Abstract
Rationale: Following acute respiratory distress syndrome (ARDS), joblessness is common but poorly understood.
Objectives: To evaluate the timing of return to work after ARDS, and associated risk factors, lost earnings, and changes in healthcare coverage
Methods: Over 12-month longitudinal follow-up, ARDS survivors from 43 U.S. ARDSNet hospitals provided employment and healthcare coverage data via structured telephone interviews. Factors associated with the timing of return to work were assessed using Fine and Gray regression analysis. Lost earnings were estimated using Bureau of Labor Statistics data.
Measurements and Main Results: Of 922 consenting survivors, 386 (42%) were employed before ARDS (56% male; mean ± SD age, 45 ± 13 yr), with seven dying by 12-month follow-up. Of 379 previously employed 12-month survivors, 166 (44%) were jobless at 12-month follow-up. Accounting for competing risks of death and retirement, half of enrolled and previously employed survivors returned to work by 13 weeks after hospital discharge, with 68% ever returning by 12 months. Delays in return to work were associated with longer hospitalization and older age among nonwhite survivors. Over 12-month follow-up, 274 (71%) survivors accrued lost earnings, averaging $26,949 ± $22,447 (60% of pre-ARDS annual earnings). Jobless survivors experienced a 14% (95% confidence interval, 5–22%; P = 0.002) absolute decrease in private health insurance (from 44% pre-ARDS) and a 16% (95% confidence interval, 7–24%; P < 0.001) absolute increase in Medicare and Medicaid (from 33%).
Conclusions: At 12 months after ARDS, nearly one-half of previously employed survivors were jobless. Post-ARDS joblessness is associated with readily identifiable patient and hospital variables and accompanied by substantial lost earnings and a shift toward government-funded healthcare coverage.
Keywords: employment, intensive care unit, income, health insurance
At a Glance Commentary
Scientific Knowledge on the Subject
Survivors of acute respiratory distress syndrome (ARDS) commonly experience joblessness. However, there are little data regarding the timing of return to work after ARDS, and associated risk factors, lost earnings, and changes in healthcare coverage.
What This Study Adds to the Field
Of 386 previously employed patients with ARDS, 379 (98%) survived to 12-month follow-up, with 166 (44%) of these survivors jobless at 12 months. Accounting for competing risks of death and retirement, half of enrolled and previously employed survivors returned to work by 13 weeks after hospital discharge, with 68% ever returning to work by 12 months. Factors associated with delayed return to work were longer hospitalization and older age among nonwhite survivors. Over 12-month follow-up, 274 (71%) nonretired survivors accrued lost earnings, averaging nearly $27,000, or 60% of pre-ARDS annual earnings, with jobless, nonretired survivors experiencing a large shift from private health insurance to government-funded healthcare coverage.
Survivors of acute respiratory distress syndrome (ARDS) frequently experience long-term physical, cognitive, and mental health impairments after discharge from the intensive care unit (ICU) (1–7). Such impairments contribute to joblessness, with only half of ARDS survivors employed 1 year later (2, 8), which can have an important economic impact on patients, families, employers, and society (9).
To date, no study has performed a longitudinal evaluation of joblessness after ARDS, including evaluation of associated lost earnings and shifts in healthcare coverage. Such research is needed to help inform survivors and their families, identify at-risk populations, and tailor interventions to prevent joblessness after ARDS. In addition, these data are important to more completely understand the economic impact of ARDS. Hence, via a national, multicenter prospective study of ARDS survivors over 12-month follow-up, this research aimed to evaluate (1) employment status and joblessness after ARDS, (2) patient- and hospital-related factors associated with the timing of return to work, (3) estimated lost earnings, and (4) changes in healthcare coverage.
Methods
Study Population
This evaluation was conducted as part of ALTOS (ARDS Network [ARDSNet] Long-Term Outcome Study), a national multicenter prospective study longitudinally evaluating survivors 6 and 12 months after ARDS. ALTOS participants were enrolled from four recent ARDSNet clinical trials evaluating ICU-based therapies for ARDS (10–13). Between 2006 and late 2014, patients were enrolled from 43 ARDSNet hospitals within 48 hours of ARDS onset and 72 hours of initiation of mechanical ventilation, and followed for 12 months thereafter. ARDSNet exclusion criteria have been described previously, and include preexisting severe malnutrition, lung, liver, or neuromuscular diseases; or limitations in life support at time of study eligibility (10–13). Participants were further excluded from longitudinal follow-up in ALTOS if they were younger than 18 years old, non–English speaking, homeless, or had potential cognitive impairment before admission (as per review of medical records or patient/proxy report). The study was approved by the institutional review boards at all participating hospitals. Informed consent was obtained from the patient or a proxy when the patient was incapable of consent.
Baseline Patient- and Critical Illness–related Exposure Variables
Baseline patient-related exposure variables included age, sex, race, body mass index, and preexisting comorbidity (specifically, diabetes mellitus, cardiovascular disease, chronic pulmonary disease, and alcohol misuse). Alcohol misuse was defined by zones 3 and 4 from the Alcohol Use Disorders Identification Test, indicating alcohol consumption exceeding recommended limits (14). Hospital-related exposures were grouped into three categories. (1) baseline intensive care data: admission to a medical (vs. surgical) ICU, Acute Physiology and Chronic Health Evaluation (APACHE) III score, and ARDS risk factor of sepsis (vs. nonsepsis); (2) daily ICU data: organ failure status (maximum organ failure score, derived from the daily Brussels organ failure score for cardiovascular, pulmonary, coagulation, renal, and hepatic systems [15]), and average daily PaO2/FiO2 ratio; and (3) other ICU data: duration of mechanical ventilation, ICU and hospital length of stay, and discharge location (home with unassisted breathing vs. other).
Measures of Employment and Return to Work
Data were collected at 6- and 12-month follow-up, via structured interviews using a self-report employment instrument that was similar to prior research (16). Data collection included current employment status (e.g., full-time, part-time, unemployed, disabled, retired, or paid sick leave), type of residence, hours working per week, timing of return to work (reported as weeks to return to work after hospital discharge), perceived effectiveness at work (0–100% scale), and major change in occupation (defined as a change in U.S. Bureau of Labor Statistics occupational profile [www.bls.gov/oes/current/oes_stru.htm]). Employment status before ARDS was collected retrospectively during follow-up interview. Data were obtained from patients (94% of assessments) or from proxies when patients were unable to complete the interview (6%), via telephone (98%) or in-person or mail (2%).
Estimated Lost Earnings and Healthcare Coverage
Lost earnings, evaluated as a measure of lost work capacity, were estimated for all survivors who were employed before ARDS. In the primary analysis (described later) and post hoc sensitivity analyses (see later and the online supplement), estimated lost earnings after ARDS were defined as the difference between estimated and potential earnings. In the primary analysis, estimated earnings were calculated for each survivor using age- and sex-matched weekly wage data from the U.S. Bureau of Labor Statistics (www.bls.gov), as done in prior research (17). Next, BLS.gov weekly wages were divided by 40 hours per week to determine hourly wages, and subsequently multiplied by patient-reported hours working per week. Similarly, estimated potential earnings were calculated by multiplying estimated hourly wages by the number of hours working before hospitalization. All weekly wages were multiplied by 50 weeks to derive annual estimates. Patients reporting unemployment or disability status were assumed to incur lost earnings, whereas those who were retired or dead were assumed to incur no lost earnings. All earnings data were scaled to 2016 U.S. dollars using the U.S. consumer price index (www.bls.gov/cpi). In post hoc sensitivity analyses, different methods were used to calculate lost wages, including imputing wages based on sex and self-reported occupation; considering full- and part-time employment as 40 and 20 hours per week, respectively; and excluding survivors who were retired and/or greater than or equal to 65 years of age (see online supplement).
