Abstract
Pim kinases phosphorylate and regulate a number of key AML cell survival proteins, and Pim inhibitors have recently entered clinical trial for hematological malignancies. AZD1208 is a small molecule pan-Pim kinase inhibitor and AZD1208 treatment resulted in growth inhibition and cell size reduction in AML cell lines including FLT3-WT (OCI-AML-3, KG-1a, MOLM-16) and FLT3-ITD mutated (MOLM-13, MV-4-11). There was limited apoptosis induction (<10% increase) in the AML cell lines evaluated with up to 3 μM AZD1208 for 24h, suggesting that growth inhibition is not through apoptosis induction. Using reverse phase protein array (RPPA) and immunoblot analysis, we identified that AZD1208 resulted in suppression of mTOR signaling, including inhibition of protein phosphorylation of mTOR(Ser2448), p70S6K(Thr389), S6(Ser235/236) and 4E-BP1(Ser65). Consistent with mTOR inhibition, there was also a reduction in protein synthesis that correlated with cell size reduction and growth inhibition with AZD1208; our study provide insights into the mechanism of AZD1208.
Keywords: Pim kinase, AML, growth inhibition, mTOR, protein translation
Introduction
Acute myeloid leukemia is an aggressive difficult-to-treat hematological malignancy that has a 5-year survival rate of approximately 20%. Patients who do respond to front-line therapy frequently relapse and develop resistance thus new treatments are urgently needed. Although mutations such as FLT3, IDH1, IDH2, NPM and DNMT have emerged as key players in AML progression, agents that could target all forms of AML would be advantageous.
There are three Pim kinases proteins (Pim-1, Pim-2 and Pim-3), which are highly homologous with overlapping functions and substrate specificities[1,2]. There are numerous protein substrates of Pim kinases, including regulators of cell survival, cell cycle, transcription, translation, drug resistance and signaling with the microenvironment[2]. Due to their multiple functions and multitude of targets, we proposed inhibition of Pim kinases using a small molecule for patients with AML.
While Pim kinases alone are considered weak oncogenes, they have been demonstrated to have strong synergism with MYC and other oncogenes to drive tumorigenesis and lymphomagenesis in animal models[3]. In an AML mouse model, transplantation of murine bone marrow co-expressing MYC with any of the three Pim kinases resulted in the rapid development of lethal myeloid leukemia[4]. This correlates with clinical data as Pim expression and activity was correlated with poorer survival in patients with AML. At the molecular level, Pim kinases phosphorylate a number of proteins associated with poor prognosis in AML that are involved with multiple aspects of cell survival and proliferation[2]. For example, Pim-1 has been reported to phosphorylate and stabilize FLT3 and activate STAT5 signaling in AML with FLT3-ITD mutation[5]. Pim-1 kinase also regulates MET receptor tyrosine kinase levels and signaling via translation through phosphorylation of eIF4B[6]. More recently, Pim inhibition has been demonstrated to suppress STAT5 activation and reduce MYC protein half-life in higher CD25-expressing subpopulations of AML cell lines[7]. Thus, inhibition of Pim kinases may be an avenue to target AML cell survival.
Recently, a new class of kinase inhibitors targeting a family of Pim kinases have been developed and investigated in both hematological malignancies as well as solid tumors (reviewed in[8] ). The first Pim kinase inhibitor to enter clinical trial was SGI-1776 for refractory prostate cancer and relapsed/refractory non-Hodgkin's lymphoma (http://clinicaltrials.gov/ct2/show/NCT00848601). Induction of apoptosis by SGI-1776 was demonstrated by our group in cell lines and in primary cells from patients with chronic lymphocytic leukemia (CLL)[9], AML, mantle cell lymphoma and multiple myeloma[10–12]. SGI-1776 treatment in cell lines has also been shown to reduce the expression of cell surface drug resistance proteins[13]. However, SGI-1776 also inhibits FLT3 and haspin, thus the role of Pim inhibition alone on cytotoxicity is unclear.
AZD1208 is a pan-Pim kinase inhibitor that targets all three Pim kinases at nanomolar levels (IC50: Pim-1 (0.4 nM), Pim-2 (5.0 nM) and Pim-3 (1.9 nM)) but does not inhibit FLT3[14]. Given the significance of FLT3 in AML, we hypothesized that investigating the effects of AZD1208 on AML cells may shed light on the significance of Pim kinase inhibition alone on AML cell survival. Furthermore, protein profiling will identify which signaling axis is impacted following Pim inhibition.
Materials and Methods
Drugs
AZD1208 was obtained from AstraZeneca (Waltham, MA) and was dissolved in DMSO and stored at −20°C. All experiments including a vehicle control were conducted using 0.1 % DMSO. In cell culture media containing 10 % FBS, Pim inhibitor AZD1208 is 92.2% bound and 7.8% as free drug.
Cell lines
Five AML cell lines were used (Table I). For all cell lines, experiments were conducted in media supplemented with 10% FBS, maintained in mid-log phase growth at 37°C in 5% CO2 in a fully humidified incubator, and routinely tested for Mycoplasma by using a kit (Gen-Probe Inc., San Diego, CA). Cell number and mean cell volume were determined using a Coulter channelyzer (Coulter Electronics, Hialeah, FL).
Table I.
AML cell lines, their FLT3 and p53 mutation status and culture conditions
| Cell line | FLT3 | p53 | Source | Medium | %FBS |
|---|---|---|---|---|---|
| OCI-AML-3 | wt/wt | wt | DSMZ | RPMI | 10 |
| KG-1a | wt/wt | mut | ATCC | IMDM | 20 |
| MOLM-16 | wt/wt | mut | DSMZ | RPMI | 20 |
| MOLM-13 | ITD/wt | wt | DSMZ | RPMI | 10 |
| MV-4-11 | ITD/ITD | mut | ATCC | IMDM | 10 |
Abbreviations: wt, wild-type; mut, mutated; ITD, internal tandem duplication.
Cell death/apoptosis assay
Cells were treated with DMSO or AZD1208 and for each sample, 10,000 cells were measured using a Becton Dickinson FACSCalibur flow cytometer (San Jose, CA) as previously described[9] .
