and to optimize risk-adjustment of patients at the very beginning of their treatment course, we characterized patients’ ED clinical status based on detailed patient data from their first hour after registration. By including vital signs and treatment patterns within the first hour, we sought to digitally recapitulate, and adjust for, the clinical context that motivated decisions about antibiotic timing by emergency providers. In the first hour, we quantified the total number of vital signs recorded (heart rate, respiratory rate, systolic blood pressure, diastolic blood pressure, temperature) and the number of instances with patients’ respiratory rate greater than or equal to 22 breaths per minute, systolic blood pressure less than or equal to 90 mm Hg, and heart rate greater than or equal to 100 beats per minute. We then calculated the mean systolic blood pressure, heart rate, and respiratory rate values in the first hour. We also determined whether patients required invasive or noninvasive ventilation and counted the number of intravenous vasopressors required at 1 and 6 hours. Finally, we used illness acuity ratings assigned at the time of ED presentation based on the Emergency Severity Index (including resuscitative, emergent, urgent, less urgent, or nonurgent categories).
To further quantify sepsis-related organ dysfunction, we evaluated patient laboratory data within their first 6 hours after ED registration within binary categories, including band forms greater than or equal to 10%, platelets less than or equal to 100,000/μl, serum creatinine greater than or equal to 2 mg/dl, total bilirubin greater than or equal to 2 mg/dl, and international normalized ratio greater than or equal to two; missing values (ranging from 1.9% for creatinine to 71.2% for band forms) were imputed as normal. We used each patient’s first serum lactate value if collected within 6 hours; missing lactate values (n = 1,144; 3.3%) were imputed to the median based on severity strata. Finally, we determined abnormal mentation based on prior methods for evaluating ED Glasgow Coma Scores and/or nursing flowsheet entries (41).
Sepsis Severity Strata
We grouped patients into three levels of sepsis severity based on prevalent definitions in 2013: (1) septic shock, (2) severe sepsis, and (3) sepsis. We classified patients as having septic shock if they required vasopressors or had a first serum lactate value greater than or equal to 4 mmol/L. In the remaining sample, we classified patients as having severe sepsis if they had a lactate value greater than or equal to 2 mmol/L, had greater than or equal to one instance of hypotension, required invasive or noninvasive mechanical ventilation, or had laboratory-determined organ dysfunction (as described previously). We classified the remaining patients as having sepsis. We selected all variables describing clinical, organ failure, and severity strata characteristics a priori.
Antibiotic Administration
We calculated the time from ED registration to the administration of the first intravenous or enteral antibiotic in hours. We also determined the number of unique antibiotics administered within the first 6 hours. For multivariable regression analyses, we grouped patients’ antibiotic administration times within 30-minute increments from 0–6 hours after ED registration. For the purposes of graphical demonstration, we grouped antibiotic administration within hourly increments over the 6-hour interval.
Statistical Analysis
Continuous data are presented as mean ± SD or median (interquartile range). Categorical data are presented as number (%). We compared characteristics between patients based on sepsis severity strata with analysis of variance or chi-square tests. We displayed time to antibiotic administration using kernel density plots and compared time to antibiotics between sepsis severity strata with the Kruskal-Wallis rank test.
We estimated the impact of antibiotic timing on risk-adjusted hospital mortality using logistic regression based on the clinical variables described previously. We assessed for collinearity between variables and removed those with a correlation coefficient greater than or equal to 0.6 (predicted hospital mortality and vital sign counts). Our fully adjusted model included patient characteristics and severity of illness (age, sex, LAPS2, Comorbidity Point Score 2, Emergency Severity Index category, code status), treatments (vasopressors, invasive ventilation, or noninvasive ventilation at 1 h), mean vital sign values, sepsis severity strata, the presence of abnormal laboratory values, and hospital facility. To assess how the association between the timing of antibiotics and mortality differed across sepsis severity groups, we assessed the fully adjusted model within each severity strata subgroup separately. To evaluate how potential antibiotic appropriateness impacted outcomes, we conducted a post hoc subgroup analysis of two cohorts determined based on the administration of a broad versus a narrow first antibiotic (see Table E1 in the online supplement). We conducted analyses using STATA/SE version 13.1 (StataCorp, College Station, TX) and considered a P value less than or equal to 0.05 to be significant.
Results
Of the 35,000 patients in our sample, 13.3% (n = 4,668) met criteria for septic shock and 52.0% (n = 18,210) met criteria for severe sepsis (Table 1). Observed mortality was 3.9%, 8.8%, and 26.0% in patients with sepsis, severe sepsis, and septic shock, respectively. Including only full code patients, mortality was 2.4%, 8.5%, and 21.6%, respectively. All comparisons between groups were highly significant. For example, the frequency of elevated band forms was 10.1% (n = 1,229) in sepsis compared with 31.4% (n = 1,466) in septic shock. The time to the first lactate value was shortest in septic shock (0.8 [0.5–1.7] h); shock patients also had the highest mean lactate value (4.6 [4.0–5.9] mmol/L). Among patients with septic shock, 2.4% and 43.4% had vasopressors initiated within 1 and 6 hours, respectively. Among patients with septic shock who were full code at admission, 81.3% were admitted directly to the intensive care unit.
Table 1.
