Abstract
Rationale: Regional hypoventilation in bronchoconstricted patients with asthma is spatially associated with reduced perfusion, which is proposed to result from hypoxic pulmonary vasoconstriction (HPV).
Objectives: To determine the role of HPV in the regional perfusion redistribution in bronchoconstricted patients with asthma.
Methods: Eight patients with asthma completed positron emission tomographic/computed tomographic lung imaging at baseline and after bronchoconstriction, breathing either room air or 80% oxygen (80% O2) on separate days. Relative perfusion, specific ventilation (sV), and gas fraction (Fgas) in the 25% of the lung with the lowest specific ventilation (sVlow) and the remaining lung (sVhigh) were quantified and compared.
Measurements and Main Results: In the sVlow region, bronchoconstriction caused a significant decrease in sV under both room air and 80% O2 conditions (baseline vs. bronchoconstriction, mean ± SD, 1.02 ± 0.20 vs. 0.35 ± 0.19 and 1.03 ± 0.20 vs. 0.32 ± 0.16, respectively; P < 0.05). In the sVlow region, relative perfusion decreased after bronchoconstriction under room air conditions and also, to a lesser degree, under 80% O2 conditions (1.02 ± 0.19 vs. 0.72 ± 0.08 [P < 0.001] and 1.08 ± 0.19 vs. 0.91 ± 0.12 [P < 0.05], respectively). The Fgas increased after bronchoconstriction under room air conditions only (0.99 ± 0.04 vs. 1.00 ± 0.02; P < 0.05). The sVlow subregion analysis indicated that some of the reduction in relative perfusion after bronchoconstriction under 80% O2 conditions occurred as a result of the presence of regional hypoxia. However, relative perfusion was also significantly reduced in sVlow subregions that were hyperoxic under 80% O2 conditions.
Conclusions: HPV is not the only mechanism that contributes to perfusion redistribution in bronchoconstricted patients with asthma, suggesting that another nonhypoxia mechanism also contributes. We propose that this nonhypoxia mechanism may be either direct mechanical interactions and/or unidentified intercellular signaling between constricted airways, the parenchyma, and the surrounding vasculature.
Keywords: bronchoconstriction, methacholine, hyperoxia, positron emission tomography, pulmonary vascular physiology
At a Glance Commentary
Scientific Knowledge on the Subject
In bronchoconstricted subjects with asthma, perfusion redistribution occurs from poorly ventilated regions into regions with well-maintained ventilation. This perfusion redistribution is generally considered to be due to hypoxic pulmonary vasoconstriction (HPV), where regional hypoxia stimulates constriction of the local pulmonary vasculature.
What This Study Adds to the Field
HPV explains the majority of the regional perfusion redistribution that occurs in bronchoconstricted patients with asthma. However, some perfusion redistribution occurs in poorly ventilated regions that are not hypoxic and are not hyperinflated, suggesting that HPV is not the only mechanism that can redistribute perfusion in bronchoconstricted patients with asthma.
Bronchoconstriction in asthma causes severe regional hypoventilation (1–5), which can spatially coincide with regional reductions in perfusion (2, 6, 7). On the basis of whole-lung studies in subjects with asthma (8–10), reduced perfusion within hypoventilating regions is hypothesized to be driven by hypoxic pulmonary vasoconstriction (HPV) (6); however, no direct evidence of this mechanism currently exists. Furthermore, it is unclear to what degree other factors, such as the hypoventilating region size, regional lung inflation, and vertical gradient, may influence the redistribution of pulmonary perfusion and HPV (11, 12). Understanding the role of HPV in asthma is essential to recognizing how bronchoconstriction leads to perfusion redistribution and systemic hypoxemia.
HPV is an active mechanism in which alveolar hypoxia causes local pulmonary vasoconstriction, thereby reducing local perfusion and redirecting perfusion toward normoxic lung regions (13). HPV thus improves matching and reduces hypoxemia (14, 15). However, on the basis of animal studies, it is known that as the fraction of lung that is hypoxic exceeds 0.4, the extent of perfusion distribution relative to hypoxia decreases. This limit to the effect of HPV occurs because of increased pulmonary arterial pressure (11, 12, 16), which counteracts the vasoconstriction. Specifically, as the fraction of lung that is vasoconstricted increases, the pulmonary arterial pressure also rises in response to the elevated pulmonary vascular resistance. The elevated pulmonary arterial pressure may partially overcome the increased local resistance generated by HPV, and therefore the extent of perfusion redistribution may be less. However, these animal studies (11, 12, 16) have limited applicability to human asthma because the experimentally generated hypoventilating regions had homogeneously reduced ventilation and were both large (lung or lobe sized) and contiguous (11, 12). These are not features common to hypoventilating regions in adults with asthma (17–19), in whom hypoventilating regions have heterogeneous ventilation, are smaller than lobes, and are diffusely located (17, 20–22).
There are also passive mechanisms that may impact perfusion redistribution in asthma. Regional hypoventilation itself may act to reduce regional perfusion by increasing alveolar pressure (regional hyperinflation) (23, 24) above the vascular luminal pressure, thereby causing direct mechanical compression of the regional pulmonary vasculature, reducing local perfusion (i.e., West’s zone 1) (6, 25). This mechanism highlights the potential for factors other than HPV to influence the alveolar/vascular pressure. Additionally, hydrostatic pressure due to gravity, along with anatomic differences in vessel conductance (26), result in a vertical gradient in perfusion (25, 27–31), which may mean that HPV is also influenced by the vertical gradient. For example, regions with greater hydrostatic pressure (dependent lung) may experience less change in perfusion with the same increase in resistance from HPV.
We aimed to determine the role of HPV in the regional redistribution of perfusion in bronchoconstricted subjects with asthma and to assess whether the size of the hypoventilating region, the presence of regional hyperinflation, or the vertical gradient modulates any HPV-driven perfusion redistribution. We hypothesized that HPV would be the dominant cause of regional perfusion redistribution after bronchoconstriction in subjects with asthma. Furthermore, we hypothesized that larger hypoventilating regions would exhibit less perfusion redistribution than smaller regions and that the dependent lung would have a lesser degree of perfusion redistribution than the nondependent lung. Some of the results of these studies were previously reported in the form of abstracts (32, 33).
Methods
Eight subjects with asthma completed the study (Institutional Review Board 2007P002386). The screening visit included spirometry (seated) and a methacholine challenge test (34) to assess airway hyperresponsiveness (provocative concentration of methacholine causing a 20% fall in FEV1 [PC20]). Two imaging visits were held (1 wk apart), one under normoxic conditions (room air) and one under hyperoxic conditions (80% oxygen [80% O2]; balanced room air) with the order randomized. The imaging visit included supine spirometry, functional 13NN-saline positron emission tomographic/computed tomographic (PET-CT) lung imaging, supine methacholine administration to induce bronchoconstriction (five inspiratory capacity breaths at PC20), repeat functional imaging, and repeat supine spirometry. Additional information on recruitment and enrollment is included in the online supplement.
13NN-Saline PET-CT Functional Imaging
Perfusion and specific ventilation images at baseline and after bronchoconstriction were generated from the 13NN-saline PET scan (35). The degree of lung inflation was quantified as the gas fraction (Fgas), or the fraction of volume (voxel or region of interest) occupied by gas, as has been used by others (36–38). Specifically, Hounsfield units (HU) as determined from the high-resolution CT scans were converted to Fgas using Equation 1:
| (1) |
where the blood plus tissue density was defined as 65 HU and the air density was defined as −1,000 HU, as prescribed by the Apollo analysis software (VIDA Diagnostics, Coralville, IA). For ease of comparison between HU and Fgas, a measure of −700 HU equates to an Fgas of 0.72 and −900 HU equates to an Fgas of 0.9. In addition, an Fgas of 0.72 means that in that area or region, 72% of the total volume is gas, with the remaining 28% being a combination of blood and tissue. For a detailed outline of image instrumentation, processing, and analysis, see the online supplement.
