Abstract
Rationale: The impact of a broad range of occupational exposures on subclinical interstitial lung disease (ILD) has not been studied.
Objectives: To determine whether occupational exposures to vapors, gas, dust, and fumes (VGDF) are associated with high-attenuation areas (HAA) and interstitial lung abnormalities (ILA), which are quantitative and qualitative computed tomography (CT)-based measurements of subclinical ILD, respectively.
Methods: We performed analyses of participants enrolled in MESA (Multi-Ethnic Study of Atherosclerosis), a population-based cohort aged 45–84 years at recruitment. HAA was measured at baseline and on serial cardiac CT scans in 5,702 participants. ILA was ascertained in a subset of 2,312 participants who underwent full-lung CT scanning at 10-year follow-up. Occupational exposures were assessed by self-reported VGDF exposure and by job-exposure matrix (JEM). Linear mixed models and logistic regression were used to determine whether occupational exposures were associated with log-transformed HAA and ILA. Models were adjusted for age, sex, race/ethnicity, education, employment status, tobacco use, and scanner technology.
Measurements and Main Results: Each JEM score increment in VGDF exposure was associated with 2.64% greater HAA (95% confidence interval [CI], 1.23–4.19%). Self-reported vapors/gas exposure was associated with an increased odds of ILA among those currently employed (1.76-fold; 95% CI, 1.09–2.84) and those less than 65 years old (1.97-fold; 95% CI, 1.16–3.35). There was no consistent evidence that occupational exposures were associated with progression of HAA over the follow-up period.
Conclusions: JEM-assigned and self-reported exposures to VGDF were associated with measurements of subclinical ILD in community-dwelling adults.
Keywords: epidemiology, community-based study, occupational exposures, subclinical, interstitial lung disease
At a Glance Commentary
Scientific Knowledge on the Subject
The recognition of subclinical forms of interstitial lung disease (ILD) on computed tomography provides a unique opportunity to study potential risk factors for ILD in a population-based cohort. Exposures to certain environmental antigens and fibrogenic particulates are well established causes of hypersensitivity pneumonitis, asbestosis, and other subtypes of ILD. The impact of a broad range of occupational exposures on the burden of ILD is unknown.
What This Study Adds to the Field
Self-reported and objectively assigned exposures to vapors, gas, dusts, and fumes were associated with subclinical ILDs in a cohort of community-dwelling adults enrolled in the Multi-Ethnic Study of Atherosclerosis.
The interstitial lung diseases (ILDs) encompass a diverse group of chronic lung diseases, characterized by recurrent alveolar injury, parenchymal inflammation, and extracellular fibrosis. Despite a low incidence rate, the ILDs are associated with significant morbidity and mortality, and remain the primary indication for lung transplantation in the United States (1). As symptoms typically present late in the disease course, the inciting cause of injury is often unclear and difficult to distinguish from other chronic and mixed exposures.
Although the underlying etiology of the ILDs is largely unknown, occupational exposures are important risk factors, and specific disease subtypes are characteristically associated with certain exposures. For example, specific fibrogenic particulates, such as asbestos, silica or coal dust are prerequisites for the development of the pneumoconioses (2). Inhalational exposures to a range of environmental and occupational organic antigens precipitate the immunologic reaction that characterizes hypersensitivity pneumonitis. There is also evidence suggesting that occupational exposures may contribute to the pathobiology of idiopathic forms of disease (3). Individuals with occupational dust exposure, particularly wood and metal, have an increased risk of idiopathic pulmonary fibrosis (4–6).
Identification of subclinical radiographic forms of ILD provides a unique opportunity to study potential antecedent causes of disease in an asymptomatic population. Interstitial lung abnormalities (ILA), a qualitative assessment of early interstitial changes in nondependent portions of the lung, and high-attenuation areas (HAA), a quantitative computed tomography (CT) attenuation-based phenotype, are two validated measurements of subclinical ILD (7–9). Even in the absence of clinical ILD, populations with subclinical ILD have more respiratory symptoms, physiologic decrements, and higher mortality (8, 10, 11).
The association between a broad range of self-reported and independently assigned occupational exposures and subclinical ILD has not been previously investigated in a population-based study. We hypothesized that exposure to vapors, gas, dust, and fumes (VGDF) would be associated with qualitative and quantitative measurements of subclinical ILD in community-dwelling adults.
Some of these results have been previously reported in the form of an abstract (12).
Methods
Study Design and Participant Selection
MESA (Multi-Ethnic Atherosclerosis Study) is a prospective cohort study funded by the NHLBI to investigate subclinical cardiovascular disease. MESA and ancillary studies, MESA Lung and MESA Air, are described in depth elsewhere, and served as the sampling frame for this study (8, 13–15). Informed consent was obtained for all participants, and the study was approved by the institutional review boards at collaborating centers.
Briefly, MESA enrolled participants that were free of known cardiovascular disease from six centers around the United States (Baltimore, MD; Chicago, IL; Los Angeles County, CA; New York, NY; St. Paul, MN; and Winston Salem, NC). A total of 6,814 participants, ages 45–84 years, were recruited between 2000 and 2002 and underwent questionnaires regarding demographics, family history, medical history, lifestyle habits, and psychosocial factors. At enrollment and during the four subsequent exams, participants had noninvasive assessment of cardiovascular status, including cardiac CT scans. By design, all returning participants had a repeat cardiac CT scan at either exam 2 (2,955 participants) or 3 (2,805 participants); 30% of the cohort (1,405 participants) had a third cardiac CT scan at exam 4. A total of 3,200 participants underwent cardiac CT scan again at exam 5 during years 2010–2012, nearly all of whom also had a full CT scan at this time.
The sampling scheme of participants included in our qualitative and quantitative assessments of subclinical ILD are described in detail in the Appendix in the online supplement.
ILA
Full-lung MESA CT scans were acquired at suspended full inspiration using the MESA Lung/SPIROMICS protocol, as previously described (8, 16). One of five board-certified radiologists reviewed the full-lung CT scans for ILA, which was defined as the presence of ground-glass, reticular abnormality, diffuse centrilobular nodularity, honeycombing, traction bronchiectasis, nonemphysematous cysts, or architectural distortion in at least 5% of nondependent portions of the lung (interreader κ = 0.4706; 95% confidence interval [CI], 0.1368–0.8044) (17).
HAA
HAA was measured on noncontrast cardiac CT scans performed at the MESA baseline visit and selected follow-up exams using standardized protocols (18). These cardiac CT scans image approximately 65–70% of the total lung volume, capture most of the lower lobes, and exclude much of the lung apexes. Quantitative image attenuation was measured by trained readers using a modified version of the Pulmonary Analysis Software developed by E.A.H. at the University of Iowa (Iowa City, IA). HAA was defined as the percentage of the imaged lung volume having attenuation values between −600 and −250 Hounsfield units (HU) (8, 9). This range of CT lung attenuation includes ground-glass and reticular abnormalities, and excludes denser areas that characterize atelectasis, medium and large blood vessels, and pulmonary nodules. Percent emphysema was defined as the percentage of voxels below −950 HU.