Healthcare coverage data were collected as part of the structured interviews at 6- and 12-month follow-up, with prehospitalization data collected during the follow-up interview. Healthcare coverage was categorized into private insurance, Medicaid, Medicare (which included dually eligible Medicare and Medicaid beneficiaries, for which Medicare is the primary payer [18]), and no coverage. For patients younger than 65 years old surviving to 12-month follow-up and reporting healthcare coverage data, changes in healthcare coverage from prehospitalization to 12-month follow-up were computed using a multinomial logistic regression model with the main effect of time and a robust variance estimate for patient-level clustering of healthcare coverage status.
Quality of Life
Six and 12 months after ARDS, survivors’ health-related quality of life status was evaluated using the EQ-5D and Medical Outcomes Study Short-Form 36 version 2.
Statistical Analysis
We used survival analysis methods to evaluate the primary outcome of the timing of return to work after hospital discharge among patients who were employed before ARDS. Before returning to work, patients may experience one of two competing risks: retirement or death. Patients who neither returned to work nor experienced either competing risk were censored at 52 weeks. With retirement and death treated as competing risks, the timing of returning to work was explored using cumulative incidence functions, including stratification by age and race (19); additionally, Fine and Gray regression models were used to evaluate the association of baseline patient- and hospital-related exposure variables with return to work, with hazard ratios (HRs) <1 indicating longer time to return to work (20). Separate bivariable regression models evaluated the association of each exposure variable (individual independent variables) with the timing of return to work (dependent variable), and all exposure variables with a bivariable association of P < 0.20 were included in the multivariable regression model. Pairwise statistical interactions were assessed in the multivariable model for preselected demographic (age, sex, race, body mass index) and ICU (cardiovascular disease, diabetes, APACHE III score, hospital length of stay) variables. A significant statistical interaction was noted between age and race, and therefore included in the final multivariable model. Post hoc sensitivity analyses were performed to evaluate for potential effects of age-related changes in employment, and to evaluate a full multivariable model that included all exposure variables, rather than variable selection based on bivariable associations (see online supplement).
The linearity of each exposure variable was confirmed using locally weighted scatterplot smoothing (LOWESS) of Martingale residuals from the regression models. A Schoenfeld residual plot for each variable confirmed no violation of the proportional hazards assumption (21). Multicollinearity was assessed using variance inflation factors, with multicollinearity (defined as variance inflation factor ≥10) demonstrated between mechanical ventilation duration, ICU length of stay, and hospital length of stay, with the latter variable retained in the final model.
A two-sided P < 0.05 denoted statistical significance. All statistical analyses were performed using Stata version 13.1 (StataCorp, College Station, TX).
Results
Patient Characteristics and Employment Status
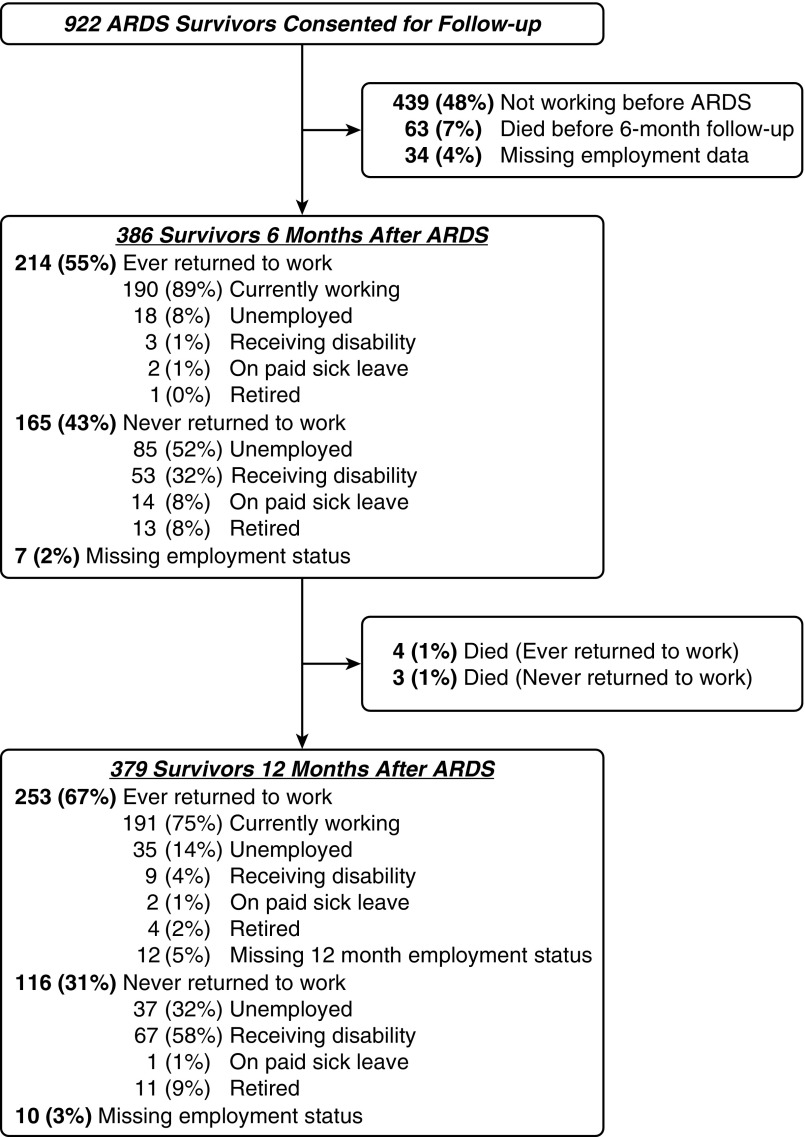
Among 922 survivors who consented for follow-up, 386 (42%) reported full- or part-time employment before ARDS (Figure 1). These 386 enrolled and previously-employed survivors were 44% female, and 82% white, with a mean ± SD age of 45 ± 13 years and APACHE III score of 83 ± 26 (Table 1). Fourteen (4%) of these survivors were greater than or equal to 65 years old. A total of 299 (77%) had sepsis as an ARDS risk factor. These enrolled and previously employed survivors had a mean duration of mechanical ventilation of 11 ± 10 days and hospital length of stay of 22 ± 16 days. As compared with survivors who were not employed before ARDS, previously employed survivors were younger, predominantly male, and had fewer baseline comorbidities, with similar values across their ICU variables.
Figure 1.
Patient flow chart. Some percentages do not add up to 100% because of rounding. ARDS = acute respiratory distress syndrome.
Table 1.