Each perfusion, specific ventilation, and Fgas value was normalized by the mean perfusion-specific ventilation and Fgas value of the imaged lung, respectively. For example, each voxel in the specific ventilation image was divided by the mean specific ventilation in that image (see Figures 1A and 1B). The resulting mean normalized images allow straightforward regional comparisons to be made between perfusion, specific ventilation, and Fgas images without concern for units. It follows that a regional mean normalized specific ventilation of 1.2 indicates that region to have 20% greater specific ventilation than the average of the lung. In the following sections, mean normalized specific ventilation, perfusion, and Fgas are referred to as relative specific ventilation, perfusion, and Fgas, respectively.
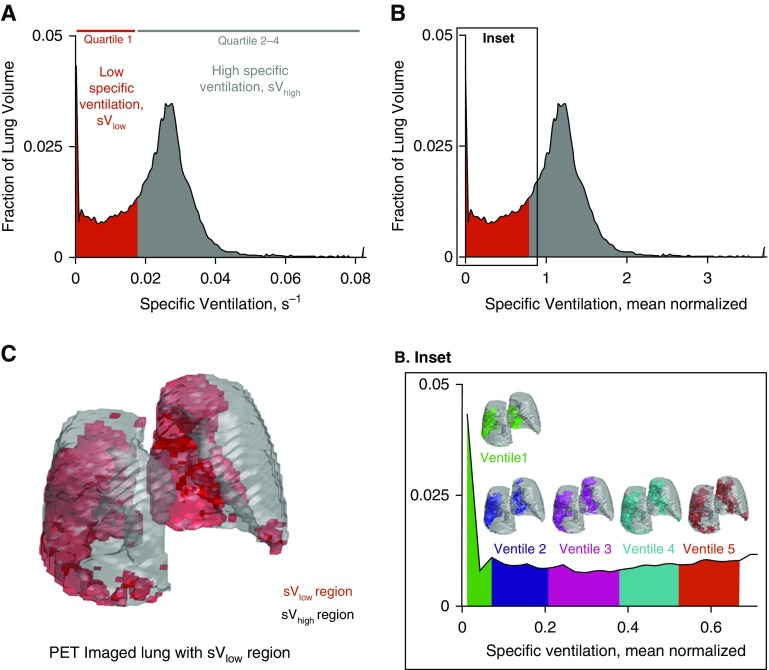
Figure 1.
Overview of key methods. (A) Histogram of postmethacholine specific ventilation (s−1) in subject 8. The red region represents the 25% of the lung with lowest specific ventilation (sVlow; quartile 1). Quartile 1 includes those specific ventilation values that make up the sVlow region. The gray region represents the specific ventilation values that make up the remaining lung region with high specific ventilation (sVhigh). (B) Histogram of mean normalized specific ventilation illustrating the mean normalization process when compared with A. The value 1 represents the mean specific ventilation in this subject. (Inset) For the subregion analysis, quartile 1, the sVlow region, was further subdivided into five ventiles, each representing 1/20th of the imaged lung volume. Here we illustrate how each ventile corresponds to the relative specific ventilation values on the histogram (zoomed in) and their spatial locations within the lung. Green = ventile 1, subregion with lowest specific ventilation; purple = ventile 2; pink = ventile 3; aqua = ventile 4; orange = ventile 5. (C) The spatial location of the sVlow and sVhigh lung regions in the imaged lung, illustrated in three dimensions. PET = positron emission tomography.
Functional Imaging Outcomes
To assess how methacholine and 80% O2 altered the distribution of perfusion, we first separated the postmethacholine lung into two regions: lung with low specific ventilation (sVlow) and the remaining lung with high specific ventilation (sVhigh). The sVlow region was defined as the 25% of the lung volume with the lowest relative specific ventilation after methacholine. The sVhigh region was defined as the remaining 75% of the lung (Figure 1C). To ensure that the same regions of the lung were assessed under baseline and postmethacholine conditions, the sVlow and sVhigh regions were mapped onto the baseline lung space using an isometric mapping algorithm (see Figure E1 in the online supplement). Quantitative analysis of the accuracy of the mapping algorithm was performed (Figures E2 and E3). At baseline and after methacholine under both room air and 80% O2 conditions, the average relative specific ventilation, the relative perfusion, and the relative Fgas in the sVlow and sVhigh regions were quantified. In all instances, the alveolar oxygen partial pressure (PaO2) within the sVlow and sVhigh regions was estimated (see online supplement).
By design, the sVlow region is defined as a proportion of the lung and therefore will contain lung with varying degrees of hypoventilation after bronchoconstriction. We therefore also performed subregion analysis in the sVlow region. Specifically, the sVlow region was further subdivided into five regions of equal lung volume. The resulting sVlow subregions represented 20th quantiles of ventilation (ventiles) within each region. Each subregion contains 5% of the total lung volume (see Figure 1B, inset) and represents approximately 2,000–2,500 voxels. In each of these subregions, the percentage change in relative specific ventilation, perfusion, and Fgas after bronchoconstriction was determined and compared under room air and 80% O2 conditions. In addition, the percentage change in each variable was compared with the postmethacholine estimated PaO2 under room air and 80% O2 conditions. Regional hypoxia and hyperoxia were defined as regions with a PaO2 less than 83 mm Hg (39) and PaO2 greater than 150 mm Hg, respectively. The methods used to assess the impact of the vertical gradient and the size of the individual sVlow regions on the perfusion redistribution are outlined in the online supplement.
The dispersion of the global distribution, a measure of global perfusion heterogeneity, was quantified as the log10 SD of perfusion on the scale (10, 14, 15); comparisons were made across all conditions. For specific methods used for the distribution calculation, see the online supplement.
Statistical Analysis
The effect of methacholine and 80% O2 on the functional imaging data was assessed with two-way repeated-measures analysis of variance and Student-Newman-Keuls post hoc paired comparisons. Under all conditions, baseline and postmethacholine PaO2 were compared with paired Student’s t-tests. sVlow subregion analysis was performed with one-way analysis of variance. Data are presented as mean ± SD, and statistical significance was accepted at P < 0.05. Statistical analysis was performed using SigmaPlot software (Systat Software, San Jose, CA).
Results
Subject Characteristics
All subjects had airway hyperresponsiveness and normal baseline lung function (Table 1). Baseline supine lung function was not different between the two imaging visits, nor was the decrease in FEV1 after methacholine different between the screening visit (seated) and the two imaging visits (supine) (Table 2). No significant arterial oxygen desaturation or increase in heart rate or systolic blood pressure occurred after methacholine under room air or 80% O2 conditions, with one exception: diastolic blood pressure increased after methacholine (P < 0.05).
Table 1.
Subject Characteristics and Lung Function
| Mean ± SE* | |
|---|---|
| Characteristics | |
| Age, yr | 24.5 ± 3.3 |
| n (M:F) | 8 (4:4) |
| Height, cm | 172.7 ± 3.1 |
| Weight, kg | 70.1 ± 4.4 |
| BMI, kg/m2 | 23.5 ± 1.3 |
| Baseline lung function | |
| FEV1, L; % predicted | 3.8 ± 0.3; 92.9 ± 6.0% |
| FVC, L; % predicted | 4.8 ± 0.5; 98.6 ± 6.5% |
| FEF25–75%, L/s; % predicted | 3.8 ± 0.5; 87.4 ± 10.8% |
| PEF, L/s; % predicted | 8.2 ± 0.5; 95.3 ± 3.9% |
| FEV1/FVC | 0.80 ± 0.03 |
| PC20, mg/ml | 1.9 ± 1.0 |
Definition of abbreviations: BMI = body mass index; FEF25–75% = forced expiratory flow, midexpiratory phase; PC20 = provocative concentration of methacholine causing a 20% fall in FEV1; PEF = peak expiratory flow.
Percentage predicted values were calculated using data from Hankinson and colleagues (54).
Except where otherwise indicated.
Table 2.