RNA/protein synthesis assay
AML cell lines were incubated with AZD1208 for 24 h. Cell cultures were incubated for 1 h with 1 μCi/culture [3H]uridine for RNA synthesis or 5 μCi/culture [3H]leucine (Moravek Biochemicals Brea, CA) for protein synthesis, in triplicate per experiment. The cell cultures then were transferred onto Whatman GF/C glass microfiber filters (GE Healthcare) pre-treated with 1% aqueous sodium pyrophosphate on a multiscreen vacuum assay system (Millipore Corp., Bedford, MA). The filters were washed with cold PBS, then twice with 0.4N perchloric acid, and dried with 70% ethanol. The glass filters then placed in scintillation vials with 7mL scintillation fluid, and the level of radioactivity was measured using a liquid scintillation analyzer (Packard Instrument Co., Downer’s grove, IL). Radioactivity was measured from control and AZD1208-treated cells and expressed as percent of control.
Immunoblot analysis
Cellular lysates were prepared and immunoblot analysis was performed as previously described[9]. The antibodies used are listed in Table II, and membranes were probed with infrared-labeled secondary antibodies and visualized using a LI-COR Odyssey Infrared Imager (LI-COR Inc.) as previously described[9].
Table II.
Antibodies used for immunoblot analysis.
| Antibody | Supplier | Species |
|---|---|---|
| PARP | Enzo Life Sciences | mouse IgG |
| RNA Pol2 (Ser5) | Enzo Life Sciences | mouse IgM |
| RNA Pol2 | Enzo Life Sciences | mouse IgG |
| mTOR | Millipore | mouse IgG |
| mTOR (Ser2448) | Cell Signaling | rabbit IgG |
| p70S6K (Thr389) | Cell Signaling | mouse IgG |
| p70S6K | Cell Signaling | rabbit IgG |
| S6 (Ser235/236) | Cell Signaling | rabbit IgG |
| S6 | Cell Signaling | mouse IgG |
| Mcl-1 | Cell Signaling | rabbit IgG |
| Bad (Ser112) | Cell Signaling | mouse IgG |
| Bad | Cell Signaling | rabbit IgG |
| 4E-BP1 (Ser65) | Cell Signaling | rabbit IgG |
| 4E-BP1 (Thr37/46) | Cell Signaling | rabbit IgG |
| 4E-BP1 | Santa Cruz | goat IgG |
| p27 | Santa Cruz | mouse IgG |
| STAT5 | Santa Cruz | rabbit IgG |
| β-actin | Sigma | mouse IgG |
| GAPDH | Novus | mouse IgG |
| Lamin B1 | Abcam | rabbit IgG |
Reverse-phase protein array (RPPA)
Three independent experiments each consisting of five AML cell lines treated with DMSO or 3 μM AZD1208 for 3, 6 and 24 hours were harvested and submitted for RPPA to measure protein level changes across a set (n = 171) of antibodies (http://www.mdanderson.org/education-and-research/resources-for-professionals/scientific-resources/core-facilities-and-services/functional-proteomics-rppa-core/index.html), then analyzed using bioinformatics methods.
Statistical analysis
Protein levels by RPPA were measured in triplicate at four different time points (0, 3, 6, and 24 h following AZD1208 treatment) and levels between early (0, 3 h) and late time points (6, 24 h) were compared using t-tests (two-sided Welch Two Sample t-tests). Due to the small sample size, permutations were carried out to obtain more precise p-values as well (permutation size = 700). With permutation test p-values, we found a significant difference in expression levels for several proteins using the Benjamini-Hochberg method, which are shown in Table III.
Table III.
Statistically significant changes in cellular proteins from AZD1208 in AML cell lines by RPPA analysis
| Cell line | Benjamin-Hoschberg at 5% | Using p-values computed from permutation distribution | Change |
|---|---|---|---|
| FLT3-WT | |||
| OCI-AML-3 | 4 proteins | Akt, eEF2 | decrease |
| Chk2 (Thr68), SF2 | increase | ||
| KG-1a | 11 proteins | GATA3, GSK3α/β(Ser9), mTOR (Ser2448), p70S6K (Thr389) SCD1, | decrease |
| DJ-1, Rb, p38-MAPK, Raptor, Stathmin, TSC1 | increase | ||
| MOLM-16 | 19 proteins | 4E-BP1 (Thr37/46), ACC (Ser79), c-Jun (Ser73), Chk1, Cyclin B1, EGFR, eIF4G, JNK (Thr183/185), mTOR (Ser2448), p38-MAPK, Rictor (Thr1135) | decrease |
| AR, Bim, Chk2 (Thr68), DJ-1, EGFR, ER.alpha (Ser118), Smad1, Tuberin (Thr1462), XRCC1 | increase | ||
| FLT3-ITD | |||
| MOLM-13 | 3 proteins | BRCA2 | decrease |
| c-Myc, Chk2 (Thr68) | increase | ||
| MV-4-11 | 2 proteins | Myosin-IIA (Ser1943) | decrease |
| Rictor | increase | ||
Results
Growth inhibition with limited apoptosis induction by AZD1208 in both wild-type FLT3 and FLT3-ITD AML cell lines
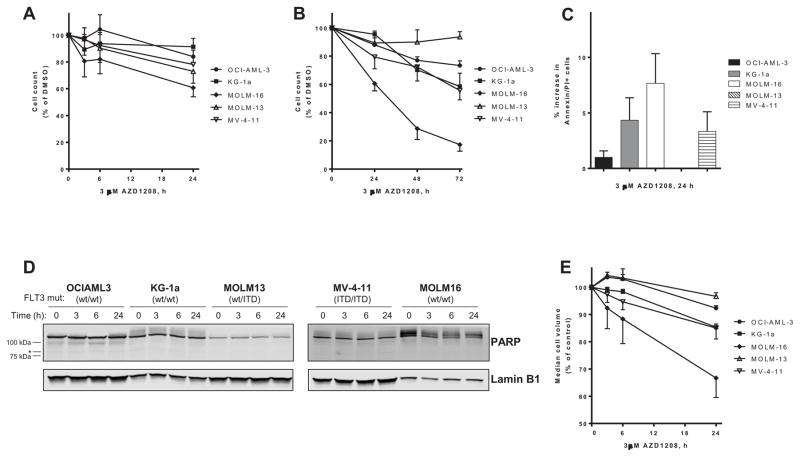
Five AML cell lines with varying mutation status (Table I) were incubated with 3 μM AZD1208 for shorter (3, 6 and 24 h) and longer exposures (24, 48, and 72 h) and cell density was measured at various times and compared with vehicle control (0.1% DMSO). After 24h, growth inhibition was the most in MOLM-16, which was reduced to ~60% of control (Figure 1A). MV-4-11 and MOLM-13 were reduced to ~80% of control and there was limited effect on OCI-AML-3 and KG-1a after 24 h. However, after longer exposure to AZD1208, growth inhibition was measured in OCI-AML and reduced to ~70% of control, and in KG-1a and MV-4-11, which both reduced to ~60% of control (Figure 1B).
