To the Editor:
There is growing interest in the potential vascular rarefaction of the right ventricular (RV) microcirculation to explain RV failure due to pulmonary hypertension (PH). Published data, reliant on measures of stained capillary area in selected RV fields, indicated a potential capillary drop, possibly of 50 or 60% versus control values, in decompensated RVs in PH induced by SU5416-hypoxia and monocrotaline (1, 2); supportive qualitative data were reported in human RV by immunostaining (2). However, accurate assessment of the vasculature in the normal and diseased RV requires an unbiased approach, with tools including systematic uniform random sampling and assessment of the pertinent reference volume (i.e., RV tissue) (3). Using these approaches, the hypoxia mouse and SU5416-hypoxia rat models of PH exhibited increased absolute vascular length that parallels the increase in RV cell mass (4, 5), leading to maintenance of the ratio between myocyte volume and capillary length. In this pilot study, we report a systematic unbiased analysis of human RV specimens using a rigorous stereologic approach. Our data indicate that in advanced PH, there was a significant increase of the RV vasculature, whereas the average radius of RV myocyte served per vessel increased by 17% over control subjects.
Methods
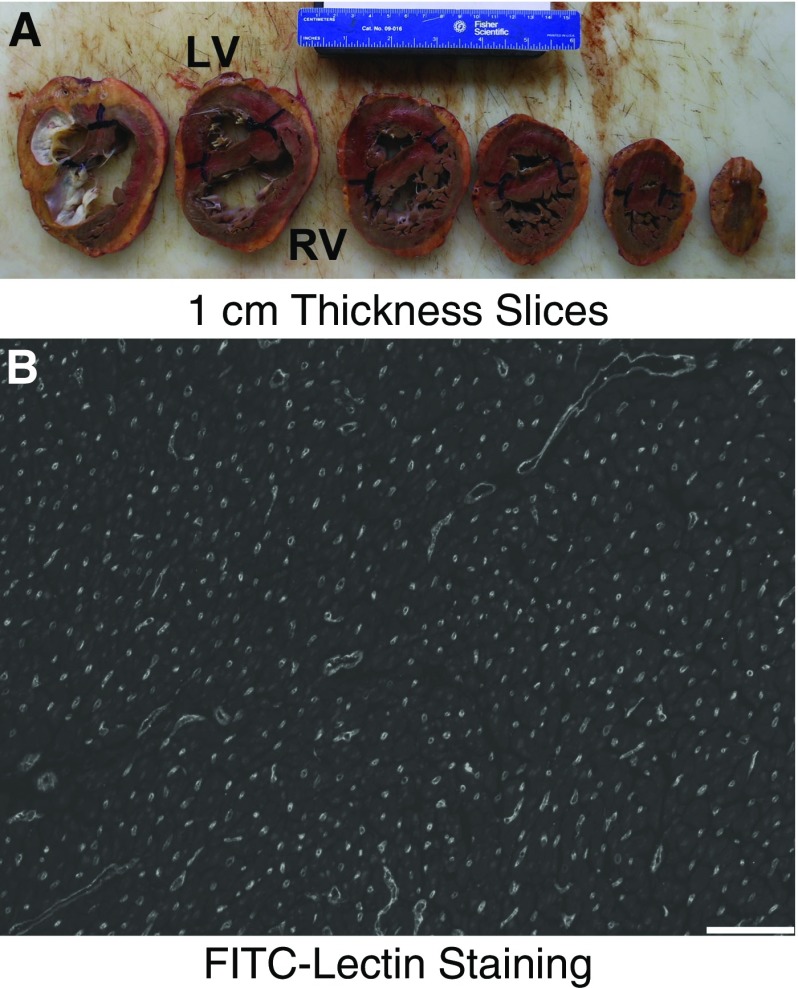
All studies were approved by the Colorado Multiple Institutional Review Board (Protocol #08-0462). Cardiac tissue was collected at autopsy and processed by sectioning the heart in 1-cm-thick slices (Figure 1A), starting at the apex, at a depth between 1 and 10 mm as determined by computerized random number generator, and continued every 1 cm to the atrioventricular junction. The slices were laid down and imaged adjacent to a ruler. A plastic sheet with 5-mm holes at 1-cm intervals was placed over the tissue. Eight uniformly random locations were identified, with a random start for the first location (determined by computerized random number generator) and at regular intervals thereafter (6). Each location was marked with ink, and a 5-mm core biopsy was performed at each site. Isotropic orientation of the biopsies was performed with the Isector technique (7), with each sample formalin-fixed and embedded in a paraffin ball, which was rolled. In the final orientation, the ball was placed into a slicer matrix (Zivic, Pittsburgh, PA) for cutting into 3-mm interval slices. Slices with tissue were laid down, reembedded into a paraffin block, and sectioned at 4 μm.