Supine Lung Function
| Room Air |
80% Oxygen |
Two-Way RM ANOVA |
|||||
|---|---|---|---|---|---|---|---|
| Baseline | Methacholine | Baseline | Methacholine | Room Air vs. 80% Oxygen | Baseline vs. Methacholine | Interaction | |
| FEV1, % predicted, % decrease from baseline | 83.3 ± 5.0 | 66.3 ± 4.7*, 18.9 ± 16.6% | 83.4 ± 5.2 | 66.8 ± 5.5*, 19.3 ± 15.4% | n.s. | P < 0.05 | n.s. |
| FVC, % predicted, % decrease from baseline | 90.1 ± 5.0 | 82.4 ± 5.9, 8.5 ± 14.5% | 91.4 ± 4.9 | 82.6 ± 5.4, 7.9 ± 15.4% | n.s. | n.s. | n.s. |
| FEV1/FVC, % | 78.1 ± 3.1 | 68.4 ± 3.8† | 77.0 ± 3.6 | 68.3 ± 3.8† | n.s. | P < 0.01 | n.s. |
| Oxygen saturation, % | 99.4 ± 1.0 | 98.5 ± 1.4 | 99.7 ± 1.0 | 99.7 ± 0.5 | n.s. | n.s. | n.s. |
| Heart rate, bpm | 64.5 ± 6.0 | 72.1 ± 16.1 | 64.3 ± 10.9 | 66.6 ± 10.7 | n.s. | n.s. | n.s. |
| Blood pressure, systolic/diastolic, mm Hg | 102/56 ± 9/10 | 109/63* ± 5/10 | 107/61 ± 13/14 | 108/60 ± 11/11 | n.s. | Diastolic: P < 0.05 | n.s. |
| Mean lung volume, L | 3.2 ± 1.1 | 3.6 ± 0.9 | 3.2 ± 0.9 | 3.7 ± 1.1 | n.s. | P < 0.05 | n.s. |
Definition of abbreviations: bpm = beats per minute; n.s. = not significant; RM ANOVA = repeated-measures analysis of variance.
Percentage predicted values were calculated on the basis of publication by Hankinson and colleagues (54).
P < 0.01 significant difference between baseline and after methacholine using post hoc analysis.
P < 0.001 significant difference between baseline and after methacholine using post hoc analysis.
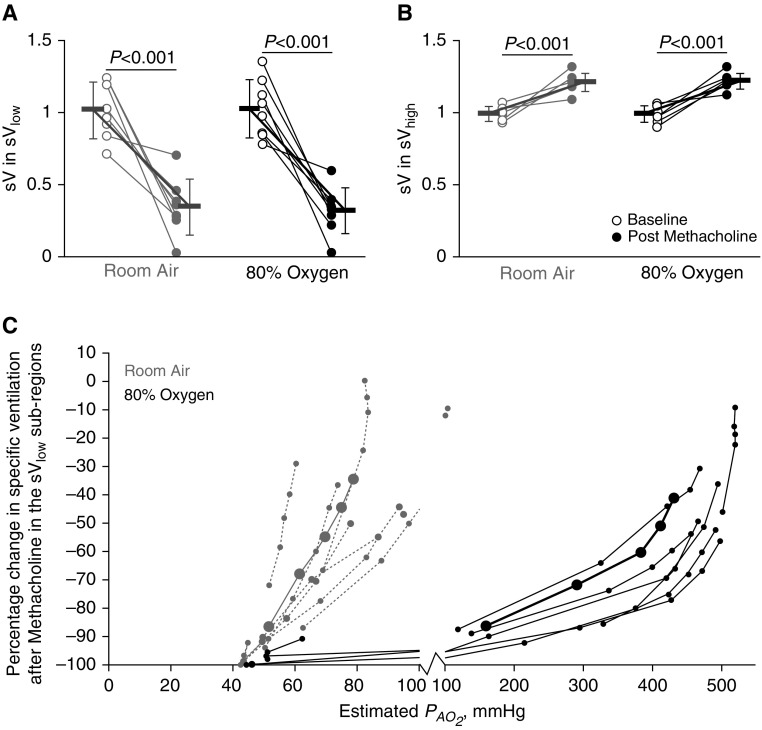
Regional Specific Ventilation: Effect of Methacholine and 80% O2
After methacholine, regardless of whether under room air or 80% O2 conditions, relative specific ventilation significantly decreased in the sVlow region and increased in the sVhigh region (main effect, P < 0.001 for both conditions) (Figures 2A and 2B). The sVlow subregion analysis showed that under room air conditions, greater reductions in relative specific ventilation had lower estimated PaO2 values, as expected (Figure 2C). Specifically, under room air, all subjects had at least one sVlow subregion become hypoxic (PaO2 < 83 mm Hg) after methacholine; the mean reduction in relative specific ventilation in those regions was 70.1 ± 26.3%. Surprisingly, under 80% O2 conditions, four of eight subjects also had at least one hypoxic sVlow subregion; the mean reduction in relative specific ventilation in those hypoxic subregions was 97.1 ± 2.9%.
Figure 2.
Regional specific ventilation: effect of methacholine and 80% oxygen. Gray markers indicate room air conditions, and black indicates 80% oxygen. Open circles represent baseline data, and closed circles represent postmethacholine data. P values reflect the results from the pairwise comparisons. (A) The relative specific ventilation in the 25% of the lung with lowest specific ventilation, sVlow region, decreased significantly after methacholine under both room air and 80% oxygen conditions. (B) Relative specific ventilation in the remaining lung with high specific ventilation, sVhigh region, was significantly increased after methacholine under both room air and 80% oxygen conditions. (C) The percentage change in relative specific ventilation after methacholine within the sVlow subregions plotted against the predicted postmethacholine regional alveolar oxygen partial pressure (PaO2) are plotted in gray for room air conditions and in black for 80% oxygen conditions. In A and B, the bold lines with error bars represent mean and SD values within each subregion. In C, the bold black and solid gray lines with large closed circles represent mean values. Under room air conditions, the majority of subjects and subregions were hypoxic (PaO2 < 83 mm Hg). Under 80% oxygen conditions, the lowest-ventilating subregions in four of eight subjects had a PaO2 less than 83 mm Hg. sV = relative specific ventilation.
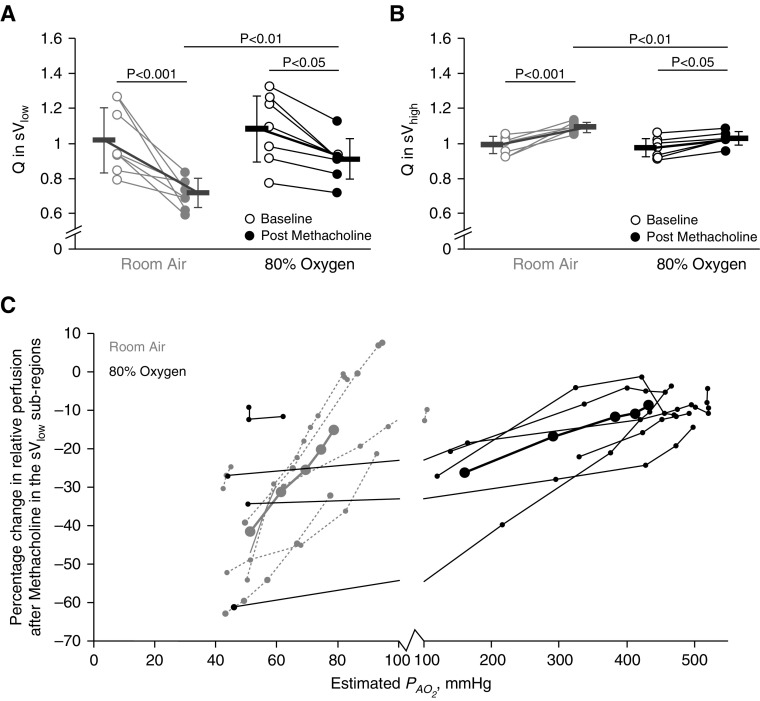
Regional Perfusion: Effect of Methacholine and 80% O2
Relative perfusion in the sVlow and the sVhigh regions were both significantly affected by methacholine and by 80% O2 (sVlow main effect, P < 0.001 for methacholine, P < 0.05 for 80% O2; sVhigh main effect, P < 0.001 for methacholine, P < 0.05 for 80% O2) (Figures 3A and 3B). Pairwise comparisons showed that under room air conditions, relative perfusion in the sVlow region decreased after methacholine (Figure 3A). Under 80% O2 conditions, relative perfusion in the sVlow region also decreased after methacholine, though the magnitude of the decrease was less (Figure 3A). In the sVhigh regions, methacholine and 80% O2 also significantly affected perfusion (main effect, P < 0.001 for methacholine, P < 0.05 for 80% O2) (Figure 3B). Perfusion in the sVhigh region increased after methacholine under both room air and 80% O2 conditions. Again, the magnitude of the increase in relative perfusion in the sVhigh region was lower under 80% O2 conditions than with room air.