Baseline and Intensive Care Data by Return to Work Status by 12 Months after ARDS
| Variable* | Not Employed before ARDS (n = 439) | Employed before ARDS (n = 386) |
||||
|---|---|---|---|---|---|---|
| Total (n = 386) | Ever Returned to Work (n = 257) | Never Returned to Work (n = 105) | Retired or Died (n = 14) | Missing Data (n = 10)† | ||
| Baseline patient data | ||||||
| Age, mean (SD), yr | 54 (15) | 45 (13) | 43 (13) | 48 (12) | 56 (11) | 48 (12) |
| Age ≥65 yr, n (%) | 111 (25) | 14 (4) | 7 (3) | 4 (4) | 3 (21) | 0 (0) |
| Female, n (%) | 257 (59) | 171 (44) | 102 (40) | 56 (53) | 9 (64) | 4 (40) |
| White, n (%) | 353 (82) | 308 (82) | 215 (85) | 75 (77) | 11 (79) | 7 (78) |
| BMI, mean (SD), kg/m2 | 31 (9) | 30 (8) | 30 (7) | 31 (8) | 32 (7) | 33 (9) |
| Cardiovascular disease, n (%)‡ | 230 (52) | 148 (38) | 88 (34) | 45 (43) | 9 (64) | 6 (60) |
| Diabetes, n (%) | 127 (29) | 67 (17) | 35 (14) | 27 (26) | 1 (7) | 4 (40) |
| Chronic pulmonary disease, n (%)‡ | 72 (16) | 28 (7) | 17 (7) | 8 (8) | 1 (7) | 2 (20) |
| Alcohol misuse, n (%)‡ | 78 (20) | 81 (23) | 53 (22) | 23 (23) | 2 (14) | 3 (33) |
| Baseline intensive care data | ||||||
| Admission to medical ICU, n (%) | 274 (62) | 206 (53) | 136 (53) | 58 (55) | 7 (50) | 5 (50) |
| APACHE III, mean (SD) | 88 (26) | 83 (26) | 82 (26) | 86 (26) | 85 (35) | 86 (32) |
| Sepsis as ARDS risk factor, n (%) | 357 (81) | 299 (77) | 194 (75) | 84 (80) | 13 (93) | 8 (80) |
| Daily intensive care data | ||||||
| Maximum organ failure, mean (SD)§ | 2 (1) | 2 (1) | 2 (1) | 3 (1) | 2 (1) | 3 (1) |
| PaO2/FiO2, mean (SD) | 200 (72) | 208 (80) | 207 (76) | 210 (87) | 211 (88) | 207 (61) |
| Other intensive care data | ||||||
| Ventilation duration, mean (SD), d | 10 (9) | 11 (10) | 9 (8) | 14 (13) | 13 (14) | 10 (10) |
| ICU length of stay, mean (SD), d | 13 (10) | 15 (12) | 13 (10) | 19 (14) | 18 (16) | 14 (10) |
| Hospital length of stay, mean (SD), d | 21 (13) | 22 (16) | 19 (13) | 29 (18) | 31 (22) | 25 (18) |
| Discharge to home with unassisted breathing, n (%) | 408 (93) | 373 (97) | 253 (98) | 96 (91) | 14 (100) | 10 (100) |
Definition of abbreviations: APACHE III = Acute Physiology and Chronic Health Evaluation III; ARDS = acute respiratory distress syndrome; BMI = body mass index; ICU = intensive care unit.
Missing data for each variable (n; %): race (12; 3%), BMI (2; 0%), alcohol misuse (65; 8%), APACHE III (24; 3%), maximum organ failure (1; 0%), PaO2/FiO2 (29; 4%), ventilation duration (25; 3%), ICU length of stay (6; 1%), and hospital length of stay (6; 1%).
Did not report return to work status within 12-month follow-up period.
Cardiovascular and chronic pulmonary disease status was measured as part of the APACHE III questionnaire. Alcohol misuse was defined by zone 3 and 4 from the Alcohol Use Disorders Identification Test, which indicates alcohol consumption exceeded recommended limits.
Maximum number of organ failures during ICU stay, using the Brussels scoring system (15) for the following five organ systems (with definition of organ failure): cardiac (systolic blood pressure ≤90 mm Hg or use of vasopressor), pulmonary (PaO2/FiO2 ratio ≤300), coagulation (platelets ≤80 × 109/L), renal (creatinine ≥2.0 mg/dl), and hepatic (bilirubin ≥2.0 mg/dl).
Return to Work after ARDS
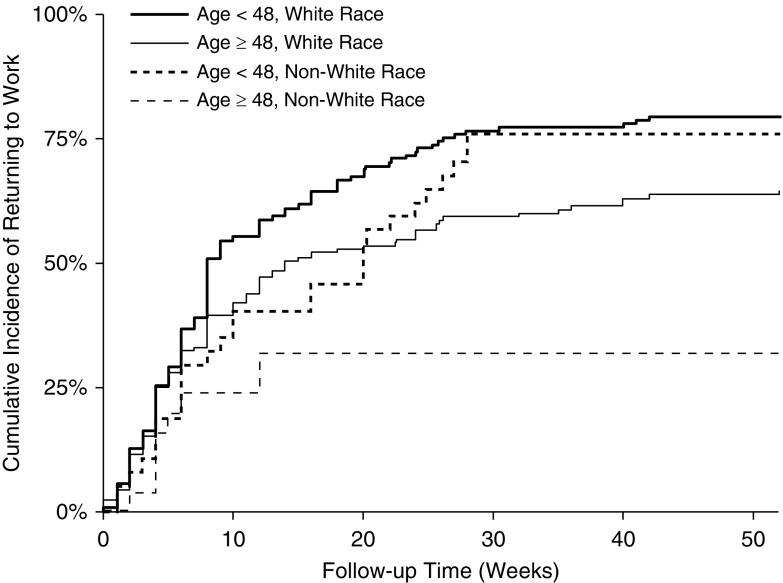
Among previously employed surviving patients at 6- and 12-month follow-up, 189 of 386 (49%) and 166 of 379 (44%), respectively, were jobless (Figure 1). Accounting for competing risks of death and retirement, half of enrolled and previously employed survivors returned to work by 13 weeks after hospital discharge, with 68% ever returning by 12 months. When stratified by age (at the median, 48 yr) and race (nonwhite vs. white), the 12-month cumulative incidence of returning to work was 76% and 32% among nonwhite survivors less than 48 and greater than or equal to 48 years old, respectively, and 79% and 64% for white survivors less than 48 and greater than or equal to 48 years old (Figure 2). Among the 257 eligible and previously employed survivors ever returning to work, 111 (43%) never returned to their previous hours worked, 69 (27%) reported reduced effectiveness at work (mean ± SD self-reported effectiveness score, 77% ± 22%), and 62 (24%) subsequently lost their jobs during 12-month follow-up. Finally, of these 257 survivors, 62 of 215 (29%) previously full-time workers and 17 of 42 (40%) previously part-time workers experienced a major occupation change. Those surviving workers with versus without a major occupation change worked fewer hours per week after ARDS (31 of 59 [53%] vs. 51 of 127 [40%]; P = 0.11) at 12-month follow-up.
Figure 2.
Cumulative incidence of returning to work over 12-month follow-up, stratified by age and race, with retirement and death treated as competing risks.
Among the 166 survivors who were jobless 12 months after ARDS, 72 (43%) were unemployed, 76 (46%) receiving disability, three (2%) on paid sick leave, and 15 (9%) retired. Most of these survivors (98%) were living at home, whereas three (2%) were hospitalized and one (1%) at a nursing home. Of 72 unemployed survivors, 41 (57%) were unable to work because of other health-related issues, whereas 16 (22%) were actively looking for work, five (7%) laid-off, six (8%) in school, two (3%) were in a healthcare facility, and two (3%) homemakers.
In the multivariable Fine and Gray regression model, longer hospital length of stay was associated with longer time to return to work (HR, 0.81 per week; 95% confidence interval [CI], 0.74–0.88; P < 0.001) (Table 2). Older nonwhite survivors also were estimated to have a longer time to return to work (HR, 0.68 per 10-yr increase; 95% CI, 0.54–0.84; P = 0.001), whereas among white survivors, age was not significantly associated with return to work (HR, 0.91; 95% CI, 0.81–1.02; P = 0.114). In all post hoc sensitivity analyses, there were no important differences in results (see Tables E1–E3 in the online supplement).
Table 2.