Figure 1. Growth inhibition with limited apoptosis induction by AZD1208 in both wild-type FLT3 and FLT3-ITD AML cell lines.
FLT3 wild-type OCI-AML-3 (circles), KG-1a (squares), MOLM-16 (diamonds), and FLT3-ITD mutated MOLM-13 (open triangles), and MV-4-11 (inverted open triangle) were either treated with control 0.1% DMSO alone or 3 μM AZD1208. Cell density was measured in AZD1208-treated cells and expressed as percent of DMSO-treated control cells after (A) 3, 6 and 24 h or (B) after 24, 48 and 72 h. Data are the average of three independent experiments ± SEM. (C) Induction of apoptosis was measured by flow cytometry of AML cell lines stained with annexin V-FITC/propidium iodide. The results represent an average of triplicate experiments ± SEM. (D) AML cell lines treated with 3 μM AZD1208 were harvested after 3, 6, and 24 h and lysed, then analyzed by immunoblot for PARP protein. An asterisk (*) denotes the gel migration size of cleaved PARP protein. β-actin was used as a loading control and the data are representative of two independent experiments. (E) Cell volume was measured in AZD1208-treated cells and expressed as percent of DMSO-treated control cells after 3, 6 and 24 h. Data are the average of three independent experiments ± SEM.
To evaluate the effect on AZD1208 on the clonogenic potential of AML cells, AML cell lines were cultured in methylcellulose with 3 μM AZD1208 for 5–10 days until colonies were formed and then quantified. There was suppression of colony formation with AZD1208 in the three cell lines evaluated, OCI-AML-3, MOLM-13 and MV-4-11 (Supplemental Figure S1A). Although there was limited growth inhibition in MOLM-13, there was suppression of colony formation with AZD1208 and the colonies formed were visually smaller than those cultured without AZD1208 (Supplemental Figure S1B).
Although there was growth inhibition in the five cell lines evaluated, there was limited induction of apoptosis by AZD1208 (Figure 1C). The five AML cell lines were treated with DMSO or 3 μM AZD1208 and evaluated by flow cytometry analysis of annexin-V/propidium iodide staining following 24 h treatment. There was an increase in apoptosis of approximately 5–10% in MOLM-16, but less than 5% increase in OCI-AML-3, KG-1a and MV-4-11. After 24 h treatment with AZD1208, there was no increase in apoptosis in MOLM-13 compared with DMSO alone. Induction of apoptosis was also evaluated by immunoblot analysis of cellular proteins, and there was no detectable PARP protein cleavage in the AML cell lines treated with 3 μM AZD1208 for 24h (Figure 1D). However, the median cell volume decreased following treatment with 3 μM AZD1208 for 24 h in all five cell lines evaluated (Figure 1E). The reduction in cell volume correlated with growth inhibition sensitivity, and MOLM-16 cells were reduced by ~35%, KG-1a and MV-4-11 by 15%, and OCI-AML-3 and MOLM-13 by 5–10%. There was also a dose-dependent correlation between cell volume and inhibition of cell growth in KG-1a cells treated with increasing concentrations of AZD1208 over 24 h (Supplemental Figure S2A).
RPPA analysis of AML cells treated with AZD1208
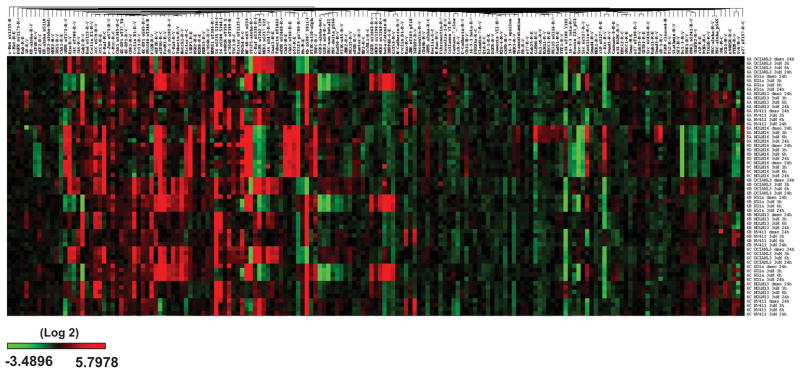
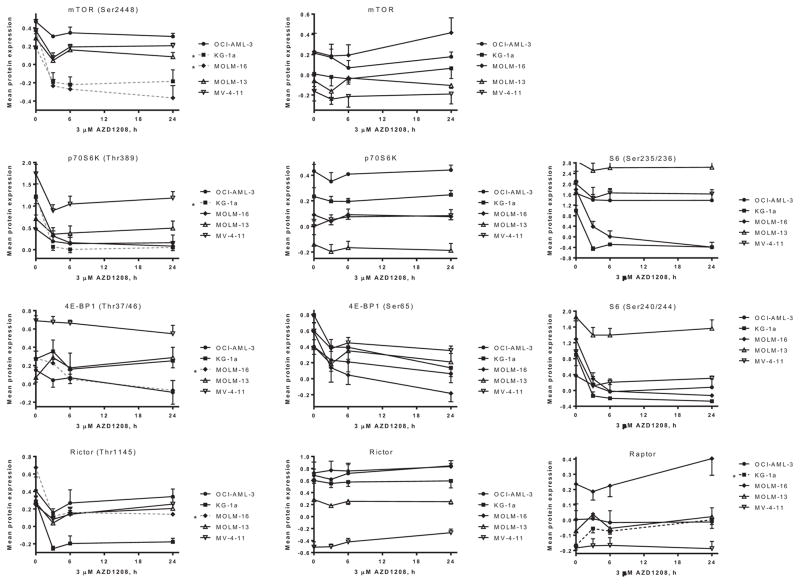
To investigate the changes in cellular protein levels across a broad set of pathways, RPPA analysis was used to evaluate changes in 171 protein levels in five AML cell lines with heterogeneous genetic mutations treated with DMSO or 3 μM AZD1208 for 3, 6 and 24 hours. The cells were harvested and cellular protein level changes were measured across a set of 171 antibodies. There was variation amongst the five cell lines and the RPPA data was subjected to bioinformatics analysis to determine the statistical significance of the protein level changes (Figure 2–3). The protein level changes that were statistically significant are summarized in Table III, and components of the mTOR pathway emerged as key molecules affected by AZD1208 in the various AML cell lines. The more sensitive cell line, MOLM-16, was the line with the most proteins affected by AZD1208 treatment (19 proteins) that were statistically significant (Table III), followed by KG-1a (11 proteins). In FLT3-wild-type OCI-AML-3, the levels of four proteins were significantly changed. In FLT3-ITD mutated cell lines MOLM-13 and MV-4-11 the levels of three and two proteins, respectively, were statistically significantly changed . Of the pathways evaluated, the mTOR pathway was most affected by AZD1208 and the phosphorylation of a number of key pathway proteins were inhibited (Figure 3). Phosphorylation of mTOR (Ser2448) was reduced in a statistically significant manner in the two more sensitive cell lines, MOLM-16 and KG-1a, but also in the other three cell lines. Likewise, in at least one cell line, phosphorylation of p70S6K (Thr389), Rictor (Thr1145) and 4E-BP1 (Thr37/46) were all inhibited, while levels of total mTOR, p70S6K, and Rictor were not significantly changed (Figure 3). Although not statistically significant, phosphorylation of Pim-2 target 4EBP-1(Ser65) was reduced in all five cell lines. Similarly, p70S6K-downstream target ribosomal S6 phosphorylation at both Ser235/236 and Ser240/244 was also decreased in all five cell lines (Figure 3), which is consistent with measured decrease in cell size by AZD1208 (Figure 1E).
