Abstract
In this study, we combined approaches from media psychology and neuroscience to ask whether brain activity in response to online antismoking messages can predict smoking behavior change. In particular, we examined activity in subregions of the medial prefrontal cortex linked to self- and value-related processing, to test whether these neurocognitive processes play a role in message-consistent behavior change. We observed significant relationships between activity in both brain regions of interest and behavior change (such that higher activity predicted a larger reduction in smoking). Furthermore, activity in these brain regions predicted variance independent of traditional, theory-driven self-report metrics such as intention, self-efficacy, and risk perceptions. We propose that valuation is an additional cognitive process that should be investigated further as we search for a mechanistic explanation of the relationship between brain activity and media effects relevant to health behavior change.
Keywords: neuroimaging, behavior change, smoking, brain-as-predictor, cognitive neuroscience
Cigarette smoking is the most prominent cause of preventable death in the United States (US Department of Health and Human Services, 2014). Smoking cigarettes increases the odds of developing the most frequently diagnosed cancers (American Cancer Society, 2012), and accounts for one of five deaths each year (Centers for Disease Control and Prevention, 2014). Although the prevalence of cigarette smoking has dropped drastically since the 1960s, it is estimated that 18% of adults in the United States still smoke (Centers for Disease Control and Prevention, 2014). For decades, researchers in tobacco control have studied how smoking attitudes and behaviors are affected by exposure to antitobacco mass media campaigns, and there is strong evidence that such campaigns have played a role in reducing the number of adults who smoke (Emery et al., 2012; Wakefield et al., 2008; Wakefield, Loken, & Hornik, 2010).
Using Neural Activity to Predict Behavior Change
Major theories of behavior change that suggest factors promoting message-driven behavior change, such as the theory of reasoned action, the theory of planned behavior, and the health belief model, largely rely on self-reported measures of variables like intentions to change a behavior, self-efficacy, or beliefs about a behavior (Ajzen, 1985, 1991; Ajzen & Fishbein, 1980; Becker, 1974; Janz & Becker, 1984; Rosenstock, 1974; Rosenstock, Stretcher, & Becker, 1988). Although these measures are certainly related to future behavior change, they are not perfect predictors (Armitage & Conner, 2001; Webb & Sheeran, 2006). Additional information from sources such as neuroimaging could improve our understanding of the relationship between media exposure and actual behavior change (Berkman & Falk, 2013). Neuroimaging allows us a glimpse of what is happening in the brain while individuals are viewing health-relevant media, and may give us access to important variables that are outside conscious awareness and therefore missed by self-report measurements (Dijksterhuis, 2004; Nisbett & Wilson, 1977). As such, neuroimaging has the potential to improve the mechanistic understanding of media effects on behavior, and of what cognitive processes might differentiate those viewers who do or don’t subsequently change their behavior (Cascio, Dal Cin, & Falk, 2013; Falk, 2013).
A small but growing body of work has begun to address whether behavior change at the population and individual level can be predicted from individuals’ brain activity during exposure to media (Chua et al., 2011; Falk, Berkman, & Lieberman, 2012; Falk, Berkman, Mann, Harrison, & Lieberman, 2010; Falk, Berkman, Whalen, & Lieberman, 2011; Wang et al., 2013). This “brain as predictor” approach to analyzing neuroimaging data is relatively novel (see Berkman & Falk, 2013, for full discussion). Traditionally, the goal of neuroimaging studies has been to map where in the brain simple and controlled cognitive processes occur (face recognition, reading text, etc.). More recent work has incorporated more sophisticated designs and naturalistic stimuli, but shares the use of brain activity as a dependent variable. In the brain-as-predictor approach, brain activity is considered an independent variable, and is used to predict longitudinal, real-world outcomes, such as behavior change. Brain regions selected as potential predictors should be hypothesis-driven, and selected on the basis of previous brain mapping work. This approach has been used to predict consumer decisions (Berns & Moore, 2012; Levy, Lazzaro, Rutledge, & Glimcher, 2011; Tusche, Bode, & Haynes, 2010), disease states (Costafreda, Khanna, Mourao-Miranda, & Fu, 2009; E. B. McClure et al., 2006; Paulus, Tapert, & Schuckit, 2005), and the success of health media interventions (Chua et al., 2011; Falk et al., 2011, 2012; Wang et al., 2013).
Candidate Cognitive Processes Underlying the Brain–Behavior Relationship
In the context of smoking cessation, studies utilizing the brain-as-predictor approach have identified a relationship between exposure to antismoking media, health behavior change, and the brain. Despite differences in whether smokers were seeking treatment, and in the use of different forms of media (text, videos), all of these studies have reported a relationship between behavior change and activity in subregions of the brain’s medial prefrontal cortex (MPFC) during message exposure (Chua et al., 2011; Falk et al., 2011, 2012; Wang et al., 2013). However, several questions about the precise nature of this brain–behavior relationship have yet to be answered. Thus, we aimed to conceptually replicate and extend past research by bringing together more precise localization of potential neurocognitive mechanisms that may explain variance in health behavior change in response to externally valid, real-world quit-smoking media. To most effectively leverage neuroimaging methods to advance communication theory, it is important to establish the extent to which neural and self-report variables explain the same or different variance in health behavior change, and what psychological processes are represented by the neural activity observed. To achieve these goals, in this investigation we examined the relationship between smoking behavior change and activity in three subregions of MPFC. The use of multiple regions, which have been linked to different cognitive processes, allowed us to ask whether there was evidence for the involvement of each component process in predicting behavior change (although we must be cautious about reverse-inference; Poldrack, 2006a). In the present study, first, we used a region defined as predictive of behavior change in another domain (sun-screen use; Falk et al., 2010), to verify that we could replicate previous results across health domains. We then used a well-validated functional localizer to identify the subregion of MPFC recruited while participants were making self-related judgments (about personality traits). Finally, we considered an additional type of cognitive processing, value-related processing, suggested by current neuroimaging research.
Relationships Between Self-Related Neural Processing and Health Behavior Change
Extant studies have largely hypothesized that the brain–behavior relationship in MPFC is the result of self-related processing. In line with major theories of persuasion and behavior change (Fishbein, 2001; Petty & Cacioppo, 1986), it is certainly plausible that those who think more about how a message relates to them might be those who go on to change their behavior, and that messages eliciting higher average levels of self-related thought across individuals might have wider-ranging success. Consistent with these ideas, studies have demonstrated that messages that are tailored to individual smokers, or that are rated to be more self-relevant, are more likely to change intentions and behaviors (Brug, Steenhuis, van Assema, & de Vries, 1996; Chua et al., 2011; Chua, Liberzon, Welsh, & Strecher, 2009; Noar, Benac, & Harris, 2007; Strecher, 1999; Strecher, Shiffman, & West, 2006). This line of reasoning is consistent with the MPFC being the region most frequently cited as predicting behavior change, and the region most commonly observed in studies that involve processing of self-relevant stimuli and judgments of self-relevance (Amodio & Frith, 2006; Denny, Kober, Wager, & Ochsner, 2012; Lieberman, 2010; Northoff et al., 2006; Schmitz & Johnson, 2007).
Individual differences in explicit ratings of message self-relevance and other self-related processing variables (e.g., changes in self-efficacy and changes in intentions to modify one’s own behavior) have not been found to mediate the MPFC–behavior relationship, however (Falk et al., 2010, 2011). What might explain this lack of mediation of the MPFC–behavior relationship by seemingly self-related self-reports? One possibility is that the type of self-processing captured by neuroimaging tasks of self-relevance may also be engaged during message processing, but its effects are not captured by previously used retrospective self-reports. One approach to testing this hypothesis is to more precisely identify the subregion of MPFC most strongly engaged during self-related processing using a well-validated task, and then ask whether activity within this region during message exposure can be used to predict behavior change, independent of self-report metrics. This approach is called functional localization – experimenters can first collect data using a task that will capture the specific cognitive process of interest (Poldrack, 2006b; Saxe, Brett, & Kanwisher, 2006), and the resulting functionally localized regions of interest can then be interrogated in another independent task (e.g., during media exposure).
A second possibility is that the key neural processes engaged during messaging are not identical to those captured in basic-science investigations of self-related processing, and that a different function, also executed within subregions of MPFC, is engaged during message exposure. This presents an opportunity and a challenge to consider what is known about the function of MPFC that may be able to offer insight into variables explaining variance that is not optimally captured by theory-driven constructs previously measured. One such candidate process is valuation of behaviors relative to the self.
Potential Role of Value-Related Processing
Many studies in the nascent field of neuroeconomics have demonstrated that an area of the ventral MPFC plays a key role in representing the personal, or subjective, value of many types of stimuli during decision making. Activity in this region scales positively with subjective value across many decision categories, from primary rewards such as food, drink, and touch (Grabenhorst, Rolls, Margot, da Silva, & Velazco, 2007; Kringelbach, O’Doherty, Rolls, & Andrews, 2003; S. M. McClure, Ericson, Laibson, Loewenstein, & Cohen, 2007; Rolls et al., 2003), to secondary and abstract rewards such as money, music, and social rewards (Berns & Moore, 2012; Chib, Rangel, Shimojo, & O’Doherty, 2009; King-Casas et al., 2005; Knutson, 2005; Menon & Levitin, 2005; Montague & Lohrenz, 2007; Sanfey, Rilling, Aronson, Nystrom, & Cohen, 2003). The ventral MPFC has therefore been hypothesized to carry a common currency signal, which allows for decision making across domains (Kable & Glimcher, 2009; Levy & Glimcher, 2012).
The literatures on self-related processing and valuation have, for the most part, evolved separately. Although some elements of value/expectancy have been incorporated in theories of health behavior change (Becker, 1974; Rosenstock et al., 1988), those most closely resembling valuation as studied by neuroeconomists have not. Importantly for the current investigation, there is overlap in the areas of the brain that support self-related processing (Denny et al., 2012; Northoff et al., 2006; Schmitz & Johnson, 2007) and value-related processing (Bartra, McGuire, & Kable, 2013; Clithero & Rangel, 2014; Denny et al., 2012; Levy & Glimcher, 2012; Northoff et al., 2006; Schmitz & Johnson, 2007). Although there are likely some subcomponents of self- and value-related processing that are distinct, the relationship of MPFC activity to both self- and value-related processing may indicate that some elements are overlapping. In relation to health messaging, it is very likely that the value, or relative importance, of the content in a persuasive message is a component of how relevant and persuasive the message is to a given person. For example, if an ad focuses on the limitations an unhealthy behavior places on physical abilities, those who feel that playing sports is an important and valuable part of life may be more persuaded than those who are not physically inclined.