Exposure Assessment
Occupational exposures were assessed using two methods: (1) by self-reported exposure to VGDF; and (2) by a job-exposure matrix (JEM) previously created by the National Institute for Occupational Safety and Health (NIOSH) (19). Self-reported exposures were obtained during MESA exam 4 and included presence/absence, duration, and severity of exposure to VGDF. Participants were asked separately about exposure to dusts, fumes, and vapors/gas (combined into one category). Demographic questionnaires at each exam ascertained current occupation if participants were employed, or the occupation where last employed if the participants were retired (see Appendix in the online supplement). These reported occupations were coded using Bureau of Census 2002 occupational codes by trained staff from NIOSH. One industrial hygienist assigned an initial score based on the four-digit U.S. Census Occupation Code, representing the likelihood and severity of exposure (low, intermediate, or high) to vapors/gas, fumes, dust, subcategories of dust (inorganic vs. organic), and combined VGDF exposure. The other two hygienists reviewed the preliminary scoring and reached a final consensus score.
Statistical Analysis
All statistical tests were performed in SAS version 9.3 (SAS Institute, Cary, NC) using a two-tailed P value, with an α of 0.05 to define statistical significance.
Occupational risk factors were defined according to self-reported exposures, and the exposures established using the NIOSH JEM. Occupational exposure variables that were individually evaluated in models included: self-reported exposures to VGDF; any VGDF exposure (none vs. any); severity of VGDF exposure (none, mild, moderate, or severe); years of exposure to VGDF; and JEM-assigned VGDF exposure scores (low, intermediate, or high). In the main analyses, exposures with more than one category were treated as ordinal variables, and duration of exposure to VGDF was treated as a continuous variable. As the difference in severity between categories of exposures may not be linear, these exposure variables were treated as dummy variables in sensitivity analyses.
Multivariable logistic regression was used to examine associations between occupational exposures and the odds of ILA on full-lung CT scan at exam 5. Models were adjusted for age, sex, ethnicity, tobacco use (current smoking status and pack-years), and site. We assessed for effect modification through models stratified on age, sex, ethnicity, employment status, and smoking status. Where stratified models showed differential effects, we tested for statistical significance with nested models, including an interaction term between the exposure and potential effect modifier.
A linear mixed model was used to analyze the cross-sectional association between occupational exposures and HAA at baseline examination and the relationship between occupational exposures and the rate of progression of HAA. HAA was log transformed in the model and back transformed to obtain an estimate of percent change. Repeat measurements were modeled as a function of study time, and time-varying exposures modeled as an interaction with time to examine the associations with the linear rate of change of HAA over time. Participant-specific random intercepts and slopes were included. Models were adjusted for potential confounders selected a priori: age; sex; educational attainment; employment status; height; body mass index; waist circumference; smoking status; cigarette pack-years; glomerular filtration rate; total volume of imaged lung; percent emphysema on CT scan; scanner type; and study site. Potential effect modification was examined with stratification based on smoking status, sex, ethnicity, age, and employment status.
Results
A total of 6,813 MESA participants underwent a cardiac CT scan that was assessed for HAA at baseline, and 5,965 participants had at least one follow-up cardiac CT scan. The average number of cardiac CT scans per participant was 2.4, with mean follow-up time of 5.9 years (range, 0.9–11.4 yr). After excluding participants missing adjustment covariates or occupational exposures, 5,702 participants were included in the longitudinal assessment of progression of HAA (see Figure E1 in the online supplement).
A total of 3,137 MESA participants, including some of the participants recruited under MESA Air, had a full-lung CT scan that was assessed by a radiologist, 128 were not read for ILA, and 34 had unreadable scans. As previously done, we further excluded 491 full-lung CT scans that were read as “indeterminate” for ILA (17). Additional participants that were missing occupational exposures or other covariates were excluded from final ILA analyses (see Figure E2), leaving 2,312 participants for analysis.
Baseline characteristics were similar in the analytic groups used for ILA and HAA analyses (Table 1). A total of 2,528 (44.3%) of the participants included in the HAA analysis had never smoked, whereas 1,287 (55.7%) of the participants in the ILA analysis had never smoked. The mean age of the MESA cohort at baseline was 62 years (SD, 10), and 3,214 (47%) were male. Racial/ethnic differences were based on study design: 2,621 (39%) participants were white; 1,893 (28%) were African American; 1,496 (22%) were Hispanic; and 803 (12%) were Asian (Chinese).
Table 1.
Baseline Characteristics at Participant Recruitment
| HAA Measured at Exam 1 | Cohort Included in HAA Model | Cohort Included in ILA Model* | |
|---|---|---|---|
| Participants, n | 6,813 | 5,702 | 2,312 |
| Age, yr | 62.2 ± 10.2 | 61.7 ± 10.1 | 59.5 ± 9.3 |
| Male | 3,214 (47.2) | 2,813 (49.3) | 1,108 (47.9) |
| Race | |||
| White | 2,621 (38.5) | 2,304 (40.4) | 934 (40.4) |
| African American | 1,893 (27.8) | 1,566 (27.5) | 617 (26.7) |
| Hispanic | 1,496 (22.0) | 1,198 (21.0) | 461 (19.9) |
| Asian (Chinese) | 803 (11.8) | 634 (11.1) | 300 (13.0) |
| BMI, kg/m2 | 28.3 ± 5.5 | 28.3 ± 5.4 | 28.3 ± 5.3 |
| Height, cm | 166.4 ± 10.0 | 166.8 ± 10.0 | 167.0 ± 10.0 |
| Weight, kg | 79.7 ± 17.3 | 79.1 ± 17.2 | 79.1 ± 17.1 |
| Smoking status | |||
| Never-smokers | 3,085 (45.0) | 2,528 (44.3) | 1,287 (55.7) |
| Former smokers | 2,761 (41.0) | 2,370 (41.5) | 776 (33.6) |
| Current smokers | 967 (14.0) | 804 (14.1) | 249 (10.8) |
| Cigarette pack-years† | 13 (2.0–31.5) | 13.5 (2.2–31.0) | 13.5 (4.0–30.0) |
| Socioeconomic status | |||
| Education | |||
| ≤High school | 2,460 (36.2) | 1,878 (32.9) | 675 (29.2) |
| Some college | 1,937 (28.5) | 1,669 (29.3) | 675 (29.2) |
| ≥College | 2,393 (35.2) | 2,155 (37.8) | 962 (41.6) |
| Income, $‡ | |||
| <25,000 | 2,059 (31.5) | 1,560 (28.3) | 536 (23.8) |
| 25,000–74,999 | 3,003 (45.9) | 2,606 (47.3) | 1,118 (49.7) |
| ≥75,000 | 1,478 (22.6) | 1,348 (24.4) | 596 (26.5) |
| Percent emphysema | 4.2 ± 4.5 | 4.3 ± 4.5 | 4.0 ± 4.0 |
| Study site | |||
| Winston Salem, NC | 1,077 (15.8) | 937 (16.4) | 439 (19.0) |
| New York, NY | 1,102 (16.2) | 963 (16.9) | 408 (17.7) |
| Baltimore, MD | 1,086 (15.9) | 872 (15.3) | 305 (13.2) |
| St. Paul, MN | 1,066 (15.7) | 921 (16.2) | 330 (14.3) |
| Chicago, IL | 1,164 (17.1) | 1,010 (17.7) | 467 (20.2) |
| Los Angeles, CA | 1,318 (19.4) | 999 (17.5) | 363 (15.7) |
Definition of abbreviations: BMI = body mass index; HAA = high-attenuation areas; ILA = interstitial lung abnormalities.