Figure 2. RPPA heatmap: Modulation of protein levels by AZD1208 in five AML cell lines measured by RPPA.
FLT3 wild-type (OCI-AML-3, KG-1a, MOLM-16) and FLT3-ITD mutated (MOLM-13 and MV-4-11) AML cell lines were treated with DMSO or 3 μM AZD1208. Cells were harvested after 3, 6 and 24 h, and relative cellular protein levels were measured by RPPA.
Figure 3. RPPA: Suppression of mTOR signaling in five AML cell lines.
Graphical representation of relative log2-transformed protein levels normalized by median-centering. The results represent the average level of triplicate experiments ± SEM of proteins where changes determined to be statistically significant.
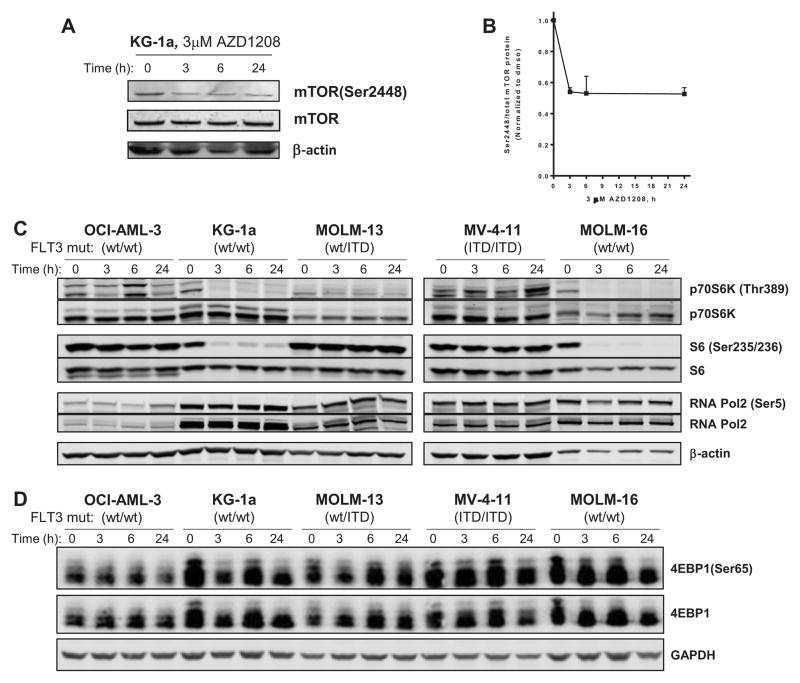
The RPPA results were validated by immunoblot analysis. AML cell lines were treated with 3 μM AZD1208 over the same time-course and the cellular protein levels were evaluated. There was a decrease in mTOR(Ser2448) phosphorylation in KG-1a cells treated with 3μM AZD1208 that was detected within 3h, which was sustained after 24 h (Figure 4A–B). Similar results were obtained in MOLM-16 (Supplemental Figure S3), and there was a decrease in mTOR phosphorylation to less than 50% of control after 6 h that was further reduced to ~30% of control after 24 h. There was also a decrease in total Mcl-1 protein levels in MOLM-16 that was not detected in the other four cell lines. As with mTOR protein itself, the mTOR pathway proteins that were affected by AZD1208 as determined by RPPA (Figure 3) were also decreased as analyzed by immunoblot (Figure 4). In various cell lines, p70S6K (Thr389), S6(Ser235/236) and 4E-BP1 (Ser65) phosphorylation were decreased following AZD1208 treatment (Figure 4C–D), which correlated with response to AZD1208. In addition, in KG-1a the decrease in cell size, S6 (Ser235/236) and 4E-BP1 (Ser65) phosphorylation was dose-dependent (Supplemental Figure S2A–C). Even with 0.1 μM AZD1208 for 24h, S6 phosphorylation was reduced to ~10% of control and undetectable at 0.1 μM (Supplemental Figure S2B). We also validated the specificity of AZD1208 in AML cell lines. After the three Pim kinases, the next kinase that was found to bind AZD1208 was CDK7, although at 43-fold higher concentration. To evaluate whether off-target effects could be contributing to AZD1208 mechanism in AML, we evaluated phosphorylation of CDK7 target RNA Pol2(Ser5) phosphorylation, and no detectable change was observed (Figure 4C).
Figure 4. Inhibition of mTOR signaling in AML cells by AZD1208.
AML cells were cultured with DMSO or 3 μM AZD1208 for 3, 6 or 24 h, then cellular proteins were extracted and analyzed by immunoblot. β-actin was used as a loading control. (A) Immunoblot analysis of mTOR(Ser2448) in KG-1a cells treated with AZD1208. Data are representative of three independent experiments. (B) The phospho-protein levels of mTOR(Ser2448) were analyzed using immunoblot and normalized to total mTOR protein levels. Data are the average of three independent experiments ± SEM. (C) Immunoblot analysis of p70S6K(Thr389), S6 (Ser235/236) and 4E-BP1(Ser65), and RNAPol2, RNAPol2 (Ser2) in OCI-AML-3, KG-1a, MOLM-13, MV-4-11 and MOLM-16 cells treated with AZD1208. Data are representative of two independent experiments. (D) Immunoblot analysis of total and phospho-4E-BP1(Ser65), and GAPDH was used as a loading control. Data are representative of two independent experiments.