The domains in which studies of valuation have come closest to topics relevant to health media effects are within social influence and marketing. Such studies contain evidence of a valuation signal in MPFC predicting preference change. For example, studies have demonstrated that exposure to the opinions of others can change preferences for facial attractiveness (Klucharev, Hytonen, Rijpkema, Smidts, & Fernandez, 2009; Zaki, Schirmer, & Mitchell, 2011) and abstract symbols (Mason, Dyer, & Norton, 2009), and that these preference changes are linked to activity in MPFC. This domain-general valuation process (i.e., value to the self), then, may also extend to the valuation of ideas put forth in a persuasive health message, and we tested that possibility in this report. We utilized the results of a recent meta-analysis of subjective valuation studies to identify the brain region most likely to be involved in valuation.
We found that the independently identified self- and value-related regions partially, but not completely, overlapped with each other, and together covered a region of MPFC previously found to be predictive of behavior change across domains (sunscreen use; Falk et al., 2010; smoking reduction; Falk et al., 2011). We examined the relationship of each of these three regions to smoking reduction in response to a set of quit-smoking messages. In doing so, we considered the role of both overlapping and nonoverlapping subregions of MPFC involved in considering one’s own attributes as well as the value of that message to the self. We suggest that both are likely components of the persuasiveness of a message reflected in studies using MPFC to predict behavior change.
Methods
Participants
Fifty smokers participated in this functional magnetic resonance imaging (fMRI) study. All participants were consented in accordance with the procedures of the Institutional Review Board of the University of Michigan. Four participants were excluded due to excessive head motion (n = 3) or data corruption (n = 1). The remaining 46 participants included 27 men and 19 women, with a mean age of 32.06 years (SD = 12.61, range 19–64 years). Thirty of the participants reported being of White/European-American ethnic background, five were African American, five were Hispanic/Latino, and six selected the “mixed” ethnicity category. Ten participants had a bachelor’s degree or postgraduate degree, three participants had an associate degree from a 2-year college, 12 participants were currently attending a 4-year college, and 21 participants had a high school education or less.
Participants were recruited from the general population using Craigslist and UMClinicalStudies.org. Interested participants completed an eligibility screening phone call. To participate in the study, participants had to report smoking at least five cigarettes per day for the past month, having been a smoker for at least 12 months, and being between the ages of 18 and 65. In addition, participants had to meet standard fMRI eligibility criteria, including having no metal in their body, no history of psychiatric or neurological disorders, and currently not taking any psychiatric or illicit drugs. Participants were also required to be right-handed.
Tasks
Study Timeline
Once enrolled in the study, participants completed three appointments. The first was an intake appointment (Session 1), during which participants gave their informed consent and completed baseline self-report surveys, including the Fagerström Test for Nicotine Dependence, among other surveys that were not the focus of this investigation. This session lasted approximately 1 hr. The fMRI scanning appointment (Session 2) took place an average of 5 days (SD = 4 days) after the intake session, and lasted approximately 3 hr. Participants completed both prescan and post-scan self-report measures, as well as 1 hr of tasks inside the fMRI scanner. The follow-up appointment (Session 3) was conducted over the phone, an average of 40 days (SD = 9 days) after the scanning appointment (Session 2).
At all appointments, participants reported the number of cigarettes they smoked per day. As a reference, they were told that a pack contains 20 cigarettes. Self-report measures are commonly used to track smoking behavior change (Chua et al., 2011; Jasinska et al., 2010), and have been shown to have a moderate to high correlation with physiological metrics such as expired CO (Falk et al., 2011; Vogt, Selvin, Widdowson, & Hulley, 1977), and saliva, urine, and serum cotinine (Etter, Due, & Perneger, 2000; Klebanoff, Levine, Clemens, DerSimonian, & Wilkins, 1998; Pickett, Rathouz, Kasza, Wakschlag, & Wright, 2005; Pokorski, Chen, & Bertholf, 1994).
At each time point, participants were also asked whether they were currently enrolled in any kind of quit-smoking program, and whether they had a planned quit date. Of our 42 final participants, on the day of the scan, one participant was enrolled in a quit-smoking program, and one had a planned quit date. At the follow-up appointment, two participants were enrolled in quit-smoking programs, and four had planned quit dates. Hence, we infer that the majority of the change in participants’ smoking behavior was not a result of external professional interventions.
Smoking Questionnaires
At all appointments, participants answered a series of questions about their intentions to quit or reduce their smoking, self-efficacy concerning quitting smoking, and perceived risks of smoking (Schneider, Gadinger, & Fischer, 2012; Wong & Cappella, 2009; Wright, French, Weinman, & Marteau, 2006). Three intention questions asked participants about their intentions to quit, reduce, or refrain from smoking in the next 3 months. The intention ratings were made on a 4-point scale (anchors: 1 = definitely will not, 2 = probably will not, 3 = probably will, to 4 = definitely will). The self-efficacy questions asked how confident participants were that they would be able to stop smoking in the next 3 months, and how easy it would be for them to stop smoking in the next 3 months. Self-efficacy ratings were made on a 7-point scale (anchors: 1 = not at all confident/easy, 4 = confident/easy, to 7 = extremely confident/easy). The perceived risk questions asked how much participants thought that smoking can harm or had already harmed their health, and how concerned they were that smoking had affected their own health or someone else’s health (5-point scale; anchors: 1 = not at all, 2 = a little bit, 3 = somewhat, 4 = quite a bit, to 5 = very much). Participants were also asked how likely it was that they would get a serious smoking-related disease in their lifetime if they didn’t quit smoking (5-point scale; anchors: 1 = very unlikely, 2 = unlikely, 3 = somewhat likely, 4 = likely, to 5 = very likely).
fMRI Self-Localizer Task
The self-localizer task was the first task performed during the scanning session. Participants completed two runs of an adapted version of a well-validated self-related processing task (Chua et al., 2011; Schmitz & Johnson, 2006). Participants judged the self-relevance and valence of trait adjectives taken from the Anderson word trait list (Anderson, 1968). The task contained five conditions (each condition was repeated in six blocks, each containing six trials, for a total of 36 trials per condition): you_you (from your own perspective, judging yourself), you_friend (from your perspective, judging a friend), friend_you (from a friend’s perspective, judging you), friend_friend (from a friend’s perspective, judging a friend), and valence (is the word positive or negative).
Each block of judgments consisted of six trials: three trials with positive words and three with negative words. The same 36 words (18 negative and 18 positive) were judged in each condition, and presentation of positive or negative words first in each condition was counterbalanced. Each block was preceded by a 3-s orientation screen identifying the condition participants were in, and blocks were separated by 2 s of fixation.
fMRI Banner Ads Task
Our main task of interest asked participants to watch and rate 23 animated banner ads, created as part of the American Legacy Foundation’s EX campaign. The target audience for the campaign was adults who are considering or had recently tried to quit smoking. Some ads encouraged smokers to relearn how to handle common smoking triggers (i.e., dealing with stress, drinking coffee) without cigarettes. Others empathized with the difficulty of quitting and suggested resources to help smokers quit (i.e., http://www.becomeanex.org). All ads contained movement, some as cartoons illustrating trigger situations and others as dynamic cartoon text suggesting quit resources. None contained sound.
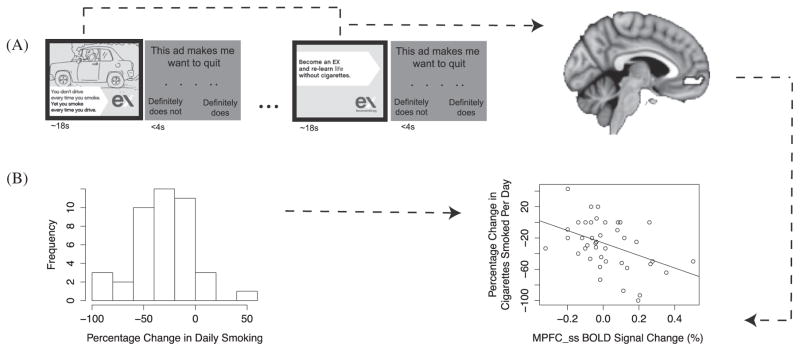
This was the last task completed during the scan, following the self-localizer task described above and two other tasks which were not the focus of the current investigation (one of which contained exposure to smoking-relevant images). Banner ads were presented in random order, and were mean of 17.7-s long (range 13.9–30 s, SD = 3.9 s). Immediately following each ad, participants were presented with a response screen with the statement “This makes me want to quit” and a 5-point rating scale (1 = definitely does not, 2 = does not, 3 = neutral, 4 = does, to 5 = definitely does; this rating was referred to as QUIT). They were allowed 4 s on the response screen, which was followed by fixation with a jittered inter-trial interval (M = 4.1 s, range 3.1–7.5 s, SD = 1.1). See Figure 1A for an illustration of the task design.
Figure 1.
Task and analysis design. (A) Participants viewed 23 banner ads, which averaged 18 s each in duration. After watching each video, they were asked to rate how much the ad made them want to quit smoking, on a 5-point scale. This was followed by an intertrial fixation period. We extracted activity during the time period participants were watching the banner ads, from our regions of interest in medial prefrontal cortex (MPFC). (B) Histogram of the percentage change in daily smoking across the final sample (negative value = reduction in smoking). Activity in each region of interest was used to predict the percentage change in daily smoking.