Data are presented as mean ± SD or n (%) unless otherwise stated. All parameters were collected at MESA (Multi-Ethnic Study of Atherosclerosis) baseline visit in years 2000–2002, unless otherwise stated.
Includes demographics from 53 MESA Air new recruits at time of recruitment, with the exception of age, which is backdated to the year 2000.
Median (interquartile range) among ever-smokers.
Missing data on 188 participants in the HAA cohort and 62 in the ILA cohort.
Occupational exposures are presented in Table 2. In the entire MESA cohort, 1,454 (37%) participants reported exposure to dust, 735 (18.8%) reported exposure to vapors or gases, and 929 (23.7%) reported exposures to fumes. According to the JEM, 16.4% of participants had intermediate exposures and 7.5% of participants had high exposures to VGDF, which was similar to self-reported severity of exposures. At the start of the study, 3,220 (48.1%) participants were employed and 2,543 (38%) were retired. Of the participants studied at exam 5, 2,059 (44%) were still employed and 1,968 (42.3%) had retired (data not shown). There was a low Spearman correlation (0.20; P < 0.001) between self-reported severity of exposures and exposures assigned per JEM (see Table E1).
Table 2.
Occupational Characteristics of Participants at Recruitment
| HAA Measured at Exam 1 | Cohort Included in HAA Model | Cohort Included in ILA Model | |
|---|---|---|---|
| Participants, n | 6,813 | 5,702 | 2,312 |
| Employment status* | |||
| Homemaker | 774 (11.6) | 391 (6.9) | 151 (6.5) |
| Employed | 3,220 (48.1) | 2,973 (52.1) | 1,385 (59.9) |
| Unemployed | 154 (2.3) | 127 (2.2) | 45 (1.9) |
| Retired | 2,543 (38.0) | 2,211 (38.8) | 731 (31.6) |
| Job exposure matrix–assigned exposures† | |||
| VGDF score | |||
| Low | 4,913 (76.2) | 4,389 (77.0) | 1,816 (78.6) |
| Intermediate | 1,055 (16.4) | 901 (15.8) | 341 (14.8) |
| High | 481 (7.5) | 412 (7.2) | 155 (6.7) |
| Gas/vapor‡ | 1,180 (18.3) | 1,008 (17.7) | 379 (16.4) |
| Dust‡ | 910 (14.1) | 782 (13.7) | 281 (12.2) |
| Inorganic | 397 (6.2) | 350 (6.1) | 125 (5.4) |
| Organic | 468 (7.3) | 393 (6.9) | 146 (6.3) |
| Fumes‡ | 306 (4.7) | 262 (4.6) | 98 (4.2) |
| Self-reported exposures§ | |||
| Dust | 1,454 (37.0) | 1,398 (38.1) | 776 (38.9) |
| Gas/vapor | 735 (18.8) | 709 (19.4) | 418 (21.1) |
| Fumes | 929 (23.7) | 895 (24.4) | 502 (25.2) |
| Any VGDF exposure‖ | 1,713 (43.5) | 1,651 (44.9) | 923 (46.3) |
| Severity¶ | |||
| None | 2,212 (56.4) | 2,017 (55.0) | 1,070 (53.7) |
| Mild | 977 (24.9) | 946 (25.8) | 537 (27.0) |
| Moderate | 537 (13.7) | 514 (14.0) | 286 (14.4) |
| Severe | 196 (5.0) | 188 (5.1) | 99 (5.0) |
| No. of VGDF agents** | |||
| 0 | 2,212 (56.7) | 2,017 (55.4) | 1,070 (54.0) |
| 1 | 798 (20.5) | 772 (21.2) | 429 (21.7) |
| 2 | 386 (9.9) | 370 (10.2) | 197 (10.0) |
| 3 | 503 (12.9) | 484 (13.3) | 284 (14.3) |
| Length of exposure to VGDF, yr | 18.2 ± 12.7 | 18 ± 12.8 | 17.6 ± 12.5 |
Definition of abbreviations: HAA = high-attenuation areas; ILA = interstitial lung abnormalities; VGDF = vapors, gas, dust, and fumes.
Occupational characteristics obtained from questionnaires administered during exam 4. Data are presented as mean ± SD or n (%) unless otherwise stated.
Employment status at recruitment. Data are missing for 122 at exam 1.
Job exposure matrix–assigned exposures missing for 364 at exam 1.
Exposure score dichotomized into “low” versus “intermediate/high.”
Self-reported exposures missing for dust in 2,883, vapors/gas in 2,901, and fumes in 2,891 at exam 1; dust in 2,029, vapors/gas in 2,047, and fumes in 2,037 in the HAA cohort; and dust in 317, vapors/gas in 327, and fumes in 323 in the ILA cohort.
Self-reported exposure to any VGDF agent missing for 2,877 at exam 1, 2,024 in the HAA cohort, and 332 in the ILA cohort.
Self-reported severity missing for 2,891 at exam 1, 2,037 in the HAA cohort, and 320 in the ILA cohort.
Total number of VGDF-constituent agents, calculated from self-reported exposure. Data are missing for 2,914 at exam 1, 2,059 in the HAA cohort, and 322 in the ILA cohort.
Association with HAA
At recruitment, MESA participants had a mean HAA of 5.1% (3.1% SD) with a range of 1.4–46.6%. HAA decreased slightly (0.35% per year; 95% CI, 0.21–0.48%) over follow-up.