Clinical Characteristics, Stratified by Sepsis Severity Level
| Overall (n = 35,000) | Sepsis Severity Strata |
|||
|---|---|---|---|---|
| Sepsis (n = 12,122) | Severe Sepsis (n = 18,210) | Septic Shock (n = 4,668) | ||
| Age, yr | 73 (60–83) | 72 (56–83) | 74 (62–83) | 73 (61–83) |
| Male | 16,961 (48.5) | 5,235 (43.2) | 9,322 (51.2) | 2,404 (51.5) |
| Full code (42) | 25,671 (73.4) | 9,133 (75.3) | 13,130 (72.1) | 3,408 (73.0) |
| LAPS2 value | 100 (74–129) | 80 (59–104) | 104 (81–129) | 149 (122–177) |
| COPS2 value | 56.9 ± 51.1 | 46.3 ± 45.5 | 63.5 ± 52.9 | 58.8 ± 53.3 |
| Predicted mortality, % | 9.0 ± 12.3 | 4.4 ± 6.3 | 8.6 ± 10.6 | 22.2 ± 19.2 |
| ED acuity level | |
|||
| Resuscitative | 1,086 (3.1) | 84 (0.7) | 478 (2.6) | 524 (11.2) |
| Emergent | 14,248 (40.7) | 3,766 (31.1) | 7,743 (42.5) | 2,739 (58.7) |
| No. of instances in 1 h | |
|
||
| Vital signs recorded | 15 (9–19) | 11 (9–17) | 15 (9–20) | 18 (12–28) |
| SBP <90 mm Hg | 0 (0–0) | 0 (0–0) | 0 (0–0) | 0 (0–1) |
| RR ≥22 breaths/min | 0 (0–1) | 0 (0–1) | 0 (0–1) | 1 (0–2) |
| HR ≥90 beats/min | 1 (0–2) | 1 (0–2) | 1 (0–2) | 2 (1–3) |
| Mechanical ventilation | 495 (1.4) | 0 (0.0) | 195 (1.1) | 300 (6.4) |
| Noninvasive ventilation | 1,208 (3.5) | 0 (0.0) | 858 (4.7) | 350 (7.5) |
| Vasopressor use | 110 (0.3) | 0 (0.0) | 0 (0.0) | 110 (2.4) |
| Mean values in first hour | |
|||
| SBP, mm Hg | 127.3 ± 25.5 | 133.3 ± 21.4 | 127.0 ± 25.9 | 113.4 ± 27.7 |
| HR, beats/min | 101.8 ± 21.0 | 101.5 ± 19.1 | 100.9 ± 21.1 | 106.4 ± 24.5 |
| RR, breaths/min | 21.4 ± 5.1 | 20.6 ± 4.3 | 21.5 ± 5.1 | 23.4 ± 6.3 |
| Laboratory abnormalities | |
|||
| Bands ≥10% | 5,550 (15.9) | 1,229 (10.1) | 2,855 (15.7) | 1,466 (31.4) |
| Creatinine ≥2.0 mg/dl | 5,593 (16.0) | 0 (0.0) | 4,181 (23.0) | 1,412 (30.3) |
| INR ≥1.5 | 4,757 (13.6) | 0 (0.0) | 3,690 (20.3) | 1,067 (22.9) |
| Platelets ≤100,000 | 2,661 (7.6) | 0 (0.0) | 2,006 (11.0) | 655 (14.0) |
| Bilirubin ≥2.0 g/dl | 1,974 (5.6) | 0 (0.0) | 1,394 (7.7) | 580 (12.4) |
| First lactate value, mmol/L | 1.8 (1.2–2.7) | 1.3 (1.0–1.5) | 2.2 (1.5–2.7) | 4.6 (4.0–5.9) |
| Time to first lactate, h | 1.0 (0.6–2.1) | 1.1 (0.6–2.6) | 0.9 (0.6–2.0) | 0.8 (0.5–1.7) |
| First non-ED hospital unit | |
|||
| Intensive care | 7,221 (20.6) | 760 (6.3) | 3,112 (17.1) | 3,349 (71.7) |
| Hospital mortality | 3,285 (9.4) | 474 (3.9) | 1,596 (8.8) | 1,215 (26.0) |
Definition of abbreviations: COPS2 = Comorbidity Point Score, version 2; ED = emergency department; HR = heart rate; INR = international normalized ratio; LAPS2 = Laboratory and Acute Physiology Score, version 2; RR = respiratory rate; SBP = systolic blood pressure.
Continuous data are presented as mean ± SD or median (interquartile range). Categorical data are presented as number (%). All comparisons between sepsis severity strata were significant to a P < 0.001. ED acuity level is based on the Emergency Severity Index.