MRI Image Acquisition
Neuroimaging data were acquired using a 3-Tesla GE Signa MRI scanner. Two functional runs for the self-localizer task (288 volumes total) were collected at the start of the scan, and one functional run of the banner ads task (304 volumes total) was acquired at the end of the scan for each participant, separated by other tasks that were not the focus of the current investigation. Functional images were recorded using a reverse spiral sequence (repetition time (TR) = 2,000 ms, echo time (TE) = 30 ms, flip angle = 90°, 43 axial slices, field of view (FOV) = 220 mm, slice thickness = 3 mm); sequential descending slice acquisition; voxel size = 3.44 × 3.44 × 3.0 mm). We also acquired in-plane T1-weighted images (43 slices; slice thickness = 3 mm; voxel size = 0.86 × 0.86 × 3.0 mm) and high-resolution T1-weighted images (SPGR; 124 slices; slice thickness = 1.02 × 1.02 × 1.2 mm) for use in coregistration and normalization.
Imaging Data Analysis
Functional data were preprocessed and analyzed using Statistical Parametric Mapping (SPM8, Wellcome Department of Cognitive Neurology, Institute of Neurology, London, UK). To allow for the stabilization of the BOLD signal, the first five volumes (10 s) of each run were discarded prior to analysis. Functional images were despiked using the 3dDespike program as implemented in the AFNI tool-box. Next, data were corrected for differences in the time of slice acquisition using sinc interpolation; the first slice served as the reference slice. Data were then spatially realigned to the first functional image. We then coregistered the functional and structural images using a two-stage procedure, each stage being six parameter affine. First, in-plane T1 images were registered to the mean functional image. Next, high-resolution T1 images were registered to the in-plane image (12 parameter affine). After coregistration, high-resolution structural images were skull-stripped using the VBM8 (voxel-based morphometry) toolbox for SPM (http://dbm.neuro.uni-jena.de/vbm), and then normalized to the skull-stripped Montreal Neurological Institute (MNI) template provided by FSL (FMRIB Software Library; MNI152_T1_1mm_brain.nii). Finally, functional images were smoothed using a Gaussian kernel (8 mm full width at half maximum). The fMRI data were modeled for each participant for each task using fixed effects models within the general linear model as implemented in SPM8. The six rigid-body translation and rotation parameters derived from spatial realignment were also included as nuisance regressors in all first-level models. Data were high-pass filtered with a cutoff of 128 s. Data were modeled at the first level using the general linear model as implemented in SPM8, using SPM’s canonical difference of gammas hemodynamic response function (HRF). Random effects models for each task were also implemented in SPM8.
Self-Localizer Task
The self-localizer task was modeled using a single boxcar function for each 18-s block. Fixation and condition preparation periods were included with baseline rest. The contrast of interest examined conditions in which the participant was the target of judgment (you_you & friend_you) versus conditions in which the judgment was word valence. The resulting contrast images were combined using a random effects model in SPM8 and the resulting image map (cluster corrected familywise error, p < .05) was used to identify a subregion of MPFC that was most robustly associated with self-related processing across participants. This cluster was converted to a functionally defined region of interest (ROI) using MarsBaR (Brett, Anton, Valabregue, & Poline, 2002) and served as an ROI in the subsequent banner ads task.
Banner Ads Task
The duration of the presentation of each ad was modeled with a single variable epoch task regressor. We modeled the response period as the length of time the participant took to answer the QUIT question for each ad, and combined the response periods into a single additional regressor of no interest. If participants did not make a rating, the duration of the banner ad and response period was modeled as a separate missed trial regressor of no interest. Fixation rest periods constituted an implicit baseline. Neural activity during the banner ads was compared with this implicit baseline. The resulting contrast images were combined using a random effects model in SPM8. From each a priori ROI, average parameter estimates of activity during the banners task were extracted at the group level using Marsbar and converted to percentage signal change by dividing each set of parameter estimates by the constant.
A Priori Regions of Interest
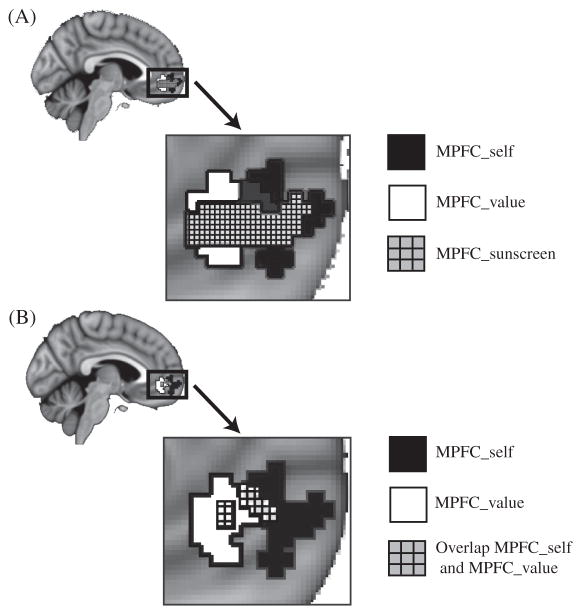
Three ROIs were identified, each addressing a different question about the relationship between activity in MPFC and behavior change. The first ROI was taken from a previous study that predicted changes in sunscreen use from neural activity (Falk et al., 2010), as well as smoking behavior change in an independent sample (Falk et al., 2011), to replicate previous work. This ROI included a ventral region of MPFC that was associated with this behavior change (MPFC_ss; volume = 1,232.00 mm3). The second ROI was identified by the self-localizer task, described above, as the region of MPFC most active during self-related judgments, as compared with valence judgments (MPFC_self; volume = 1,878.81 mm3). Finally, we examined valuation as another cognitive process that might contribute to the explanation of the observed MPFC–behavior relationship. The ROI corresponding to subjective valuation was taken from Bartra et al. (2013): MPFC_sv; volume = 3,582.00 mm3. This was a quantitative meta-analysis of 206 studies that reported subjective value-related neural signals during decision making. The region used here was reported in Figure 9 of that paper (Bartra et al., 2013), and is the conjunction of several valuation-relevant contrasts. We also separately examined the voxels that overlapped between the self and value ROIs (Intersection; volume = 352 mm3), and the voxels unique to the ROIs for self (MPFC_self only; volume = 1,384.00 mm3) or value (MPFC_sv only; volume = 3,240.00 mm3). See Figure 2 for the overlap of all three ROIs, and Figure 3 for each ROI individually. The relationship between activity from the ROIs and behavior change was examined in a separate model for each ROI given the high degree of collinearity between ROIs.
Figure 2.
Regions of interest. (A) Self- and value-related regions of interest overlap with predictive region from prior work. The MPFC_ss region (medial prefrontal cortex [MPFC] region identified by prior sunscreen use study) is in crosshatch; the MPFC_self region (identified by the self-localizer) is in black; and the MPFC_sv region (valuation region identified by meta-analysis) is in white. (B) Self- and value-related regions overlap with each other.
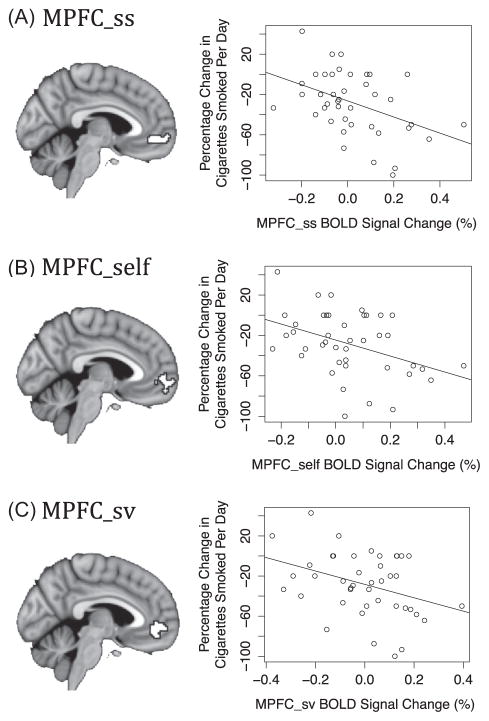
Figure 3.
Neural activity during online antismoking ads predicts smoking behavior change. Proportional behavior change is plotted against percentage signal change in activity from the: (A) MPFC sunscreen region of interest (ROI; Falk et al., 2010), r2 = 0.18; (B) MPFC self-localizer ROI, r2 = 0.16; and (C) MPFC subjective value ROI (Bartra et al., 2013), r2 = 0.13.
Specificity of Predictive Effects
Additional analyses were performed to test whether the effects of interest were selective for activity in these ROIs during exposure to the banner ads, or might reflect more general sensitivity in these regions across tasks. To test this, we examined whether activity in our target ROIs during two conditions of the self-localizer task could also predict behavior change. For example, one concern might be that any form of thinking about the self could predict behavior change, if participants have been primed to think about cessation by answering the prescan questionnaires about smoking behavior. To address this concern, we examined activity in all three of the ROIs during conditions of the self-localizer in which participants were making judgments about themselves (you_you and friend_you), versus judgments of word valence. A second concern could be that activity in these regions reflecting mentalizing could be predictive of behavior change. In response to this, we examined the condition in which participants judged their friend’s personality traits, from the friend’s perspective (friend_friend). From each a priori ROI, average parameter estimates of activity during the contrasts of interest were extracted at the group level using Marsbar and converted to percentage signal change.
Whole Brain Analysis
After completing our planned ROI analyses, we conducted an exploratory whole brain search for regions associated with behavior change outside of our hypothesized target ROIs. In the whole brain analysis of the banner ads task, parameter estimates of activity during the ads compared with rest were correlated at the group level, with each participant’s proportional reduction in smoking. More specifically, models were computed for each participant at the single subject as described above. Next, a random effects model was computed at the group level using the multiple regression function in SPM8. Because this was an exploratory post hoc analysis, the image map was thresholded at a liberal p < .01, with a cluster threshold of k = 20 voxels. Regions that remained significant at p < .005, k = 20 were also indicated.
Behavioral Data Analysis
The main dependent variable of interest in this study was the change in the number of cigarettes participants smoked per day following the fMRI scanning session. The behavior change metric used in the analysis was the proportional reduction in daily cigarette smoking (Figure 1B). This was calculated as cigarettes per day at the follow-up session minus cigarettes per day at the scanning session, divided by cigarettes per day at the scanning session. If participants reported a range of cigarettes smoked per day, the average was used. A negative behavior change corresponded to a reduction in smoking, and a positive behavior change to an increase in smoking (at the follow-up appointment relative to the scanning session). We chose to use the smoking report at the scanning session because of its proximity to the intervention, but reports of daily smoking at intake and the scanning session were very consistent (r = .94).