Impact of AZD1208 on AML primary blasts
The effects of AZD1208 on AML primary blasts were also investigated. AML blasts were isolated from the peripheral blood from a patient with AML and cultured with 3 μM AZD1208 in vitro for 24 h. There were no significant changes in phospho-protein levels of 4EBP1, Bad, RNA Pol2, nor were there significant changes in total STAT5, p27, Mcl-1 or in PARP cleavage (Supplemental Figure S4A). However, there was a reduction in cell volume following treatment with AZD1208 (Supplemental Figure S4B). The levels of total and phosphorylated S6 were not detected, and S6 protein appears to be more labile in primary leukemia cells (unpublished observations).
Correlation between RNA and protein synthesis inhibition and growth inhibition by AZD1208
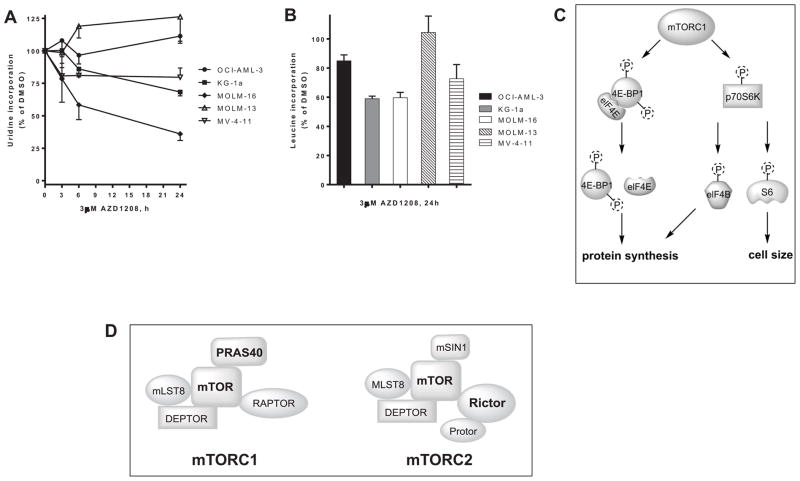
Investigations of first-generation Pim inhibitor, SGI-1776, in CLL primary cells and AML cell lines and primary cells identified inhibition of RNA and protein synthesis[9,10]. To evaluate the effect of Pim kinase inhibition in the absence of FLT3 inhibition, AML cell lines were treated with DMSO or 3 μM AZD1208 for 3, 6 and 24 hours, then pulsed with [3H]-labeled uridine to measure RNA synthesis. There was a decrease in RNA synthesis in the 3 cell lines that were more sensitive to growth inhibition (MV-4-11, KG-1a and MOLM-16) but not OCI-AML-3 or MOLM-13 (Figure 5A). RNA synthesis was reduced by 20–30% in KG-1a and MV-4-11 after 24 h. The most sensitive cell line, MOLM-16, exhibited the most RNA synthesis inhibition and was reduced by 20% after only 3h, which further decrease by 40% and 60% after 6 h and 24h, respectively.
Figure 5. Inhibition of RNA and protein synthesis in AML cell lines treated with AZD1208.
OCI-AML-3 (circles), KG-1a (squares), MOLM-16 (diamonds), MOLM-13 (open triangles) MV-4-11 (inverted open triangle) cell lines were incubated with 0.1% DMSO or 3 μM AZD1208 for 24 h, then 1 h prior to harvesting the cells (A) [3H]uridine was added to the cell culture or (B) [3H]leucine was added to the cell culture as described in Materials and Methods. The results represent an average of triplicate experiments ± SEM. mTOR pathway proteins. (C) mTOR signaling as a driver of protein synthesis and cell size. (D) General scheme of mTORC1 and mTORC2 complex components. Protein phosphorylation decreased by Pim kinase inhibition with AZD1208 are shown in bold.
To evaluate the impact on protein synthesis, AML cell lines were treated with DMSO or 3 μM AZD1208 for 24 hours then pulsed with [3H]-labeled leucine. There was a decrease in protein synthesis in all the cell lines except MOLM-13 (Figure 5B), which was also the cell line with the least growth inhibition and RNA synthesis inhibition. There was ~15% and 30% reduction in protein synthesis in OCI-AML-3 and MV-4-11, respectively, and in the two more sensitive cell lines MOLM-16 and KG-1a synthesis was reduced by ~40%. Thus, both RNA and protein synthesis correlated with growth inhibition sensitivity to AZD1208 and changes in biomacromolecular synthesis may be a hallmark of Pim kinase inhibition.
Discussion
The primary objectives of the present project were to evaluate the biological effect of AZD1208 in AML cells with different genetic background and to identify the key molecular pathways impacted by this Pim kinase inhibitor. Our data demonstrate that inhibition of cell growth, reduction of cell size, and inhibition of protein synthesis as the primary biological consequences and identifies that AZD1208 impacts mTOR pathway signaling.
The effects of AZD1208 vary between cell types and AZD1208 was reported to induce apoptosis as measured by caspase 3 cleavage in prostate cancer graft specimens[15]. Likewise, in mice with myeloid leukemia from co-expression of MYC and PIM, AZD1208 suppressed the synergism between MYC and PIM in vivo and extended the life of the treated mice[4]. AZD1208 has been previously reported to induce apoptosis in AML cell lines[16], however, apoptosis induction was only demonstrated in MOLM-16. Consistent with this report, in the four additional AML cell lines analyzed in our investigations, AZD1208 was cytostatic with little induction of apoptosis. Limited apoptosis appears to be a common feature of Pim kinase inhibitors, and growth inhibition may be through other mechanisms.[9–12,17,18] While limited apoptosis was observed, cell size, cell growth and clonogenicity were reduced in the AML cell lines evaluated. The cell size change correlated with inhibition of protein synthesis (Figure 5B), which is consistent with mTOR inhibition[19].