Figure 1.
Stereologic approach for analysis of right ventricle (RV) vasculature. (A) The ventricles are sectioned at 1-cm thickness with a random start. (B) Uniform, random, isotropically oriented sections are stained with fluorescein isothiocyanate–lectin (FITC-Lectin) (scale bar: 100 μm). LV = left ventricle.
RV absolute volume was calculated using Cavalieri’s method on the fresh tissue image (8). The outline of the RV tissue was drawn (Photoshop; Adobe, San Jose, CA), and cross-sectional area determined. The absolute volume was calculated as the sum of RV cross-sectional areas multiplied by the slice thicknesses (1 cm).
Each section underwent antigen retrieval (Vector H3300; Vector Laboratories, Burlingame, CA), Tris-buffered saline with Tween 20 rinse, staining overnight at 4°C with fluorescein isothiocyanate–labeled lectin from Griffonia simplicifolia (Sigma L9381; Sigma-Aldrich, St. Louis, MO; 1:75 dilution in Tris-buffered saline), and coverslipping (Vector H1400). Four images of each section were randomly acquired using a microscope (Nikon, Tokyo, Japan) with a black-and-white camera (Photometrics, Tucson, AZ) (Figure 1B). Two specimens had poor lectin signal, and anti-CD31 immunostaining (ab28364; Abcam, Cambridge, MA) was performed instead.
Vascular length (Lv) was determined using the equation (9, 10), where Q is the sum of vessel profiles that intersect a test plane, P is the sum of test points that intersect the tissue, and A is the plane area divided by the number of test points. The test plane we used had a sampling area of 0.550 mm2 and 1,426 test points, for an A of 386 μm2.
Each image was digitally thresholded (Metamorph; Molecular Devices, Sunnyvale, CA) on tissue autofluorescence to identify all RV tissue and determine P. The image was then rethresholded on the lectin signal, and the number of vessel profiles Q that intersected the test plane (but not the exclusion boundary) was determined. The sum of all Ps and all Qs for each specimen was used to calculate Lv. Lv was corrected for tissue shrinkage by formalin fixation (estimated at 20% for each dimension, although variability in this factor may introduce bias [3]). Lv was multiplied by absolute volume to determine absolute vascular length for each specimen.
We also calculated the average cross-sectional area of cardiac tissue supplied by a single vessel, or volume of tissue per vessel length (1/Lv), and then the effective radius of tissue supplied by each vessel ().
Results
Tissue was collected and analyzed from three control subjects and four subjects with advanced PH. Absolute RV volume, absolute length of the vasculature in the RV, and the average radius of RV tissue served per vessel were determined (Figure 2). Total vascular length in the RV tissue was highly correlated with absolute RV volume across all control and PH specimens (P < 0.001). The average radius of tissue served per vessel was 14.4 μm in control RVs and 16.9 μm in PH RVs (P < 0.05).
Figure 2.
Analysis of right ventricle (RV) vessels in three control and four pulmonary hypertension (PH) specimens. (A) Total RV volume, measured by Cavalieri’s method, versus absolute length of vessels in the RV tissue, measured by stereology (n = 3–4/group; linear regression). (B) Average radius of RV tissue served per vessel (n = 3–4/group; t test).
Discussion
We applied a stringent stereologic approach to analyze human RV vasculature, in line with American Thoracic Society guidelines for stereological assessment of lung structure (3). This approach is similar to that previously reported in the hypoxic mouse and SU5416-hypoxia rat models (4, 5).
In this pilot study, we observed a strong correlation between total vascular length and myocyte volume, consistent with significant compensatory angiogenesis in the PH RV. This likely occurs in homeostatic adaptation to support RV contractile function in the setting of increased afterload and muscle mass.