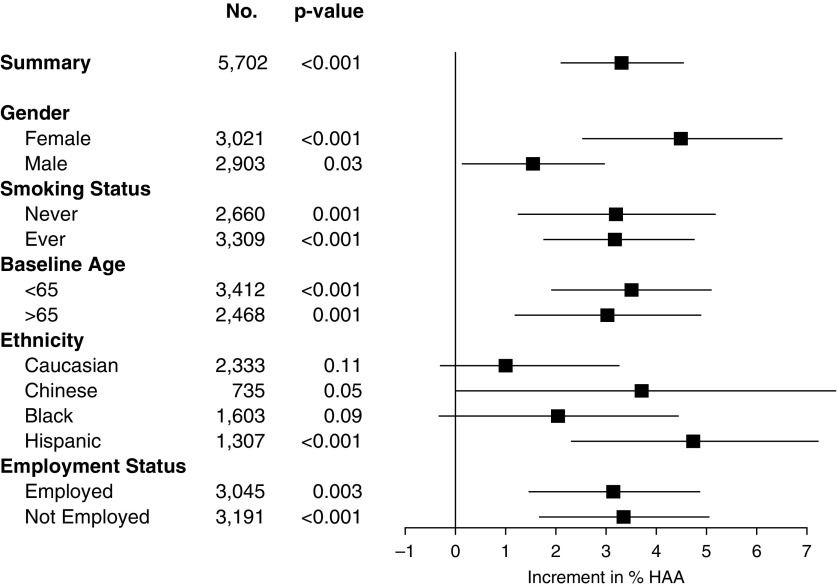
In our mixed model that adjusted for potential confounders (Table 3, Figure 1), occupational exposures were significantly associated with higher measurements of HAA in the entire cohort. With increasing exposure to VGDF, as assigned by the JEM, HAA increased by 2.39% (95% CI, 1.23–3.57%; P < 0.001; Figure 1) per exposure category (low to intermediate to high). Sex may be an effect modifier (P value for interaction with sex < 0.001): in females, HAA increased by 3.93% (95% CI, 1.94–5.96%; P < 0.001) per exposure category, compared with males, where HAA increased 1.05% (95% CI, −0.30 to 2.42%; P = 0.13) per exposure category. Sensitivity analyses treating JEM exposures as dummy variables had similar results: in general, associations were strongest in the higher- compared with lower-exposure categories (see the online supplement).
Table 3.
Association between Job-Exposure Matrix–assigned and Self-reported Exposure to VGDF and High-Attenuation Areas
| Overall [Difference in % HAA (95% CI)] | Females [Difference in % HAA (95% CI)] | Males [Difference in % HAA (95% CI)] | Sex (P Interaction) | Currently Employed [Difference in % HAA (95% CI)] | Not Employed [Difference in % HAA (95% CI)] | Employment (P Interaction) | |
|---|---|---|---|---|---|---|---|
| JEM-assigned exposure | |||||||
| VGDF | 2.39 (1.23 to 3.57) | 3.93 (1.94 to 5.96) | 1.05 (−0.30 to 2.42) | <0.001 | 2.45 (0.82 to 4.10) | 2.29 (0.64 to 3.97) | 0.77 |
| Gas/vapor | 1.82 (0.57 to 3.09) | 4.80 (2.41 to 7.25) | 0.54 (−0.84 to 1.94) | <0.001 | 1.70 (0 to 3.46) | 1.76 (0 to 3.58) | 0.99 |
| Combined dust | 1.36 (−0.07 to 2.82) | 4.09 (1.11 to 7.15) | −0.02 (−1.54 to 1.53) | 0.002 | −0.63 (−2.61 to 1.40) | 3.04 (1.00 to 5.12) | 0.02 |
| Inorganic dust | 2.29 (0.25 to 4.37) | 10.1 (2.85 to 17.9) | 1.16 (−0.81 to 3.17) | 0.03 | −1.21 (−4.07 to 1.73) | 4.88 (2.00 to 7.83) | 0.05 |
| Organic dust | 1.82 (−0.28 to 3.96) | 3.78 (0.19 to 7.50) | 0.25 (−0.54 to 2.72) | 0.009 | 0.34 (−2.73 to 3.50) | 2.81 (−0.07 to 5.76) | 0.29 |
| Fumes | 0.14 (−1.91 to 2.23) | −0.76 (−5.13 to 3.85) | 0.17 (−1.98 to 2.37) | 0.45 | 0.48 (−2.62 to 3.68) | −0.09 (−2.84 to 2.73) | 0.52 |
| Self-reported exposure | |||||||
| Gas/vapor | 0.33 (−1.63 to 2.32) | −2.36 (−3.23 to 2.93) | −0.24 (−2.41 to 1.98) | 0.55 | 1.05 (−1.36 to 3.52) | −0.21 (−3.32 to 3.01) | 0.78 |
| Dust | 0.28 (−1.34 to 1.93) | 0.81 (−1.57 to 3.25) | −0.33 (−2.35 to 1.75) | 0.87 | −0.25 (−2.27 to 1.81) | 0.69 (−1.84 to 3.30) | 0.63 |
| Fumes | 0.07 (−1.14 to 2.57) | 0.57 (−2.33 to 3.56) | 0.05 (−2.10 to 2.24) | 0.43 | 0.80 (−1.49 to 3.15) | 0.58 (−2.29 to 3.54) | 0.99 |
| Any VGDF exposure | −0.14 (−1.72 to 1.46) | −0.33 (−2.33 to 1.71) | −0.33 (−2.33 to 1.71) | 0.89 | −0.69 (−2.67 to 1.34) | 0.13 (−2.30 to 2.61) | 0.70 |
| Severity | −0.19 (−1.05 to 0.69) | −0.44 (−1.71 to 0.85) | −0.13 (−1.22 to 0.98) | 0.42 | −0.67 (−1.80 to 0.48) | 0.14 (−1.16 to 1.46) | 0.53 |
| Per 10-yr exposure | −0.06 (−0.98 to 0.87) | −1.40 (−2.88 to 0.10) | 0.64 (−0.46 to 1.76) | 0.42 | −0.15 (−1.39 to 0.28) | −0.02 (−1.37 to 1.35) | 0.88 |
Definition of abbreviations: CI = confidence interval; HAA = high-attenuation areas; JEM = job-exposure matrix; VGDF = vapors, gas, dust, and fumes.
Estimates of the association between JEM-assigned and self-reported exposures to VGDF and HAA. Shown is a cross-sectional association of JEM-assigned and self-reported VGDF exposures and percent change in HAA, from linear mixed models adjusted for age, sex, race/ethnicity, educational attainment, employment status, height, body mass index, waist circumference, smoking status, cigarette pack-years, glomerular filtration rate, total volume of lung imaged, percent emphysema on computed tomography (CT) scan, CT scanner type, and study site. Bold numbers signify effect estimates that are statistically significant.
Figure 1.
Forest plot of multivariable-adjusted associations between job-exposure matrix–assigned exposures and high-attenuation areas (HAA), stratified on selected clinical and demographic variables. Boxes represent point estimates; whiskers are 95% confidence intervals. P values for stratified analyses are shown. P values for interactions are less than 0.001, 0.96, 0.88, 0.87, and 0.77 for sex, smoking status, baseline age, ethnicity, and employment status, respectively.
