Abstract
Objective
To assess whether strict adherence to quality metrics by hospitals could explain the association between hospital volume and survival for ovarian cancer.
Methods
We used the National Cancer Data Base to perform a retrospective cohort study of women with ovarian cancer from 2004 to 2013. Hospitals were stratified by annual case volume into quintiles (≤2, 2.01–5, 5.01–9, 9.01–19.9, ≥20 cases) and by adherence to ovarian cancer quality metrics into quartiles. Hospital-level adjusted 2- and 5-year survivals were compared based on volume and adherence to the quality metrics.
Results
A total of 100,725 patients at 1268 hospitals were identified. Higher-volume hospitals were more likely to adhere to the quality metrics. Both 2- and 5-year survival increased with hospital volume and with adherence to the measured quality metrics. For example, 2-year survival increased from 64.4% (95% CI, 62.5–66.4%) at low volume to 77.4% (95% CI, 77.0–77.8%) at high-volume centers and from 66.5% (65.5–67.5%) at low-quality to 77.3% (95% CI, 76.8–77.7%) at high-quality hospitals (P<0.001 for both). For each hospital volume category, survival increased with increasing adherence to the quality metrics. For example, in the lowest-volume hospitals (≤2 cases annually), adjusted 2-year survival was 61.4% (95% CI, 58.4–64.5%) at hospital with the lowest adherence to quality metrics and rose to 65.8% (95% CI, 61.2–70.8%) at the hospitals with highest adherence to the quality metrics (P<0.001). However, lower-volume hospitals with higher-quality scores still had survival that was lower than higher-volume hospitals.
Conclusion
While both hospital volume and adherence to quality metrics are associated with survival for ovarian cancer, low-volume hospitals that provide high-quality care still have survival rates that are lower than high-volume centers.
Introduction
The association between procedure volume and outcomes has long been recognized; patients operated on at high volume hospitals and by high volume surgeons have improved outcomes.1,2 The volume-outcome paradigm is strongest for high-risk surgeries that are associated with substantial morbidity.1,2 Numerous factors likely contribute to the volume-outcome relationship including superior technical prowess, adherence to evidence-based guidelines, decreased complication rates and improved management of complications.3,4
Both hospital and physician procedural volume are associated with outcomes for ovarian cancer.5–12 One analysis found that management of ovarian cancer patients at low-volume centers was associated with a 14% increase in mortality compared to high-volume hospitals.7 Studies have consistently shown that women managed at high-volume hospitals are more likely to receive treatment consistent with evidence-based guidelines.6–9,13,14 In turn, adherence to recommended treatment guidelines is independently associated with improved survival and may in part explain the relationship between volume and outcomes.6
While referral to high-volume centers is a consideration for ovarian cancer, this is often not feasible. An alternative strategy is to improve the quality of care at low-volume centers. However, it remains unknown whether strict adherence to treatment guidelines at low-volume centers can result in the same outcomes achieved at high-volume hospitals. The objective of our study was to assess the relative importance of hospital procedural volume and adherence to quality guidelines on survival for ovarian cancer. Specifically, we examined whether lower volume hospitals that are compliant with quality metrics recognized by the National Comprehensive Cancer Network can achieve outcomes similar to higher volume hospitals.
Materials and Methods
Data from the National Cancer Data Base (NCDB) was used to perform a retrospective cohort study. The NCDB is a registry developed by the American College of Surgeons and American Cancer Society that captures hospitalized patients from across the United States.15,16 The NCDB collects data on all patients with newly diagnosed invasive cancers from over 1500 Commission on Cancer (CoC) affiliated hospitals. Data elements include patient demographics, tumor characteristics, treatment, and survival.15,16 Incident tumor cases are abstracted by trained registrars and the data is audited regularly to ensure accuracy. The analysis utilized de-identified data and the Columbia University Institutional Review Board deemed this study exempt.
We identified women with invasive epithelial ovarian cancer diagnosed from 2004 to 2013. The cohort included only women with ovarian cancer as their first cancer diagnosis and those with histologic confirmation. After patient selection, we identified all hospitals that treated at least 1 patient during the study period. For each facility, we calculated the annualized hospital volume, defined as the total number of patients divided by the years in which a given hospital treated at least one patient. Volume estimates were then visually inspected and stratified approximately based on the number of hospitals into quintiles. Based on prior work, we defined a high-volume hospital as a center with ≥20 cases.17 High-intermediate volume hospitals had 9.01–19.9 cases, intermediate-volume hospitals 5.01–9 cases, intermediate-low hospitals 2.01–5, cases and low-volume hospitals ≤2 cases annually.
To measure quality, we defined hospital level rates of five quality metrics. All of these quality metrics are based on high-quality data and accepted by the National Comprehensive Cancer Network (NCCN) as standard treatment for ovarian cancer.18 We examined lymph node dissection performed for patients with stage I-IIIB tumors19,20, performance of omentectomy or cytoreduction for patients with advanced stage tumors (IIA, IIB, IIINOS, IIIA, IIIB, IIIC, IV)19,20, use of chemotherapy among patients with early-stage, high risk tumors (stage IA or IB and grade 3; stage IC; or any stage I and clear cell histology)21,22, omission of chemotherapy for women with early stage low-risk tumors (stage IA or IB, grade 1, and serous, mucinous, endometrioid, or transitional cell histology)23 and use of chemotherapy (either as neoadjuvant or adjuvant therapy) for women with advanced stage disease (stage III–IV).18 For each metric, we determined the rate of hospital-level compliance for all eligible patients (patients who adhered to the quality metric divided by patients eligible for the metric). A composite variable of overall quality was also derived incorporating all five metrics. For the composite, the total adherence to each quality metric was estimated for each hospital as the percentage of patients meeting the quality metrics divided by the number of eligible patients. Hospitals were then stratified based on the overall quality metric into quartiles: low quality, medium low quality, medium high quality, and high quality.