We also planned to examine whether self-report measures of intentions, self-efficacy, and perceived risks were associated with behavior change, and/or with MPFC activity during exposure to the ads. Thus, participants answered a number of questions related to smoking intentions, self-efficacy, and perceived risk at all appointments. A composite of questions relating to each of these categories was used in analysis. The averages of three questions relating to intentions, two for self-efficacy, and five for perceived risk were taken for each appointment’s questions. We subtracted the intake composite scores from the follow-up composite scores to obtain differences in smoking intentions, self-efficacy, and perceived risk. A positive difference corresponded to an increase at the follow-up appointment relative to the intake appointment. These metrics were collected immediately postscan as well, but we chose to use the follow-up reports to match the timing of the smoking reports.
We examined Cronbach’s alphas on these composite measures. Cronbach’s alphas were as follows: intake intention (.94), self-efficacy (.61), and perceived risks (.67); follow-up intention (.87), self-efficacy (.55), and perceived risks (.78). The low alphas in the self-efficacy composite were most likely due to the small number of averaged items (n = 2). To ensure that we were not missing any significant relationships by using the composites, however, we examined all relationships reported in the paper using each self-efficacy question separately. All relationships reported of the composite were true of each metric individually, with one exception — the correlation between change in intentions and change in self-efficacy noted in Table 1 was due to change in the confidence in ability to quit, not to how easy it would be to quit.
Table 1.
Changes in smoking intentions, self-efficacy, and perceived risks
| Intentions* | Self-efficacy* | Perceived risks | |
|---|---|---|---|
| Intake (Session 1) | 2.42 (SD = 0.83) | 2.65 (SD = 1.24) | 3.27 (SD = 0.69) |
| Follow-up (Session 3) | 3.08 (SD = 0.74) | 3.95 (SD = 1.43) | 3.25 (SD = 0.79) |
| Change (Session 3–1) | 0.66 (SD = 0.78) | 1.30 (SD = 1.37) | −0.02 (SD = 0.64) |
Note. Measures that were significantly different between appointments are marked with an asterisk (p < .005).
Imaging and Behavioral Data Attrition
Beyond those noted in the main description of participant characteristics, participants were excluded from the self task (n = 1) due to missing data specific to that task, and from the banners task (n = 1) due to excessive head motion specific to that task. In addition, one participant could not be reached for the follow-up appointment, and hence we did not have a behavior change score or end point self-report measures for this participant. Two participants fell greater than 2.5 standard deviations above the mean on proportional behavior change, and were excluded from further analysis (results with the full sample are given in the footnotes 1 and 3, below). This brought the total number of participants in the main analysis of interest to 42.
Results
Smoking Behavior Change
Participants reported the number of cigarettes they smoked on a typical day, given the reference value of a pack containing 20 cigarettes. At the scanning appointment (Session 2), participants smoked an average of 13.17 (SD = 6.93) cigarettes per day. The average score on the Fagerström Test for Nicotine Dependence was 4.72 (SD = 1.3), indicating low to moderate addiction. At the follow-up appointment (Session 3), which took place an average of 40 days later, participants smoked an average of 8.92 (SD = 6) cigarettes per day. This was a significant decline in daily smoking, paired t(41) = 5.32, p < .001. A histogram of smoking reduction can be found in Figure 1B.
Neural Activity During Ad Exposure Predicts Smoking Behavior Change
Our primary interest was whether neural activity in our a priori MPFC regions during exposure to the health messages was associated with behavior change. (See Figure 1 for illustration of the task and analysis design.) Three regions of interest were identified, each of which addressed a question about the nature of the relationship between activity in MPFC and behavior change. First, for the purposes of replication, we examined a region identified by previous work (Falk et al., 2010) as correlated with sun-screen and smoking behavior change (denoted MPFC_ss). Next, we examined whether behavior change might be associated with activity in regions that were specifically identified in the current sample as being involved in self-related processing focused on assessing one’s own attributes. We used a functional localizer to identify the area of MPFC involved in self-related processing in these participants (see Methods for details; denoted MPFC_self). In addition, given the logic outlined above with respect to the possibility that additional cognitive processes might help explain previously observed MPFC–behavior relationships, we examined another possible cognitive process known to be encoded in MPFC. More specifically, we examined the possibility that value-related processing could be an additional factor, using a recent meta-analysis to identify the region of MPFC most often associated with subjective valuation (denoted MPFC_sv). Both the self-and value-related processing regions overlap with the region identified by previous work as predicting behavior change (Figure 2A), consistent with the hypothesis that activity predictive of behavior change may involve both processes.
We compared neural activity during the presentation of the banner ads with rest, and then extracted average activity estimates from each of our three ROIs to use as predictors of behavior change. Each model predicting behavior change included activity from a single ROI. Activity in each of these regions separately predicted reductions in smoking, such that higher activity in the subregions of MPFC previously implicated in health behavior change, self-related processing, and valuation each led to a larger proportional reduction in reported cigarettes smoked per day, MPFC_ss: β = −.8, t(40) = −2.93, p = .006; MPFC_self: β = −.79, t(40) = −2.8, p = .008; MPFC_sv: β = −.67, t(40) = −2.42, p = .02. Activity estimates and proportional behavior change for each participant are plotted in Figure 3, in each ROI.
To examine whether the voxels that overlapped between the MPFC_self and MPFC_sv ROIs were driving these effects, we created ROIs that were unique to MPFC_self and MPFC_sv, and created an additional ROI of the overlapping voxels between them (Intersection). Activity in all of these ROIs predicted reductions in smoking, MPFC_self only: β = −.8, t(40) = −2.79, p = .008; MPFC_sv only: β = −.66, t(40) = −2.39, p = .02; Intersection: β = −.62, t(40) = −2.56, p = .014. See Figure 2B for the overlap between the self and value ROIs.
Next, we sought to establish whether the effects observed were selective for activity in our ROIs during message exposure, or reflected more general sensitivity within these regions across tasks. To test whether activity in these ROIs during general self-related thought could predict behavior change, we examined MPFC activity during conditions of the self-localizer in which participants were making judgments about themselves, versus judgments about word valence (i.e., the same task condition used to localize the self-ROI). Activity in none of the ROIs during this self task condition predicted behavior change, MPFC_ss: β = −.25, t(39) = −0.68, p = .499; MPFC_self: β = −.36, t(39) = −1.13, p = .266; MPFC_sv: β = −.06, t(39) = −0.19, p = .85. To test whether general mentalizing after answering smoking-relevant questions predicted behavior change, we examined MPFC activity during the condition of the self-localizer in which the participant needed to take the perspective of a friend to make a judgment about that friend’s personality. Again, activity in none of the ROIs predicted behavior change, MPFC_ss: β = −.07, t(39) = −0.22, p = .83; MPFC_self: β = −.25, t(39) = −0.84, p = .41; MPFC_sv: β = .002, t(39) = 0.01, p = .99.
Predicting Behavior Change Using Self-Report and Neural Responses
We also examined whether neural activity in these ROIs was related to participants’ self-reported changes in intentions, self-efficacy, or beliefs from intake (Session 1) to follow-up (Session 3), or to self-reports of their average quit ratings during the task. In addition, we examined whether neural activity within the selected neural ROIs predicted the same or different variance as our self-report variables.
Participants’ reports of intentions and self-efficacy significantly increased from intake to follow-up, although reports of perceived risks did not (see Table 1).1,2 Only the change in smoking intentions was correlated with behavior change. Neural activity in MPFC was not significantly related to any of these behavioral measurements, ruling out the possibility of mediation. We compared the R2 of models predicting behavior change from intention change alone (r2 = 0.1) with those including both intention and MPFC_ss activity (r2 = 0.28), MPFC_self activity (r2 = 0.23), and MPFC_sv activity (r2 = 0.22), and found that neural activity explained significant variance above and beyond changes in self reported intentions. The increases in explained variance were significant, MPFC_ss: F(1, 39) = 9.5, p = .004; MPFC_self: F(1, 39) = 6.7, p = .01; MPFC_sv: F(1, 39) = 5.7, p = .02. See Table 2 for correlations between these metrics, MPFC activity, and behavior change.3
Table 2.
Correlations between MPFC activity and changes in behavior and self-report metrics
| 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 | ||
|---|---|---|---|---|---|---|---|---|---|
| Behavior brain | 1. Δ Smoking | ||||||||
| 2. MPFC_ss | −0.42* | ||||||||
| 3. MPFC_self | −0.40* | 0.89** | |||||||
| 4. MPFC_sv | −0.36* | 0.87** | 0.86** | ||||||
| Self-report | 5. Δ Intentions | −0.32* | 0.00 | 0.14 | 0.06 | ||||
| 6. Δ Self-efficacy | −0.19 | 0.05 | −0.04 | 0.01 | 0.37* | ||||
| 7. Δ Perceived risk | −0.27 | 0.18 | 0.07 | 0.17 | 0.34* | 0.36* | |||
| 8. Ad quit rating | −0.16 | −0.03 | 0.05 | −0.08 | 0.02 | 0.10 | −0.09 |
Notes. MPFC = medial prefrontal cortex.
p < .05;
p < .005.
Participants also made ratings about each ad. After viewing each ad in the scanner, participants rated how much the ad made them want to quit smoking, on a 5-point scale (1 = definitely does not, 3 = neutral, to 5 = definitely does). The average within-participant rating was 2.8 (SD = 0.84). As described above for behavior change, we examined whether activity in each of the three ROIs during presentation of the ads was correlated with these averaged quit ratings. Average quit ratings were not significantly correlated with activity in any of the three ROIs. This average quit rating was also not correlated significantly with behavior change or with changes in self-reported intentions, self-efficacy, or perceived risks (see Table 2).