When JEM-assigned exposures were separated into constituent agents, associations with HAA were strongest with dust and gas exposure (Table 3, Table E3). HAA increased by 2.29% (95% CI, 0.25–4.37%; P < 0.001) per exposure category (low to intermediate to high) of inorganic dust and by 1.82% (95% CI, −0.28 to 3.96%; P = 0.09) per exposure category of organic dust.
Overall, there was no consistent association between self-reported exposures and HAA or of occupational exposures and progression of HAA over time. In ethnicity-stratified models, self-reported VGDF exposure was associated with an increased rate of progression of HAA in white individuals (P value for interaction with race = 0.16, Table 4).
Table 4.
Association between Job-Exposure Matrix–assigned and Self-reported VGDF Exposures and Progression of High-Attenuation Areas
| Overall [Annual % Change in HAA (95% CI)] | White Individuals [Annual % Change in HAA (95% CI)] | Race P Interaction | |
|---|---|---|---|
| JEM exposure | |||
| VGDF | 0 (−0.20 to 0.19) | 0.13 (−0.16 to 0.4) | 0.35 |
| Gas/vapor | 0.03 (−0.19 to 0.24) | 0.07 (−0.24 to 0.38) | 0.97 |
| Combined dust | 0.15 (−0.09 to 0.39) | 0.12 (−0.20 to 0.44) | 0.51 |
| Inorganic dust | 0.15 (−0.19 to 0.49) | 0.32 (−0.11 to 0.76) | 0.56 |
| Organic dust | −0.19 (−0.78 to 0.40) | −0.09 (−0.61 to 0.43) | 0.56 |
| Fumes | 0.21 (−0.15 to 0.57) | 0.18 (−0.30 to 0.67) | 0.66 |
| Self-reported exposure | |||
| Gas/vapor | 0.37 (−0.34 to 1.09) | 0.58 (0.16 to 1.01) | 0.66 |
| Dust | 0.04 (−0.23 to 0.31) | 0.42 (0.07 to 0.78) | 0.12 |
| Fumes | 0.19 (−0.12 to 0.50) | 0.61 (0.21 to 1.01) | 0.13 |
| Any VGDF exposure | 0.05 (−0.10 to 0.19) | 0.53 (0.19 to 0.88) | 0.16 |
| Severity | 0.08 (−0.17 to 0.35) | 0.31 (0.12 to 0.51) | 0.11 |
| Per 10-yr exposure | 0.01 (−0.15 to 0.16) | 0 (0 to 0.3) | 0.17 |
Definition of abbreviations: CI = confidence interval; HAA = high-attenuation areas; JEM = job-exposure matrix; VGDF = vapors, gas, dust, and fumes.
Estimates of the association between occupational exposures to VGDF and progression of HAA. Linear longitudinal association of JEM-assigned and self-reported VGDF exposures with percent change in HAA, from linear mixed models adjusted for age, sex, race/ethnicity, educational attainment, employment status, height, body mass index, waist circumference, smoking status, cigarette pack-years, glomerular filtration rate, total volume of lung imaged, percent emphysema on computed tomography scan, computed tomography scanner type, and study site. Bold numbers signify effect estimates that are statistically significant.
Association with ILA
A total of 310 (9.9%) of the 3,137 participants who underwent full-lung CT scan had ILA: 289 (9.2%) had scans read as suspicious for ILD and 21 (0.67%) had scans that met standard criteria for usual interstitial pneumonia pattern with bilateral fibrosis associated with honeycombing and traction bronchiectasis in a subpleural distribution.
In our multivariable-adjusted analyses, overall estimates did not show a significant association between occupational exposures and odds of ILA at exam 5 (Table 5). However, there was an increasing trend in the odds of ILA with exposures to VGDF, both per self-report and per JEM. Among those currently employed, there was a 1.39-fold increase in odds of ILA (95% CI, 0.94–2.08) for each increase in exposure category. In the a priori specified subgroup analyses, self-reported exposure to vapor or gas was associated with a 1.76-fold increase in odds of ILA (95% CI, 1.09–2.84; P = 0.02; P value for interaction with age = 0.7) in participants who were less than 65 years of age and a 1.97-fold increase in odds of ILA (95% CI, 1.16–3.35; P = 0.01; P value for interaction with employment status = 0.22) in participants who were employed during exam 5.
Table 5.
Association between Job-Exposure Matrix–assigned and Self-reported VGDF Occupational Exposures and Interstitial Lung Abnormalities
| Overall [OR (95% CI)] | Baseline Age < 65 yr [OR (95% CI)] | Baseline Age ≥ 65 yr [OR (95% CI)] | Currently Employed* [OR (95% CI)] | Not Employed* [OR (95% CI)] | |
|---|---|---|---|---|---|
| JEM-assigned exposure | |||||
| VGDF | 1.04 (0.83–1.30) | 1.22 (0.85–1.73) | 0.95 (0.71–1.29) | 1.39 (0.94–2.08) | 0.95 (0.72–1.25) |
| Combined dust | 1.10 (0.82–1.46) | 1.22 (0.75–2.00) | 1.08 (0.75–1.56) | 1.57 (0.93–2.64) | 0.98 (0.68–1.40) |
| Inorganic dust | 1.21 (0.82–1.81) | 0.45 (0.12–1.75) | 1.52 (0.96–2.39) | 0.48 (0.12–1.86) | 1.47 (0.95–2.29) |
| Organic dust | 0.92 (0.59–1.42) | 1.76 (0.84–3.69) | 0.91 (0.42–1.96) | 2.33 (1.13–4.80) | 0.60 (0.33–1.12) |
| Self-reported exposure | |||||
| Gas/vapor | 1.30 (0.93–1.83) | 1.76 (1.09–2.84) | 1.02 (0.61–1.68) | 1.97 (1.16–3.35) | 0.99 (0.63–1.57) |
| Dust | 0.94 (0.69–1.28) | 0.96 (0.62–1.48) | 0.93 (0.60–1.42) | 1.14 (0.69–1.87) | 0.85 (0.56–1.26) |
| Fumes | 1.13 (0.81–1.58) | 1.39 (0.86–2.25) | 0.87 (0.53–1.41) | 1.57 (0.90–2.72) | 0.97 (0.63–1.49) |
| Any VGDF agents† | 0.93 (0.70–1.25) | 0.90 (0.58–1.38) | 0.96 (0.63–1.42) | 1.17 (0.72–1.92) | 0.83 (0.57–1.20) |
| Severity | 1.00 (0.86–1.17) | 1.07 (0.84–1.35) | 0.95 (0.77–1.19) | 1.12 (0.86–1.48) | 0.95 (0.76–1.16) |
| Per 10-yr exposure | 0.92 (0.78–1.09) | 1.17 (0.91–1.51) | 0.97 (0.95–1.00) | 1.08 (0.81–1.44) | 0.98 (0.96–1.01) |
Definition of abbreviations: CI = confidence interval; JEM = job-exposure matrix; OR = odds ratio; VGDF = vapors, gas, dust, and fumes.