Demographic data included age at diagnosis (<40, 40–49, 50–59, 60–69, 70–79, ≥80 years), race (white, black, Hispanic, other, unknown), year of diagnosis, and insurance status (private, Medicaid, Medicare, uninsured, other governmental/unknown). Income was measured by median household income in the patients’ zip code and classified as <$38,000, $38,000–$47,999, $48,000–$62,999, over $63,000, or unknown. Education was measured by percentage of adults in a patient's zip code who did not graduate from high school, and classified as ≥21%, 13–20%, 7.0–12.9%, <7%, or unknown. Location was estimated by matching the patients’ state and county code to rural-urban continuum codes from the United States Department of Agriculture Economic Research Service, and classified as metropolitan, urban, rural, and unknown. Comorbidity was measured using the Deyo classification of the Charlson comorbidity score, and grouped as 0, 1, or ≥2.24 Tumor characteristics included tumor stage (INOS, IA, IB, IC, IINOS, IIA, IIB, IIINOS, IIIA, IIIB, IIIC, IV, unknown), histology (serous, mucinous, endometrioid, clear cell, transitional cell, NOS), and grade (well, moderate, poorly, unknown).
Hospital characteristics included facility region (eastern, midwest, south, west, unknown) and facility type as defined by the American Cancer Society’s Commission on Cancer Accreditation program criteria, classified as academic centers, community centers or comprehensive community cancer centers.16
The primary outcome of the analysis was overall survival at 2 and 5 years. Frequency distributions based on hospital volume quintiles and hospital quality metric quartiles were compared using χ2 tests at the patient level. Adherence to each quality metric is reported as a mean with standard deviations for each hospital volume category and compared using ANOVA.
We fit Cox proportional hazards models to examine risk-adjusted survival rates by hospital volume and quality, adjusting for age, race, insurance status, education, location, comorbidity, year of diagnosis, stage, grade, histology, and lymph node dissection. The models were stratified by volume, or by both volume and overall quality. Estimates were reported as the predicted 2-year and 5-year risk-adjusted survival rates with 95% confidence intervals using the average covariate method.25,26 Survival rates at 2 and 5-years were compared across the volume and quality categories using ANOVA. All analyses were conducted using SAS version 9.4 (SAS Institute Inc, Cary, North Carolina). All hypothesis testing was two-sided and a P-value of <0.05 was considered statistically significant.
Results
A total of 100,725 patients treated at 1268 hospitals were identified. The analysis included 299 (23.6%) low volume hospitals, 465 (36.7%) with low intermediate volume, 157 (12.4%) with intermediate volume, 194 (15.3%) with high intermediate and 153 (12.1%) with high volume centers (Table 1). Patients treated at high volume centers were younger, more often non-white, more frequently had private insurance, were residents of metropolitan areas and lived in census tracts with higher median incomes than those treated at low volume hospitals.
Table 1.
Clinical and demographic characteristics of the cohort stratified by hospital volume.
| Low Volume |
Low Intermediate Volume |
Intermediate Volume |
High Intermediate Volume |
High Volume |
||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
|
|
||||||||||||
| N | (%) | N | (%) | N | (%) | N | (%) | N | (%) | P-value | ||
| All, patients | 2,789 | (2.8) | 12,926 | (12.8) | 10,082 | (10.0) | 27,079 | (26.9) | 47,849 | (47.5) | ||
| All, hospitals | 299 | (23.6) | 465 | (36.7) | 157 | (12.4) | 194 | (15.3) | 153 | (12.1) | ||
| Age (years) | <0.001 | |||||||||||
| <40 | 124 | (4.4) | 635 | (4.9) | 568 | (5.6) | 1,336 | (4.9) | 2,598 | (5.4) | ||
| 40–49 | 326 | (11.7) | 1,700 | (13.2) | 1,405 | (13.9) | 3,900 | (14.4) | 7,189 | (15.0) | ||
| 50–59 | 544 | (19.5) | 2,940 | (22.7) | 2,507 | (24.9) | 6,991 | (25.8) | 13,071 | (27.3) | ||
| 60–69 | 709 | (25.4) | 3,178 | (24.6) | 2,523 | (25.0) | 7,154 | (26.4) | 12,678 | (26.5) | ||
| 70–79 | 648 | (23.2) | 2,731 | (21.1) | 1,983 | (19.7) | 5,277 | (19.5) | 8,684 | (18.1) | ||
| ≥80 | 438 | (15.7) | 1,742 | (13.5) | 1,096 | (10.9) | 2,421 | (8.9) | 3,629 | (7.6) | ||
| Race | <0.001 | |||||||||||
| White | 2,404 | (86.2) | 10,827 | (83.8) | 8,111 | (80.5) | 22,060 | (81.5) | 39,267 | (82.1) | ||
| Black | 219 | (7.9) | 944 | (7.3) | 766 | (7.6) | 1,935 | (7.1) | 3,417 | (7.1) | ||
| Hispanic | 79 | (2.8) | 660 | (5.1) | 707 | (7.0) | 1,581 | (5.8) | 2,688 | (5.6) | ||
| Other | 69 | (2.5) | 422 | (3.3) | 442 | (4.4) | 1,196 | (4.4) | 1,795 | (3.8) | ||
| Unknown | 18 | (0.6) | 73 | (0.6) | 56 | (0.6) | 307 | (1.1) | 682 | (1.4) | ||