Finally, in an exploratory post hoc analysis, we examined the relationship between behavior change and neural activity across the entire brain. Additional regions outside of MPFC in which activity was correlated with behavior change included the parahippocampal gyrus and temporoparietal junction (see full list in Table 3). Because of the exploratory nature of this analysis, and its utility for the generation of future ROIs, we present results at a liberal threshold; regions that survived a more stringent threshold are also noted.
Table 3.
Whole brain exploratory analysis of correlations with behavior change
| Region | Lat. | Size | Peak T | X, Y, Z |
|---|---|---|---|---|
| Medial prefrontal cortex | L (/R) | 92 | −3.54 | −16, 56, −8 |
| Temporoparietal junction* | L | 46 | −3.35 | −44, −40, 28 |
| Medial temporal lobe* | L | 31 | −4.59 | −13, −5, −38 |
| Parahippocampal gyrus* | −2.42 | −18, −5, −36 | ||
| Parahippocampal gyrus* | L | 47 | −3.89 | −20, −26, −14 |
| Lateral occipital cortex | R | 25 | −3.63 | 35, −84, 1 |
| Cerebellum | L | 27 | −4.01 | −33, −30, −35 |
Notes. Negative activations were associated with reduced smoking. No regions showed a positive effect in this contrast (i.e., no regions were associated with increased smoking).
p < .005, k = 20.
Discussion
In this study, we utilized neuroimaging to examine mechanisms predicting health behavior change in response to anti-smoking messages. Although theory-based self-report metrics have had success in predicting health behavior change (Armitage & Conner, 2001; Webb & Sheeran, 2006), recent neuroimaging work has found that activity in MPFC explains additional variance in behavior change after media exposure (Falk et al., 2010, 2011), suggesting the value of using neural data to aid in further theory development. However, the specific psychological mechanism for this relationship has yet to be elucidated. To further the understanding of observed MPFC–behavior relationships, we replicated previous results with an improved experimental design, and identified an additional cognitive process relevant to explaining this relationship.
We examined theory-based self-report metrics, as well as activity during exposure to antismoking banner ads in three subregions of MPFC. These regions were a subregion found to be predictive of behavior change in prior work (Falk et al., 2010, 2011), a region identified by self-localizer task in this group of participants, and a region identified by meta-analysis to be involved in valuation (Bartra et al., 2013). Although we did observe that changes in participants’ self-reported intentions were predictive of reductions in their smoking, none of the intention, self-efficacy, or perceived risk measures was correlated with activity in our MPFC regions. These theory-based self-report metrics, then, are capturing different variance in behavior change than neural activity is, suggesting that other forms of cognitive processing may contribute to their ultimate effects.
Several previous studies have identified a link between neural activity in MPFC during health messaging and behavior change, and propose that this link is mediated by self-related processing (Chua et al., 2011; Falk et al., 2010, 2011). This hypothesis is supported by neuroimaging research demonstrating that the MPFC is involved in tasks requiring self-related reflection or judgments (Amodio & Frith, 2006; Denny et al., 2012; Northoff et al., 2006; Schmitz & Johnson, 2007). To strengthen this hypothesis, we utilized a functional localizer to identify the region of MPFC recruited during self-judgments in our participant pool, and found that activity in this subregion of MPFC does predict behavior change. The MPFC subregion identified by prior work partially overlaps with this functionally localized self-related subregion, but not entirely.
Although one other study has taken advantage of a self-localizer task, that work did not use naturalistic health media designed for mass consumption, but rather demonstrated increased self-related processing in response to individually tailored text statements compared with non-tailored statements (Chua et al., 2011). In addition, the ROI used by Chua and colleagues was the result of a conjunction between a self-localizer (which encompassed a large area of MPFC, including the regions used here) and another contrast concerning the tailoring of message content. Another paper predicting behavior change from MPFC activity used a dorsal MPFC region, which was identified not through self-related processing but through other message components (Wang et al., 2013). The present study is unique in predicting behavior change from a functionally localized self-processing subregion of MPFC to identify self-related processes in response to mass media, as well as in exploring another candidate cognitive process.
More specifically, another candidate process for the variance captured by the region of MPFC reported in prior studies, which we investigate here, is valuation. We examined a subregion of MPFC identified by a large quantitative meta-analysis to be involved in subjective valuation. We found that this subregion overlapped with our self-localizer area as well as with the subregion of MPFC reported in prior studies (Falk et al., 2010, 2011). Activity in the valuation subregion during exposure to the banner ads was also predictive of behavior change, suggesting that valuation might play a role in explaining this MPFC–behavior relationship as well.
The literatures on self-related processing in social neuroscience and valuation in the field of neuroeconomics have largely evolved separately. The study of value in neuroeconomics largely focuses on the personal value of external stimuli, such as money, food, or other goods (Bartra et al., 2013; Clithero & Rangel, 2014; Levy & Glimcher, 2012; Rangel, Camerer, & Montague, 2008), rather than more abstract ideas, personal qualities, or goals. However, the relationship of the MPFC to both self- and value-related processing suggests that this region may perform a broader function that overlaps with both of these cognitive processes, such as the valuation of higher-level self-relevant ideas or goals (D’Argembeau, 2013; Northoff & Hayes, 2011; Roy, Shohamy, & Wager, 2012). It may be this intersection of self- and value-related processing that we are detecting in the relationship between MPFC activity and health behavior change. Work in social psychology and persuasion has alluded to this idea; the elaboration likelihood model, for example, discusses issue involvement as an important factor in persuasion (Petty & Cacioppo, 1984, 1986), and individual level factors interact with message features to determine effectiveness (Weber, Westcott-Baker, & Anderson, 2013). Our neuroimaging results thus help bring together research from social psychology and behavioral economics. Further work in health messaging may be able to design self-report or behavioral task metrics that tap into this type of cognition and examine whether those metrics mediate the MPFC–behavior relationship, as well as more precisely specifying the degree to which they are neurally dissociable or overlapping processes.
Future research may also help to elucidate relationships between additional regions and networks of brain regions in predicting behavior change. For example, in our exploratory whole brain analysis, we find that several regions beyond MPFC are associated with behavior change. Of particular interest, we observe that increased activation in the parahippocampal gyrus is associated with behavior change. The hippocampus has been linked to memory as well as to prospection, or imagining the future (Buckner & Carroll, 2007; Schacter, Addis, & Buckner, 2007; Schacter et al., 2012; Spreng, Mar, & Kim, 2009). It could be the case that people who more spontaneously imagine themselves performing a future behavior in line with the message (Gollwitzer, 1999), or who can call to mind autobiographical memories that are relevant to the future process of quitting smoking, are more likely to change their behavior. This could be something that acts at the individual difference level (e.g., people could be more or less proficient at vividly envisioning future scenarios) or could be a property of ad design (such as featuring identifiable scenarios or individuals). Several studies examining the persuasiveness of health messages find that more persuasive messages are associated with higher activity in the medial temporal lobe (Falk et al., 2009; Langleben et al., 2009; Ramsay, Yzer, Luciana, Vohs, & MacDonald, 2013), providing evidence for the latter possibility. Further work could explore individual-difference level effects in the hippocampus and medial temporal lobe.
More broadly, the processes of self-referential thinking, valuation, memory, and prospection have been linked to what is known as the default mode network (DMN), a group of brain regions very often observed to be more active while participants are at rest as compared with engaged in cognitive goal-directed tasks (Buckner, Andrews-Hanna, & Schacter, 2008; Gusnard & Raichle, 2001; Raichle et al., 2001). The DMN is thought to consist of midline regions, such as MPFC and the posterior cingulate, that are active during self-relevant and affective processing; as well as regions in the medial temporal lobe, which are active during memory- and prospection-based tasks (Andrews-Hanna, Reidler, Sepulcre, Poulin, & Buckner, 2010; Buckner et al., 2008; Spreng et al., 2009; Spreng & Grady, 2010). Evidence suggests that while participants are not engaged in specific tasks, they are likely engaged in some form of self-referential thinking, perhaps remembering or imagining personally significant or valuable events (Andrews-Hanna, 2012; Andrews-Hanna et al., 2010; D’Argembeau et al., 2005; Mason et al., 2007; Spreng, 2012; Whitfield-Gabrieli et al., 2011). One possible explanation of the link between the regions observed here and the DMN is that messages are more effective when people tap into these highly practiced processes during message exposure.
Limitations and Future Directions
The current investigation conceptually replicates and substantially extends prior work. Strengths of the current investigation included the combination of theory-driven data from neuroimaging of MPFC with longitudinal behavior change. As with any study, however, limitations in the current design suggest opportunities for future research.
Experimental designs in future neuroimaging work on health messaging would benefit from the inclusion of a between-subjects control condition. Although our design did not include a between-subjects control group, participants did complete another fMRI task within subjects (the self-localizer) before exposure to the banner ads. The lack of a predictive relationship between activity in several relevant conditions in the self-localizer (self-reflection and perspective taking) and behavior change provides support for the specificity of the relationship of MPFC during message exposure and behavior change, though this cannot establish causal media effects on subsequent behavior.
One primary avenue for future research will be further investigation of the overlap, both spatial and cognitive, between self- and value-related processing in MPFC. We observed considerable spatial overlap in the current investigation. However, a comparison between two localizer tasks in the same individual would provide even stronger evidence. We used a functional localizer for self-related processing, but used the results of a meta-analysis to identify our valuation MPFC subregion. There is not currently a single widely used value localizer task, but future work could pursue this.
We noted in the results section that although the ROIs for self-related processing and valuation are overlapping, the relationship between activity in those ROIs and behavior change is significant when restricted only to voxels unique to each region. It is likely that there are both neural and cognitive elements of self- and value-related processing that overlap, as well as elements that do not. Future research that carefully disentangles self- and value-related thought can address the extent to which the two processes are truly overlapping or distinct in the brain, perhaps utilizing neuroimaging analysis techniques such as multivoxel pattern analysis (MVPA) to examine the spatial representations of self- and value-related processing more closely.