Estimates of the association between occupational exposures to VGDF and risk of interstitial lung abnormalities. Shown is a cross-sectional association of JEM-assigned and self-reported exposures to VGDF with the odds of interstitial lung abnormalities, from stratified multivariable logistic regression models adjusted for age, sex, ethnicity, tobacco use (current smoking status and pack-years), and site. Bold numbers signify effect estimates that are statistically significant.
Employment status at exam 5.
Self-reported exposure to vapors/gas, dust, or fumes.
Discussion
We found that occupational exposures to vapors/gas, dusts, and fumes were associated with quantitative and, among those currently employed, qualitative subclinical ILD phenotypes. Exposure based on JEM score was associated with increased HAA and demonstrated evidence of a dose–response relationship, with higher estimated exposure levels associated with increased HAA. There was a trend toward increased ILA with occupational exposures, which was strongest in the subgroup of younger participants and those who were currently employed.
This is the first study to show that a broad range of occupational exposures, categorized as VGDF, is linked to markers of lung inflammation and fibrosis. With the exception of specific exposures that are known causes of ILD, such as welding fumes or tobacco smoke, vapors, fumes, and gases have been traditionally classified as risk factors for obstructive lung disease (20). However, it is common for similar environmental triggers to produce different pathologic responses in the genetically susceptible host.
Although the biologic pathways leading to pulmonary fibrosis are still poorly understood, current evidence suggests that ILD arises from recurrent epithelial injury and dysregulated repair. Animal models of pneumoconiosis have led to important insights into lung injury and disease mechanisms, demonstrating how inhaled particles are taken up by alveolar epithelial cells, sequestered in the lung and cause parenchymal injury. When animals are exposed to aerosolized silica, the particles are transported into the interstitium and enter the lymphatics, where they induce oxidative stress and initiate an inflammatory cascade characterized by T cell and macrophage activation (21). Similarly, rats that are exposed to high doses of intratracheal instilled welding fumes show nodular aggregates containing particulate matter in the alveolar and alveolar ductal regions (22). These models provide evidence that a variety of environmental insults produce the same pathologic response that can lead to fibrinogenesis.
The advantage of using a broad exposure metric, such as VGDF, is the ability to capture multiple, mixed exposures that may act synergistically to cause disease over time. When JEM-assigned exposures were separated into constituent agents, the associations with HAA were most robust with dust exposure, and, in particular, inorganic dust exposure. This observation is consistent with the known pathogenicity of fibrogenic dusts, such as asbestos, silica, and coal dust. Interestingly, associations with self-reported exposures were strongest with vapors/gas and fume exposure. The ability to draw conclusions about the relative pathogenicity of these different agents is limited by small sample size and different exposure assessment techniques.
One of the more interesting aspects of our study was the suggestion of a temporal relationship between VGDF exposure and subclinical ILD. Subclinical ILD is often presumed to be a progressive process, and a recent study found that 20–46% of individuals with ILA showed worsening imaging abnormalities over time (10). In our study, however, we only saw a significant association between some self-reported exposures and progression of HAA in white individuals. This association was not seen with JEM-assigned exposures, in other stratified analyses, or in the overall cohort.
A potential explanation for the lack of a consistent temporal progression that we observed may be attributed to the demographics of the population studied, with a relatively advanced age (mean of 62 yr at recruitment) and, subsequently, a higher rate of retirement (38% at recruitment) than the general population. Consequently, we presume that the heaviest burden of occupational exposure in the MESA cohort took place before study recruitment. This would suggest that damage to the lungs from VGDF is more likely to occur early in the exposure, rather than after a prolonged latency period. Without ongoing inhalational exposures to incite inflammation and alveolar injury, some phenotypes of subclinical ILD may stabilize or partially resolve.
This hypothesis is supported by the observation that the odds of ILA were greatest in the subgroup of younger participants and those who were actively employed. Both of these groups were more likely to have current inhalational exposures. Although we did not find statistical evidence of effect modification by age or employment status, our ability to detect a difference was limited by sample size.
In contrast, we found the opposite relationship in some of the JEM-assigned exposures where associations were stronger in females, older participants, and those who were not actively employed. This may reflect the heterogeneity of the underlying disease process and susceptible population: subclinical ILD may represent early changes in a diverse group of chronic lung diseases, from autoimmune diseases to pneumoconioses. Fibrogenic dust inhalation typically leads to slowly progressive disease that manifests after a prolonged latency period. In this exposure category, we would expect associations to be stronger in those with remote exposures. In addition, there are significant temporal trends in exposure levels as stricter worker regulations have led to dramatic reductions in dust exposure over the past several decades. Although provocative, it is difficult to draw a definitive conclusion, due to the inherent limitations of observation studies and the specific limitations to our study.
Exposure assessment in occupational epidemiology research is an important source of error, with concerns for both over- and underreporting biasing estimates (23). To mitigate this limitation, we used two separate methods of VGDF assessment: self-reported exposure and JEM-assigned exposure. JEM-assigned exposure was assessed at baseline for each participant, based on the participant’s current or most recent occupation. Change of employment status was also recorded at each subsequent exam and adjusted for in the analyses. This method of exposure assessment did not capture remote exposures from prior jobs, or the longest-held position.
The self-reported VGDF questionnaire was administered during MESA exam 4: a median of 4.7 years (range, 3.5–6.8 yr) after exam 1, during which the baseline HAA was assessed. The questionnaire (relevant questions are replicated in the online supplement) assessed for VGDF exposure at any time during the employment history, not just the most current occupation. Despite the lag time between baseline HAA assessment and the self-exposure questionnaire, we do not feel that this is likely to be a major source of exposure misclassification. This assumption is based on: (1) the advanced age of the MESA cohort with a high proportion of retired participants at the study start; and (2) the prolonged exposures reported on the VGDF questionnaire (median duration of 16 yr).
Self-reported exposures may be subject to recall bias with significant inter- and intrarespondent inconsistency and inaccuracy. The occupational questionnaires used in this study were intended to capture a more comprehensive exposure history by asking separately about kinds of exposures, severity, and duration. Nonetheless, participants may have been unaware of certain exposures or unable to distinguish between technical classes of exposures (for example, the difference between vapors/gas and fumes). In addition, where participants perceived a work-related health outcome, they may have systemically overreported exposures.