Bivariable and Multivariable Risk Factors of the Timing of Return to Work after ARDS
| Variable | Bivariable Hazard Ratio (95% CI)* | P Value | Multivariable Hazard Ratio (95% CI)* | P Value |
|---|---|---|---|---|
| Baseline patient data | ||||
| Female | 0.76 (0.59–0.98) | 0.033 | 0.81 (0.62–1.07) | 0.136 |
| White | 1.46 (1.03–2.06) | 0.033 | ||
| Age, per 10 yr† | 0.86 (0.79–0.94) | 0.001 | ||
| White | 0.91 (0.81–1.02) | 0.114 | ||
| Nonwhite | 0.68 (0.54–0.84) | 0.001 | ||
| BMI, per 10 kg/m2 | 0.87 (0.74–1.01) | 0.074 | 0.97 (0.82–1.16) | 0.773 |
| Cardiovascular disease | 0.75 (0.58–0.97) | 0.027 | 1.16 (0.85–1.60) | 0.351 |
| Diabetes | 0.63 (0.44–0.91) | 0.013 | 0.79 (0.54–1.15) | 0.212 |
| Chronic pulmonary disease | 1.00 (0.60–1.66) | 0.996 | ||
| Alcohol misuse | 1.11 (0.81–1.54) | 0.514 | ||
| Baseline intensive care data | ||||
| Admission to medical ICU | 1.01 (0.79–1.28) | 0.964 | ||
| APACHE III, per 20 | 0.91 (0.83–1.00) | 0.059 | 1.01 (0.91–1.13) | 0.841 |
| Sepsis as ARDS risk factor | 0.91 (0.70–1.18) | 0.456 | ||
| Daily intensive care data | ||||
| Maximum organ failure score | 0.87 (0.76–1.00) | 0.051 | 0.94 (0.80–1.09) | 0.411 |
| PaO2/FiO2, per 10 | 0.99 (0.97–1.01) | 0.238 | ||
| Other intensive care data | ||||
| Ventilation duration, per week | 0.79 (0.70–0.89) | <0.001 | ||
| ICU length of stay, per week | 0.79 (0.72–0.88) | <0.001 | ||
| Hospital length of stay, per week | 0.80 (0.74–0.87) | <0.001 | 0.81 (0.74–0.88) | <0.001 |
| Discharge to home with unassisted breathing | 3.60 (1.21–10.72) | 0.021 | 1.65 (0.49–5.55) | 0.418 |
Definition of abbreviations: APACHE III = Acute Physiology and Chronic Health Evaluation III; ARDS = acute respiratory distress syndrome; BMI = body mass index; CI = confidence interval; ICU = intensive care unit.
Calculated using Fine and Gray regression models, with hazard ratios <1 indicating a longer time to return to work. Variables with bivariable P < 0.20 were included in the multivariable model. Ventilator duration and ICU length of stay were collinear with hospital length of stay and were excluded from the final multivariable model. All significant associations (P < 0.05) in multivariable models are highlighted in bold. The overall model Wald test was P < 0.001. In this model, 344 of 386 (89%) previously employed survivors were included; 29 (8%) had missing timing of return to work data and 13 (3%) had missing data among included covariates. There were no important differences in baseline or intensive care variables when comparing individuals included versus not included in the multivariable model.
Assessed using an interaction term for age and race (white vs. nonwhite) in the multivariable model.
Estimated Lost Earnings after ARDS
Over 12-month follow-up, 274 (71%) of nonretired survivors accrued lost earnings, totaling an estimated $7,384,062 and averaging $26,949 ± 22,447, representing 60% of their estimated pre-ARDS annual income ($44,784) (Table 3). When averaged across all previously employed ARDS survivors (n = 386), mean estimated lost earnings totaled $19,130 ± 22,522. All post hoc sensitivity analyses demonstrated results similar to the primary analysis (see online supplement).
Table 3.
Earnings and Lost Earnings after ARDS (n = 386)*
| First 6 Months after ARDS (0–6 mo) |
Second 6 Months after ARDS (6–12 mo) |
|||||
|---|---|---|---|---|---|---|
| n (%) | Earnings ($)† (Mean ± SD) | Lost Earnings ($)† (Mean ± SD) | n (%) | Earnings ($)† (Mean ± SD) | Lost Earnings ($)† (Mean ± SD) | |
| Employment status | ||||||
| Working greater or equal hours per week than before ARDS | 110 (28) | 21,963 ± 7,875 | — | 106 (27) | 22,738 ± 8,055 | — |
| Working fewer hours per week than before ARDS | 80 (21) | 16,083 ± 9,651 | 9,497 ± 6,985 | 85 (22) | 19,020 ± 9,621 | 8,298 ± 7,407 |
| Unemployed‡ | 103 (27) | 2,561 ± 7,211 | 18,602 ± 12,228 | 72 (19) | 6,598 ± 11,228 | 15,529 ± 13,150 |
| Receiving disability‡ | 56 (15) | 910 ± 4,338 | 23,124 ± 11,468 | 76 (20) | 129 ± 1,121 | 20,920 ± 11,132 |
| Paid sick leave | 16 (4) | 22,441 ± 3,601 | — | 3 (1) | 33,076 ± 14,274 | — |
| Retired‡ | 14 (4) | 2,350 ± 8,791 | — | 15 (4) | 3,396 ± 8,982 | — |
| Unknown employment status‡ | 7 (2) | — | — | 22 (6) | — | — |
| Died during period | N/A§ | — | — | 7 (2) | — | — |
| Total among nonretired survivors who accrued lost earnings|| | 233 (60) | $6,271 ± 9,773 | $17,042 ± 11,647 | 222 (59) | $8,432 ± 11,385 | $15,375 ± 11,685 |
| Total among all survivors in cohort† | 386 (100) | $11,423 ± 11,832 | $10,287 ± 12,305 | 379 (100) | $12,301 ± 12,596 | $9,006 ± 11,719 |
| Cumulative, nonretired who accrued lost earnings | 274 (71) | $18,034 ± 21,840 | $26,949 ± 22,447 | |||
| Cumulative, all subjects | 386 (100) | $23,501 ± 22,633 | $19,130 ± 22,522 | |||
Definition of abbreviations: ARDS = acute respiratory distress syndrome; N/A = not applicable.
All estimated earnings are adjusted to 2016 U.S. dollars using the consumer price index. Only study participants with full- or part-time work before ARDS are included. Estimates are based on hours of reported work and age- and sex-based hourly wages from the Bureau of Labor statistics. Based on this earnings estimation, patients reporting full-time (n = 312 of 386; 81%) and part-time (n = 74 of 386; 19%) work had $15,801,932 (mean ± SD, $50,647 ± 17,583) and $1,484,554 ($20,062 ± 9,643), respectively, of estimated annual earnings immediately before ARDS (for entire group [n = 386]: $44,784 ± 20,315).
Calculated for 6-month period.
Survivors who worked but were subsequently unemployed, disabled, or retired at the time of follow-up were assumed to accrue earnings during that period. Among 6- and 12-month survivors who were unemployed (n = 103 and 72, respectively), disabled (n = 56 and 76), and retired (n = 14 and 15), 18 (17%) and 27 (38%), three (5%) and one (1%), and one (7%) and two (13%) accrued earnings over the prior 6 months. Survivors who had retired, had unknown employment status, or died at each time point were assumed to have zero potential earnings and therefore zero lost earnings.
Death during 0-to-6-month follow-up period is not applicable because survival until 6 months was required for inclusion in the study cohort.
Includes only survivors who accrued lost earnings. At 6- and 12-month follow-up, 6 (five unemployed and one disabled) and 11 (all unemployed) survivors, respectively, reported work during the preceding 6-month period that was the same or greater than hours worked before ARDS, and were therefore assumed to accrue $0 lost earnings.
Healthcare Coverage after ARDS
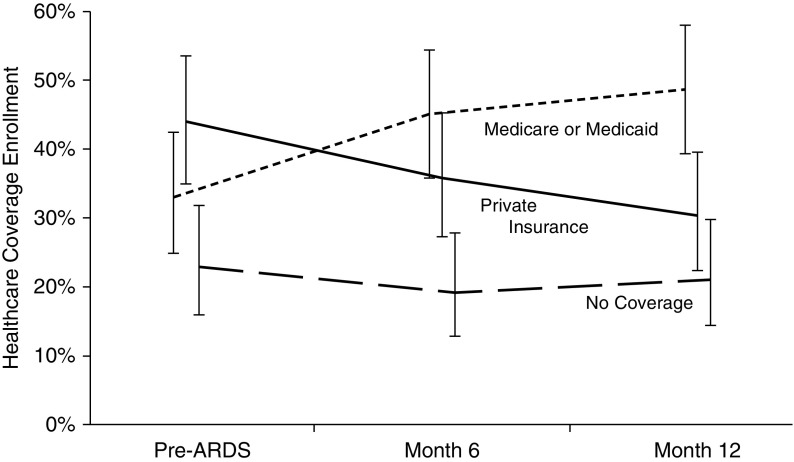
Among survivors who were unemployed or disabled, there was a marked decline in private health insurance (44% to 30%; difference = −14%; 95% CI, −22% to −5%; P = 0.002) and a concomitant rise in Medicare and Medicaid (33% to 49%; difference = 16%; 95% CI, 7–24%; P < 0.001) coverage, with little change in those with no healthcare coverage (23% to 21%; difference = −2%; 95% CI, −10% to 7%; P = 0.67) (Figure 3). Conversely, previously employed survivors who were working exhibited little change from pre-ARDS baseline in private health insurance (78% to 79%; difference = 1%; 95% CI, −3% to 6%; P = 0.59), Medicare and Medicaid (10% to 12%; difference = 2%; 95% CI, −3% to 7%; P = 0.41), and no coverage (12% to 9%; difference = −3%; 95% CI, −8% to 1%; P = 0.16).