| Year of diagnosis | <0.001 | |||||||||||
| 2004 | 266 | (9.5) | 1,497 | (11.6) | 986 | (9.8) | 2,541 | (9.4) | 4,185 | (8.7) | ||
| 2005 | 281 | (10.1) | 1,404 | (10.9) | 988 | (9.8) | 2,608 | (9.6) | 4,539 | (9.5) | ||
| 2006 | 256 | (9.2) | 1,369 | (10.6) | 990 | (9.8) | 2,534 | (9.4) | 4,751 | (9.9) | ||
| 2007 | 273 | (9.8) | 1,239 | (9.6) | 943 | (9.4) | 2,684 | (9.9) | 4,738 | (9.9) | ||
| 2008 | 279 | (10.0) | 1,269 | (9.8) | 1,014 | (10.1) | 2,816 | (10.4) | 4,856 | (10.1) | ||
| 2009 | 307 | (11.0) | 1,223 | (9.5) | 993 | (9.8) | 2,722 | (10.1) | 4,885 | (10.2) | ||
| 2010 | 257 | (9.2) | 1,226 | (9.5) | 1,042 | (10.3) | 2,747 | (10.1) | 4,754 | (9.9) | ||
| 2011 | 271 | (9.7) | 1,254 | (9.7) | 1,026 | (10.2) | 2,713 | (10.0) | 4,996 | (10.4) | ||
| 2012 | 291 | (10.4) | 1,261 | (9.8) | 1,073 | (10.6) | 2,767 | (10.2) | 5,034 | (10.5) | ||
| 2013 | 308 | (11.0) | 1,184 | (9.2) | 1,027 | (10.2) | 2,947 | (10.9) | 5,111 | (10.7) | ||
| Insurance status | <0.001 | |||||||||||
| Private | 1,092 | (39.2) | 5,422 | (41.9) | 4,794 | (47.6) | 13,375 | (49.4) | 24,692 | (51.6) | ||
| Medicaid | 147 | (5.3) | 829 | (6.4) | 702 | (7.0) | 1,603 | (5.9) | 2,575 | (5.4) | ||
| Medicare | 1,335 | (47.9) | 5,723 | (44.3) | 3,815 | (37.8) | 10,171 | (37.6) | 16,798 | (35.1) | ||
| Other government/Unknown | 91 | (3.3) | 320 | (2.5) | 214 | (2.1) | 657 | (2.4) | 1,806 | (3.8) | ||
| Uninsured | 124 | (4.4) | 632 | (4.9) | 557 | (5.5) | 1,273 | (4.7) | 1,978 | (4.1) | ||
| Income | <0.001 | |||||||||||
| <$38,000 | 523 | (18.8) | 2,079 | (16.1) | 1,367 | (13.6) | 4,696 | (17.3) | 7,115 | (14.9) | ||
| $38,000–$47,999 | 797 | (28.6) | 3,570 | (27.6) | 1,988 | (19.7) | 6,088 | (22.5) | 10,421 | (21.8) | ||
| $48,000–$62,999 | 728 | (26.1) | 3,600 | (27.9) | 2,945 | (29.2) | 7,229 | (26.7) | 12,328 | (25.8) | ||
| $63,000+ | 697 | (25.0) | 3,424 | (26.5) | 3,609 | (35.8) | 8,620 | (31.8) | 16,912 | (35.3) | ||
| Unknown | 44 | (1.6) | 253 | (2.0) | 173 | (1.7) | 446 | (1.6) | 1,073 | (2.2) | ||
| Education | <0.001 | |||||||||||
| ≥21% | 456 | (16.3) | 2,059 | (15.9) | 1,656 | (16.4) | 4,527 | (16.7) | 6,925 | (14.5) | ||
| 13–20% | 895 | (32.1) | 3,504 | (27.1) | 2,249 | (22.3) | 6,681 | (24.7) | 11,481 | (24.0) | ||
| 7.0–12.9% | 897 | (32.2) | 4,500 | (34.8) | 3,332 | (33.0) | 8,897 | (32.9) | 15,208 | (31.8) | ||
| <7% | 498 | (17.9) | 2,617 | (20.2) | 2,676 | (26.5) | 6,536 | (24.1) | 13,186 | (27.6) | ||
| Unknown | 43 | (1.5) | 246 | (1.9) | 169 | (1.7) | 438 | (1.6) | 1,049 | (2.2) | ||
| Location | <0.001 | |||||||||||
| Metropolitan | 1,899 | (68.1) | 9,847 | (76.2) | 8,646 | (85.8) | 22,248 | (82.2) | 38,493 | (80.4) | ||
| Urban | 701 | (25.1) | 2,222 | (17.2) | 978 | (9.7) | 3,394 | (12.5) | 6,423 | (13.4) | ||
| Rural | 83 | (3.0) | 311 | (2.4) | 113 | (1.1) | 530 | (2.0) | 722 | (1.5) | ||
| Unknown | 106 | (3.8) | 546 | (4.2) | 345 | (3.4) | 907 | (3.3) | 2,211 | (4.6) | ||
| Comorbidity score | <0.001 | |||||||||||
| 0 | 2,170 | (77.8) | 10,290 | (79.6) | 8,060 | (79.9) | 21,849 | (80.7) | 39,450 | (82.4) | ||
| 1 | 473 | (17.0) | 2,046 | (15.8) | 1,597 | (15.8) | 4,190 | (15.5) | 6,867 | (14.4) | ||
| ≥2 | 146 | (5.2) | 590 | (4.6) | 425 | (4.2) | 1,040 | (3.8) | 1,532 | (3.2) | ||
| Facility region | <0.001 | |||||||||||
| Eastern | 663 | (23.8) | 2,444 | (18.9) | 2,396 | (23.8) | 4,733 | (17.5) | 8,616 | (18.0) | ||
| Midwest | 1,008 | (36.1) | 4,803 | (37.2) | 3,085 | (30.6) | 8,391 | (31.0) | 14,595 | (30.5) | ||
| South | 788 | (28.3) | 2,984 | (23.1) | 1,853 | (18.4) | 7,448 | (27.5) | 14,141 | (29.6) | ||
| West | 206 | (7.4) | 2,060 | (15.9) | 2,180 | (21.6) | 5,171 | (19.1) | 7,899 | (16.5) | ||
| Unknown | 124 | (4.4) | 635 | (4.9) | 568 | (5.6) | 1,336 | (4.9) | 2,598 | (5.4) | ||
| Facility type | <0.001 | |||||||||||
| Community cancer | 1,948 | (69.8) | 3,465 | (26.8) | 355 | (3.5) | 136 | (0.5) | 241 | (0.5) | ||
| Comprehensive community cancer | 670 | (24.0) | 8,151 | (63.1) | 6,672 | (66.2) | 13,851 | (51.2) | 12,726 | (26.6) | ||
| Academic/research | 47 | (1.7) | 636 | (4.9) | 2,351 | (23.3) | 10,759 | (39.7) | 26,162 | (54.7) | ||
| Integrated network cancer | 0 | (0.0) | 39 | (0.3) | 136 | (1.3) | 997 | (3.7) | 5,976 | (12.5) | ||
| Other/unknown | 124 | (4.4) | 635 | (4.9) | 568 | (5.6) | 1,336 | (4.9) | 2,744 | (5.7) | ||
| Stage | <0.001 | |||||||||||
| INOS | 47 | (1.7) | 219 | (1.7) | 179 | (1.8) | 310 | (1.1) | 466 | (1.0) | ||
| IA | 238 | (8.5) | 1,093 | (8.5) | 1,062 | (10.5) | 2,641 | (9.8) | 4,868 | (10.2) | ||
| IB | 12 | (0.4) | 107 | (0.8) | 92 | (0.9) | 253 | (0.9) | 440 | (0.9) | ||
| IC | 182 | (6.5) | 854 | (6.6) | 703 | (7.0) | 2,082 | (7.7) | 3,728 | (7.8) | ||
| IINOS | 12 | (0.4) | 82 | (0.6) | 69 | (0.7) | 142 | (0.5) | 218 | (0.5) | ||