In addition, although our results suggested that self- and value-related processing during messaging were key predictors of behavior change, we must be careful to avoid overstating this result. In other words, we must be cautious with interpretations that rely on reverse inference, a limitation that is much-discussed in the neuroimaging literature (Poldrack, 2006a, 2011). It is tempting to conclude that because activation in a given brain region is observed, a cognitive process that has previously been linked to that region is engaged. However, if brain regions are involved in multiple cognitive processes, as they nearly always are, it is possible to misattribute activation to an inappropriate cognitive function. Here we have demonstrated that subregions of MPFC are activated by a self-localizer task and by subjective value tasks, and that activity in these regions during health messaging goes on to predict behavior change. However, we cannot conclude definitively that self- and value-related processes are occurring during health messaging and mediating behavior change. Further work could test this hypothesis, perhaps through use of additional self-report or implicit metrics that clearly involve these processes, and testing whether those metrics mediate the MPFC–behavior relationship, or by directly altering known self-relevant or value-relevant attributes of messages.
An important next step in understanding the MPFC–behavior relationship will be to examine networks of regions, rather than the MPFC in isolation. Our exploratory whole brain analysis, as well as the whole brain results of other papers, suggests some candidate regions of interest, such as the hippocampus, posterior cingulate cortex/precuneus, and supplementary motor area (Falk et al., 2011; Langleben et al., 2009; Ramsay et al., 2013). Examining whether other regions explain additional variance in predicting behavior change would aid in identifying and understanding the cognitive processes important for behavior change.
Future work could utilize different neuroimaging techniques to gain even more information about cognitive processing during messaging. For example, in our analysis we averaged brain activity throughout the duration of ad exposure. It would be possible to examine the brain’s response to messaging at a finer timescale, perhaps to examine whether there is activity during a particular event in an ad that most differentiates people who will subsequently change their behavior from those who will not. It is also possible to examine convergence in brain activity between individuals across the time course of an ad, to ask whether dynamic patterns of response during the ad differentiate those who do or do not change their behavior (Hasson, Ghazanfar, Galantucci, Garrod, & Keysers, 2012; Hasson, Nir, Levy, Fuhrmann, & Malach, 2004). Such examinations of the time course of activation during an ad could identify different sets of cognitive processes that are involved across time while viewing media, and explore the degree of, and interactions between, those processes.
A final topic for future work could be to examine brain activity during repeated exposures to the same media. Much experimental work involves just one exposure to any given message, at one time point. In practice, people are likely to be exposed to the same messaging on multiple occasions, and it is thought that repeated exposure produces stronger effects (Hornik, 2002). Observing the brain’s response to repeated presentations of the same ads, perhaps even across time, could prove very useful in understanding why repeated exposure is of such importance.
Conclusions
We found that although some of the most common theorydriven self-report variables are significant predictors of health behavior change, they capture a different element of cognitive processing than activity in MPFC does. We probed the MPFC–behavior relationship using three theory-driven ROIs in MPFC. We replicated prior results linking MPFC to behavior change and found that behavior change is significantly related to activity in both self- and value-related subregions of MPFC. Our data provide support for two potential neurocognitive processes responsible for the MPFC–behavior relationship, and also suggest great benefit in examining the extent to which these processes are psychologically similar or distinct. Self-report and neuroimaging approaches can be synergistic, and future work utilizing both will likely make the most progress in identifying a mechanistic explanation for a wide range of media effects, and health behavior change in particular. Further work to identify the precise cognitive mechanism that is being captured by brain activity in the MPFC during health messaging will greatly improve our understanding of the success of media messages in producing health behavior change. Although antitobacco media has contributed significantly to a large reduction in the prevalence of tobacco use, the burden of tobacco, in lives and cost, remains formidable. Continuing improvements in the effectiveness of health communications could be speeded by combining well-validated measures such as self-reports with newer methodologies, such as neuroimaging.
Acknowledgments
We acknowledge Richard Gonzalez, Sonya Dal Cin, Victor Strecher, and Lawrence An for collaboration on a larger study relevant to this work; Francis Tinney Jr., Kristin Shumaker, Li Chen, Nicolette Gregor, Becky Lau, Larissa S. Svintsitski, and Cole Schaffer for assistance with data collection; Lynda Lin for assistance with data processing; and Robert Hornik for helpful discussion; as well as Donna Vallone, Jeffrey Costantino, Amanda Richardson, Aaron Mushro, and the American Legacy Foundation for provision of study stimuli. This work was supported in part by grants from The Michigan Center of Excellence in Cancer Communication Research/NIH-P50 CA101451 (PI Strecher), and an NIH New Innovator Award/NIH 1DP2DA03515601 (PI Falk).
Biographies
 Nicole Cooper is a postdoctoral researcher at the University of Pennsylvania’s Annenberg School for Communication. Her research investigates the neural mechanisms of decision making and health behavior change. Her work focuses particularly on the role of the medial prefrontal cortex in these processes.
Nicole Cooper is a postdoctoral researcher at the University of Pennsylvania’s Annenberg School for Communication. Her research investigates the neural mechanisms of decision making and health behavior change. Her work focuses particularly on the role of the medial prefrontal cortex in these processes.
 Matthew Brook O’Donnell is a research assistant professor at the Annenberg School for Communication at the University of Pennsylvania. His research background includes corpus linguistics, natural language processing, and data mining. He is interested in combining linguistic analyses of media language and persuasive discourse with behavioral and neuroscience approaches.
Matthew Brook O’Donnell is a research assistant professor at the Annenberg School for Communication at the University of Pennsylvania. His research background includes corpus linguistics, natural language processing, and data mining. He is interested in combining linguistic analyses of media language and persuasive discourse with behavioral and neuroscience approaches.
 Steve Tompson is a PhD student in psychology at the University of Michigan. He is interested in using neuroimaging to understand social cognitive processes mediating sociocultural differences in self-appraisal and decision making. He is also interested in extending this research to examine the contribution of self-appraisal to attitude and behavior change.
Steve Tompson is a PhD student in psychology at the University of Michigan. He is interested in using neuroimaging to understand social cognitive processes mediating sociocultural differences in self-appraisal and decision making. He is also interested in extending this research to examine the contribution of self-appraisal to attitude and behavior change.
 Emily Falk is an assistant professor of communication at the University of Pennsylvania’s Annenberg School for Communication. She is interested in predicting behavior change following exposure to persuasive messages and in understanding what makes successful ideas spread (e.g., through social networks, cultures), using methods from communication science, neuroscience, and psychology.
Emily Falk is an assistant professor of communication at the University of Pennsylvania’s Annenberg School for Communication. She is interested in predicting behavior change following exposure to persuasive messages and in understanding what makes successful ideas spread (e.g., through social networks, cultures), using methods from communication science, neuroscience, and psychology.
Footnotes
Two participants were excluded from this analysis for having behavior change reductions greater than 2.5 standard deviations from the mean. When included, there remained a significant increase in intentions and self-efficacy (p < .005) but not perceived risks.
Using self-reports from the intake session and immediately after the scanning session, changes in self-reported intentions, self-efficacy, and perceived risks did not predict behavior change.
Two participants were excluded from this analysis for having behavior change reductions greater than 2.5 standard deviations from the mean. With all subjects included, brain activity in only MPFC_SV was predictive of behavior change (p < .005). Self-reported changes in intentions were significantly correlated with behavior change (p < .01), but changes in self-efficacy, changes in perceived threat, and quit ratings were not, nor were these metrics correlated with brain activity.