Antibiotic Timing and Use
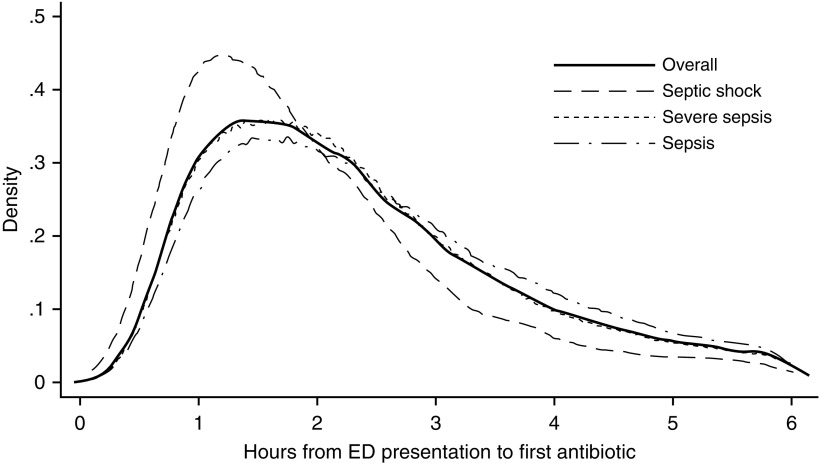
Overall, the median time to antibiotic administration was 2.1 hours (interquartile range, 1.4–3.1 h) (Figure 1); this timing did not differ across years. The median time to antibiotics was shortest in patients with septic shock (1.7 h) and longest in patients with sepsis (2.3 h; P < 0.001). Patients receiving earlier antibiotics had greater severity of illness compared with those receiving later antibiotics based on acuity level, acute severity of illness (LAPS2), vital signs, and laboratory values (see Table E1). They also had the highest unadjusted mortality (11.4% and 9.5% for Hour 1 and Hour 2 patients, respectively). In total, 42.2% of patients received one antibiotic and 42.5% received two antibiotics (Table 2). The frequency of receiving two or more antibiotics increased as sepsis severity increased (52.0% in sepsis vs. 71.7% in septic shock; P < 0.01). The most common antibiotic used in all groups was ceftriaxone, and the second most common antibiotic varied among azithromycin (sepsis), vancomycin (severe sepsis), and pipercillin-tazobactam (septic shock).
Figure 1.
Kernel density plot showing time to first antibiotic administration from emergency department registration. Distribution in the overall cohort is shown with a solid line, the septic shock cohort is shown in a dashed line, the severe sepsis cohort with a dotted line, and the sepsis cohort with a dashed-dotted line. ED = emergency department.
Table 2.
Antibiotic Usage (Number and Percentage) in the Cohort Stratified by Sepsis Severity level
| Overall (n = 35,000) | Sepsis Severity |
|||
|---|---|---|---|---|
| Sepsis (n = 12,122) | Severe Sepsis (n = 18,210) | Septic Shock (n = 4,668) | ||
| Unique antibiotics administered within 6 h, n (%) | ||||
| One | 14,767 (42.2) | 5,815 (48.0) | 7,632 (41.9) | 1,320 (28.3) |
| Two | 14,869 (42.5) | 5,053 (41.7) | 7,796 (42.8) | 2,020 (43.3) |
| Three or more | 5,364 (15.3) | 1,254 (10.3) | 2,782 (15.3) | 1,328 (28.5) |
| Most common antibiotics (n; %) | ||||
| First | Ceftriaxone (16,796; 48.0) | Ceftriaxone (5,846; 48.2) | Ceftriaxone ( 8,754; 48.1) | Ceftriaxone (2,196; 47.0) |
| Second | Vancomycin (8,840; 25.3) | Azithromycin (2,370; 19.6) | Vancomycin (4,721; 25.9) | Pip/Tazo (1,819; 39.0) |
| Third | Pip/Tazo (8,131; 23.2) | Vancomycin (2,348; 19.4) | Pip/Tazo (4,264; 23.4) | Vancomycin (1,771; 37.9) |
| Fourth | Azithromycin (6,706; 19.2) | Pip/Tazo ( 2,048; 16.9) | Azithromycin (3,438; 18.9) | Azithromycin (898; 19.2) |
| Fifth | Ciprofloxacin (5,435; 15.5) | Ciprofloxacin (1,961; 16.2) | Ciprofloxacin (2,753; 15.1) | Ciprofloxacin (721; 15.4) |
Definition of abbreviation: Pip/Tazo = pipercillin-tazobactam.
Hospital Mortality
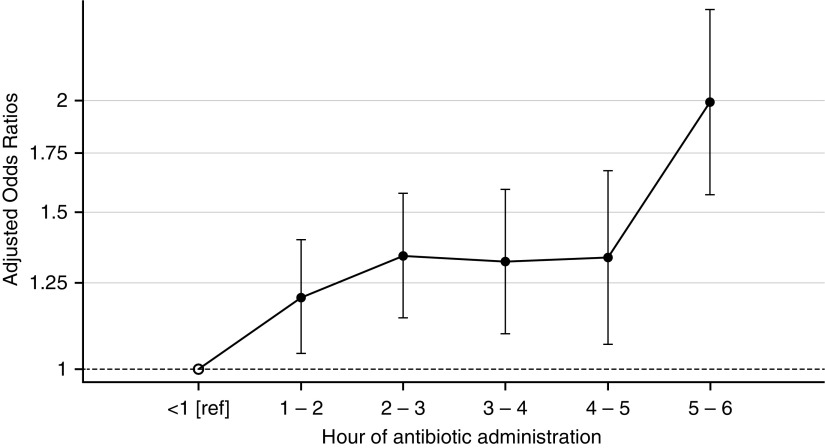
The fully adjusted odds ratio for hospital mortality based on antibiotic timing was 1.09 (95% confidence interval [CI], 1.05–1.13) per elapsed hour after ED presentation (Table 3). The odds ratios were similar in patients with sepsis (1.09; 95% CI, 1.00–1.19; P = 0.046) and severe sepsis (1.07; 95% CI, 1.01–1.24; P = 0.014), whereas they were increased in septic shock (1.14; 95% CI, 1.06–1.23; P = 0.001). The absolute increase in mortality associated with an hour’s delay in antibiotic administration was 0.3% (95% CI, 0.01–0.6%; P = 0.04) for sepsis, 0.4% (95% CI, 0.1–0.8%; P = 0.02) for severe sepsis, and 1.8% (95% CI, 0.8–3.0%; P = 0.001) for shock. Figure 2 displays the adjusted odds ratios based on hourly increments of antibiotic administration time each compared with the reference value of less than 1 hour. In subgroup analysis, delays in broad antibiotic administration were associated with an increased effect size (1.08; 95% CI, 1.01–1.16; P = 0.02) compared with delays in narrow antibiotic administration (odds ratio, 1.05; 95% CI, 1.01–1.10; P = 0.03).