| IIA | 33 | (1.2) | 174 | (1.3) | 131 | (1.3) | 399 | (1.5) | 725 | (1.5) | ||
| IIB | 107 | (3.8) | 542 | (4.2) | 466 | (4.6) | 1,545 | (5.7) | 2,758 | (5.8) | ||
| IIINOS | 55 | (2.0) | 256 | (2.0) | 218 | (2.2) | 465 | (1.7) | 636 | (1.3) | ||
| IIIA | 32 | (1.1) | 198 | (1.5) | 185 | (1.8) | 535 | (2.0) | 964 | (2.0) | ||
| IIIB | 58 | (2.1) | 345 | (2.7) | 299 | (3.0) | 857 | (3.2) | 1,559 | (3.3) | ||
| IIIC | 412 | (14.8) | 2,437 | (18.9) | 2,539 | (25.2) | 8,113 | (30.0) | 15,928 | (33.3) | ||
| IV | 499 | (17.9) | 2,244 | (17.4) | 1,606 | (15.9) | 3,956 | (14.6) | 7,019 | (14.7) | ||
| Unknown | 1,102 | (39.5) | 4,375 | (33.8) | 2,533 | (25.1) | 5,781 | (21.3) | 8,540 | (17.8) | ||
| Histology | <0.001 | |||||||||||
| Serous | 1,323 | (47.4) | 6,666 | (51.6) | 5,538 | (54.9) | 16,696 | (61.7) | 30,576 | (63.9) | ||
| Mucinous | 234 | (8.4) | 1,029 | (8.0) | 840 | (8.3) | 1,941 | (7.2) | 3,309 | (6.9) | ||
| Endometrioid | 283 | (10.1) | 1,445 | (11.2) | 1,267 | (12.6) | 3,428 | (12.7) | 5,909 | (12.3) | ||
| Clear cell | 164 | (5.9) | 723 | (5.6) | 681 | (6.8) | 1,947 | (7.2) | 3,640 | (7.6) | ||
| Transitional cell | 14 | (0.5) | 44 | (0.3) | 53 | (0.5) | 115 | (0.4) | 209 | (0.4) | ||
| Epithelial tumor NOS | 771 | (27.6) | 3,019 | (23.4) | 1,703 | (16.9) | 2,952 | (10.9) | 4,206 | (8.8) | ||
| Grade | <0.001 | |||||||||||
| Well | 220 | (7.9) | 1,044 | (8.1) | 850 | (8.4) | 2,427 | (9.0) | 4,189 | (8.8) | ||
| Moderate | 406 | (14.6) | 1,975 | (15.3) | 1,571 | (15.6) | 4,229 | (15.6) | 6,866 | (14.3) | ||
| Poorly | 1,139 | (40.8) | 5,654 | (43.7) | 4,936 | (49.0) | 14,669 | (54.2) | 27,624 | (57.7) | ||
| Unknown | 1,024 | (36.7) | 4,253 | (32.9) | 2,725 | (27.0) | 5,754 | (21.2) | 9,170 | (19.2) | ||
NOS: not otherwise specified. Income was measured by median household income in the patients’ zip code and classified as <$38,000, $38,000–$47,999, $48,000–$62,999, over $63,000, or unknown. Education was measured by percentage of adults in a patient's zip code who did not graduate from high school, and classified as ≥21%, 13–20%, 7.0–12.9%, <7%, or unknown.wq21qaq1
Chi-square tests were used to make the comparison by hospital volume groups.
Compliance with the quality metrics generally increased with hospital volume (Table 2). Lymph node dissection for early-stage tumors was performed in 50.9% (SD=38.2%) of patients at low volume hospitals and increased with each volume category to 78.2% (SD=11.6%) in the highest volume hospitals (P<0.001). Similar trends of increased compliance with the quality metrics associated with increasing hospital volume were seen for cytoreduction for advanced stage tumors (P<0.001) and use of chemotherapy for advanced stage tumors (P=0.01); no trends were evident for use of chemotherapy for high risk, early stage tumors (P=0.28). In contrast, there was a trend for higher volume hospitals to administer chemotherapy for low risk, early stage tumors (P=0.16). Compliance with the composite, overall quality metric was noted in 64.2% (SD=24.5%) of low volume centers and increased with each volume category to 82.2% (SD=7.7%) at the highest volume hospitals.
Table 2.
Compliance with each quality metric stratified by hospital volume.
| Low Volume |
Low Intermediate Volume |
Intermediate Volume |
High Intermediate Volume |
High Volume |
P-value | |||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
|
| ||||||||||||
| All | Hospitals | 299 | 465 | 157 | 194 | 153 | ||||||
| Lymph node dissection for early-stage tumors | Hospitals | 252 | 454 | 157 | 194 | 153 | ||||||
| Mean (SD) | 50.9% | (38.2%) | 58.1% | (27.1%) | 68.6% | (19.5%) | 72.6% | (15.9%) | 78.2% | (11.6%) | <0.001 | |
| Cytoreduction for advanced stage tumors | Hospitals | 272 | 465 | 157 | 194 | 153 | ||||||
| Mean (SD) | 59.7% | (33.0%) | 65.7% | (22.7%) | 75.4% | (15.4%) | 80.3% | (13.0%) | 82.2% | (12.4%) | <0.001 | |
| Avoidance of chemotherapy for low risk, early stage tumors | Hospitals | 50 | 202 | 123 | 187 | 150 | ||||||
| Mean (SD) | 90.0% | (30.3%) | 86.5% | (30.7%) | 87.9% | (26.6%) | 82.2% | (23.4%) | 83.9% | (17.2%) | 0.16 | |
| Chemotherapy for high risk, early stage tumors | Hospitals | 117 | 327 | 149 | 193 | 153 | ||||||
| Mean (SD) | 69.2% | (43.3%) | 69.3% | (36.8%) | 71.5% | (27.6%) | 72.5% | (23.8%) | 75.7% | (17.8%) | 0.28 | |
| Advanced stage chemotherapy | Hospitals | 269 | 465 | 157 | 194 | 153 | ||||||
| Mean (SD) | 79.0% | (27.5%) | 80.5% | (17.1%) | 81.0% | (14.0%) | 82.8% | (12.9%) | 85.2% | (10.0%) | 0.01 | |
| Overall quality | Hospitals | 287 | 465 | 157 | 194 | 153 | ||||||
| Mean (SD) | 64.2% | (24.5%) | 69.6% | (14.7%) | 76.0% | (11.4%) | 79.2% | (9.5%) | 82.2% | (7.7%) | <0.001 | |
The number of patients and hospitals varied by quality measures. ANOVA tests were used to make the comparison by hospital volume groups.