Figure 3.
Regional perfusion: effect of methacholine and 80% oxygen. Gray markers indicate room air conditions, and black indicates 80% oxygen. Open circles represent baseline, and closed circles represent postmethacholine data. P values reflect the results from pairwise comparisons. (A) The mean relative perfusion in the lowest quartile of ventilating lung (sVlow) at baseline and after methacholine under both room air and 80% oxygen conditions. During room air, perfusion in the sVlow region significantly decreased after methacholine. The same effect was seen under 80% oxygen, but to a lesser degree. (B) The perfusion in the median and highest quartiles of ventilating lung (sVhigh) increased after methacholine under both room air and 80% oxygen conditions. The postmethacholine perfusion in the sVhigh regions was greater under room air conditions than with 80% oxygen. (C) The percentage change in relative perfusion after methacholine within the sVlow subregions plotted against the predicted postmethacholine regional alveolar oxygen partial pressure (PaO2) are plotted in gray for room air conditions and in black for 80% oxygen conditions. In A and B, the bold lines with error bars represent mean and SD values within each subregion. In C, the bold black and solid gray lines with large closed circles represent mean values. Under room air conditions, in the hypoxic subregions (PaO2 < 83 mm Hg), the average reduction in perfusion was 33.5 ± 15.7%. Under 80% oxygen conditions, the hypoxic subregions had an average reduction in perfusion of 21.7 ± 18.5%. In the hyperoxic regions (PaO2 > 150 mm Hg), the reduction in perfusion was 12.2 ± 8.3%. The lowest ventilating subregions had a significantly greater reduction in perfusion under room air than with 80% oxygen conditions. = mean relative perfusion.
In the subregion analysis (Figure 3C), the decrease in relative perfusion was greater under room air than with 80% O2 conditions across all the subregions and was significant in the lowest two ventiles (ventile 1, P < 0.01; ventile 2, P < 0.05) (see Figure 1B, inset, for description of ventiles). Under room air conditions, the hypoxic subregions had an average reduction in perfusion of 33.5 ± 15.7% (P < 0.001); subregions with a PaO2 greater than 83 mm Hg had a reduction of 6.9 ± 9.8%. Under 80% O2 conditions, the hypoxic subregions (four subjects) had an average reduction in perfusion of 21.7 ± 18.5% (P < 0.001). Under 80% O2 conditions, in the hyperoxic subregions (estimated PaO2 > 150 mm Hg), the average reduction in perfusion reduction was 12.1 ± 8.3% (P < 0.001). Representative transverse images of regional perfusion under each condition with sVlow and sVhigh regions outlined are included in Figure 4.
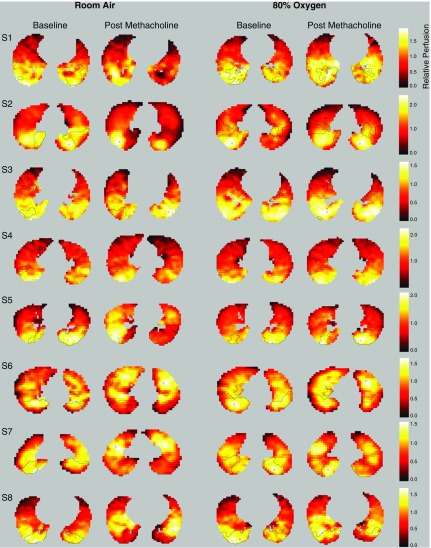
Figure 4.
Representative images of regional perfusion. Representative transverse images of relative perfusion at baseline and after methacholine under room air and 80% oxygen conditions in each of the eight subjects (one subject per row, S1–S8). Brighter colors, white and yellow, represent higher relative perfusion. Darker colors, red and black, represent lower relative perfusion. Outlined regions represent the boundary of the lowest quartile of ventilating lung (sVlow) regions in each image. The lung outside the sVlow boundaries is the median and highest quartiles of ventilating lung, sVhigh lung region. In many cases, relative perfusion is dramatically lower within the sVlow region after methacholine under room air conditions than under 80% oxygen, in particular S2 and S5. Furthermore, in many slices, the areas of lower relative perfusion are clearly demarcated within the sVlow region (see room air postmethacholine slices for S2, S5, S6, and S8).
Regional Lung Inflation: Effect of Methacholine and 80% O2
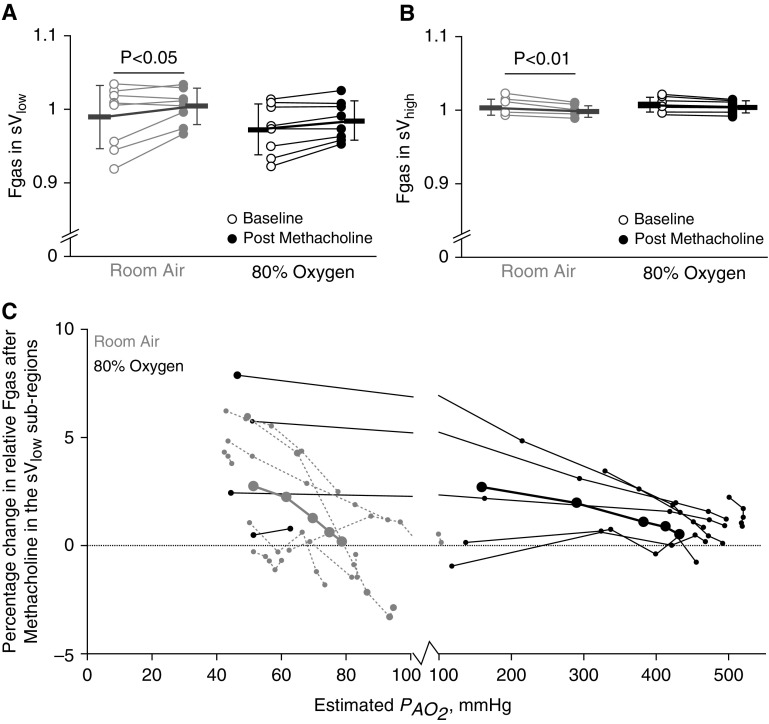
The Fgas within the sVlow region was significantly influenced by methacholine (main effect, P < 0.05) (Figure 5A), but not under 80% O2 conditions. Specifically, pairwise comparisons showed that Fgas significantly increased after methacholine under room air conditions (P < 0.05) and not under 80% O2 (P = 0.07). In the sVhigh region, Fgas was found to be significantly influenced by methacholine (main effect, P < 0.01) (Figure 5B). Pairwise comparisons in the sVlow region showed that methacholine caused a significant decrease in relative Fgas under room air (P < 0.01) but a nonsignificant decrease under 80% O2 (P = 0.07). Under room air, hypoxic subregions had an average increase in Fgas of 2.0 ± 2.7% (Figure 5C). Under 80% O2, hypoxic subregions had an average increase in Fgas of 2.3 ± 2.9%, and hyperoxic subregions had an Fgas increase of 1.2 ± 1.1%.
Figure 5.