Table 3.
Odds Ratios for Hospital Mortality Based on the Time of Antibiotic Administration in Unadjusted and Adjusted Logistic Regression Models
| Model | Odds Ratio for Hospital Mortality, per Elapsed Hour until Antibiotic Administration | 95% CI | P Value |
|---|---|---|---|
| Unadjusted | 0.89 | 0.86–0.91 | <0.001 |
| + Sepsis severity strata | 0.96 | 0.93–0.99 | 0.013 |
| + Severity of illness | 1.08 | 1.04–1.12 | <0.001 |
| + Demographics | 1.09 | 1.05–1.13 | <0.001 |
| Fully adjusted model, in each subgroup |
|||
| Sepsis only | 1.09 | 1.00–1.19 | 0.046 |
| Severe sepsis only | 1.07 | 1.01–1.24 | 0.014 |
| Septic shock only | 1.14 | 1.06–1.23 | 0.001 |
Definition of abbreviation: CI = confidence interval.
Beyond the unadjusted model, each subsequent model includes an additional set of covariates, including sepsis severity strata (categorized as sepsis, severe sepsis, or septic shock), severity of illness (Laboratory and Acute Physiology Score, version 2; Emergency Severity Index; mean vital sign values; presence of altered mental status; laboratory data; need for direct intensive care unit transfer; number of vasopressors given within the first h; and number of antibiotics given within 6 h), and demographics (age; sex; code status; Comorbidity Point Score, version 2; and facility). The results of the fully adjusted model within each sepsis severity subgroup are shown at the bottom of the table.
Figure 2.
Adjusted odds ratios for hospital mortality comparing patients within each hourly antibiotic administration group with the reference group of patients given antibiotics in <1 hour. The y-axis is on logarithmic scale and the error bars represent 95% confidence intervals.
Discussion
In this study, we used a large, multicenter, and contemporary sample of patients with sepsis to evaluate the association between early antibiotic timing and hospital mortality. We found that each elapsed hour between ED registration and antibiotic administration was associated with a 9% increase in the odds of mortality. This relative effect was similar for patients with sepsis and severe sepsis, whereas it was largest for patients with septic shock.
Although no one recommends delaying antibiotics for patients with sepsis, the existing evidence supporting the mortality benefits of earlier antibiotic administration is mixed (14–30, 32). In a frequently cited study, Kumar and coworkers (19) retrospectively evaluated 2,154 critically ill patients with septic shock between 1989 and 2004. After controlling for measures of illness severity and management decisions, they found that increasing time intervals between the first episode of persistent hypotension and the administration of effective antibiotics was associated with increased mortality. Notably, however, the median time from hypotension to antibiotic administration was 6 hours after the recognition of shock and the overall mortality rate was 56.2%, likely representing the much less aggressive approach to sepsis care from a prior era and heavy selection criteria to enter the cohort.
More recently, Ferrer and coworkers (20) conducted a retrospective analysis of Surviving Sepsis Campaign data including 17,990 patients from 165 intensive care units between 2005 and 2010. The adjusted odds of hospital mortality increased as the time from patient triage or sepsis identification to antibiotics increased. This international study also captured a more contemporary approach to sepsis care, with only 12% of patients receiving antibiotics greater than 6 hours after presentation and a 29.7% overall mortality rate. Although one of the study’s strengths was that it considered patients with sepsis identified in a variety of different hospital settings, it was nonetheless limited to patients eventually admitted to the intensive care unit. As a result, the study only addresses antibiotic timing in the most severely ill patients with sepsis, who make up a modest fraction of all sepsis inpatients (35).
In a recently published metaanalysis of 11 studies by Sterling and coworkers (15), the authors found no significant association between early antibiotics and improved mortality. Including data drawn from more than 16,000 patients in six studies, the authors found that the odds ratio for mortality among patients receiving antibiotics more than 3 hours after triage time was 1.16 (P = 0.21) compared with patients receiving antibiotics in less than 3 hours. However, the lack of patient-level data, heterogeneity in the eligible studies, and a smaller sample size may have limited the power to detect statistical significance for the point estimates, which favored earlier antibiotics and could still be associated with meaningful absolute population-level mortality benefits given sepsis’ high prevalence. Other smaller studies have reported similar findings (14, 17, 18, 28, 29, 31, 32, 43).
The current study seeks to address the limitations of prior studies. First, we evaluated a multicenter sample of patients treated within the contemporary framework of a sepsis quality improvement program. We sought to evaluate whether antibiotic timing continued to show an association with improved outcomes in the modern era of care, especially because some earlier elements of sepsis care no longer seem to impact patient outcomes (44). We further chose to limit our evaluation to patients who received antibiotics within 6 hours because, in the context of aggressive screening and treatment, patients who receive antibiotics later than 6 hours are likely to have demonstrated diagnostic uncertainty or received potentially delayed care (1). Even in the setting where the median time to antibiotics was 2.1 hours from ED registration, early antibiotics were significantly associated with improved survival.