Survival increased with increasing hospital volume. Two-year adjusted survival rose sequentially from 64.4% (95% CI, 62.5–66.4%) at low-volume hospitals to 77.4% (95% CI, 77.0–77.8%) at high-volume hospitals (P<0.001) (Table 3). Likewise, five-year survival rose with each volume category from 39.3% (95% CI, 37.0–41.7%) at the low volume centers to 51.0% (50.4–51.6%) at the high-volume hospitals (P<0.001). Similarly, survival increased with adherence to the quality metrics. Two-year survival rose from 66.5% (95% CI, 65.5–67.5%) at the low-quality quartile hospitals to 77.3% (95% CI, 76.8–77.7%) (P<0.001) at the highest-quality quartile centers, while five-year survival rose from 42.6% (95% CI, 41.4–43.8%) to 50.3% (95% CI, 49.7–51.0%) (P<0.001), respectively.
Table 3.
Two and five-year adjusted survival rates stratified by hospital-level volume and adherence to quality metrics.
| Hospital Volume | P-value | |||||
|
|
||||||
| Low Volume | Low Intermediate Volume | Intermediate Volume | High Intermediate Volume | High Volume | ||
|
| ||||||
| 2-year adjusted survival rate (95% CI) | 64.4 (62.5–66.4) | 66.9 (66.0–67.7) | 72.1 (71.2–73.1) | 74.4 (73.9–75.0) | 77.4 (77.0–77.8) | <0.001 |
| 5-year adjusted survival rate (95% CI) | 39.3 (37.0–41.7) | 41.8 (40.7–42.8) | 46.8 (45.6–48.0) | 48.4 (47.6–49.2) | 51.0 (50.4–51.6) | <0.001 |
|
| ||||||
| Hospital-Level Adherence to Quality Metrics | P-value | |||||
|
|
||||||
| Low Quality | Medium Low Quality | Medium High Quality | High Quality | Unknown Quality | ||
|
| ||||||
| 2-year adjusted survival rate (95% CI) | 66.5 (65.5–67.5) | 72.7 (72.0–73.3) | 74.5 (74.0–75.0) | 77.3 (76.8–77.7) | 60.0 (45.0–80.0) | <0.001 |
| 5-year adjusted survival rate (95% CI) | 42.6 (41.4–43.8) | 47.0 (46.1–47.9) | 48.8 (48.1–49.5) | 50.3 (49.7–51.0) | 45.3 (29.4–69.9) | <0.001 |
CI: confidence interval. P-value from ANOVA. The adjusted survival rates by hospital volume were estimated using stratified Cox proportional hazard model adjusted for age, race, year of diagnosis, insurance status, education, location, comorbidity, tumor stage, histology, grade, lymph node dissection, and stratified by hospital volume. The p-values were estimated from the p-value of hospital volume using the Cox proportional hazard model adjusted for age, race, year of diagnosis, insurance status, education, location, comorbidity, tumor stage, histology, grade, lymph node dissection, and hospital volume. P-values were from test for linear trends in proportions.
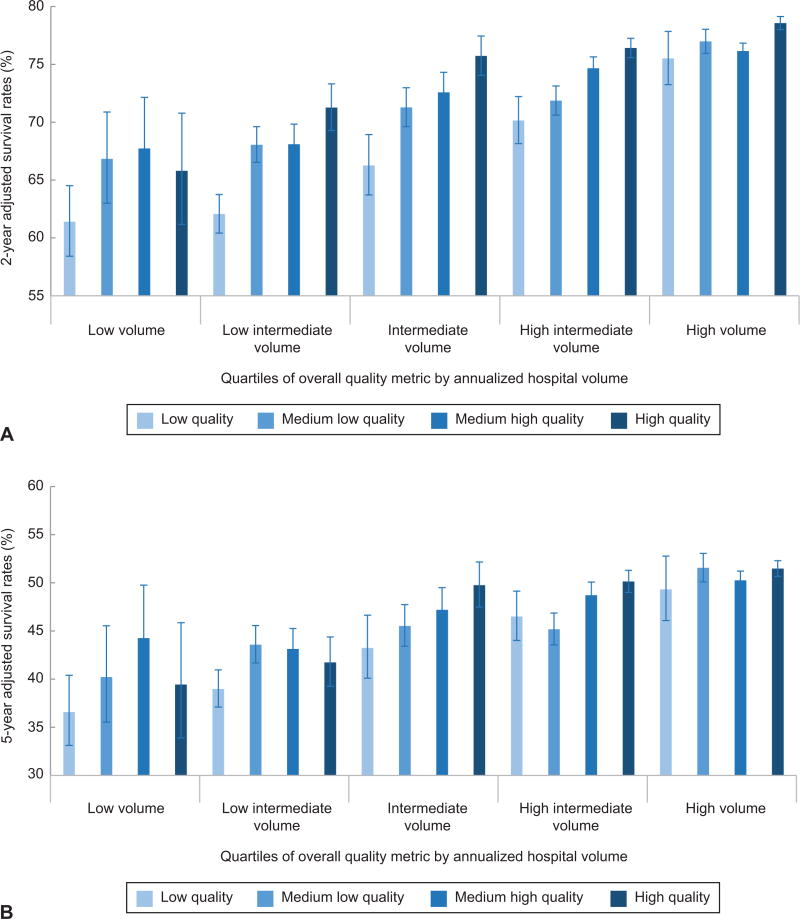
The association between volume and quality was then examined (Table 4, Figure 1A). For each volume category, survival increased with increasing adherence to the quality metrics. For example, in the low volume hospitals, adjusted two-year survival was 61.4% (95% CI, 58.4–64.5%) at hospital with the lowest adherence to quality metrics and rose to 65.8% (95% CI, 61.2–70.8%) at the highest-quality hospitals (P<0.001). At the highest volume hospitals, two-year adjusted survival rose from 75.5% (95% CI, 73.2–77.8%) at the lowest quality hospitals to 78.6% (95% CI, 78.0–79.1%) at the highest quality hospitals (P<0.001). Two-year survival at the intermediate volume hospitals with the highest adherence to quality metrics (75.7%; 95% CI, 74.0–77.5%) was similar to survival at high volume hospitals with the lowest adherence to the quality metrics (75.5%; 95% CI, 73.2–77.8%). Similar trends were noted for five-year survival; however the relationship between adherence to the quality metrics and survival was less consistent for the low, low intermediate, and high intermediate volume hospitals (Figure 1B).