Although JEM exposure was assigned by independent experts, there was also a risk of substantial misclassification. There is considerable heterogeneity in exposures within the same job title: not all road workers are substantially exposed to dust, whereas some office employees may have vapors/gas exposures (24). These sources of misclassification in JEM-assigned exposures would be expected to bias toward the null and attenuate the estimates that we observed.
Both methods of exposure assessment in our study were associated with different sources of exposure misclassification, yet each captured different aspects of VGDF exposure. By using both methods, we hoped to achieve a more comprehensive assessment of the participant’s exposure history. Although there was low correlation between exposure levels calculated by self-report versus JEM-assignment, a similar discordance has been previously reported in many other studies, and is representative of the difficulty in assigning occupational exposures in epidemiologic studies (25, 26).
In this study, exposure levels predicted by the JEM were more consistently associated with HAA than self-reported exposures on questionnaires. This pattern is in contrast to epidemiologic studies that have compared the risk of asthma and chronic obstructive pulmonary disease with multiple approaches to the assessment of occupational exposures. These usually find more significant associations with self-reported exposures rather than JEM-assigned exposures (19, 23, 24, 27). Once again, this could be attributed to limited power in our study, especially because a smaller number of participants answered the self-reported exposure questionnaires in comparison to the number of participants assigned an exposure through the JEM.
In addition to potential errors from exposure misclassification, our study had some additional limitations. It is possible that the cohort that completed the entire study, from recruitment to MESA exam 5, had fewer comorbidities than participants who were censored due to death or loss to follow-up. It is somewhat reassuring that the baseline characteristics of the participants who had a full-lung CT at exam 5 were similar to those of the overall cohort; however, there may be some unmeasured effects due to survivorship bias in our estimates. Another limitation of this study was the use of cardiac, rather than full-lung, CT scans to measure HAA. Although this method could potentially miss some areas of affected lung, we previously showed that HAA on cardiac CT scan strongly agrees with HAA on full-lung CT scan (9).
Despite these limitations, this observational study has several novel findings. We observed significant associations with two different approaches to exposure assessment and two separate subclinical ILD phenotypes. There was evidence of a dose–response relationship and a possible temporal association between exposures and the outcome. In combination with a plausible biologic mechanism, these findings suggest a relationship between occupational exposures and subclinical ILD. More studies are needed to determine the significance of this association, distinguish the importance between different exposures classes, and follow disease progression in those with occupational exposures over time.
Footnotes
Supported by NHLBI contracts HHSN268201500003I, N01-HC-95159, N01-HC-95160, N01-HC-95161, N01-HC-95162, N01-HC-95163, N01-HC-95164, N01-HC-95165, N01-HC-95166, N01-HC-95167, N01-HC-95168, and N01-HC-95169; by NHLBI grants R01-HL-103676, K24-HL-131937, R01-HL-077612, R01-HL-093081, RC1-HL100543, and T32HL007287; and by National Center for Research Resources/National Center for Advancing Translational Sciences grants UL1-TR-000040 and UL1-TR-001079. The coding of occupational information was conducted by the National Institute for Occupational Safety and Health (NORA FY08 CRN SLB8). This project was funded in part by the Pulmonary Fibrosis Foundation and the Rocco Guinta Research Fund.
This report has not been formally reviewed by the U.S. Environmental Protection Agency (EPA). The findings and conclusions in this report are those of the authors, and do not necessarily represent the views of the EPA or the National Institute for Occupational Safety and Health. The EPA and National Institute for Occupational Safety and Health do not endorse any products or commercial services mentioned in this publication.
Author Contributions: C.S.S., J.D.K, D.J.L., and B.C.D. conceived and designed the study; A.J.P., E.A.H., R.G.B., J.D.K., B.C.D., and D.J.L. acquired the data; C.S.S., A.J.P., B.C.D., J.D.K., and D.J.L. analyzed the data; C.S.S. drafted the initial manuscript; all authors contributed to interpretation of the data and drafting or critical revision of the manuscript for important intellectual content and approved the final version submitted.
This article has an online supplement, which is accessible from this issue’s table of contents at www.atsjournals.org
Originally Published in Press as DOI: 10.1164/rccm.201612-2431OC on July 28, 2017
Author disclosures are available with the text of this article at www.atsjournals.org.
References
- 1.Raghu G, Chen SY, Yeh WS, Maroni B, Li Q, Lee YC, Collard HR. Idiopathic pulmonary fibrosis in US Medicare beneficiaries aged 65 years and older: incidence, prevalence, and survival, 2001–11. Lancet Respir Med. 2014;2:566–572. doi: 10.1016/S2213-2600(14)70101-8. [DOI] [PubMed] [Google Scholar]
- 2.American Thoracic Society; European Respiratory Society. American Thoracic Society/European Respiratory Society international multidisciplinary consensus classification of the idiopathic interstitial pneumonias. Am J Respir Crit Care Med. 2002;165:277–304. doi: 10.1164/ajrccm.165.2.ats01. [DOI] [PubMed] [Google Scholar]
- 3.Taskar V, Coultas D. Exposures and idiopathic lung disease. Semin Respir Crit Care Med. 2008;29:670–679. doi: 10.1055/s-0028-1101277. [DOI] [PubMed] [Google Scholar]
- 4.Baumgartner KB, Samet JM, Coultas DB, Stidley CA, Hunt WC, Colby TV, Waldron JA Collaborating Centers. Occupational and environmental risk factors for idiopathic pulmonary fibrosis: a multicenter case-control study. Am J Epidemiol. 2000;152:307–315. doi: 10.1093/aje/152.4.307. [DOI] [PubMed] [Google Scholar]
- 5.Hubbard R. Occupational dust exposure and the aetiology of cryptogenic fibrosing alveolitis. Eur Respir J Suppl. 2001;32:119s–121s. [PubMed] [Google Scholar]
- 6.Hubbard R, Lewis S, Richards K, Johnston I, Britton J. Occupational exposure to metal or wood dust and aetiology of cryptogenic fibrosing alveolitis. Lancet. 1996;347:284–289. doi: 10.1016/s0140-6736(96)90465-1. [DOI] [PubMed] [Google Scholar]