Second, we evaluated patients presenting with variable sepsis severity, most of who were not treated in critical care settings. Although critically ill patients with sepsis have high mortality, they comprise a relatively small proportion of all patients with sepsis based on 2001 consensus definitions (34). We sought to demonstrate whether the biologically plausible principle of early infection control with antibiotics would show consistent benefits for all infected patients with systemic inflammation. We found that early antibiotics were associated with improved survival among all patients with sepsis, a finding that has broad implications for a large cohort of inpatients whom together comprise as many as half of all hospital deaths in the United States (35). However, the increasing odds of mortality associated with later antibiotics were most prominent among patients with septic shock for whom each hourly delay was associated with a 1.8% increase in hospital mortality.
Finally, we addressed prior limitations by using inpatient data characterized by breadth (drawn from a large population sample of 35,000 hospitalizations) and depth (including detailed physiologic and treatment measures). We also included a wide variety of predictors that would be clinically relevant for emergency providers in the midst of early decision-making about antibiotic administration. Our findings demonstrate the benefits of leveraging already available electronic medical record data from narrow time intervals to address confounding and reliably evaluate highly time-sensitive outcomes.
Our findings support currently held beliefs that administering early antibiotics to infected patients with systemic inflammation is beneficial for reducing mortality. Our study also helps address prior conflicting evidence and redefines what constitutes equipoise about the exact timing thresholds that are necessary to ensure optimal care. This is especially relevant because a clinical trial that randomizes patients with sepsis to delayed antibiotics is unlikely to be deemed ethical, at least while the harms of indiscriminate antibiotics remain incompletely characterized.
The current study does not resolve all questions about antibiotic timing (e.g., are antibiotics given at 2 h more beneficial than those given at 3 or 4 h) because the odds ratio confidence limits we observed between 2 and 5 hours are overlapping. These data could suggest that among patients with clear evidence of septic shock, earliest antibiotics confer the greatest mortality benefits. However, among patients with less diagnostic certainty for sepsis, modest delays in antibiotics may not substantially increase mortality. This finding has important implications for antibiotic timing when it is placed within the larger context of competing ED priorities and resource needs. Clinical trials that examine antibiotic timing intervals when sepsis is uncertain and/or cost-effectiveness studies evaluating the costs and benefits of accelerated antibiotic pathways may prove highly useful.
Our study was limited in several important ways. First, we evaluated a sample of patients treated at a network of hospitals with an existing sepsis performance improvement program. The mortality among full code patients with septic shock (21.6%) was similar to that reported in recent clinical trials (44–47). Thus, our results may be less generalizable to hospitals where sepsis care occurs outside of focused sepsis improvement programs. Second, we were not able to adjust for concomitant sepsis treatments administered to patients along with antibiotics. For example, patients receiving earlier antibiotics may have also received other treatments, such as fluid resuscitation, earlier, such that early antibiotics are only a marker of an overall higher quality of sepsis care. We were also not able to adjust for patients who received preexisting antibiotics. Third, we did not specifically evaluate the adequacy of antibiotics based on microbiologic results and specific susceptibility patterns. Fourth, we limited our evaluation to patients who received antibiotics within 6 hours of ED presentation because this represents a contemporary and guideline-concordant standard of sepsis care. Fifth, we identified patients with sepsis with diagnostic codes that may lack sensitivity for certain patient subgroups (e.g., low-risk patients with sepsis). Finally, we did not evaluate the impact of antibiotic timing outside of the ED because the recognition and treatment of sepsis in other hospital settings is highly variable and less amenable to robust analysis.
In summary, in a large, contemporary, multicenter sample of patients with sepsis admitted through the ED, we found that each elapsed hour between presentation and antibiotic administration was associated with a 9% increase in the odds of mortality in patients with sepsis of all severity strata. Although antibiotics given within the first hour of registration were associated with the greatest benefit, antibiotics given between hours 2 and 5 were associated with similar odds of mortality. Earlier antibiotics conferred the greatest absolute benefit in patients with septic shock.
Acknowledgments
Acknowledgment
The authors thank the hundreds of clinicians and support staff engaged in ongoing sepsis quality improvement work across Kaiser Permanente Northern California.
Footnotes
Supported by the Permanente Medical Group and Kaiser Foundation Hospitals, National Institute of General Medical Sciences (K23GM112018, V.X.L.), and Veterans Affairs Health Services Research and Development Service (IIR 13-079, T.J.I.). This work does not necessarily represent the views of the U.S. Government or the Department of Veterans Affairs.
Author Contributions: Study conception and design, V.X.L., V.F.-S., T.J.I., and G.J.E. Acquisition, analysis, or interpretation of data, V.X.L., V.F.-S., J.D.G., J.M.B., T.J.I., J.B., and G.J.E. Drafting and revision of the work as well as final approval of the submitted manuscript, all authors.
This article has an online supplement, which is accessible from this issue's table of contents at www.atsjournals.org
Originally Published in Press as DOI: 10.1164/rccm.201609-1848OC on March 27, 2017
Author disclosures are available with the text of this article at www.atsjournals.org.