The RPPA results suggest that there are additional targets of Pim, or alternatively that AZD1208 affects other proteins, or likely a combination of both. MOLM-16 was the only cell line where moderate apoptosis was induced by AZD1208, and was also the cell line with the largest number of proteins that changed significantly (19 proteins). OCI-AML-3 and MOLM-13 were the cell lines that demonstrated the least growth inhibition, and had few proteins that were significantly changed (3–4 proteins). Of these proteins, only an increase Chk2 (Thr68) phosphorylation was common to both cell lines, which was also measured in MOLM-16. Increased Chk2 phosphorylation is associated with S-phase delay or arrest, however, MOLM-16 cells treated with 0.1 or 1 μM AZD1208 for 72 h resulted in increased sub-G1 and G0/G1 cell populations[14]. Likewise, the other sensitive cell lines KG-1a and EOL-1 were reported to not exhibit cell cycle arrest following AZD1208 treatment. While MV-4-11 was relatively sensitive to AZD1208, only two proteins were found to be significantly altered, one of which was a decrease in Myosin-IIA (Ser1943) phosphorylation. Myosin-IIA has not been reported as a Pim target to date, nor is the role of Myosin-IIA (Ser1943) phosphorylation in AML known, however phosphorylation at this site has been reported to regulate cell motility in MDA-MB-231 breast cancer cell line[20].
Although the proteins (n = 171) covered by RPPA may not identify all the pathways and proteins affected by AZD1208, the data from MOLM-16 and KG-1a suggest that the mTOR signaling pathway may be key to AZD1208 effect in AML cells, given the inhibition of mTOR, Rictor, p70S6K and 4E-BP1 phosphorylation (Figure 5C). Immunoblot assays support that AZD1208 impacts mTOR pathway, as mTOR(Ser2448), p70S6K(Thr389), S6(Ser235/236) and 4E-BP1(Ser65) phosphorylation was reduced following treatment (Figure 4, Supplemental Figure S2–S3). Phosphorylation of ribosomal S6 protein has been reported to be a determinant of cell size[21], which is consistent with the reduction in cell size in AZD1208-treated AML cells (Figure 1E, Supplemental Figure S4B). Similarly, inhibition of mTOR signaling is consistent with protein synthesis inhibition measured by leucine incorporation following AZD1208 (Figure 5B).
AZD1208 impact in AML cell lines can affect components of both mTORC1 and mTORC2 complexes depending on the cell-type (Figure 5C–D). Reduction of phosphorylation of mTOR targets were detected for both mTORC1 (p70S6K and 4E-BP1) as well as mTORC2 (Rictor) pathways (Figure 3). Interestingly, protein levels of Raptor increased following AZD1208 treatment in KG1a in a statistically significant manner, but also in MOLM-16 and MV-4-11 (Figure 3). Raptor is required for mTOR phosphorylation of 4E-BP1 and enhances mTOR phosphorylation of p70S6K.[22] Both 4E-BP1 and p70S6K phosphorylation is inhibited by AZD1208 (Figure 4), and increased Raptor may be a cellular compensatory mechanism for disruption of mTOR signaling. mTOR affects cell size[19], and our data are in agreement with mTORC1 inhibition as there was a reduction in cell size in all five cell lines (Figure 1E) as well as in AML primary blasts treated with AZD1208 (Supplemental Figure S4B). Protein synthesis inhibition may also be in part through eIF4B, as AZD1208 was recently reported to inhibit eIF4B (Ser406) phosphorylation and reduce MET expression[6].
Regulation of mTOR activity by Pim kinase has been reported.[23] In concert, our results suggest that mTOR axis will be blunted by AZD1208, and further inhibition of this pathway would suggest synergy. In fact, dual inhibition of Pim kinases and mTORC1/2 using AZD1208 in combination with AZD2014 resulted in synergistic inhibition of AML cell growth[24]. mTORC1/2 is activated in CML cells[25] and a recent report on SGI-1776 indicated inhibition of mTOR pathway as well as augmentation of imatinib colony formation suppression by SGI-1776 or AZD1208[26]. It is interesting to note that in addition to inhibition of mTOR pathway protein phosphorylation there was also increase in protein DJ-1 (gene PARK7) in the two relatively more AZD1208-sensitive cell lines, MOLM-16 and KG-1a. DJ-1 is involved with response to oxidative stress (reviewed in[27]) as well as promoting cancer cell invasion[28–30]. DJ-1 activation may be a survival mechanism against blockade of Pim kinase and/or mTOR pathway signaling. The decrease in Chk1 protein levels following AZD1208 treatment was statistically significant in MOLM-16, however the decline was also seen in the other cell lines. Pim kinases have been reported to catalyze Chk1 Ser280 phosphorylation and regulate its function in AML cells[31]. Although Chk1 phosphorylation at Ser280 has been reported to be associated with ubiquitination and cytoplasmic localization[32], the effect of Ser280 phosphorylation of Chk1 protein stability has not been reported in AML cells, thus the mechanism of Chk1 protein decrease by AZD1208 in AML cells is not clear.
PIM inhibitors have been investigated in AML cells as single agents as well as in combination, with varying potencies and cellular consequences[5,7,10,14,17,31,33–44]. Downstream mTOR targets (4E-BP1, PRAS40, eIF4B)[23,45,46] and upstream regulators (TSC2) [47] have been identified as Pim targets, and our RPPA investigations support the reports of Pim activity on mTOR signaling. However, to date, mTOR itself has not been reported as a direct Pim-target and further investigation may shed light on the direct interactions between Pim and mTOR proteins and signaling (Figure 5C–D). Our results suggest that there may be additional Pim kinase targets that play a role in AML cell survival and proliferation, as well as previously unreported compensatory mechanisms as cellular responses to Pim kinase inhibition.
Supplementary Material
Acknowledgments
This work is supported by a grant from the Translational Research Program of the Leukemia and Lymphoma Society of America (#TRP-R6011-14) and a Sponsored Research Agreement from AstraZeneca.
Footnotes
Conflict of Interest Statement.
V.G. received a Sponsored Research Agreement from AstraZeneca. No other authors have any conflicts of interest to disclose.