- 7.Doyle TJ, Hunninghake GM, Rosas IO. Subclinical interstitial lung disease: why you should care. Am J Respir Crit Care Med. 2012;185:1147–1153. doi: 10.1164/rccm.201108-1420PP. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Podolanczuk AJ, Oelsner EC, Barr RG, Hoffman EA, Armstrong HF, Austin JH, Basner RC, Bartels MN, Christie JD, Enright PL, et al. High attenuation areas on chest computed tomography in community-dwelling adults: the MESA study. Eur Respir J. 2016;48:1442–1452. doi: 10.1183/13993003.00129-2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Lederer DJ, Enright PL, Kawut SM, Hoffman EA, Hunninghake G, van Beek EJ, Austin JH, Jiang R, Lovasi GS, Barr RG. Cigarette smoking is associated with subclinical parenchymal lung disease: the Multi-Ethnic Study of Atherosclerosis (MESA)–lung study. Am J Respir Crit Care Med. 2009;180:407–414. doi: 10.1164/rccm.200812-1966OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Araki T, Putman RK, Hatabu H, Gao W, Dupuis J, Latourelle JC, Nishino M, Zazueta OE, Kurugol S, Ross JC, et al. Development and progression of interstitial lung abnormalities in the Framingham Heart Study. Am J Respir Crit Care Med. 2016;194:1514–1522. doi: 10.1164/rccm.201512-2523OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Putman RK, Hatabu H, Araki T, Gudmundsson G, Gao W, Nishino M, Okajima Y, Dupuis J, Latourelle JC, Cho MH, et al. Evaluation of COPD Longitudinally to Identify Predictive Surrogate Endpoints (ECLIPSE) Investigators; COPDGene Investigators. Association between interstitial lung abnormalities and all-cause mortality. JAMA. 2016;315:672–681. doi: 10.1001/jama.2016.0518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Sack C, Doney B, Podolanczuk A, Hooper LG, Seixas N, Hoffman EA, Kawut S, Vedal S, Raghu G, Barr RG, et al. Occupational exposures and subclinical interstitial lung disease: the Multi-Ethnic Study of Atherosclerosis (MESA) Air-Lung Study [abstract] Am J Respir Crit Care Med. 2017;195:A5428. doi: 10.1164/rccm.201612-2431OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kaufman JD, Adar SD, Barr RG, Budoff M, Burke GL, Curl CL, Daviglus ML, Diez Roux AV, Gassett AJ, Jacobs DR, Jr, et al. Association between air pollution and coronary artery calcification within six metropolitan areas in the USA (the Multi-Ethnic Study of Atherosclerosis and Air Pollution): a longitudinal cohort study. Lancet. 2016;388:696–704. doi: 10.1016/S0140-6736(16)00378-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Barr RG, Ahmed FS, Carr JJ, Hoffman EA, Jiang R, Kawut SM, Watson K. Subclinical atherosclerosis, airflow obstruction and emphysema: the MESA Lung Study. Eur Respir J. 2012;39:846–854. doi: 10.1183/09031936.00165410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Bild DE, Bluemke DA, Burke GL, Detrano R, Diez Roux AV, Folsom AR, Greenland P, Jacob DR, Jr, Kronmal R, Liu K, et al. Multi-Ethnic Study of Atherosclerosis: objectives and design. Am J Epidemiol. 2002;156:871–881. doi: 10.1093/aje/kwf113. [DOI] [PubMed] [Google Scholar]
- 16.Sieren JP, Newell JD, Jr, Barr RG, Bleecker ER, Burnette N, Carretta EE, Couper D, Goldin J, Guo J, Han MK, et al. SPIROMICS protocol for multicenter quantitative computed tomography to phenotype the lungs Am J Respir Crit Care Med 2016194794–806 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Washko GR, Hunninghake GM, Fernandez IE, Nishino M, Okajima Y, Yamashiro T, Ross JC, Estépar RS, Lynch DA, Brehm JM, et al. COPDGene Investigators. Lung volumes and emphysema in smokers with interstitial lung abnormalities. N Engl J Med. 2011;364:897–906. doi: 10.1056/NEJMoa1007285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Hoffman EA, Jiang R, Baumhauer H, Brooks MA, Carr JJ, Detrano R, Reinhardt J, Rodriguez J, Stukovsky K, Wong ND, et al. Reproducibility and validity of lung density measures from cardiac CT scans—the Multi-Ethnic Study of Atherosclerosis (MESA) Lung Study. Acad Radiol. 2009;16:689–699. doi: 10.1016/j.acra.2008.12.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Doney B, Hnizdo E, Graziani M, Kullman G, Burchfiel C, Baron S, Fujishiro K, Enright P, Hankinson JL, Stukovsky KH, et al. Occupational risk factors for COPD phenotypes in the Multi-Ethnic Study of Atherosclerosis (MESA) Lung Study. COPD. 2014;11:368–380. doi: 10.3109/15412555.2013.813448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Balmes J, Becklake M, Blanc P, Henneberger P, Kreiss K, Mapp C, Milton D, Schwartz D, Toren K, Viegi G Environmental and Occupational Health Assembly, American Thoracic Society. American Thoracic Society Statement: occupational contribution to the burden of airway disease. Am J Respir Crit Care Med. 2003;167:787–797. doi: 10.1164/rccm.167.5.787. [DOI] [PubMed] [Google Scholar]
- 21.Adamson IY, Prieditis H. Silica deposition in the lung during epithelial injury potentiates fibrosis and increases particle translocation to lymph nodes. Exp Lung Res. 1998;24:293–306. doi: 10.3109/01902149809041536. [DOI] [PubMed] [Google Scholar]
- 22.Hicks R, Al-Shamma KJ, Lam HF, Hewitt PJ. An investigation of fibrogenic and other toxic effects of arc-welding fume particles deposited in the rat lung. J Appl Toxicol. 1983;3:297–306. doi: 10.1002/jat.2550030605. [DOI] [PubMed] [Google Scholar]
- 23.Quinlan PJ, Earnest G, Eisner MD, Yelin EH, Katz PP, Balmes JR, Blanc PD. Performance of self-reported occupational exposure compared to a job-exposure matrix approach in asthma and chronic rhinitis. Occup Environ Med. 2009;66:154–160. doi: 10.1136/oem.2008.040022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Blanc PD, Eisner MD, Balmes JR, Trupin L, Yelin EH, Katz PP. Exposure to vapors, gas, dust, or fumes: assessment by a single survey item compared to a detailed exposure battery and a job exposure matrix. Am J Ind Med. 2005;48:110–117. doi: 10.1002/ajim.20187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Delclos GL, Gimeno D, Arif AA, Benavides FG, Zock JP. Occupational exposures and asthma in health-care workers: comparison of self-reports with a workplace-specific job exposure matrix. Am J Epidemiol. 2009;169:581–587. doi: 10.1093/aje/kwn387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Teschke K, Olshan AF, Daniels JL, De Roos AJ, Parks CG, Schulz M, Vaughan TL. Occupational exposure assessment in case–control studies: opportunities for improvement. Occup Environ Med. 2002;59:575–593, discussion 594. doi: 10.1136/oem.59.9.575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Marchetti N, Garshick E, Kinney GL, McKenzie A, Stinson D, Lutz SM, Lynch DA, Criner GJ, Silverman EK, Crapo JD COPDGene Investigators. Association between occupational exposure and lung function, respiratory symptoms, and high-resolution computed tomography imaging in COPDGene. Am J Respir Crit Care Med. 2014;190:756–762. doi: 10.1164/rccm.201403-0493OC. [DOI] [PMC free article] [PubMed] [Google Scholar]