Table 4.
Two and five-year adjusted survival rates stratified by adherence to overall quality metrics and hospital volume.
| Hospital volume | P-value | |||||
|---|---|---|---|---|---|---|
|
|
||||||
| Low | Low intermediate | Intermediate | High intermediate | High | ||
| 2-year adjusted survival (95% CI) | ||||||
| Overall quality | ||||||
| Low | 61.4 (58.4–64.5) | 62.1 (60.4–63.7) | 66.3 (63.7–68.9) | 70.1 (68.1–72.2) | 75.5 (73.2–77.8) | <0.0001 |
| Medium Low | 66.8 (63.0–70.9) | 68.0 (66.5–69.6) | 71.3 (69.6–73.0) | 71.9 (70.6–73.1) | 77.0 (75.9–78.0) | <0.0001 |
| Medium High | 67.7 (63.6–72.2) | 68.1 (66.4–69.8) | 72.6 (70.9–74.3) | 74.7 (73.7–75.7) | 76.1 (75.5–76.8) | <0.0001 |
| High | 65.8 (61.2–70.8) | 71.3 (69.3–73.3) | 75.7 (74.0–77.5) | 76.4 (75.6–77.2) | 78.6 (78.0–79.1) | <0.0001 |
| P-value | <0.0001 | <0.0001 | <0.0001 | <0.0001 | <0.0001 | |
| 5-year adjusted survival (95% CI) | ||||||
| Overall quality | ||||||
| Low | 36.6 (33.1–40.4) | 39.0 (37.1–41.0) | 43.2 (40.1–46.6) | 46.5 (44.0–49.1) | 49.3 (46.1–52.8) | <0.0001 |
| Medium Low | 40.2 (35.5–45.5) | 43.6 (41.7–45.6) | 45.5 (43.4–47.7) | 45.2 (43.6–46.9) | 51.6 (50.1–53.1) | <0.0001 |
| Medium High | 44.3 (39.4–49.7) | 43.1 (41.1–45.3) | 47.2 (45.0–49.5) | 48.7 (47.4–50.1) | 50.3 (49.3–51.2) | <0.0001 |
| High | 39.4 (33.9–45.9) | 41.7 (39.2–44.4) | 49.8 (47.5–52.2) | 50.1 (49.0–51.3) | 51.5 (50.6–52.3) | <0.0001 |
| P-value | <0.0001 | <0.0001 | <0.0001 | <0.0001 | <0.0001 | |
CI: confidence interval. P-value from ANOVA. The adjusted survival rates by hospital volume were estimated using stratified Cox proportional hazard model adjusted for age, race, year of diagnosis, insurance status, education, location, comorbidity, tumor stage, histology, grade, lymph node dissection, and stratified by hospital volume and adherence to quality metrics. P-values were from test for linear trends in proportions.
Figure 1.
A. Two-year adjusted survival rates stratified by volume and adherence to quality measures. B. Five-year adjusted survival rates stratified by volume and adherence to quality measures. The error bars indicate 95% confidence intervals.
Discussion
These findings suggest that hospital volume and adherence to quality metrics are both associated with survival for ovarian cancer. Although survival is improved at low-volume centers that are compliant with quality metrics, these hospitals still have survival rates that are lower than high-volume hospitals.
The association between higher hospital volume and improved outcomes for ovarian cancer has been demonstrated in several studies.5–12 We found that improved adherence to evidence-based guidelines may be one mechanism underlying this association. A prior study that examined the NCDB found that lower volume hospitals were more likely to render non-guideline based care and that guideline-based care was an independent predictor of survival.10 We found a similar association; high-volume hospitals met all of the quality metrics we examined in 82% of patients compared to only 64% compliance at low-volume centers.
Given the association between higher surgical volume and improved outcomes, efforts to regionalize the care of high-risk surgeries to high-volume centers are attractive.11,12,27–30 For some procedures, this appears to be a reasonable strategy. After national efforts to concentrate some surgical procedures to high-volume hospitals, Finks and colleagues reported that from 1999–2008 the number of hospitals performing four high-risk cancer operations decreased, while the median volume of those hospitals performing the procedures increased. Importantly, mortality decreased for all of the procedures and a significant proportion of the reduction in mortality was attributed to the concentration of procedures at higher volume centers.27 Similar results were noted for ovarian cancer in a population-based registry in Sweden. After centralization of care in 2011, the relative 3-year survival increased from 40% to 61%.11
While regionalization of care to high-volume centers may improve outcomes, there are a number of practical difficulties with such strategies.27 First, patients prefer to receive care locally, and are often unwilling to travel to a regional center, even if it would result in a significant reduction in mortality.31 Second, regionalization of care often exacerbates disparities in access to care and may adversely impact low-volume hospitals..32 Lastly, access to high-volume centers is not available in some regions.33 For example, a recent report suggested that 9% of the U.S. female population had geographic barriers to receiving care from a gynecologic oncologist.34
Given the potential causal pathway of higher hospital volume, increased quality, and improved outcomes, an important question is whether lower volume facilities that deliver high-quality care can achieve the same outcomes as high-volume centers. One report of patients who underwent coronary artery bypass grafting found that low-volume hospitals that met all of the quality metrics examined had mortality rates similar to high-volume hosptials.35 Given the difficulties associated with regionalization of care, strategies to raise the quality of care at low volume centers have many advocates.36 We noted that although outcomes improve at low volume centers that are highly compliant with the quality metrics examined, survival at these centers is still lower than at high-volume centers. These data imply that factors other than just guideline adherence play a role in the effect of hospital volume on outcomes for ovarian cancer.