References
- 1.Dellinger RP, Levy MM, Rhodes A, Annane D, Gerlach H, Opal SM, Sevransky JE, Sprung CL, Douglas IS, Jaeschke R, et al. Surviving Sepsis Campaign Guidelines Committee including the Pediatric Subgroup. Surviving sepsis campaign: international guidelines for management of severe sepsis and septic shock: 2012. Crit Care Med. 2013;41:580–637. doi: 10.1097/CCM.0b013e31827e83af. [DOI] [PubMed] [Google Scholar]
- 2.Cohen J, Vincent JL, Adhikari NK, Machado FR, Angus DC, Calandra T, Jaton K, Giulieri S, Delaloye J, Opal S, et al. Sepsis: a roadmap for future research. Lancet Infect Dis. 2015;15:581–614. doi: 10.1016/S1473-3099(15)70112-X. [DOI] [PubMed] [Google Scholar]
- 3.Seymour CW, Rosengart MR. Septic shock: advances in diagnosis and treatment. JAMA. 2015;314:708–717. doi: 10.1001/jama.2015.7885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Angus DC, van der Poll T. Severe sepsis and septic shock. N Engl J Med. 2013;369:2063. doi: 10.1056/NEJMc1312359. [DOI] [PubMed] [Google Scholar]
- 5.Seymour CW, Cooke CR, Heckbert SR, Spertus JA, Callaway CW, Martin-Gill C, Yealy DM, Rea TD, Angus DC. Prehospital intravenous access and fluid resuscitation in severe sepsis: an observational cohort study. Crit Care. 2014;18:533. doi: 10.1186/s13054-014-0533-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Seymour CW, Rea TD, Kahn JM, Walkey AJ, Yealy DM, Angus DC. Severe sepsis in pre-hospital emergency care: analysis of incidence, care, and outcome. Am J Respir Crit Care Med. 2012;186:1264–1271. doi: 10.1164/rccm.201204-0713OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Nanayakkara PW. Prehospital Antibiotics Against Sepsis Trial (PHANTASi). NCT01988428. [accessed 2016 Jan 10]. Available from: https://clinicaltrials.gov/
- 8.Rhee C, Gohil S, Klompas M. Regulatory mandates for sepsis care: reasons for caution. N Engl J Med. 2014;370:1673–1676. doi: 10.1056/NEJMp1400276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Wachter RM, Flanders SA, Fee C, Pronovost PJ. Public reporting of antibiotic timing in patients with pneumonia: lessons from a flawed performance measure. Ann Intern Med. 2008;149:29–32. doi: 10.7326/0003-4819-149-1-200807010-00007. [DOI] [PubMed] [Google Scholar]
- 10.National Action Plan for Combating Antibiotic-Resistant Bacteria. [accessed 2016 Jan 10]. Available from: https://www.cdc.gov/drugresistance/pdf/national_action_plan_for_combating_antibotic-resistant_bacteria.pdf.
- 11.Davies J, Davies D. Origins and evolution of antibiotic resistance. Microbiol Mol Biol Rev. 2010;74:417–433. doi: 10.1128/MMBR.00016-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Neuhauser MM, Weinstein RA, Rydman R, Danziger LH, Karam G, Quinn JP. Antibiotic resistance among gram-negative bacilli in US intensive care units: implications for fluoroquinolone use. JAMA. 2003;289:885–888. doi: 10.1001/jama.289.7.885. [DOI] [PubMed] [Google Scholar]
- 13.Pines JM, Hollander JE, Lee H, Everett WW, Uscher-Pines L, Metlay JP. Emergency department operational changes in response to pay-for-performance and antibiotic timing in pneumonia. Acad Emerg Med. 2007;14:545–548. doi: 10.1197/j.aem.2007.01.022. [DOI] [PubMed] [Google Scholar]
- 14.Bruce HR, Maiden J, Fedullo PF, Kim SC. Impact of nurse-initiated ED sepsis protocol on compliance with sepsis bundles, time to initial antibiotic administration, and in-hospital mortality. J Emerg Nurs. 2015;41:130–137. doi: 10.1016/j.jen.2014.12.007. [DOI] [PubMed] [Google Scholar]
- 15.Sterling SA, Miller WR, Pryor J, Puskarich MA, Jones AE. The impact of timing of antibiotics on outcomes in severe sepsis and septic shock: a systematic review and meta-analysis. Crit Care Med. 2015;43:1907–1915. doi: 10.1097/CCM.0000000000001142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Wisdom A, Eaton V, Gordon D, Daniel S, Woodman R, Phillips C. INITIAT-E.D.: Impact of timing of INITIation of Antibiotic Therapy on mortality of patients presenting to an Emergency Department with sepsis. Emerg Med Australas. 2015;27:196–201. doi: 10.1111/1742-6723.12394. [DOI] [PubMed] [Google Scholar]
- 17.Ryoo SM, Kim WY, Sohn CH, Seo DW, Koh JW, Oh BJ, Lim KS. Prognostic value of timing of antibiotic administration in patients with septic shock treated with early quantitative resuscitation. Am J Med Sci. 2015;349:328–333. doi: 10.1097/MAJ.0000000000000423. [DOI] [PubMed] [Google Scholar]
- 18.Gaieski DF, Mikkelsen ME, Band RA, Pines JM, Massone R, Furia FF, Shofer FS, Goyal M. Impact of time to antibiotics on survival in patients with severe sepsis or septic shock in whom early goal-directed therapy was initiated in the emergency department. Crit Care Med. 2010;38:1045–1053. doi: 10.1097/CCM.0b013e3181cc4824. [DOI] [PubMed] [Google Scholar]