Figure 3.
Primary healthcare coverage reported by acute respiratory distress syndrome (ARDS) survivors younger than 65 years old who were unemployed or disabled at each post-ARDS time point. Plotted values are proportions (and 95% confidence intervals) of 12-month survivors enrolled in each healthcare coverage category at each of these three time points: pre-ARDS baseline, 6 months after ARDS, and 12 months after ARDS.
Quality of Life
Survivors who never versus ever returned to work by 6- or 12-month follow-up consistently reported worse health-related quality of life (see Table E4).
Discussion
In this multicenter, prospective longitudinal study of 386 ARDS survivors employed before hospitalization, nearly one-half were jobless at 12-month follow-up, and among those who returned to work, one-fourth became jobless during the follow-up period. Older, nonwhite survivors, and those experiencing longer hospitalization, experienced significantly greater delays in return to work. In addition, 71% of previously employed, nonretired survivors incurred lost earnings, averaging nearly $27,000 in lost earnings over 12 months, representing nearly two-thirds of their pre-ARDS annual income. Moreover, jobless, nonretired survivors experienced a marked shift from private to government-funded healthcare coverage.
In our multicenter national study, 42% (386 of 922) of survivors were employed before hospitalization, similar to a prior single-center U.S. ARDS study (54%) (22) and consistent with the 23–58% employment rates reported in other U.S. ICU survivor studies (23–28). We found that 68% of enrolled, previously employed, and nonretired survivors returned to work within 12 months of ARDS, which was higher than the 48–56% reported in two smaller (n = 82 and n = 27) single-center North American ARDS studies (2, 8) and the 49–58% reported in international studies (n = 67–363) involving ICU survivors without ARDS (28–31). These differences may be due to a national, multicenter design; a relatively young mean age (49 yr old); and patient recruitment as part of trials with multiple exclusion criteria.
Notably, of the ARDS survivors who returned to work (n = 257), 43% (n = 111) never returned to their previous hours worked, 31% (n = 79) experienced a major occupation change, 27% (n = 69) reported reduced effectiveness at work, and 24% (n = 62) subsequently lost their jobs, demonstrating that return to work after ARDS may fall short of previous levels or be short-lived. Among previously employed survivors not returning to work during 12-month follow-up, 58% were receiving disability, suggesting functional impairments as a contributor to unemployment. Prior post-ARDS research has suggested that impairments in memory and attention may contribute to delayed return to work (32), with survivors also reporting that fatigue and weakness (1) contributed to joblessness. In addition, depressive symptoms have been associated with unemployment 2 years after ARDS (33). Conversely, a recent study demonstrated no independent association of cognitive and physical function with 12-month post-ICU employment status in critical illness survivors (28). Notably, we found that survivors who never returned to work by 6 and 12 months reported worse quality of life, although the directionality of this cross-sectional association is unknown. Additional longitudinal studies are needed to explore the associations of post-ICU impairments with return to work and impact on quality of life.
In addition, we demonstrated that white patients of different ages had similar timing of return to work, whereas older (vs. younger) nonwhite patients experienced longer delays in returning to work. The reason for this racial difference is unclear, but may be caused by differences in type of work (i.e., office vs. manual work), socioeconomic status, and/or access to rehabilitation services. In addition, longer hospital length of stay was associated with delayed return to work, regardless of age and race, potentially because of worse physical impairments occurring with longer hospitalization (7, 34). Future studies should evaluate the efficacy and cost-effectiveness of targeting occupational rehabilitation interventions toward these at-risk patient groups to reduce delays in returning to work after ARDS.
To our knowledge, this analysis was the first to evaluate lost earnings and changes in healthcare coverage after ARDS. We demonstrated that 71% of nonretired survivors accrued lost earnings over 12-month follow-up, averaging $26,949, representing nearly two-thirds of estimated pre-ARDS annual earnings. These lost earnings totals were substantial, and comparable with traumatic brain injury and stroke (35, 36). In addition, over 12 months, jobless, nonretired survivors experienced a substantial decline in private insurance and rise in government-funded healthcare coverage, an important finding given the substantial healthcare utilization of ARDS survivors (37, 38).
Strengths of this study include its national, multicenter longitudinal design; relatively large sample size; high participant retention rate (>94%); and evaluation of patient- and hospital-related risk factors, estimated lost earnings, and shifts in healthcare coverage. However, this study has potential limitations. First, similar to most studies in the field (39), employment status was determined via self-report. However, sensitivity analyses did not materially change the primary results; nonetheless, the true magnitude of any measurement error if not known. Second, lost earnings were calculated based on estimated, rather than actual, earnings. However, our analysis was conducted using a national ARDS survivor cohort, estimating lost earnings using age- and sex-matched Bureau of Labor Statistics data. Hence, these results may be more nationally representative, thus enhancing generalizability. In addition, because we focused on earnings as a measure of lost work capacity, our calculation did not account for disability benefits and other sources of disposable income (i.e., personal or retirement savings), which may have offset the financial hardship created by lost earnings (40). Moreover, we did not consider lost earnings while patients were hospitalized, lost earnings accrued by informal caregivers, costs of ongoing medical care, and other economic and noneconomic issues associated with patient and informal caregiver joblessness (41).
Third, there may be other important variables, not included in this study, which may be associated with joblessness after ARDS, including baseline physical and cognitive function and potential ICU-related variables (e.g., delirium), along with expected declines in employment potential with age. However, a sensitivity analysis adjusting for advancing age did not change our overall results, and in a recent study, neither baseline neurocognitive function nor delirium in the ICU was associated with 12-month employment status (28). Further studies are needed to evaluate the association of postdischarge factors and post-ARDS employment. Fourth, our study population included relatively young ARDS survivors and findings may not generalize to all ICU populations. However, enrollment from 43 sites and comparability of results with prior literature help support generalizability. Finally, as an observational cohort study, we could not assess the causality of ARDS with joblessness.
In conclusion, in this multicenter longitudinal study, we found that nearly one-half of previously employed survivors were jobless at 12-month post-ARDS follow-up, with older, nonwhite survivors and those with a longer hospital stay having a greater risk of delayed return to work. Jobless, nonretired survivors also accrued substantial lost earnings, amounting to two-thirds of their pre-ARDS earnings, and experienced a substantial decline in private healthcare coverage and rise in Medicare and Medicaid. These findings highlight the major economic consequences of joblessness after ARDS and identify at-risk groups for future evaluation of occupational rehabilitation interventions.
Acknowledgments
Acknowledgment
The authors thank all of the patients and their proxies who participated in this study. They thank Caroline Chessare, Mardee Merrill, Mariela Pinedo, Kyle Schneck, Stacey Schoonmaker, Kristin Sepulveda, Marcella Shrout, Cassie Wicken, Melissa McCullough, Jonathan Gellar, Elizabeth Vayda, Gita Byraiah, Laura Methvin, Vanessa Stan, Shirani Rajan, Cassie Wicken, Meg Shanahan, Elizabeth Baer, and Anita Chandra who assisted with data collection; and Lin Chen, William Flickinger, Christopher Mayhew, and Bharat Kamdar who assisted with data management. They also thank Akshay S. Desai, M.D., M.P.H., and Jennifer L. Martin, Ph.D. for their thoughtful review of a draft of the manuscript.