We recognize a number of limitations. The quality metrics we examined focus on care during initial treatment. Women with ovarian cancer are often treated over the course of several years and we are unable to capture downstream care. Second, NCDB lacks information on modifiable hospital factors such as staffing and infrastructure and there are undoubtedly other unmeasured confounders that influenced treatment and outcomes. Third, although the quality of data in NCDB has been validated, we cannot exclude misclassification in a small number of patients. Likewise, some hospitals did not treat patients who would be eligible for a given quality metric and thus are not included in some analyses. Fourth, 13.7% patients received treatment at multiple facilities. However, in sensitivity analyses including only these patients, our results were largely unchanged. Fifth, while the volume cutpoints we chose were based on prior work, there is often some variation in outcomes within volume strata.37 We tested a variety of different volume cutpoints in a series of sensitivity analyses and our findings were largely unchanged.
These findings have important implications for patients, hospitals, and policy makers. As the best outcomes appear to be achieved at high-volume hospitals, efforts to promote volume-based referral for women with ovarian cancer are reasonable.38 However, from a practical standpoint, there are many women who will not be able to receive care at high-volume centers. For low-volume centers, targeted quality improvement efforts and strict adherence to quality guidelines may help to optimize outcomes for women with ovarian cancer.
Acknowledgments
Dr. Wright has served as a consultant for Tesaro and Clovis Oncology. Dr. Neugut has served as a consultant to Pfizer, Teva, Otsuka, and United Biosource Corporation. He is on the medical advisory board of EHE, Intl.
Dr. Wright (NCI R01CA169121-01A1) and Dr. Hershman (NCI R01 CA166084) are recipients of grants from the National Cancer Institute. Dr. Hershman is the recipient of a grant from the Breast Cancer Research Foundation/Conquer Cancer Foundation. Dr. Tergas is a recipient of an NCI Diversity Supplement (CA197730).
Footnotes
Financial Disclosure:
The other authors did not report any potential conflicts of interest.
References
- 1.Birkmeyer JD, Siewers AE, Finlayson EV, et al. Hospital volume and surgical mortality in the United States. N Engl J Med. 2002;346:1128–37. doi: 10.1056/NEJMsa012337. [DOI] [PubMed] [Google Scholar]
- 2.Birkmeyer JD, Stukel TA, Siewers AE, Goodney PP, Wennberg DE, Lucas FL. Surgeon volume and operative mortality in the United States. N Engl J Med. 2003;349:2117–27. doi: 10.1056/NEJMsa035205. [DOI] [PubMed] [Google Scholar]
- 3.Ghaferi AA, Birkmeyer JD, Dimick JB. Variation in hospital mortality associated with inpatient surgery. N Engl J Med. 2009;361:1368–75. doi: 10.1056/NEJMsa0903048. [DOI] [PubMed] [Google Scholar]
- 4.Wright JD, Herzog TJ, Siddiq Z, et al. Failure to rescue as a source of variation in hospital mortality for ovarian cancer. J Clin Oncol. 2012;30:3976–82. doi: 10.1200/JCO.2012.43.2906. [DOI] [PubMed] [Google Scholar]
- 5.Vernooij F, Heintz AP, Witteveen PO, van der Heiden-van der Loo M, Coebergh JW, van der Graaf Y. Specialized care and survival of ovarian cancer patients in The Netherlands: nationwide cohort study. J Natl Cancer Inst. 2008;100:399–406. doi: 10.1093/jnci/djn033. [DOI] [PubMed] [Google Scholar]
- 6.Bristow RE, Chang J, Ziogas A, Anton-Culver H. Adherence to treatment guidelines for ovarian cancer as a measure of quality care. Obstet Gynecol. 2013;121:1226–34. doi: 10.1097/AOG.0b013e3182922a17. [DOI] [PubMed] [Google Scholar]
- 7.Bristow RE, Palis BE, Chi DS, Cliby WA. The National Cancer Database report on advanced-stage epithelial ovarian cancer: impact of hospital surgical case volume on overall survival and surgical treatment paradigm. Gynecol Oncol. 2010;118:262–7. doi: 10.1016/j.ygyno.2010.05.025. [DOI] [PubMed] [Google Scholar]
- 8.Bristow RE, Zahurak ML, Diaz-Montes TP, Giuntoli RL, Armstrong DK. Impact of surgeon and hospital ovarian cancer surgical case volume on in-hospital mortality and related short-term outcomes. Gynecol Oncol. 2009;115:334–8. doi: 10.1016/j.ygyno.2009.08.025. [DOI] [PubMed] [Google Scholar]
- 9.Vernooij F, Heintz AP, Coebergh JW, Massuger LF, Witteveen PO, van der Graaf Y. Specialized and high-volume care leads to better outcomes of ovarian cancer treatment in the Netherlands. Gynecol Oncol. 2009;112:455–61. doi: 10.1016/j.ygyno.2008.11.011. [DOI] [PubMed] [Google Scholar]
- 10.Cliby WA, Powell MA, Al-Hammadi N, et al. Ovarian cancer in the United States: contemporary patterns of care associated with improved survival. Gynecol Oncol. 2015;136:11–7. doi: 10.1016/j.ygyno.2014.10.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Dahm-Kahler P, Palmqvist C, Staf C, Holmberg E, Johannesson L. Centralized primary care of advanced ovarian cancer improves complete cytoreduction and survival - A population-based cohort study. Gynecol Oncol. 2016;142:211–6. doi: 10.1016/j.ygyno.2016.05.025. [DOI] [PubMed] [Google Scholar]
- 12.Eggink FA, Mom CH, Kruitwagen RF, et al. Improved outcomes due to changes in organization of care for patients with ovarian cancer in the Netherlands. Gynecol Oncol. 2016;141:524–30. doi: 10.1016/j.ygyno.2016.04.012. [DOI] [PubMed] [Google Scholar]
- 13.Goff BA, Matthews BJ, Larson EH, et al. Predictors of comprehensive surgical treatment in patients with ovarian cancer. Cancer. 2007;109:2031–42. doi: 10.1002/cncr.22604. [DOI] [PubMed] [Google Scholar]
- 14.Bristow RE, Puri I, Diaz-Montes TP, Giuntoli RL, Armstrong DK. Analysis of contemporary trends in access to high-volume ovarian cancer surgical care. Ann Surg Oncol. 2009;16:3422–30. doi: 10.1245/s10434-009-0680-5. [DOI] [PubMed] [Google Scholar]
- 15.The National Cancer Data Base. at https://www.facs.org/qualityprograms/cancer/ncdb.)