References
- Ajzen I. From intentions to actions: A theory of planned behavior. In: Beckman J, editor. Action-control: From cognition to behavior. Heidelberg: Springer; 1985. pp. 11–39. [Google Scholar]
- Ajzen I. The theory of planned behavior. Organizational Behavior and Human Decision Processes. 1991;50:179–211. [Google Scholar]
- Ajzen I, Fishbein M. Understanding attitudes and predicting social behavior. Englewood Cliffs, NJ: Prentice Hall; 1980. [Google Scholar]
- Amodio DM, Frith CD. Meeting of minds: The medial frontal cortex and social cognition. Nature Reviews: Neuroscience. 2006;7:268–277. doi: 10.1038/nrn1884. [DOI] [PubMed] [Google Scholar]
- Anderson NH. Likableness ratings of 555 personality-trait words. Journal of Personality and Social Psychology. 1968;9:272–279. doi: 10.1037/h0025907. [DOI] [PubMed] [Google Scholar]
- Andrews-Hanna JR. The brain’s default network and its adaptive role in internal mentation. The Neuroscientist. 2012;18:251–270. doi: 10.1177/1073858411403316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Andrews-Hanna JR, Reidler JS, Sepulcre J, Poulin R, Buckner RL. Functional-anatomic fractionation of the brain’s default network. Neuron. 2010;65:550–562. doi: 10.1016/j.neuron.2010.02.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Armitage CJ, Conner M. Efficacy of the theory of planned behaviour: A meta-analytic review. British Journal of Social Psychology. 2001;40:471–499. doi: 10.1348/014466601164939. [DOI] [PubMed] [Google Scholar]
- Bartra O, McGuire JT, Kable JW. The valuation system: A coordinate-based meta-analysis of BOLD fMRI experiments examining neural correlates of subjective value. NeuroImage. 2013;76:1–16. doi: 10.1016/j.neuroimage.2013.02.063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Becker M. The health belief model and personal health behavior. Health Education Monographs. 1974;2:324–473. [Google Scholar]
- Berkman ET, Falk EB. Beyond brain mapping: Using neural measures to predict real-world outcomes. Current Directions in Psychological Science. 2013;22(1):45–50. doi: 10.1177/0963721412469394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berns GS, Moore SE. A neural predictor of cultural popularity. Journal of Consumer Psychology. 2012;22(1):154–160. doi: 10.1016/j.jcps.2011.05.001. [DOI] [Google Scholar]
- Brett M, Anton JL, Valabregue R, Poline JB. Region of interest analysis using the MarsBar toolbox for SPM 99. NeuroImage. 2002;16:S497. [Google Scholar]
- Brug J, Steenhuis I, van Assema P, de Vries H. The impact of a computer-tailored nutrition intervention. Preventive Medicine. 1996;25:236–242. doi: 10.1006/pmed.1996.0052. [DOI] [PubMed] [Google Scholar]
- Buckner RL, Andrews-Hanna JR, Schacter DL. The brain’s default network: anatomy, function, and relevance to disease. Annals of the New York Academy of Sciences. 2008;1124(1):1–38. doi: 10.1196/annals.1440.011. [DOI] [PubMed] [Google Scholar]
- Buckner RL, Carroll DC. Self-projection and the brain. Trends in Cognitive Sciences. 2007;11(2):49–57. doi: 10.1016/j.tics.2006.11.004. [DOI] [PubMed] [Google Scholar]
- Cascio CN, Dal Cin S, Falk EB. Health communications: Predicting behavior change from the brain. New York: Springer; 2013. pp. 57–71. [DOI] [Google Scholar]
- Centers for Disease Control and Prevention. Current cigarette smoking among adults: United States, 2005–2012. Morbidity & Mortality Weekly Report. 2014;63:29–34. [PMC free article] [PubMed] [Google Scholar]
- Chib VS, Rangel A, Shimojo S, O’Doherty JP. Evidence for a common representation of decision values for dissimilar goods in human ventromedial prefrontal cortex. Journal of Neuroscience. 2009;29:12315–12320. doi: 10.1523/JNEUROSCI.2575-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chua HF, Ho SS, Jasinska AJ, Polk TA, Welsh RC, Liberzon I, Strecher VJ. Self-related neural response to tailored smoking-cessation messages predicts quitting. Nature Publishing Group. 2011;14:426–427. doi: 10.1038/nn.2761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chua HF, Liberzon I, Welsh RC, Strecher VJ. Neural correlates of message tailoring and self-relatedness in smoking cessation programming. Biological Psychiatry. 2009;65:165–168. doi: 10.1016/j.biopsych.2008.08.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clithero JA, Rangel A. Informatic parcellation of the network involved in the computation of subjective value. Social Cognitive and Affective Neuroscience. 2014;9:1289–1302. doi: 10.1093/scan/nst106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Costafreda SG, Khanna A, Mourao-Miranda J, Fu CHY. Neural correlates of sad faces predict clinical remission to cognitive behavioural therapy in depression. NeuroReport. 2009;20:637–641. doi: 10.1097/WNR.0b013e3283294159. [DOI] [PubMed] [Google Scholar]
- D’Argembeau A. On the role of the ventromedial prefrontal cortex in self-processing: the valuation hypothesis. Frontiers in Human Neuroscience. 2013;7:1–13. doi: 10.3389/fnhum.2013.00372/abstract. [DOI] [PMC free article] [PubMed] [Google Scholar]
- D’Argembeau A, Collette F, Van der Linden M, Laureys S, Del Fiore G, Degueldre C, Salmon E. Self-referential reflective activity and its relationship with rest: a PET study. NeuroImage. 2005;25:616–624. doi: 10.1016/j.neuroimage.2004.11.048. [DOI] [PubMed] [Google Scholar]
- Denny BT, Kober H, Wager TD, Ochsner KN. A meta-analysis of functional neuroimaging studies of self-and other judgments reveals a spatial gradient for mentalizing in medial prefrontal cortex. Journal of Cognitive Neuroscience. 2012;24:1742–1752. doi: 10.1162/jocn_a_00233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dijksterhuis A. Think different: The merits of unconscious thought in preference development and decision making. Journal of Personality and Social Psychology. 2004;87:586–598. doi: 10.1037/0022-3514.87.5.586. [DOI] [PubMed] [Google Scholar]
- Emery S, Kim Y, Choi YK, Szczypka G, Wakefield M, Chaloupka FJ. The effects of smoking-related television advertising on smoking and intentions to quit among adults in the United States: 1999–2007. American Journal of Public Health. 2012;102:751–757. doi: 10.2105/AJPH.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Etter JF, Due TV, Perneger TV. Saliva cotinine levels in smokers and nonsmokers. American Journal of Epidemiology. 2000;151:251–258. doi: 10.1093/oxfordjournals.aje.a010200. [DOI] [PubMed] [Google Scholar]
- Falk EB. Can neuroscience advance our understanding of core questions in Communication Studies? In: Jones S, editor. An overview of Communication Neuroscience. Communication@ the Center. New York: Hampton Press; 2013. pp. 77–94. [Google Scholar]
- Falk EB, Berkman ET, Lieberman MD. From neural responses to population behavior: Neural focus group predicts population-level media effects. Psychological Science. 2012;23:439–445. doi: 10.1177/0956797611434964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Falk EB, Berkman ET, Mann T, Harrison B, Lieberman MD. Predicting persuasion-induced behavior change from the brain. Journal of Neuroscience. 2010;30:8421–8424. doi: 10.1523/JNEUROSCI.0063-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Falk EB, Berkman ET, Whalen D, Lieberman MD. Neural activity during health messaging predicts reductions in smoking above and beyond self-report. Health Psychology. 2011;30:177–185. doi: 10.1037/a0022259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Falk EB, Rameson L, Berkman ET, Liao B, Kang Y, Inagaki TK, Lieberman MD. The neural correlates of persuasion: a common network across cultures and media. Journal of Cognitive Neuroscience. 2009;22:2447–2459. doi: 10.1162/jocn.2009.21363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fishbein M. Factors influencing behavior and behavior change. In: Baum A, Revenson TA, editors. Handbook of health psychology. Mahwah, NJ: Erlbaum; 2001. [Google Scholar]
- Gollwitzer PM. Implementation intentions: Strong effects of simple plans. American Psychologist. 1999;54:493–503. [Google Scholar]
- Grabenhorst F, Rolls ET, Margot C, da Silva MAAP, Velazco MI. How pleasant and unpleasant stimuli combine in different brain regions: odor mixtures. Journal of Neuroscience. 2007;27:13532–13540. doi: 10.1523/JNEUROSCI.3337-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gusnard DA, Raichle ME. Searching for a baseline: Functional imaging and the resting human brain. Nature Reviews: Neuroscience. 2001;2:685–694. doi: 10.1038/35094500. [DOI] [PubMed] [Google Scholar]
- Hasson U, Ghazanfar AA, Galantucci B, Garrod S, Keysers C. Brain-to-brain coupling: a mechanism for creating and sharing a social world. Trends in Cognitive Sciences. 2012;16(2):114–121. doi: 10.1016/j.tics.2011.12.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hasson U, Nir Y, Levy I, Fuhrmann G, Malach R. Intersubject synchronization of cortical activity during natural vision. Science. 2004;303(5664):1634–1640. doi: 10.1126/science.1089506. [DOI] [PubMed] [Google Scholar]
- Hornik RC. Exposure: Theory and evidence about all the ways it matters. Social Marketing Quarterly. 2002;8(3):31–37. [Google Scholar]
- Janz NK, Becker MH. The health belief model: A decade later. Health Education & Behavior. 1984;11(1):1–47. doi: 10.1177/109019818401100101. [DOI] [PubMed] [Google Scholar]
- Jasinska AJ, Chua HF, Ho SS, Polk TA, Rozek LS, Strecher VJ. Amygdala response to smoking-cessation messages mediates the effects of serotonin transporter gene variation on quitting. NeuroImage. 2010;60(1):1–8. doi: 10.1016/j.neuroimage.2011.12.064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kable JW, Glimcher PW. The neurobiology of decision: Consensus and controversy. Neuron. 2009;63:733–745. doi: 10.1016/j.neuron.2009.09.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- King-Casas B, Tomlin D, Anen C, Camerer CF, Quartz SR, Montague PR. Getting to know you: Reputation and trust in a two-person economic exchange. Science. 2005;308(5718):78–83. doi: 10.1029/2003PA000992. [DOI] [PubMed] [Google Scholar]
- Klebanoff MA, Levine RJ, Clemens JD, DerSimonian R, Wilkins DG. Serum cotinine concentration and self-reported smoking during pregnancy. American Journal of Epidemiology. 1998;148:259–262. doi: 10.1093/oxfordjournals.aje.a009633. [DOI] [PubMed] [Google Scholar]
- Klucharev V, Hytonen K, Rijpkema M, Smidts A, Fernandez G. Reinforcement learning signal predicts social conformity. Neuron. 2009;61(1):140–151. doi: 10.1016/j.neuron.2008.11.027. [DOI] [PubMed] [Google Scholar]
- Knutson B. Distributed neural representation of expected value. Journal of Neuroscience. 2005;25:4806–4812. doi: 10.1523/JNEUROSCI.0642-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kringelbach ML, O’Doherty J, Rolls ET, Andrews C. Activation of the human orbitofrontal cortex to a liquid food stimulus is correlated with its subjective pleasantness. Cerebral Cortex. 2003;13:1064–1071. doi: 10.1093/cercor/13.10.1064. [DOI] [PubMed] [Google Scholar]