Investigators and research staff from National Heart, Lung, and Blood Institute Acute Respiratory Distress Syndrome Clinical Trials Network sites that participated in this study: University of Washington, Harborview (L. Hudson*, S. Gundel, C. Hough, M. Neff, K. Sims, A. Ungar, and T. Watkins); Baystate Medical Center (J. Steingrub*, M. Tidswell, E. Braden, L. DeSouza, C. Kardos, L. Kozikowski, and S. Ouellette); Baylor College of Medicine (K. Guntupalli, V. Bandi, C. Pope, and C. Ross); Johns Hopkins University (R. Brower*, H. Fessler, D. Hager, P. Mendez-Tellez, D. Needham, and K. Oakjones); Johns Hopkins Bayview Medical Center (J. Sevransky and A. Workneh); University of Maryland (C. Shanholtz, D. Herr, H. Howes, G. Netzer, P. Rock, A. Sampaio, and J. Titus); Union Memorial Hospital (P. Sloane, T. Beck, D. Highfield, and S. King); Washington Hospital Center (B. Lee and N. Bolouri); Cleveland Clinic Foundation (H. P. Wiedemann*, R. W. Ashton, D. A. Culver, T. Frederick, J. A. Guzman, J. J. Komara, Jr., and A. J. Reddy); University Hospitals of Cleveland (R. Hejal, M. Andrews, and D. Haney); MetroHealth Medical Center (A. F. Connors, S. Lasalvia, J. D. Thornton, and E. L. Warren); University of Colorado Hospital, Aurora (M. Moss*, E. L. Burnham, L. Gray, J. Maloney, and M. Mealer); Denver Health Medical Center (I. Douglas, K. Overdier, K. Thompson, and R. Wolken); Rose Medical Center (S. Frankel and J. McKeehan); Swedish Medical Center (M. L. Warner); Saint Anthony's Hospital (T. Bost, C. Higgins, and K. Hodgin); Duke University (N. MacIntyre*, L. Brown, C. Cox, M. Gentile, J. Govert, and N. Knudsen); University of North Carolina (S. Carson, L. Chang, S. Choudhury, W. Hall, and J. Lanier); Vanderbilt University (A. P. Wheeler*, G. R. Bernard, M. Hays, S. Mogan, and T. W. Rice); Wake Forest University (R. D. Hite*, A. Harvey, P. E. Morris, and M. Ragusky); Moses Cone Memorial Hospital (P. Wright, S. Groce, J. McLean, and A. Overton); University of Virginia (J. Truwit, K. Enfield, and M. Marshall); Intermountain Medical Center (A. Morris*, C. Grissom*, A. Austin, S. Barney, S. Brown, J. Ferguson, H. Gallo, T. Graydon, E. Hirshberg, A. Jephson, N. Kumar, M. Lanspa, R. Miller, D. Murphy, J. Orme, A. Stowe, L. Struck, F. Thomas, and D. Ward); LDS Hospital (P. Bailey, W. Beninati, L. Bezdjian, T. Clemmer, S. Rimkus, R. Tanaka, and L. Weaver); McKay Dee Hospital (C. Lawton and D. Hanselman); Utah Valley Regional Medical Center (K. Sundar, W. Alward, C. Bishop, D. Eckley, D. Harris, T. Hill, B. Jensen, K. Ludwig, D. Nielsen, and M. Pearce); University of California, San Francisco (M. A. Matthay*, C. Calfee, B. Daniel, M. Eisner, O. Garcia, K. Kordesch, K. Liu, N. Shum, and H. Zhou); University of California, San Francisco, Fresno (M. W. Peterson, J. Blaauw, and K. Van Gundy); San Francisco General Hospital (R. Kallet and E. Johnson); University of California, Davis (T. Albertson, B. Morrissey, and E. Vlastelin); Louisiana State University Health Sciences Center-New Orleans (B. deBoisblanc*, A. Antoine, D. Charbonnet, J. Hunt, P. Lauto, A. Marr, G. Meyaski, and C. Romaine); Earl K. Long Medical Center (S. Brierre, J. Byrne, T. Jagneaux, C. LeBlanc, K. Moreau, and C. Thomas); Ochsner Clinic Foundation (S. Jain, D. Taylor, and L. Seoane); Our Lady of the Lake Medical Center (C. Hebert and J. Thompson); and Tulane Medical Center (F. Simeone and J. Fearon). Clinical Coordinating Center: Massachusetts General Hospital and Harvard Medical School (D. Schoenfeld*, N. Dong, M. Guha, E. Hammond, P. Lazar, R. Morse, C. Oldmixon, N. Ringwood, E. Smoot, B. T. Thompson, and R. Wilson). NHLBI: A. Harabin, S. Bredow, M. Waclawiw, and G. Weinmann. Data and Safety Monitoring Board: R. G. Spragg (chair), A. Slutsky, M. Levy, B. Markovitz, E. Petkova, and C. Weijer. Protocol Review Committee: J. Sznajder (chair), M. Begg, L. Gilbert-McClain E. Israel, J. Lewis, S. McClave, and P. Parsons.
*Principal investigators.
Footnotes
Supported by a Career Development Award from the UCLA Clinical and Translational Science Institute (National Institutes of Health–National Center for Advancing Translational Science UCLA UL1TR000124 and UL1TR001881, B.B.K.). NHLBI funded this follow-up study (N01HR56170, R01HL091760, and 3R01HL091760-02S1) and the ARDS Network trials (contracts HHSN268200536165C to HHSN268200536176C and HHSN268200536179C).
Author Contributions: M.H. and B.B.K. had full access to all of the data in the study and take full responsibility for the integrity of the data and the accuracy of the data analysis. B.B.K., M.H., V.D.D., E.C., T M.v.W., R.O.H., and D.M.N. developed the study concept and design. M.H. and B.B.K. conducted the statistical analysis, and all authors have interpreted the data. B.B.K., M.H., and D.M.N. drafted the article, and all authors have provided critical revisions for important intellectual content. This study was supervised by D.M.N. All authors have read and approved the final article.
This article has an online supplement, which is accessible from this issue's table of contents at www.atsjournals.org
Originally Published in Press as DOI: 10.1164/rccm.201611-2327OC on April 27, 2017
Author disclosures are available with the text of this article at www.atsjournals.org.