The effect of methacholine and 80% oxygen on lung inflation. Gray markers indicate room air conditions, and black indicates 80% oxygen. Open circles represent baseline, and closed circles represent postmethacholine data. P values reflect the results from pairwise comparisons. In A and B, the bold lines with error bars represent mean and SD values within each subregion. In C, the bold black and solid gray lines with large closed circles represent mean values. (A) Only under room air conditions was there a significant increase in relative gas fraction (Fgas) within the lowest quartile of ventilating lung (sVlow) region. (B) There was a significant decrease in relative Fgas in the median and highest quartiles of ventilating lung, sVhigh region, after methacholine under room air only. (C) Relative Fgas was higher in sVlow subregions with lower estimated alveolar oxygen partial pressure (PaO2).
To determine if regional differences in inflation as measured by relative Fgas independently contributed to the perfusion redistribution, forward stepwise regression was performed. The percentage changes in relative perfusion, specific ventilation, and Fgas in both the sVlow and sVhigh regions under 80% O2 conditions were included in the model. For this analysis, the 80% O2 condition was used to reduce the impact of regional hypoxia, and PaO2 was excluded from the analysis because it is derived from the specific ventilation and relative perfusion. The resulting model showed that 13% of the variance in the perfusion redistribution under 80% O2 was attributable to changes in relative Fgas (percentage change in relative specific ventilation, ΔR2 = 0.85, P < 0.001; percentage change in relative Fgas, ΔR2 = 0.13, P < 0.001).
Effect of the Vertical Gradient and sVlow Region Size
See the online supplement for detailed results regarding the effect of the vertical gradient (Figure E4) and the sVlow region size (Figure E5). In summary, the vertical gradient and the sVlow size did not significantly contribute to the change in relative perfusion independent of the change in relative specific ventilation.
Global Perfusion Redistribution
Methacholine caused a significant increase in the SD log10 under both room air and 80% O2 conditions; this increase was 28% greater under 80% O2 than with room air (Figure E6) (interaction P = 0.001). Each subject’s individual distributions under room air and 80% O2 are included in Figure E7. Of note, subject 7 had a significant proportion of lung with extremely low , with greater than 20% of perfusion going to regions with a of approximately 0.001.
Discussion
Our results demonstrate that HPV is the primary but not the sole mechanism responsible for regional perfusion redistribution in bronchoconstricted patients with asthma. Specifically, when regional hypoxia was present in bronchoconstricted patients with asthma (breathing either room air or 80% O2), perfusion decreased proportionally to the degree of regional hypoxia, as expected with HPV (39). However, when regional hypoxia was absent from hypoventilating lung regions, a degree of perfusion redistribution still occurred. We propose that this non–hypoxia-driven perfusion redistribution occurs as a result of mechanical or biological interactions between the constricted airway, vasculature, and surrounding parenchyma, leading to an increase in local vascular impedance. This non–hypoxia-driven perfusion redistribution may contribute to the difficulty in achieving adequate oxygenation in severely bronchoconstricted patients with asthma even when high concentrations of oxygen are inspired (8, 40, 41).
Bronchoconstricted patients with asthma breathing room air had a 28.4% reduction of perfusion to hypoventilating lung and a corresponding 10.4% increase in perfusion to areas with well-maintained ventilation. This 28.4% overall reduction in perfusion is less than previous reports based on animal studies, in which reductions of 50 to 65% were reported in lobe-sized regions (11, 12). However, the subregion analysis revealed that under room air conditions, the subsegment with the lowest PaO2 (ventile 1, the 5% of lung volume with lowest ventilation) (Figure 1B, inset) had an average reduction in perfusion after methacholine of 42 ± 15% with regional PaO2 of 51 ± 10 mm Hg, with three subjects having perfusion reductions between 50 and 63% (PaO2, 46 ± 4 mm Hg) (Figure 3C). Reductions in regional perfusion to this degree are equivalent to those maximal perfusion redistributions previously reported (11, 12). It follows that regional hypoventilation in bronchoconstricted patients with asthma can result in severe reductions in regional perfusion, even when the degree of bronchoconstriction would not be considered serious or life threatening and no arterial desaturation manifests (Table 2).
In these same bronchoconstricted patients with asthma, perfusion redistribution when breathing 80% O2 persisted, albeit to a lesser degree. Specifically, within hypoventilating areas, the average reduction in perfusion was 15.1%, and within well-maintained ventilation areas, the average increase in perfusion was 5.5%. Under 80% O2 conditions, the subregion analysis indicated that two factors contributed to the continued perfusion redistribution. The first was that regional hypoxia still developed in some low-ventilating subregions. A portion of the overall 15.1% reduction in perfusion in hypoventilating lung is therefore likely due to HPV. Within lung regions that were both hypoventilating and hypoxic under 80% O2 conditions (PaO2 < 83 mm Hg), the reduction in perfusion was 25.6 ± 15.9%. However, some perfusion redistribution occurred even when the hypoventilating lung regions were hyperoxic. We found that under 80% O2 conditions, the decrease in perfusion in regions that were hypoventilating and hyperoxic (PaO2 > 150 mm Hg) was 10.9 ± 5.9% (P < 0.001). On the basis of elevated PaO2 in these regions (mean PaO2, 426 ± 89 mm Hg), this reduction in perfusion cannot be the result of regional HPV; another factor must be responsible. To explain the perfusion redistribution within hypoventilating, hyperoxic lung regions, we propose that in subjects with asthma, bronchoconstriction itself can lead to increased regional vascular impedance and thereby reduce regional perfusion.
Bronchoconstriction may cause local increases in vascular impedance in a variety of ways. The first possible mechanism is that bronchoconstriction-induced regional hypoventilation subsequently leads to regional hyperinflation, which, similar to West’s zone 1 (25), compresses the pulmonary vasculature, decreasing local blood flow. Indeed, hypoventilating, hyperoxic lung regions had an increase in Fgas of 1.2 ± 1.1%, and the percentage change in relative Fgas did explain 13% of the variance in the perfusion redistribution under 80% O2 conditions. Together, these results seem to support hyperinflation as a mechanism for some degree of perfusion redistribution. However, because blood volume is a component of the Fgas measurement, these results may reflect the underlying changes in regional perfusion and not true alterations in regional lung inflation. Furthermore, 1 to 2% increases in Fgas due to hyperinflation seem unlikely to increase local pulmonary vascular resistance enough to cause a greater than 10% decrease in perfusion. However, because tissue and blood volume cannot be separated from the Fgas measurement, we cannot categorically exclude regional hyperinflation as a potential contributing factor to the persistent perfusion redistribution under 80% O2 conditions.
The second possibility is that methacholine directly causes vascular smooth muscle constriction in these hypoventilating regions (42). However, work by Perez and Sanderson (43, 44) demonstrated that airway smooth muscle contraction and relaxation with acetylcholine (a natural form of methacholine) did not result in any constriction or relaxation of the arterioles (44). Therefore, direct effects of methacholine on vascular smooth muscle are unlikely to contribute to the regional perfusion redistribution we report. However, we cannot discount the possibility of an indirect effect of bronchoconstriction on endothelial constriction via an as yet unknown cell signaling pathway.
The third possibility is that a bronchoconstricted airway may directly alter the geometry of adjacent blood vessels such that vascular impedance increases. Recent theoretical and experimental studies show that bronchoconstriction can alter both the peribronchial alveolar geometry (45) and/or cause direct translocation of the nearby pulmonary vasculature (see online video in publication by Hiorns and colleagues [46]). Either of these alterations could increase local vascular impedance by changing the vessel lumen cross-sectional shape (decreasing the vessel hydraulic diameter), increasing vascular length, or increasing the vessel branching angles. In summary, we propose that the non–hypoxia-related perfusion redistribution may be the result of local hyperinflation, unidentified cell signaling, and/or changes to the local vascular geometry as a result of interactions between the bronchoconstricted airways and the surrounding mechanical environment. Further studies are warranted to investigate the underlying mechanisms for this non–hypoxia-driven perfusion redistribution.