We observed a 2.5-μm increase in the radius of tissue supplied by each vessel, consistent with vascular rarefaction in these terminal disease samples. This increase is smaller than a predicted 9.3-μm increase in radius if there was no vascular adaptation and RV volume increased 2.7-fold (the average we observed), or a predicted 6.0-μm increase in radius if there was actually 50% dropout of vessels, as suggested by prior planimetric analyses (1, 2). We suspect a 2.5-μm increase in radius is unlikely to significantly limit O2 delivery but cannot exclude a restriction of other metabolite transport to the cardiomyocytes.
The importance of these data requires confirmation with a larger number of specimens and reinforces the need for application of rigorous stereologic techniques for unbiased tissue characteristic analysis.
Footnotes
Supported by National Institutes of Health grants P01HL014985 (B.B.G. and R.M.T.), K08HL105536, R03HL133306, and R01HL135872; the University of Colorado Department of Medicine Early Career Scholars Program (B.B.G.); and grant R24HL123767 (R.M.T.).
Author Contributions: Conception and design: B.B.G. and R.M.T.; tissue collection and processing: D.K., B.K., and R.M.T.; analysis and interpretation: B.B.G. and R.M.T.; drafting the manuscript for important intellectual content: B.B.G. and R.M.T.; approval of the final manuscript: all authors.
Originally Published in Press as DOI: 10.1164/rccm.201702-0425LE on March 9, 2017
Author disclosures are available with the text of this letter at www.atsjournals.org.
References
- 1.Bogaard HJ, Natarajan R, Henderson SC, Long CS, Kraskauskas D, Smithson L, Ockaili R, McCord JM, Voelkel NF. Chronic pulmonary artery pressure elevation is insufficient to explain right heart failure. Circulation. 2009;120:1951–1960. doi: 10.1161/CIRCULATIONAHA.109.883843. [DOI] [PubMed] [Google Scholar]
- 2.Piao L, Fang YH, Parikh K, Ryan JJ, Toth PT, Archer SL. Cardiac glutaminolysis: a maladaptive cancer metabolism pathway in the right ventricle in pulmonary hypertension. J Mol Med (Berl) 2013;91:1185–1197. doi: 10.1007/s00109-013-1064-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Hsia CCW, Hyde DM, Ochs M, Weibel ER ATS/ERS Joint Task Force on Quantitative Assessment of Lung Structure. An official research policy statement of the American Thoracic Society/European Respiratory Society: standards for quantitative assessment of lung structure. Am J Respir Crit Care Med. 2010;181:394–418. doi: 10.1164/rccm.200809-1522ST. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Kolb TM, Peabody J, Baddoura P, Fallica J, Mock JR, Singer BD, D’Alessio FR, Damarla M, Damico RL, Hassoun PM. Right ventricular angiogenesis is an early adaptive response to chronic hypoxia-induced pulmonary hypertension. Microcirculation. 2015;22:724–736. doi: 10.1111/micc.12247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Zhang L, Perez M, Serkova N, Graham B, Tuder RM. Preservation of capillary network of the right ventricle in severe experimental pulmonary hypertension. Pulm Circ. 2013;3:185. [Google Scholar]
- 6.Herring MJ, Putney LF, Wyatt G, Finkbeiner WE, Hyde DM. Growth of alveoli during postnatal development in humans based on stereological estimation. Am J Physiol Lung Cell Mol Physiol. 2014;307:L338–L344. doi: 10.1152/ajplung.00094.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Hyde DM, Harkema JR, Tyler NK, Plopper CG. Design-based sampling and quantitation of the respiratory airways. Toxicol Pathol. 2006;34:286–295. doi: 10.1080/01926230600713509. [DOI] [PubMed] [Google Scholar]
- 8.Gundersen HJ, Jensen EB. The efficiency of systematic sampling in stereology and its prediction. J Microsc. 1987;147:229–263. doi: 10.1111/j.1365-2818.1987.tb02837.x. [DOI] [PubMed] [Google Scholar]
- 9.Howard CV, Reed MG. Unbiased stereology: three-dimensional measurement in microscopy. 2nd ed. Abingdon: BIOS Scientific Publishers; 2005. [Google Scholar]
- 10.Mühlfeld C. Quantitative morphology of the vascularisation of organs: a stereological approach illustrated using the cardiac circulation. Ann Anat. 2014;196:12–19. doi: 10.1016/j.aanat.2012.10.010. [DOI] [PubMed] [Google Scholar]