Contributor Information
Collaborators: L. Hudson, S. Gundel, C. Hough, M. Neff, K. Sims, A. Ungar, T. Watkins, J. Steingrub, M. Tidswell, E. Braden, L. DeSouza, C. Kardos, L. Kozikowski, S. Ouellette, K. Guntupalli, V. Bandi, C. Pope, C. Ross, R. Brower, H. Fessler, D. Hager, P. Mendez-Tellez, D. Needham, K. Oakjones, J. Sevransky, A. Workneh, C. Shanholtz, D. Herr, H. Howes, G. Netzer, P. Rock, A. Sampaio, J. Titus, P. Sloane, T. Beck, D. Highfield, S. King, B. Lee, N. Bolouri, H. P. Wiedemann, R. W. Ashton, D. A. Culver, T. Frederick, J. A. Guzman, J. J. Komara, Jr., A. J. Reddy, R. Hejal, M. Andrews, D. Haney, A. F. Connors, S. Lasalvia, J. D. Thornton, E. L. Warren, M. Moss, E. L. Burnham, L. Gray, J. Maloney, M. Mealer, I. Douglas, K. Overdier, K. Thompson, R. Wolken, S. Frankel, J. McKeehan, M. L. Warner, T. Bost, C. Higgins, K. Hodgin, N. MacIntyre, L. Brown, C. Cox, M. Gentile, J. Govert, N. Knudsen, S. Carson, L. Chang, S. Choudhury, W. Hall, J. Lanier, A. P. Wheeler, G. R. Bernard, M. Hays, S. Mogan, T. W. Rice, R. D. Hite, A. Harvey, P. E. Morris, M. Ragusky, P. Wright, S. Groce, J. McLean, A. Overton, J. Truwit, K. Enfield, M. Marshall, A. Morris, C. Grissom, A. Austin, S. Barney, S. Brown, J. Ferguson, H. Gallo, T. Graydon, E. Hirshberg, A. Jephson, N. Kumar, M. Lanspa, R. Miller, D. Murphy, J. Orme, A. Stowe, L. Struck, F. Thomas, D. Ward, P. Bailey, W. Beninati, L. Bezdjian, T. Clemmer, S. Rimkus, R. Tanaka, L. Weaver, C. Lawton, D. Hanselman, K. Sundar, W. Alward, C. Bishop, D. Eckley, D. Harris, T. Hill, B. Jensen, K. Ludwig, D. Nielsen, M. Pearce, M. A. Matthay, C. Calfee, B. Daniel, M. Eisner, O. Garcia, K. Kordesch, K. Liu, N. Shum, H. Zhou, M. W. Peterson, J. Blaauw, K. Van Gundy, R. Kallet, E. Johnson, T. Albertson, B. Morrissey, E. Vlastelin, B. deBoisblanc, A. Antoine, D. Charbonnet, J. Hunt, P. Lauto, A. Marr, G. Meyaski, C. Romaine, S. Brierre, J. Byrne, T. Jagneaux, C. LeBlanc, K. Moreau, C. Thomas, S. Jain, D. Taylor, L. Seoane, C. Hebert, J. Thompson, F. Simeone, J. Fearon, D. Schoenfeld, N. Dong, M. Guha, E. Hammond, P. Lazar, R. Morse, C. Oldmixon, N. Ringwood, E. Smoot, B. T. Thompson, R. Wilson, A. Harabin, S. Bredow, M. Waclawiw, G. Weinmann, R. G. Spragg, A. Slutsky, M. Levy, B. Markovitz, E. Petkova, C. Weijer, J. Sznajder, M. Begg, L. Gilbert-McClain, E. Israel, J. Lewis, S. McClave, and P. Parsons
References
- 1.Herridge MS, Cheung AM, Tansey CM, Matte-Martyn A, Diaz-Granados N, Al-Saidi F, Cooper AB, Guest CB, Mazer CD, Mehta S, et al. Canadian Critical Care Trials Group. One-year outcomes in survivors of the acute respiratory distress syndrome. N Engl J Med. 2003;348:683–693. doi: 10.1056/NEJMoa022450. [DOI] [PubMed] [Google Scholar]
- 2.Herridge MS, Tansey CM, Matté A, Tomlinson G, Diaz-Granados N, Cooper A, Guest CB, Mazer CD, Mehta S, Stewart TE, et al. Canadian Critical Care Trials Group. Functional disability 5 years after acute respiratory distress syndrome. N Engl J Med. 2011;364:1293–1304. doi: 10.1056/NEJMoa1011802. [DOI] [PubMed] [Google Scholar]
- 3.Desai SV, Law TJ, Needham DM. Long-term complications of critical care. Crit Care Med. 2011;39:371–379. doi: 10.1097/CCM.0b013e3181fd66e5. [DOI] [PubMed] [Google Scholar]
- 4.Hopkins RO, Weaver LK, Collingridge D, Parkinson RB, Chan KJ, Orme JF., Jr Two-year cognitive, emotional, and quality-of-life outcomes in acute respiratory distress syndrome. Am J Respir Crit Care Med. 2005;171:340–347. doi: 10.1164/rccm.200406-763OC. [DOI] [PubMed] [Google Scholar]
- 5.Huang M, Parker AM, Bienvenu OJ, Dinglas VD, Colantuoni E, Hopkins RO, Needham DM National Institutes of Health, National Heart, Lung, and Blood Institute Acute Respiratory Distress Syndrome Network. Psychiatric symptoms in acute respiratory distress syndrome survivors: a 1-year national multicenter study. Crit Care Med. 2016;44:954–965. doi: 10.1097/CCM.0000000000001621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Needham DM, Dinglas VD, Morris PE, Jackson JC, Hough CL, Mendez-Tellez PA, Wozniak AW, Colantuoni E, Ely EW, Rice TW, et al. NIH NHLBI ARDS Network. Physical and cognitive performance of patients with acute lung injury 1 year after initial trophic versus full enteral feeding. EDEN trial follow-up. Am J Respir Crit Care Med. 2013;188:567–576. doi: 10.1164/rccm.201304-0651OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Fan E, Dowdy DW, Colantuoni E, Mendez-Tellez PA, Sevransky JE, Shanholtz C, Himmelfarb CR, Desai SV, Ciesla N, Herridge MS, et al. Physical complications in acute lung injury survivors: a two-year longitudinal prospective study. Crit Care Med. 2014;42:849–859. doi: 10.1097/CCM.0000000000000040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.McHugh LG, Milberg JA, Whitcomb ME, Schoene RB, Maunder RJ, Hudson LD. Recovery of function in survivors of the acute respiratory distress syndrome. Am J Respir Crit Care Med. 1994;150:90–94. doi: 10.1164/ajrccm.150.1.8025779. [DOI] [PubMed] [Google Scholar]
- 9.Coopersmith CM, Wunsch H, Fink MP, Linde-Zwirble WT, Olsen KM, Sommers MS, Anand KJ, Tchorz KM, Angus DC, Deutschman CS. A comparison of critical care research funding and the financial burden of critical illness in the United States. Crit Care Med. 2012;40:1072–1079. doi: 10.1097/CCM.0b013e31823c8d03. [DOI] [PubMed] [Google Scholar]
- 10.Matthay MA, Brower RG, Carson S, Douglas IS, Eisner M, Hite D, Holets S, Kallet RH, Liu KD, MacIntyre N, et al. National Heart, Lung, and Blood Institute Acute Respiratory Distress Syndrome (ARDS) Clinical Trials Network. Randomized, placebo-controlled clinical trial of an aerosolized β2-agonist for treatment of acute lung injury. Am J Respir Crit Care Med. 2011;184:561–568. doi: 10.1164/rccm.201012-2090OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Rice TW, Wheeler AP, Thompson BT, Steingrub J, Hite RD, Moss M, Morris A, Dong N, Rock P National Heart, Lung, and Blood Institute Acute Respiratory Distress Syndrome (ARDS) Clinical Trials Network. Initial trophic vs full enteral feeding in patients with acute lung injury: the EDEN randomized trial. JAMA. 2012;307:795–803. doi: 10.1001/jama.2012.137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Rice TW, Wheeler AP, Thompson BT, deBoisblanc BP, Steingrub J, Rock P NIH NHLBI Acute Respiratory Distress Syndrome Network of Investigators. Enteral omega-3 fatty acid, gamma-linolenic acid, and antioxidant supplementation in acute lung injury. JAMA. 2011;306:1574–1581. doi: 10.1001/jama.2011.1435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Truwit JD, Bernard GR, Steingrub J, Matthay MA, Liu KD, Albertson TE, Brower RG, Shanholtz C, Rock P, Douglas IS, et al. National Heart, Lung, and Blood Institute ARDS Clinical Trials Network. Rosuvastatin for sepsis-associated acute respiratory distress syndrome. N Engl J Med. 2014;370:2191–2200. doi: 10.1056/NEJMoa1401520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Babor TF, Higgins-Biddle JC, Saunders JB, Monteiro MG. AUDIT: the Alcohol Use Disorder Identification Test: guidelines for use in primary care. 2nd ed. Geneva: World Health Organization; 2001. [Google Scholar]
- 15.Bernard GR. The Brussels Score. Sepsis. 1997;1:43–44. [Google Scholar]
- 16.Radford K, Phillips J, Drummond A, Sach T, Walker M, Tyerman A, Haboubi N, Jones T. Return to work after traumatic brain injury: cohort comparison and economic evaluation. Brain Inj. 2013;27:507–520. doi: 10.3109/02699052.2013.766929. [DOI] [PubMed] [Google Scholar]
- 17.Corso P, Finkelstein E, Miller T, Fiebelkorn I, Zaloshnja E. Incidence and lifetime costs of injuries in the United States. Inj Prev. 2006;12:212–218. doi: 10.1136/ip.2005.010983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Data book: beneficiaries dually eligible for Medicare and Medicaid [accessed 2016 May]. Available from: https://www.macpac.gov/wp-content/uploads/2015/01/Jan16_MedPAC_MACPAC_DualsDataBook.pdf.