Both the overall results and the subregion analysis indicate that the decrease in ventilation after methacholine was not different under room air and 80% O2 conditions. It follows that differences in the degree of hypoventilation cannot explain the differences in the degree of perfusion redistribution under room air and 80% O2 conditions. In contrast, methacholine caused a significant increase in Fgas under room air and a nonsignificant increase under 80% O2 conditions. However, as described earlier, because blood volume/perfusion are components of the Fgas measurement, these Fgas differences do not necessarily indicate that regional hyperinflation was greater under room air than with 80% O2.
The global changes in the distribution witnessed in our study are similar to those demonstrated previously using the multiple inert gas elimination technique (MIGET) (10, 14, 15, 47–49). These MIGET studies showed increased perfusion heterogeneity, SD log10(), of between 30 and 100% (acute or chronic severe asthma) when breathing hyperoxic gas compared with room air (14, 15). Our results are concordant with these MIGET findings in that we report 31% greater SD log10() under postmethacholine 80% O2 conditions than with room air. Importantly, our study provides the first direct link between global distribution changes with 80% O2 and the regional ventilation and perfusion alterations that underlie that global response in patients with asthma. Therefore, in the case of delivering 80% O2 to bronchoconstricted patients with asthma, the global changes are the result of a regional redistribution of perfusion from hypoventilating lung to well-ventilated lung, as predicted by Ballester and colleagues (14, 15) and Harris and colleagues (6).
Vertical Gradient, sVlow Region Size, and Perfusion Redistribution
Contrary to our hypothesis, neither the vertical gradient nor the size of the low-ventilating region had an independent effect on the degree of perfusion redistribution. In relation to the vertical gradient, this is most likely because the pulmonary arterial pressure, including any vertical pressure gradient, was never large enough to overcome the activated vascular smooth muscle force. The lack of an effect of the hypoventilating region size may be because the previous work in dogs included hypoxic regions were lobe sized or larger (11, 12). In our study, the individual hypoventilating regions were almost all lobe sized or smaller. However, there was one exception, subject 7, who had a large FEV1 drop after methacholine (approximately 50% under both conditions), and a large extent (>20%) of extremely hypoventilating lung was also in the subject found to have a relatively low degree of perfusion redistribution (Figure 3).
Methodological Considerations
One source of potential experimental error comes from the mapping function that assumes an isometric lung volume change to translocate sVlow regions between images. Tests performed on the mapping function found the potential error to be 6.4 mm (1.2 voxels in x and y; 3.1 voxels in z). Given that the sVlow regions are large and contiguous (Figure 2), any mapping errors are likely to affect the perimeter voxels of the sVlow regions. The effect of the potential error in the mapping function on the average percentage change in perfusion was found to be 3.8 ± 2.6% (see online supplement); therefore, any errors in mapping are unlikely to significantly contribute to the overall perfusion redistributions we report.
The two most important assumptions made to estimate the regional PaO2 are the cardiac output and the arteriovenous oxygen difference (see online supplement for complete list). The arteriovenous oxygen difference was assumed to be 4 ml/100 ml of blood (50). In addition, the estimated cardiac output for each subject was used to predict the regional PaO2 under all conditions. Therefore, if the cardiac output of the subject changed after methacholine or between study visits, the PaO2 estimates would be inaccurate. Studies show that cardiac output may increase after bronchoconstriction secondary to arterial hypoxemia and/or lung hyperinflation (51, 52). However, arterial hypoxemia and hyperinflation were not systematic findings in this study; therefore, our model assumptions are likely valid.
Clinical Implications
For patients with asthma, the existence of a mechanism that reduces perfusion to hypoventilating lung regions without the presence of hypoxia may be beneficial in some cases and disadvantageous in others. With mild bronchoconstriction and subsequent regional mild hypoxia, such a mechanism may act to maintain matching and arterial oxygenation when HPV is not activated; this perfusion distribution would therefore be beneficial. However, in moderate to severe bronchoconstriction, where both normoxic and hyperoxic regions of hypoventilation develop, this mechanism will reduce perfusion to regions that are normoxic, which will further reduce the total oxygen uptake of the lungs and increase the susceptibility of the subject to arterial hypoxemia. Taken together with our finding that regional hypoxia can persist even when hyperoxic gas is delivered, these data may help to explain why many severely bronchoconstricted subjects with asthma fail to achieve normoxemia with supplemental oxygen (8, 40, 41) and why gas exchange and airway obstruction are poorly correlated (53).
Conclusions
We have shown that long-established alterations in whole-lung after bronchoconstriction in subjects with asthma (49), with and without supplemental oxygen (8, 10, 14, 15, 49), result from regional perfusion redistribution. The degree of perfusion redistribution was not clearly dependent on the size or the position of the hypoventilating regions relative to the vertical gradient in perfusion. Specifically, perfusion shifts away from low-ventilating lung regions into regions with well-maintained ventilation predominantly via HPV but also via a secondary mechanism that does not require regional hypoxia. We propose that this secondary nonhypoxia mechanism may be due to local hyperinflation and/or mechanical or cellular interactions between the bronchoconstricted airway and the surrounding parenchyma and pulmonary vasculature.
Acknowledgments
Acknowledgment
The authors thank the staff from the Massachusetts General Hospital departments of nuclear medicine and nuclear pharmacy for their assistance with this project, in particular Steve Weise, Michael Cournoyer, John Correia, David Lee, Peter Rice, Melissa Bruen, Erin Beloin, and Daniel Yokell. In addition, the authors extend kind thanks to Alan James (University of Western Australia), Christopher Pascoe (University of Manitoba), Jason H. Bates (Vermont Lung Institute), Jim Butler (Harvard School of Public Health), Yan Bai (Brigham and Women’s Hospital), and Xingbing Ai (Brigham and Women’s Hospital) for their helpful discussions and feedback on this work.
Footnotes
Supported by the NHLBI of the National Institutes of Health under award number R01 GM987654. Additional staff funding was provided by the American Heart Association (12POST11820025 [V.J.K.]) and the NHLBI of the National Institutes of Health (F32HL128026-02 [P.K.], T32HL7874-15 [K.A.H.], and R01 HL086717 [T.W.]).
Author Contributions: Study design: M.K., J.G.V., T.W., and R.S.H.; data acquisition: V.J.K., K.A.H., P.K., M.K., E.E.G., J.G.V., T.W., and R.S.H.; data analysis: V.J.K., T.W., and R.S.H.; data interpretation: V.J.K., K.A.H., P.K., M.K., E.E.G., J.G.V., T.W., and R.S.H.; manuscript drafting and editing: V.J.K., K.A.H., T.W., and R.S.H.; final manuscript approval: V.J.K., K.A.H., P.K., M.K., E.E.G., J.G.V., T.W., and R.S.H.; agreement to be accountable for the work: V.J.K., K.A.H., P.K., M.K., E.E.G., J.G.V., T.W., and R.S.H.
This article has an online supplement, which is accessible from this issue’s table of contents at www.atsjournals.org
Originally Published in Press as DOI: 10.1164/rccm.201612-2438OC on June 23, 2017
Author disclosures are available with the text of this article at www.atsjournals.org.