- 19.Kumar A, Roberts D, Wood KE, Light B, Parrillo JE, Sharma S, Suppes R, Feinstein D, Zanotti S, Taiberg L, et al. Duration of hypotension before initiation of effective antimicrobial therapy is the critical determinant of survival in human septic shock. Crit Care Med. 2006;34:1589–1596. doi: 10.1097/01.CCM.0000217961.75225.E9. [DOI] [PubMed] [Google Scholar]
- 20.Ferrer R, Martin-Loeches I, Phillips G, Osborn TM, Townsend S, Dellinger RP, Artigas A, Schorr C, Levy MM. Empiric antibiotic treatment reduces mortality in severe sepsis and septic shock from the first hour: results from a guideline-based performance improvement program. Crit Care Med. 2014;42:1749–1755. doi: 10.1097/CCM.0000000000000330. [DOI] [PubMed] [Google Scholar]
- 21.Ferrer R, Artigas A, Suarez D, Palencia E, Levy MM, Arenzana A, Pérez XL, Sirvent JM Edusepsis Study Group. Effectiveness of treatments for severe sepsis: a prospective, multicenter, observational study. Am J Respir Crit Care Med. 2009;180:861–866. doi: 10.1164/rccm.200812-1912OC. [DOI] [PubMed] [Google Scholar]
- 22.Larché J, Azoulay E, Fieux F, Mesnard L, Moreau D, Thiery G, Darmon M, Le Gall JR, Schlemmer B. Improved survival of critically ill cancer patients with septic shock. Intensive Care Med. 2003;29:1688–1695. doi: 10.1007/s00134-003-1957-y. [DOI] [PubMed] [Google Scholar]
- 23.Meehan TP, Fine MJ, Krumholz HM, Scinto JD, Galusha DH, Mockalis JT, Weber GF, Petrillo MK, Houck PM, Fine JM. Quality of care, process, and outcomes in elderly patients with pneumonia. JAMA. 1997;278:2080–2084. [PubMed] [Google Scholar]
- 24.Houck PM, Bratzler DW, Nsa W, Ma A, Bartlett JG. Timing of antibiotic administration and outcomes for Medicare patients hospitalized with community-acquired pneumonia. Arch Intern Med. 2004;164:637–644. doi: 10.1001/archinte.164.6.637. [DOI] [PubMed] [Google Scholar]
- 25.Iregui M, Ward S, Sherman G, Fraser VJ, Kollef MH. Clinical importance of delays in the initiation of appropriate antibiotic treatment for ventilator-associated pneumonia. Chest. 2002;122:262–268. doi: 10.1378/chest.122.1.262. [DOI] [PubMed] [Google Scholar]
- 26.Levy MM, Rhodes A, Phillips GS, Townsend SR, Schorr CA, Beale R, Osborn T, Lemeshow S, Chiche JD, Artigas A, et al. Surviving Sepsis Campaign: association between performance metrics and outcomes in a 7.5-year study. Crit Care Med. 2015;43:3–12. doi: 10.1097/CCM.0000000000000723. [DOI] [PubMed] [Google Scholar]
- 27.Karvellas CJ, Abraldes JG, Arabi YM, Kumar A Cooperative Antimicrobial Therapy of Septic Shock (CATSS) Database Research Group. Appropriate and timely antimicrobial therapy in cirrhotic patients with spontaneous bacterial peritonitis-associated septic shock: a retrospective cohort study. Aliment Pharmacol Ther. 2015;41:747–757. doi: 10.1111/apt.13135. [DOI] [PubMed] [Google Scholar]
- 28.Puskarich MA, Trzeciak S, Shapiro NI, Arnold RC, Horton JM, Studnek JR, Kline JA, Jones AE Emergency Medicine Shock Research Network (EMSHOCKNET) Association between timing of antibiotic administration and mortality from septic shock in patients treated with a quantitative resuscitation protocol. Crit Care Med. 2011;39:2066–2071. doi: 10.1097/CCM.0b013e31821e87ab. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Bloos F, Thomas-Rüddel D, Rüddel H, Engel C, Schwarzkopf D, Marshall JC, Harbarth S, Simon P, Riessen R, Keh D, et al. MEDUSA Study Group. Impact of compliance with infection management guidelines on outcome in patients with severe sepsis: a prospective observational multi-center study. Crit Care. 2014;18:R42. doi: 10.1186/cc13755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.de Groot B, Ansems A, Gerling DH, Rijpsma D, van Amstel P, Linzel D, Kostense PJ, Jonker M, de Jonge E. The association between time to antibiotics and relevant clinical outcomes in emergency department patients with various stages of sepsis: a prospective multi-center study. Crit Care. 2015;19:194. doi: 10.1186/s13054-015-0936-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Vilella AL, Seifert CF. Timing and appropriateness of initial antibiotic therapy in newly presenting septic patients. Am J Emerg Med. 2014;32:7–13. doi: 10.1016/j.ajem.2013.09.008. [DOI] [PubMed] [Google Scholar]