References
- 1.Nawijn MC, Alendar A, Berns A. For better or for worse: the role of Pim oncogenes in tumorigenesis. Nature Reviews Cancer. 2011;11:23–34. doi: 10.1038/nrc2986. [DOI] [PubMed] [Google Scholar]
- 2.Chen LS, Gandhi V. Targeted Therapy of Acute Myeloid Leukemia. Springer; 2015. PIM Kinases in AML; pp. 177–199. [Google Scholar]
- 3.Aguirre E, Renner O, Narlik-Grassow M, Blanco-Aparicio C. Genetic Modeling of PIM Proteins in Cancer: Proviral Tagging and Cooperation with Oncogenes, Tumor Suppressor Genes, and Carcinogens. Frontiers in oncology. 2014;4:109. doi: 10.3389/fonc.2014.00109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Saurabh K, Scherzer MT, Shah PP, et al. The PIM family of oncoproteins: small kinases with huge implications in myeloid leukemogenesis and as therapeutic targets. Oncotarget. 2014;5:8503–8514. doi: 10.18632/oncotarget.2330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Natarajan K, Xie Y, Burcu M, Linn DE, Qiu Y, Baer MR. Pim-1 kinase phosphorylates and stabilizes 130 kDa FLT3 and promotes aberrant STAT5 signaling in acute myeloid leukemia with FLT3 internal tandem duplication. PLoS ONE. 2013;8:e74653. doi: 10.1371/journal.pone.0074653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Cen B, Xiong Y, Song JH, et al. The Pim-1 protein kinase is an important regulator of MET receptor tyrosine kinase levels and signaling. Mol Cell Biol. 2014;34:2517–2532. doi: 10.1128/MCB.00147-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Guo Z, Wang A, Zhang W, et al. PIM inhibitors target CD25-positive AML cells through concomitant suppression of STAT5 activation and degradation of MYC oncogene. Blood. 2014;124:1777–1789. doi: 10.1182/blood-2014-01-551234. [DOI] [PubMed] [Google Scholar]
- 8.Narlik-Grassow M, Blanco-Aparicio C, Carnero A. The PIM family of serine/threonine kinases in cancer. Med Res Rev. 2014;34:136–159. doi: 10.1002/med.21284. [DOI] [PubMed] [Google Scholar]
- 9.Chen LS, Redkar S, Bearss D, Wierda WG, Gandhi V. Pim kinase inhibitor, SGI-1776, induces apoptosis in chronic lymphocytic leukemia cells. Blood. 2009;114:4150–4157. doi: 10.1182/blood-2009-03-212852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Chen LS, Redkar S, Taverna P, Cortes JE, Gandhi V. Mechanisms of cytotoxicity to Pim kinase inhibitor, SGI-1776, in acute myeloid leukemia. Blood. 2011;118:693–702. doi: 10.1182/blood-2010-12-323022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Yang Q, Chen LS, Neelapu SS, Miranda RN, Medeiros LJ, Gandhi V. Transcription and translation are primary targets of Pim kinase inhibitor SGI-1776 in mantle cell lymphoma. Blood. 2012;120:3491–3500. doi: 10.1182/blood-2012-02-412643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Cervantes-Gomez F, Chen LS, Orlowski RZ, Gandhi V. Biological effects of the Pim kinase inhibitor, SGI-1776, in multiple myeloma. Clin Lymphoma Myeloma Leuk. 2013;13(Suppl 2):S317–329. doi: 10.1016/j.clml.2013.05.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Natarajan K, Bhullar J, Shukla S, et al. The Pim kinase inhibitor SGI-1776 decreases cell surface expression of P-glycoprotein (ABCB1) and breast cancer resistance protein (ABCG2) and drug transport by Pim-1-dependent and -independent mechanisms. Biochem Pharmacol. 2013;85:514–524. doi: 10.1016/j.bcp.2012.12.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Keeton EK, McEachern K, Dillman KS, et al. AZD1208, a potent and selective pan-Pim kinase inhibitor, demonstrates efficacy in preclinical models of acute myeloid leukemia. Blood. 2014;123:905–913. doi: 10.1182/blood-2013-04-495366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kirschner AN, Wang J, van der Meer R, et al. PIM kinase inhibitor AZD1208 for treatment of MYC-driven prostate cancer. J Natl Cancer Inst. 2015:107. doi: 10.1093/jnci/dju407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Keeton E, Palakurthi S, Alimzhanov M, et al. AZD1208, a Novel, Potent and Selective Pan PIM Kinase Inhibitor, Demonstrates Efficacy in Models of Acute Myeloid Leukemia. Blood (ASH Annual Meeting Abstracts) 2011;118 doi: 10.1182/blood-2013-04-495366. p Abstract 1540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hospital MA, Green AS, Lacombe C, Mayeux P, Bouscary D, Tamburini J. The FLT3 and Pim kinases inhibitor SGI-1776 preferentially target FLT3-ITD AML cells. Blood. 2012;119:1791–1792. doi: 10.1182/blood-2011-11-393066. [DOI] [PubMed] [Google Scholar]
- 18.Yang Q, Chen LS, Neelapu SS, Gandhi V. Combination of Pim kinase inhibitor, SGI-1776 with Bendamustine in B-cell lymphoma. Clinical Lymphoma, Myeloma & Leukemia. 2013 doi: 10.1016/j.clml.2013.05.024. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Fingar DC, Salama S, Tsou C, Harlow E, Blenis J. Mammalian cell size is controlled by mTOR and its downstream targets S6K1 and 4EBP1/eIF4E. Genes Dev. 2002;16:1472–1487. doi: 10.1101/gad.995802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Dulyaninova NG, House RP, Betapudi V, Bresnick AR. Myosin-IIA heavy-chain phosphorylation regulates the motility of MDA-MB-231 carcinoma cells. Mol Biol Cell. 2007;18:3144–3155. doi: 10.1091/mbc.E06-11-1056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Ruvinsky I, Sharon N, Lerer T, et al. Ribosomal protein S6 phosphorylation is a determinant of cell size and glucose homeostasis. Genes Dev. 2005;19:2199–2211. doi: 10.1101/gad.351605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Hara K, Maruki Y, Long X, et al. Raptor, a binding partner of target of rapamycin (TOR), mediates TOR action. Cell. 2002;110:177–189. doi: 10.1016/s0092-8674(02)00833-4. [DOI] [PubMed] [Google Scholar]
- 23.Zhang F, Beharry ZM, Harris TE, et al. PIM1 protein kinase regulates PRAS40 phosphorylation and mTOR activity in FDCP1 cells. Cancer Biol Ther. 2009;8:846–853. doi: 10.4161/cbt.8.9.8210. [DOI] [PubMed] [Google Scholar]