References
- 1.Munkner T, Bundgaard A. Regional V/Q changes in asthmatics after histamine inhalation. Eur J Respir Dis Suppl. 1986;143:22–27. [PubMed] [Google Scholar]
- 2.Munkner T, Bundgaard A. Regional V/Q changes in asthmatics after antigen inhalation. Eur J Respir Dis Suppl. 1986;143:44–47. [PubMed] [Google Scholar]
- 3.Whyte KF, Ip M, Kirby T, Wathen CG, Flenley DC. Changes in regional ventilation during histamine bronchial challenge in stable asthma. Respiration. 1994;61:68–73. doi: 10.1159/000196309. [DOI] [PubMed] [Google Scholar]
- 4.Costella S, Kirby M, Maksym GN, McCormack DG, Paterson NA, Parraga G. Regional pulmonary response to a methacholine challenge using hyperpolarized 3He magnetic resonance imaging. Respirology. 2012;17:1237–1246. doi: 10.1111/j.1440-1843.2012.02250.x. [DOI] [PubMed] [Google Scholar]
- 5.Samee S, Altes T, Powers P, de Lange EE, Knight-Scott J, Rakes G, Mugler JP, III, Ciambotti JM, Alford BA, Brookeman JR, et al. Imaging the lungs in asthmatic patients by using hyperpolarized helium-3 magnetic resonance: assessment of response to methacholine and exercise challenge. J Allergy Clin Immunol. 2003;111:1205–1211. doi: 10.1067/mai.2003.1544. [DOI] [PubMed] [Google Scholar]
- 6.Harris RS, Winkler T, Tgavalekos N, Musch G, Melo MF, Schroeder T, Chang Y, Venegas JG. Regional pulmonary perfusion, inflation, and ventilation defects in bronchoconstricted patients with asthma. Am J Respir Crit Care Med. 2006;174:245–253. doi: 10.1164/rccm.200510-1634OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Melo MF, Harris RS, Layfield JD, Venegas JG. Topographic basis of bimodal ventilation-perfusion distributions during bronchoconstriction in sheep. Am J Respir Crit Care Med. 2005;171:714–721. doi: 10.1164/rccm.200409-1296OC. [DOI] [PubMed] [Google Scholar]
- 8.Rodriguez-Roisin R, Ballester E, Roca J, Torres A, Wagner PD. Mechanisms of hypoxemia in patients with status asthmaticus requiring mechanical ventilation. Am Rev Respir Dis. 1989;139:732–739. doi: 10.1164/ajrccm/139.3.732. [DOI] [PubMed] [Google Scholar]
- 9.Wagner PD, Dantzker DR, Iacovoni VE, Tomlin WC, West JB. Ventilation-perfusion inequality in asymptomatic asthma. Am Rev Respir Dis. 1978;118:511–524. doi: 10.1164/arrd.1978.118.3.511. [DOI] [PubMed] [Google Scholar]
- 10.Wagner PD, Hedenstierna G, Bylin G. Ventilation-perfusion inequality in chronic asthma. Am Rev Respir Dis. 1987;136:605–612. doi: 10.1164/ajrccm/136.3.605. [DOI] [PubMed] [Google Scholar]
- 11.Marshall BE, Marshall C. Continuity of response to hypoxic pulmonary vasoconstriction. J Appl Physiol. 1980;49:189–196. doi: 10.1152/jappl.1980.49.2.189. [DOI] [PubMed] [Google Scholar]
- 12.Marshall BE, Marshall C, Benumof J, Saidman LJ. Hypoxic pulmonary vasoconstriction in dogs: effects of lung segment size and oxygen tension. J Appl Physiol. 1981;51:1543–1551. doi: 10.1152/jappl.1981.51.6.1543. [DOI] [PubMed] [Google Scholar]
- 13.Euler US, Liljestrand G. Observations on the pulmonary arterial blood pressure in the cat. Acta Physiol Scand. 1946;12:301–320. [Google Scholar]
- 14.Ballester E, Reyes A, Roca J, Guitart R, Wagner PD, Rodriguez-Roisin R. Ventilation-perfusion mismatching in acute severe asthma: effects of salbutamol and 100% oxygen. Thorax. 1989;44:258–267. doi: 10.1136/thx.44.4.258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ballester E, Roca J, Ramis L, Wagner PD, Rodriguez-Roisin R. Pulmonary gas exchange in severe chronic asthma: response to 100% oxygen and salbutamol. Am Rev Respir Dis. 1990;141:558–562. doi: 10.1164/ajrccm/141.3.558. [DOI] [PubMed] [Google Scholar]
- 16.Benumof JL, Wahrenbrock EA. Blunted hypoxic pulmonary vasoconstriction by increased lung vascular pressures. J Appl Physiol. 1975;38:846–850. doi: 10.1152/jappl.1975.38.5.846. [DOI] [PubMed] [Google Scholar]
- 17.Svenningsen S, Guo F, Kirby M, Choy S, Wheatley A, McCormack DG, Parraga G. Pulmonary functional magnetic resonance imaging: asthma temporal-spatial maps. Acad Radiol. 2014;21:1402–1410. doi: 10.1016/j.acra.2014.08.002. [DOI] [PubMed] [Google Scholar]
- 18.Svenningsen S, Kirby M, Starr D, Coxson HO, Paterson NAM, McCormack DG, Parraga G. What are ventilation defects in asthma? Thorax. 2014;69:63–71. doi: 10.1136/thoraxjnl-2013-203711. [DOI] [PubMed] [Google Scholar]
- 19.Venegas JG, Schroeder T, Harris S, Winkler RT, Melo MF. The distribution of ventilation during bronchoconstriction is patchy and bimodal: a PET imaging study. Respir Physiol Neurobiol. 2005;148:57–64. doi: 10.1016/j.resp.2005.05.023. [DOI] [PubMed] [Google Scholar]
- 20.de Lange EE, Altes TA, Patrie JT, Battiston JJ, Juersivich AP, Mugler JP, III, Platts-Mills TA. Changes in regional airflow obstruction over time in the lungs of patients with asthma: evaluation with 3He MR imaging. Radiology. 2009;250:567–575. doi: 10.1148/radiol.2502080188. [DOI] [PubMed] [Google Scholar]
- 21.de Lange EE, Altes TA, Patrie JT, Gaare JD, Knake JJ, Mugler JP, III, Platts-Mills TA. Evaluation of asthma with hyperpolarized helium-3 MRI: correlation with clinical severity and spirometry. Chest. 2006;130:1055–1062. doi: 10.1378/chest.130.4.1055. [DOI] [PubMed] [Google Scholar]
- 22.Harris RS, Winkler T, Musch G, Vidal Melo MF, Schroeder T, Tgavalekos N, Venegas JG. The prone position results in smaller ventilation defects during bronchoconstriction in asthma. J Appl Physiol (1985) 2009;107:266–274. doi: 10.1152/japplphysiol.91386.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Tantucci C, Ellaffi M, Duguet A, Zelter M, Similowski T, Derenne JP, Milic-Emili J. Dynamic hyperinflation and flow limitation during methacholine-induced bronchoconstriction in asthma. Eur Respir J. 1999;14:295–301. doi: 10.1183/09031936.99.142. [DOI] [PubMed] [Google Scholar]
- 24.Pellegrino R, Brusasco V. On the causes of lung hyperinflation during bronchoconstriction. Eur Respir J. 1997;10:468–475. doi: 10.1183/09031936.97.10020468. [DOI] [PubMed] [Google Scholar]
- 25.West JB, Dollery CT, Naimark A. Distribution of blood flow in isolated lung; relation to vascular and alveolar pressures. J Appl Physiol. 1964;19:713–724. doi: 10.1152/jappl.1964.19.4.713. [DOI] [PubMed] [Google Scholar]
- 26.Beck KC, Rehder K. Differences in regional vascular conductances in isolated dog lungs. J Appl Physiol (1985) 1986;61:530–538. doi: 10.1152/jappl.1986.61.2.530. [DOI] [PubMed] [Google Scholar]
- 27.Hopkins SR, Henderson AC, Levin DL, Yamada K, Arai T, Buxton RB, Prisk GK. Vertical gradients in regional lung density and perfusion in the supine human lung: the Slinky effect. J Appl Physiol (1985) 2007;103:240–248. doi: 10.1152/japplphysiol.01289.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Greenleaf JF, Ritman EL, Sass DJ, Wood EH. Spatial distribution of pulmonary blood flow in dogs in left decubitus position. Am J Physiol. 1974;227:230–244. doi: 10.1152/ajplegacy.1974.227.1.230. [DOI] [PubMed] [Google Scholar]