- 16.Bilimoria KY, Stewart AK, Winchester DP, Ko CY. The National Cancer Data Base: a powerful initiative to improve cancer care in the United States. Ann Surg Oncol. 2008;15:683–90. doi: 10.1245/s10434-007-9747-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Bristow RE, Chang J, Ziogas A, Randall LM, Anton-Culver H. High-volume ovarian cancer care: survival impact and disparities in access for advanced-stage disease. Gynecol Oncol. 2014;132:403–10. doi: 10.1016/j.ygyno.2013.12.017. [DOI] [PubMed] [Google Scholar]
- 18.NCCN. Clinical Practice Guidelines in Oncology: Ovarian cancer including fallopian tube cancer and primary peritoneal cancer. Version 1.2016 2016 [Google Scholar]
- 19.Verleye L, Ottevanger PB, van der Graaf W, et al. EORTC-GCG process quality indicators for ovarian cancer surgery. Eur J Cancer. 2009;45:517–26. doi: 10.1016/j.ejca.2008.09.031. [DOI] [PubMed] [Google Scholar]
- 20.Gogoi RP, Urban R, Sun H, Goff B. Evaluation of Society of Gynecologic Oncologists (SGO) ovarian cancer quality surgical measures. Gynecol Oncol. 2012;126:217–9. doi: 10.1016/j.ygyno.2012.04.049. [DOI] [PubMed] [Google Scholar]
- 21.Trimbos JB, Parmar M, Vergote I, et al. International Collaborative Ovarian Neoplasm trial 1 and Adjuvant ChemoTherapy In Ovarian Neoplasm trial: two parallel randomized phase III trials of adjuvant chemotherapy in patients with early-stage ovarian carcinoma. J Natl Cancer Inst. 2003;95:105–12. [PubMed] [Google Scholar]
- 22.Trimbos JB, Vergote I, Bolis G, et al. Impact of adjuvant chemotherapy and surgical staging in early-stage ovarian carcinoma: European Organisation for Research and Treatment of Cancer-Adjuvant ChemoTherapy in Ovarian Neoplasm trial. J Natl Cancer Inst. 2003;95:113–25. [PubMed] [Google Scholar]
- 23.Dinkelspiel HE, Tergas AI, Zimmerman LA, et al. Use and duration of chemotherapy and its impact on survival in early-stage ovarian cancer. Gynecol Oncol. 2015;137:203–9. doi: 10.1016/j.ygyno.2015.02.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Deyo RA, Cherkin DC, Ciol MA. Adapting a clinical comorbidity index for use with ICD-9-CM administrative databases. J Clin Epidemiol. 1992;45:613–9. doi: 10.1016/0895-4356(92)90133-8. [DOI] [PubMed] [Google Scholar]
- 25.Neuberger J, Altman DG, Christensen E, Tygstrup N, Williams R. Use of a prognostic index in evaluation of liver transplantation for primary biliary cirrhosis. Transplantation. 1986;41:713–6. doi: 10.1097/00007890-198606000-00009. [DOI] [PubMed] [Google Scholar]
- 26.Nieto FJ, Coresh J. Adjusting survival curves for confounders: a review and a new method. Am J Epidemiol. 1996;143:1059–68. doi: 10.1093/oxfordjournals.aje.a008670. [DOI] [PubMed] [Google Scholar]
- 27.Finks JF, Osborne NH, Birkmeyer JD. Trends in hospital volume and operative mortality for high-risk surgery. N Engl J Med. 2011;364:2128–37. doi: 10.1056/NEJMsa1010705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Hollenbeck BK, Taub DA, Miller DC, Dunn RL, Montie JE, Wei JT. The regionalization of radical cystectomy to specific medical centers. J Urol. 2005;174:1385–9. doi: 10.1097/01.ju.0000173632.58991.a7. discussion 9. [DOI] [PubMed] [Google Scholar]
- 29.Hollenbeck BK, Dunn RL, Miller DC, Daignault S, Taub DA, Wei JT. Volume-based referral for cancer surgery: informing the debate. J Clin Oncol. 2007;25:91–6. doi: 10.1200/JCO.2006.07.2454. [DOI] [PubMed] [Google Scholar]
- 30.Gasper WJ, Glidden DV, Jin C, Way LW, Patti MG. Has recognition of the relationship between mortality rates and hospital volume for major cancer surgery in California made a difference?: A follow-up analysis of another decade. Ann Surg. 2009;250:472–83. doi: 10.1097/SLA.0b013e3181b47c79. [DOI] [PubMed] [Google Scholar]
- 31.Finlayson SR, Birkmeyer JD, Tosteson AN, Nease RF., Jr Patient preferences for location of care: implications for regionalization. Med Care. 1999;37:204–9. doi: 10.1097/00005650-199902000-00010. [DOI] [PubMed] [Google Scholar]
- 32.Finlayson SR. Delivering quality to patients. JAMA. 2006;296:2026–7. doi: 10.1001/jama.296.16.2026. [DOI] [PubMed] [Google Scholar]
- 33.Dimick JB, Finlayson SR, Birkmeyer JD. Regional availability of high-volume hospitals for major surgery. Health Aff (Millwood) 2004:VAR45–53. doi: 10.1377/hlthaff.var.45. Suppl Variation. [DOI] [PubMed] [Google Scholar]
- 34.Shalowitz DI, Vinograd AM, Giuntoli RL., 2nd Geographic access to gynecologic cancer care in the United States. Gynecol Oncol. 2015;138:115–20. doi: 10.1016/j.ygyno.2015.04.025. [DOI] [PubMed] [Google Scholar]
- 35.Auerbach AD, Hilton JF, Maselli J, Pekow PS, Rothberg MB, Lindenauer PK. Shop for quality or volume? Volume, quality, and outcomes of coronary artery bypass surgery. Ann Intern Med. 2009;150:696–704. doi: 10.7326/0003-4819-150-10-200905190-00007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Shahian DM, Normand SL. Low-volume coronary artery bypass surgery: measuring and optimizing performance. J Thorac Cardiovasc Surg. 2008;135:1202–9. doi: 10.1016/j.jtcvs.2007.12.037. [DOI] [PubMed] [Google Scholar]
- 37.Livingston EH, Cao J. Procedure volume as a predictor of surgical outcomes. Jama. 2010;304:95–7. doi: 10.1001/jama.2010.905. [DOI] [PubMed] [Google Scholar]
- 38.Aletti GD, Cliby WA. Time for centralizing patients with ovarian cancer: what are we waiting for? Gynecol Oncol. 2016;142:209–10. doi: 10.1016/j.ygyno.2016.07.004. [DOI] [PubMed] [Google Scholar]