- 24.Harada M, Benito J, Yamamoto S, et al. The novel combination of dual mTOR inhibitor AZD2014 and pan-PIM inhibitor AZD1208 inhibits growth in acute myeloid leukemia via HSF pathway suppression. Oncotarget. 2015 doi: 10.18632/oncotarget.6122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Carayol N, Vakana E, Sassano A, et al. Critical roles for mTORC2- and rapamycin-insensitive mTORC1-complexes in growth and survival of BCR-ABL-expressing leukemic cells. Proc Natl Acad Sci U S A. 2010;107:12469–12474. doi: 10.1073/pnas.1005114107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Curi DA, Beauchamp EM, Blyth GT, et al. Pre-clinical evidence of PIM kinase inhibitor activity in BCR-ABL1 unmutated and mutated Philadelphia Chromosome Ph (+) -Positive Leukemias. Oncotarget. 2015 doi: 10.18632/oncotarget.5091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Raninga PV, Di Trapani G, Tonissen KF. Cross talk between two antioxidant systems, Thioredoxin and DJ-1: consequences for cancer. Oncoscience. 2014;1:95. doi: 10.18632/oncoscience.12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.He X, Zheng Z, Li J, et al. DJ-1 promotes invasion and metastasis of pancreatic cancer cells by activating SRC/ERK/uPA. Carcinogenesis. 2012;33:555–562. doi: 10.1093/carcin/bgs002. [DOI] [PubMed] [Google Scholar]
- 29.Ismail IA, Kang HS, Lee HJ, Kim JK, Hong SH. DJ-1 upregulates breast cancer cell invasion by repressing KLF17 expression. Br J Cancer. 2014;110:1298–1306. doi: 10.1038/bjc.2014.40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Wang B, Qin H, Wang Y, et al. Effect of DJ-1 overexpression on the proliferation, apoptosis, invasion and migration of laryngeal squamous cell carcinoma SNU-46 cells through PI3K/AKT/mTOR. Oncol Rep. 2014;32:1108–1116. doi: 10.3892/or.2014.3286. [DOI] [PubMed] [Google Scholar]
- 31.Yuan LL, Green AS, Bertoli S, et al. Pim kinases phosphorylate Chk1 and regulate its functions in acute myeloid leukemia. Leukemia. 2014;28:293–301. doi: 10.1038/leu.2013.168. [DOI] [PubMed] [Google Scholar]
- 32.Puc J, Keniry M, Li HS, et al. Lack of PTEN sequesters CHK1 and initiates genetic instability. Cancer Cell. 2005;7:193–204. doi: 10.1016/j.ccr.2005.01.009. [DOI] [PubMed] [Google Scholar]
- 33.Blanco-Aparicio C, Collazo AM, Oyarzabal J, et al. Pim 1 kinase inhibitor ETP-45299 suppresses cellular proliferation and synergizes with PI3K inhibition. Cancer Lett. 2011;300:145–153. doi: 10.1016/j.canlet.2010.09.016. [DOI] [PubMed] [Google Scholar]
- 34.Pogacic V, Bullock AN, Fedorov O, et al. Structural analysis identifies imidazo[1,2-b]pyridazines as PIM kinase inhibitors with in vitro antileukemic activity. Cancer Res. 2007;67:6916–6924. doi: 10.1158/0008-5472.CAN-07-0320. [DOI] [PubMed] [Google Scholar]
- 35.Yang C, Boyson CA, Di Liberto M, et al. CDK4/6 Inhibitor PD 0332991 Sensitizes Acute Myeloid Leukemia to Cytarabine-Mediated Cytotoxicity. Cancer Res. 2015;75:1838–1845. doi: 10.1158/0008-5472.CAN-14-2486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Lin YW, Beharry ZM, Hill EG, et al. A small molecule inhibitor of Pim protein kinases blocks the growth of precursor T-cell lymphoblastic leukemia/lymphoma. Blood. 2010;115:824–833. doi: 10.1182/blood-2009-07-233445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Meja K, Stengel C, Sellar R, et al. PIM and AKT kinase inhibitors show synergistic cytotoxicity in acute myeloid leukaemia that is associated with convergence on mTOR and MCL1 pathways. Br J Haematol. 2014;167:69–79. doi: 10.1111/bjh.13013. [DOI] [PubMed] [Google Scholar]
- 38.Garcia PD, Langowski JL, Wang Y, et al. Pan-PIM kinase inhibition provides a novel therapy for treating hematologic cancers. Clin Cancer Res. 2014;20:1834–1845. doi: 10.1158/1078-0432.CCR-13-2062. [DOI] [PubMed] [Google Scholar]
- 39.Fathi AT, Arowojolu O, Swinnen I, et al. A potential therapeutic target for FLT3-ITD AML: PIM1 kinase. Leuk Res. 2012;36:224–231. doi: 10.1016/j.leukres.2011.07.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Kelly KR, Espitia CM, Taverna P, et al. Targeting PIM kinase activity significantly augments the efficacy of cytarabine. Br J Haematol. 2011;156:129–132. doi: 10.1111/j.1365-2141.2011.08792.x. [DOI] [PubMed] [Google Scholar]
- 41.Beharry Z, Zemskova M, Mahajan S, et al. Novel benzylidene-thiazolidine-2,4-diones inhibit Pim protein kinase activity and induce cell cycle arrest in leukemia and prostate cancer cells. Mol Cancer Ther. 2009;8:1473–1483. doi: 10.1158/1535-7163.MCT-08-1037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Haddach M, Michaux J, Schwaebe MK, et al. Discovery of CX-6258. A Potent, Selective, and Orally Efficacious pan-Pim Kinases Inhibitor. Acs Med Chem Lett. 2012;3:135–139. doi: 10.1021/ml200259q. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Grundler R, Brault L, Gasser C, et al. Dissection of PIM serine/threonine kinases in FLT3-ITD-induced leukemogenesis reveals PIM1 as regulator of CXCL12-CXCR4-mediated homing and migration. J Exp Med. 2009;206:1957–1970. doi: 10.1084/jem.20082074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Tao ZF, Hasvold LA, Leverson JD, et al. Discovery of 3H-benzo[4,5]thieno[3,2-d]pyrimidin-4-ones as potent, highly selective, and orally bioavailable inhibitors of the human protooncogene proviral insertion site in moloney murine leukemia virus (PIM) kinases. J Med Chem. 2009;52:6621–6636. doi: 10.1021/jm900943h. [DOI] [PubMed] [Google Scholar]
- 45.Fox CJ, Hammerman PS, Cinalli RM, Master SR, Chodosh LA, Thompson CB. The serine/threonine kinase Pim-2 is a transcriptionally regulated apoptotic inhibitor. Genes Dev. 2003;17:1841–1854. doi: 10.1101/gad.1105003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Yang J, Wang J, Chen K, et al. eIF4B phosphorylation by pim kinases plays a critical role in cellular transformation by Abl oncogenes. Cancer Res. 2013;73:4898–4908. doi: 10.1158/0008-5472.CAN-12-4277. [DOI] [PubMed] [Google Scholar]
- 47.Lu J, Zavorotinskaya T, Dai Y, et al. Pim2 is required for maintaining multiple myeloma cell growth through modulating TSC2 phosphorylation. Blood. 2013;122:1610–1620. doi: 10.1182/blood-2013-01-481457. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.