- 29.Anthonisen NR, Milic-Emili J. Distribution of pulmonary perfusion in erect man. J Appl Physiol. 1966;21:760–766. doi: 10.1152/jappl.1966.21.3.760. [DOI] [PubMed] [Google Scholar]
- 30.Treppo S, Mijailovich SM, Venegas JG. Contributions of pulmonary perfusion and ventilation to heterogeneity in Va/Q measured by PET. J Appl Physiol (1985) 1997;82:1163–1176. doi: 10.1152/jappl.1997.82.4.1163. [DOI] [PubMed] [Google Scholar]
- 31.Musch G, Layfield JDH, Harris RS, Melo MFV, Winkler T, Callahan RJ, Fischman AJ, Venegas JG. Topographical distribution of pulmonary perfusion and ventilation, assessed by PET in supine and prone humans. J Appl Physiol (1985) 2002;93:1841–1851. doi: 10.1152/japplphysiol.00223.2002. [DOI] [PubMed] [Google Scholar]
- 32.Harris RS, Kelly V, Wongviriyawong C, Winkler T, Kone M, Musch G, Vidal Melo MF, Venegas JG. Perfusion redistribution during bronchoconstriction in asthma is due in part to hypoxic pulmonary vasoconstriction [abstract] Am J Respir Crit Care Med. 2011;183:A2303. [Google Scholar]
- 33.Kelly VJ, Winkler T, Venegas JG, Wongviriyawong C, Kone M, Greenblatt EE, Hibbert KA, Gladysheva E, Vidal-Melo MF, Musch G, et al. The contribution of hypoxic pulmonary vasoconstriction and regional hyperinflation to reduced perfusion in poorly ventilated lung regions during bronchoconstriction in asthma [abstract] Am J Respir Crit Care Med. 2013;187:A4032. [Google Scholar]
- 34.Crapo RO, Casaburi R, Coates AL, Enright PL, Hankinson JL, Irvin CG, MacIntyre NR, McKay RT, Wanger JS, Anderson SD, et al. ATS Committee on Proficiency Standards for Clinical Pulmonary Function Laboratories. Guidelines for methacholine and exercise challenge testing—1999: this official statement of the American Thoracic Society was adopted by the ATS Board of Directors, July 1999. Am J Respir Crit Care Med. 2000;161:309–329. doi: 10.1164/ajrccm.161.1.ats11-99. [DOI] [PubMed] [Google Scholar]
- 35.Vidal Melo MF, Layfield D, Harris RS, O’Neill K, Musch G, Richter T, Winkler T, Fischman AJ, Venegas JG. Quantification of regional ventilation-perfusion ratios with PET. J Nucl Med. 2003;44:1982–1991. [PubMed] [Google Scholar]
- 36.Harris RS, Venegas JG, Wongviriyawong C, Winkler T, Kone M, Musch G, Vidal Melo MF, de Prost N, Hamilos DL, Afshar R, et al. 18F-FDG uptake rate is a biomarker of eosinophilic inflammation and airway response in asthma. J Nucl Med. 2011;52:1713–1720. doi: 10.2967/jnumed.110.086355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Vidal Melo MF, Winkler T, Harris RS, Musch G, Greene RE, Venegas JG. Spatial heterogeneity of lung perfusion assessed with 13N PET as a vascular biomarker in chronic obstructive pulmonary disease. J Nucl Med. 2010;51:57–65. doi: 10.2967/jnumed.109.065185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Richter T, Bellani G, Scott Harris R, Vidal Melo MF, Winkler T, Venegas JG, Musch G. Effect of prone position on regional shunt, aeration, and perfusion in experimental acute lung injury. Am J Respir Crit Care Med. 2005;172:480–487. doi: 10.1164/rccm.200501-004OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Sylvester JT, Shimoda LA, Aaronson PI, Ward JPT. Hypoxic pulmonary vasoconstriction. Physiol Rev. 2012;92:367–520. doi: 10.1152/physrev.00041.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Molfino NA, Nannini LJ, Martelli AN, Slutsky AS. Respiratory arrest in near-fatal asthma. N Engl J Med. 1991;324:285–288. doi: 10.1056/NEJM199101313240502. [DOI] [PubMed] [Google Scholar]
- 41.McFadden ER, Jr, Lyons HA. Arterial-blood gas tension in asthma. N Engl J Med. 1968;278:1027–1032. doi: 10.1056/NEJM196805092781901. [DOI] [PubMed] [Google Scholar]
- 42.Pfister SL, Deinhart DD, Campbell WB. Methacholine-induced contraction of rabbit pulmonary artery: role of platelet-endothelial transcellular thromboxane synthesis. Hypertension. 1998;31:206–212. doi: 10.1161/01.hyp.31.1.206. [DOI] [PubMed] [Google Scholar]
- 43.Perez JF, Sanderson MJ. The frequency of calcium oscillations induced by 5-HT, ACH, and KCl determine the contraction of smooth muscle cells of intrapulmonary bronchioles. J Gen Physiol. 2005;125:535–553. doi: 10.1085/jgp.200409216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Perez JF, Sanderson MJ. The contraction of smooth muscle cells of intrapulmonary arterioles is determined by the frequency of Ca2+ oscillations induced by 5-HT and KCl. J Gen Physiol. 2005;125:555–567. doi: 10.1085/jgp.200409217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Ma B, Bates JHT. Mechanical interactions between adjacent airways in the lung. J Appl Physiol (1985) 2014;116:628–634. doi: 10.1152/japplphysiol.01180.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Hiorns JE, Bidan CM, Jensen OE, Gosens R, Kistemaker LE, Fredberg JJ, Butler JP, Krishnan R, Brook BS. Airway and parenchymal strains during bronchoconstriction in the precision cut lung slice. Front Physiol. 2016;7:309. doi: 10.3389/fphys.2016.00309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Roca J, Ramis L, Rodriguez-Roisin R, Ballester E, Montserrat JM, Wagner PD. Serial relationships between ventilation-perfusion inequality and spirometry in acute severe asthma requiring hospitalization. Am Rev Respir Dis. 1988;137:1055–1061. doi: 10.1164/ajrccm/137.5.1055. [DOI] [PubMed] [Google Scholar]
- 48.Corte P, Young IH. Ventilation-perfusion relationships in symptomatic asthma: response to oxygen and clemastine. Chest. 1985;88:167–175. doi: 10.1378/chest.88.2.167. [DOI] [PubMed] [Google Scholar]
- 49.Rodriguez-Roisin R, Ferrer A, Navajas D, Agusti AG, Wagner PD, Roca J. Ventilation-perfusion mismatch after methacholine challenge in patients with mild bronchial asthma. Am Rev Respir Dis. 1991;144:88–94. doi: 10.1164/ajrccm/144.1.88. [DOI] [PubMed] [Google Scholar]
- 50.De Cort SC, Innes JA, Barstow TJ, Guz A. Cardiac output, oxygen consumption and arteriovenous oxygen difference following a sudden rise in exercise level in humans. J Physiol. 1991;441:501–512. doi: 10.1113/jphysiol.1991.sp018764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Rebuck AS, Pengelly LD. Development of pulsus paradoxus in the presence of airways obstruction. N Engl J Med. 1973;288:66–69. doi: 10.1056/NEJM197301112880203. [DOI] [PubMed] [Google Scholar]
- 52.Muñoz PA, Gómez FP, Manrique HA, Roca J, Barberà JA, Young IH, Anderson SD, Rodríguez-Roisin R. Pulmonary gas exchange response to exercise- and mannitol-induced bronchoconstriction in mild asthma. J Appl Physiol (1985) 2008;105:1477–1485. doi: 10.1152/japplphysiol.00108.2008. [DOI] [PubMed] [Google Scholar]
- 53.Wagner PD, Hedenstierna G, Rodriguez-Roisin R. Gas exchange, expiratory flow obstruction and the clinical spectrum of asthma. Eur Respir J. 1996;9:1278–1282. doi: 10.1183/09031936.96.09061278. [DOI] [PubMed] [Google Scholar]
- 54.Hankinson JL, Odencrantz JR, Fedan KB. Spirometric reference values from a sample of the general U.S. population. Am J Respir Crit Care Med. 1999;159:179–187. doi: 10.1164/ajrccm.159.1.9712108. [DOI] [PubMed] [Google Scholar]