- 32.Burnham JP, Lane MA, Kollef MH. Impact of sepsis classification and multidrug-resistance status on outcome among patients treated with appropriate therapy. Crit Care Med. 2015;43:1580–1586. doi: 10.1097/CCM.0000000000001013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Fielding-Singh VGJ, Baker JM, Escobar GJ, Liu V. The timing of early antibiotics and hospital mortality in sepsis [abstract] Am J Respir Crit Care Med. 2016;193:A2741. doi: 10.1164/rccm.201609-1848OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Levy MM, Fink MP, Marshall JC, Abraham E, Angus D, Cook D, Cohen J, Opal SM, Vincent JL, Ramsay G SCCM/ESICM/ACCP/ATS/SIS. 2001 SCCM/ESICM/ACCP/ATS/SIS International Sepsis Definitions Conference. Crit Care Med. 2003;31:1250–1256. doi: 10.1097/01.CCM.0000050454.01978.3B. [DOI] [PubMed] [Google Scholar]
- 35.Liu V, Escobar GJ, Greene JD, Soule J, Whippy A, Angus DC, Iwashyna TJ. Hospital deaths in patients with sepsis from 2 independent cohorts. JAMA. 2014;312:90–92. doi: 10.1001/jama.2014.5804. [DOI] [PubMed] [Google Scholar]
- 36.Liu V, Morehouse JW, Soule J, Whippy A, Escobar GJ. Fluid volume, lactate values, and mortality in sepsis patients with intermediate lactate values. Ann Am Thorac Soc. 2013;10:466–473. doi: 10.1513/AnnalsATS.201304-099OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Liu VX, Morehouse JW, Marelich GP, Soule J, Russell T, Skeath M, Adams C, Escobar GJ, Whippy A. Multicenter implementation of a treatment bundle for sepsis patients with intermediate lactate values. Am J Respir Crit Care Med. 2016;193:1264–1270. doi: 10.1164/rccm.201507-1489OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Escobar GJ, LaGuardia JC, Turk BJ, Ragins A, Kipnis P, Draper D. Early detection of impending physiologic deterioration among patients who are not in intensive care: development of predictive models using data from an automated electronic medical record. J Hosp Med. 2012;7:388–395. doi: 10.1002/jhm.1929. [DOI] [PubMed] [Google Scholar]
- 39.Escobar GJ, Fireman BH, Palen TE, Gardner MN, Lee JY, Clark MP, Kipnis P. Risk adjusting community-acquired pneumonia hospital outcomes using automated databases. Am J Manag Care. 2008;14:158–166. [PubMed] [Google Scholar]
- 40.Escobar GJ, Greene JD, Scheirer P, Gardner MN, Draper D, Kipnis P. Risk-adjusting hospital inpatient mortality using automated inpatient, outpatient, and laboratory databases. Med Care. 2008;46:232–239. doi: 10.1097/MLR.0b013e3181589bb6. [DOI] [PubMed] [Google Scholar]
- 41.Escobar GJ, Gardner MN, Greene JD, Draper D, Kipnis P. Risk-adjusting hospital mortality using a comprehensive electronic record in an integrated health care delivery system. Med Care. 2013;51:446–453. doi: 10.1097/MLR.0b013e3182881c8e. [DOI] [PubMed] [Google Scholar]
- 42.Kim YS, Escobar GJ, Halpern SD, Greene JD, Kipnis P, Liu V. The natural history of changes in preferences for life-sustaining treatments and implications for inpatient mortality in younger and older hospitalized adults. J Am Geriatr Soc. 2016;64:981–989. doi: 10.1111/jgs.14048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Yokota PK, Marra AR, Martino MD, Victor ES, Durão MS, Edmond MB, dos Santos OF. Impact of appropriate antimicrobial therapy for patients with severe sepsis and septic shock--a quality improvement study. PLoS One. 2014;9:e104475. doi: 10.1371/journal.pone.0104475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Angus DC, Barnato AE, Bell D, Bellomo R, Chong CR, Coats TJ, Davies A, Delaney A, Harrison DA, Holdgate A, et al. A systematic review and meta-analysis of early goal-directed therapy for septic shock: the ARISE, ProCESS and ProMISe Investigators. Intensive Care Med. 2015;41:1549–1560. doi: 10.1007/s00134-015-3822-1. [DOI] [PubMed] [Google Scholar]
- 45.Peake SL, Delaney A, Bailey M, Bellomo R, Cameron PA, Cooper DJ, Higgins AM, Holdgate A, Howe BD, Webb SA, et al. ARISE Investigators; ANZICS Clinical Trials Group. Goal-directed resuscitation for patients with early septic shock. N Engl J Med. 2014;371:1496–1506. doi: 10.1056/NEJMoa1404380. [DOI] [PubMed] [Google Scholar]
- 46.Yealy DM, Kellum JA, Huang DT, Barnato AE, Weissfeld LA, Pike F, Terndrup T, Wang HE, Hou PC, LoVecchio F, et al. ProCESS Investigators. A randomized trial of protocol-based care for early septic shock. N Engl J Med. 2014;370:1683–1693. doi: 10.1056/NEJMoa1401602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Mouncey PR, Osborn TM, Power GS, Harrison DA, Sadique MZ, Grieve RD, Jahan R, Harvey SE, Bell D, Bion JF, et al. ProMISe Trial Investigators. Trial of early, goal-directed resuscitation for septic shock. N Engl J Med. 2015;372:1301–1311. doi: 10.1056/NEJMoa1500896. [DOI] [PubMed] [Google Scholar]