- 19.Geskus RB. Cause-specific cumulative incidence estimation and the Fine and Gray model under both left truncation and right censoring. Biometrics. 2011;67:39–49. doi: 10.1111/j.1541-0420.2010.01420.x. [DOI] [PubMed] [Google Scholar]
- 20.Fine JP, Gray RJ. A proportional hazards model for the subdistribution of a competing risk. J Am Stat Assoc. 1999;94:496–509. [Google Scholar]
- 21.Schoenfeld D. Partial residuals for the proportional hazards regression model. Biometrika. 1982;69:239–241. [Google Scholar]
- 22.Weinert CR, Gross CR, Kangas JR, Bury CL, Marinelli WA. Health-related quality of life after acute lung injury. Am J Respir Crit Care Med. 1997;156:1120–1128. doi: 10.1164/ajrccm.156.4.9611047. [DOI] [PubMed] [Google Scholar]
- 23.Parno JR, Teres D, Lemeshow S, Brown RB, Avrunin JS. Two-year outcome of adult intensive care patients. Med Care. 1984;22:167–176. doi: 10.1097/00005650-198402000-00008. [DOI] [PubMed] [Google Scholar]
- 24.Goldstein RL, Campion EW, Thibault GE, Mulley AG, Skinner E. Functional outcomes following medical intensive care. Crit Care Med. 1986;14:783–788. doi: 10.1097/00003246-198609000-00005. [DOI] [PubMed] [Google Scholar]
- 25.Mundt DJ, Gage RW, Lemeshow S, Pastides H, Teres D, Avrunin JS. Intensive care unit patient follow-up. Mortality, functional status, and return to work at six months. Arch Intern Med. 1989;149:68–72. doi: 10.1001/archinte.149.1.68. [DOI] [PubMed] [Google Scholar]
- 26.Fakhry SM, Kercher KW, Rutledge R. Survival, quality of life, and charges in critically III surgical patients requiring prolonged ICU stays. J Trauma. 1996;41:999–1007. doi: 10.1097/00005373-199612000-00010. [DOI] [PubMed] [Google Scholar]
- 27.Quality of Life After Mechanized Ventilation in the Elderly Study Investigators. 2-month mortality and functional status of critically ill adult patients receiving prolonged mechanical ventilation. Chest. 2002;121:549–558. doi: 10.1378/chest.121.2.549. [DOI] [PubMed] [Google Scholar]
- 28.Norman BC, Jackson JC, Graves JA, Girard TD, Pandharipande PP, Brummel NE, Wang L, Thompson JL, Chandrasekhar R, Ely EW. Employment outcomes after critical illness: an analysis of the bringing to light the risk factors and incidence of neuropsychological dysfunction in ICU survivors cohort. Crit Care Med. 2016;44:2003–2009. doi: 10.1097/CCM.0000000000001849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Cuthbertson BH, Scott J, Strachan M, Kilonzo M, Vale L. Quality of life before and after intensive care. Anaesthesia. 2005;60:332–339. doi: 10.1111/j.1365-2044.2004.04109.x. [DOI] [PubMed] [Google Scholar]
- 30.McGee HM, Doyle F, Conroy RM, De La Harpe D, Shelley E. Impact of briefly-assessed depression on secondary prevention outcomes after acute coronary syndrome: a one-year longitudinal survey. BMC Health Serv Res. 2006;6:9. doi: 10.1186/1472-6963-6-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Myhren H, Ekeberg Ø, Stokland O. Health-related quality of life and return to work after critical illness in general intensive care unit patients: a 1-year follow-up study. Crit Care Med. 2010;38:1554–1561. doi: 10.1097/CCM.0b013e3181e2c8b1. [DOI] [PubMed] [Google Scholar]
- 32.Rothenhäusler HB, Ehrentraut S, Stoll C, Schelling G, Kapfhammer HP. The relationship between cognitive performance and employment and health status in long-term survivors of the acute respiratory distress syndrome: results of an exploratory study. Gen Hosp Psychiatry. 2001;23:90–96. doi: 10.1016/s0163-8343(01)00123-2. [DOI] [PubMed] [Google Scholar]
- 33.Adhikari NK, McAndrews MP, Tansey CM, Matté A, Pinto R, Cheung AM, Diaz-Granados N, Barr A, Herridge MS. Self-reported symptoms of depression and memory dysfunction in survivors of ARDS. Chest. 2009;135:678–687. doi: 10.1378/chest.08-0974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Needham DM, Wozniak AW, Hough CL, Morris PE, Dinglas VD, Jackson JC, Mendez-Tellez PA, Shanholtz C, Ely EW, Colantuoni E, et al. National Institutes of Health NHLBI ARDS Network. Risk factors for physical impairment after acute lung injury in a national, multicenter study. Am J Respir Crit Care Med. 2014;189:1214–1224. doi: 10.1164/rccm.201401-0158OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Johnstone B, Mount D, Schopp LH. Financial and vocational outcomes 1 year after traumatic brain injury. Arch Phys Med Rehabil. 2003;84:238–241. doi: 10.1053/apmr.2003.50097. [DOI] [PubMed] [Google Scholar]
- 36.Taylor TN, Davis PH, Torner JC, Holmes J, Meyer JW, Jacobson MF. Lifetime cost of stroke in the United States. Stroke. 1996;27:1459–1466. doi: 10.1161/01.str.27.9.1459. [DOI] [PubMed] [Google Scholar]
- 37.Ruhl AP, Lord RK, Panek JA, Colantuoni E, Sepulveda KA, Chong A, Dinglas VD, Shanholtz CB, Pronovost PJ, Steinwachs DM, et al. Health care resource use and costs of two-year survivors of acute lung injury. An observational cohort study. Ann Am Thorac Soc. 2015;12:392–401. doi: 10.1513/AnnalsATS.201409-422OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Ruhl AP, Huang M, Colantuoni E, Lord RK, Dinglas VD, Chong A, Sepulveda KA, Mendez-Tellez PA, Shanholtz CB, Steinwachs DM, et al. Healthcare resource use and costs in long-term survivors of acute respiratory distress syndrome: a 5-year longitudinal cohort study. Crit Care Med. 2017;45:196–204. doi: 10.1097/CCM.0000000000002088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Dupont J, Sollberger PA Organisation for Economic Co-operation and Development. Productivity measurement and analysis. Paris: OECD; 2008. [Google Scholar]
- 40.Dobkin C, Finkelstein A, Kluender R, Notowidigdo MJ. The economic consequences of hospital admissions. National Bureau of Economic Research; 2016 [accessed 2017 Mar 31]. Available from: https://bfi.uchicago.edu/sites/default/files/research/DFKN_hospital_admissions_0.pdf.
- 41.Cameron JI, Chu LM, Matte A, Tomlinson G, Chan L, Thomas C, Friedrich JO, Mehta S, Lamontagne F, Levasseur M, et al. RECOVER Program Investigators (Phase 1: towards RECOVER); Canadian Critical Care Trials Group. One-year outcomes in caregivers of critically ill patients. N Engl J Med. 2016;374:1831–1841. doi: 10.1056/NEJMoa1511160. [DOI] [PubMed] [Google Scholar]