- Langleben DD, Loughead JW, Ruparel K, Hakun JG, Busch-Winokur S, Strasser A, Lerman C. Reduced prefrontal and temporal processing and recall of high “sensation value” ads. NeuroImage. 2009;46(1):219–225. doi: 10.1016/j.neuroimage.2008.12.062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levy DJ, Glimcher PW. The root of all value: A neural common currency for choice. Current Opinion in Neurobiology. 2012;22:1–12. doi: 10.1016/j.conb.2012.06.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levy I, Lazzaro SC, Rutledge RB, Glimcher PW. Choice from non-choice: Predicting consumer preferences from blood oxygenation level-dependent signals obtained during passive viewing. Journal of Neuroscience. 2011;31(1):118–125. doi: 10.1523/JNEUROSCI.3214-10.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lieberman MD. Social cognitive neuroscience. In: Fiske S, Gilbert DT, Lindzey G, editors. Handbook of social psychology. 5. New York: McGraw-Hill; 2010. pp. 143–193. [Google Scholar]
- Mason MF, Dyer R, Norton MI. Neural mechanisms of social influence. Organizational Behavior and Human Decision Processes. 2009;110(2):152–159. doi: 10.1016/j.obhdp.2009.04.001. [DOI] [Google Scholar]
- Mason MF, Norton MI, Van Horn JD, Wegner DM, Grafton ST, Macrae CN. Wandering minds: The default network and stimulus-independent thought. Science. 2007;315(5810):393–395. doi: 10.1126/science.1131295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McClure EB, Adler A, Monk CS, Cameron J, Smith S, Nelson EE, Pine DS. fMRI predictors of treatment outcome in pediatric anxiety disorders. Psychopharmacology. 2006;191(1):97–105. doi: 10.1007/s00213-006-0542-9. [DOI] [PubMed] [Google Scholar]
- McClure SM, Ericson KM, Laibson DI, Loewenstein G, Cohen JD. Time discounting for primary rewards. Journal of Neuroscience. 2007;27(21):5796–5804. doi: 10.1523/JNEUROSCI.4246-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Menon V, Levitin DJ. The rewards of music listening: Response and physiological connectivity of the mesolimbic system. NeuroImage. 2005;28(1):175–184. doi: 10.1016/j.neuroimage.2005.05.053. [DOI] [PubMed] [Google Scholar]
- Montague PR, Lohrenz T. To detect and correct: Norm violations and their enforcement. Neuron. 2007;56(1):14–18. doi: 10.1016/j.neuron.2007.09.020. [DOI] [PubMed] [Google Scholar]
- Nisbett RE, Wilson TD. Telling more than we can know: Verbal reports on mental processes. Psychological Review. 1977;84:213–260. [Google Scholar]
- Noar SM, Benac CN, Harris MS. Does tailoring matter? Meta-analytic review of tailored print health behavior change interventions. Psychological Bulletin. 2007;133:673–693. doi: 10.1037/0033-2909.133.4.673. [DOI] [PubMed] [Google Scholar]
- Northoff G, Hayes DJ. Is our self nothing but reward? Biological Psychiatry. 2011;69:1019–1025. doi: 10.1016/j.biopsych.2010.12.014. [DOI] [PubMed] [Google Scholar]
- Northoff G, Heinzel A, de Greck M, Bermpohl F, Dobrowolny H, Panksepp J. Self-referential processing in our brain: A meta-analysis of imaging studies on the self. NeuroImage. 2006;31(1):440–457. doi: 10.1016/j.neuroimage.2005.12.002. [DOI] [PubMed] [Google Scholar]
- Paulus MP, Tapert SF, Schuckit MA. Neural activation patterns of methamphetamine-dependent subjects during decision making predict relapse. Archives of General Psychiatry. 2005;62:761–768. doi: 10.1001/archpsyc.62.7.761. [DOI] [PubMed] [Google Scholar]
- Petty R, Cacioppo J. The effects of involvement on responses to argument quantity and quality: Central and peripheral routes to persuasion. Journal of Personality and Social Psychology. 1984;46(1):69–81. [Google Scholar]
- Petty R, Cacioppo J. The effects of involvement on responses to argument quantity and quality: central and peripheral routes to persuasion. Journal of Personality and Social Psychology. 2001:1–13. [Google Scholar]
- Petty RE, Cacioppo JT. The elaboration likelihood model of persuasion. Advances in Experimental Social Psychology. 1986;19(1):123–205. [Google Scholar]
- Pickett KE, Rathouz PJ, Kasza K, Wakschlag LS, Wright R. Self-reported smoking, cotinine levels, and patterns of smoking in pregnancy. Paediatric and Perinatal Epidemiology. 2005;19:368–376. doi: 10.1111/j.1365-3016.2005.00660.x. [DOI] [PubMed] [Google Scholar]
- Pokorski TL, Chen WW, Bertholf RL. Use of urine cotinine to validate smoking self-reports in US Navy recruits. Addictive Behaviors. 1994;19:451–454. doi: 10.1016/0306-4603(94)90067-1. [DOI] [PubMed] [Google Scholar]
- Poldrack R. Can cognitive processes be inferred from neuroimaging data? Trends in Cognitive Sciences. 2006a;10(2):59–63. doi: 10.1016/j.tics.2005.12.004. [DOI] [PubMed] [Google Scholar]
- Poldrack RA. Region of interest analysis for fMRI. Social Cognitive and Affective Neuroscience. 2006b;2(1):67–70. doi: 10.1093/scan/nsm006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Poldrack RA. Inferring mental states from neuroimaging data: From reverse inference to large-scale decoding. Neuron. 2011;72:692–697. doi: 10.1016/j.neuron.2011.11.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raichle ME, MacLeod AM, Snyder AZ, Powers WJ, Gusnard DA, Shulman GL. A default mode of brain function. Proceedings of the National Academy of Sciences. 2001;98(2):676–682. doi: 10.1073/pnas.98.2.676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ramsay IS, Yzer MC, Luciana M, Vohs KD, MacDonald AW., III Affective and executive network processing associated with persuasive antidrug messages. Journal of Cognitive Neuroscience. 2013;25:1136–1147. doi: 10.1007/s11121-011-0212-y. [DOI] [PubMed] [Google Scholar]
- Rangel A, Camerer C, Montague PR. A framework for studying the neurobiology of value-based decision making. Nature Publishing Group. 2008;9:545–556. doi: 10.1038/nrn2357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rolls ET, O’Doherty J, Kringelbach ML, Francis S, Bowtell R, McGlone F. Representations of pleasant and painful touch in the human orbitofrontal and cingulate cortices. Cerebral Cortex. 2003;13:308–317. doi: 10.1093/cercor/13.3.308. [DOI] [PubMed] [Google Scholar]
- Rosenstock IM. Historical origins of the health belief model. Health Education Monographs. 1974;2:328–335. doi: 10.1177/109019817800600406. [DOI] [PubMed] [Google Scholar]
- Rosenstock IM, Stretcher VJ, Becker MH. Social learning theory and the health belief model. Health Education Quarterly. 1988;15(2):175–183. doi: 10.1177/109019818801500203. [DOI] [PubMed] [Google Scholar]
- Roy M, Shohamy D, Wager TD. Ventromedial prefrontal-subcortical systems and the generation of affective meaning. Trends in Cognitive Sciences. 2012;16(3):147–156. doi: 10.1016/j.tics.2012.01.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanfey AG, Rilling JK, Aronson JA, Nystrom LE, Cohen JD. The neural basis of economic decision-making in the ultimatum game. Science. 2003;300(5626):1755–1758. doi: 10.1126/science.1082976. [DOI] [PubMed] [Google Scholar]
- Saxe R, Brett M, Kanwisher N. Divide and conquer: A defense of functional localizers. NeuroImage. 2006;30:1088–1096. doi: 10.1016/j.neuroimage.2005.12.062. [DOI] [PubMed] [Google Scholar]
- Schacter DL, Addis DR, Buckner RL. Remembering the past to imagine the future: The prospective brain. Nature Reviews: Neuroscience. 2007;8:657–661. doi: 10.1038/nrn2213. [DOI] [PubMed] [Google Scholar]
- Schacter DL, Addis DR, Hassabis D, Martin VC, Spreng RN, Szpunar KK. The future of memory: Remembering, imagining, and the brain. Neuron. 2012;76:677–694. doi: 10.1016/j.neuron.2012.11.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmitz TW, Johnson SC. Self-appraisal decisions evoke dissociated dorsal—ventral aMPFC networks. NeuroImage. 2006;30:1050–1058. doi: 10.1016/j.neuroimage.2005.10.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmitz TW, Johnson SC. Relevance to self: A brief review and framework of neural systems underlying appraisal. Neuroscience and Biobehavioral Reviews. 2007;31:585–596. doi: 10.1016/j.neubiorev.2006.12.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schneider S, Gadinger M, Fischer A. Patient education and counseling. Patient education and counseling. 2012;86(1):77–83. doi: 10.1016/j.pec.2011.03.005. [DOI] [PubMed] [Google Scholar]
- Society, A. C. Cancer facts & figures 2012. Atlanta, GA: American Cancer Society; 2012. [Google Scholar]
- Spreng RN. The fallacy of a “task-negative” network. Frontiers in Psychology. 2012;3:1–5. doi: 10.3389/fpsyg.2012.00145/full. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spreng RN, Grady CL. Patterns of brain activity supporting autobiographical memory, prospection, and theory of mind, and their relationship to the default mode network. Journal of Cognitive Neuroscience. 2010;22:1112–1123. doi: 10.1162/jocn.2009.21282. [DOI] [PubMed] [Google Scholar]
- Spreng RN, Mar RA, Kim ASN. The common neural basis of autobiographical memory, prospection, navigation, theory of mind, and the default mode: A quantitative meta-analysis. Journal of Cognitive Neuroscience. 2009;21:489–510. doi: 10.1162/jocn.2008.21029. [DOI] [PubMed] [Google Scholar]
- Strecher VJ. Computer-tailored smoking cessation materials: A review and discussion. Patient Education and Counseling. 1999;36(2):107–117. doi: 10.1016/s0738-3991(98)00128-1. [DOI] [PubMed] [Google Scholar]
- Strecher V, Shiffman S, West R. Moderators and mediators of a Web-based computer-tailored smoking cessation program among nicotine patch users. Nicotine & Tobacco Research. 2006;8(1):95–101. doi: 10.1080/14622200601039444. [DOI] [PubMed] [Google Scholar]
- Tusche A, Bode S, Haynes JD. Neural responses to unattended products predict later consumer choices. Journal of Neuroscience. 2010;30:8024–8031. doi: 10.1523/JNEUROSCI.0064-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- US Department of Health and Human Services. The Health Consequences of Smoking—50 Years of Progress: A Report of the Surgeon General, 2014. 2014 Retrieved from http://www.surgeongeneral.gov/library/reports/50-years-of-progress/
- Vogt TM, Selvin S, Widdowson G, Hulley SB. Expired air carbon monoxide and serum thiocyanate as objective measures of cigarette exposure. American Journal of Public Health. 1977;67:545–549. doi: 10.2105/ajph.67.6.545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wakefield MA, Durkin S, Spittal MJ, Siahpush M, Scollo M, Simpson JA, Hill D. Impact of tobacco control policies and mass media campaigns on monthly adult smoking prevalence. American Journal of Public Health. 2008;98:1443–1450. doi: 10.2105/AJPH.2007.128991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wakefield MA, Loken B, Hornik RC. Use of mass media campaigns to change health behaviour. The Lancet. 2010;376(9748):1261–1271. doi: 10.1016/S0140-6736(10)60809-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang AL, Ruparel K, Loughead JW, Strasser AA, Blady SJ, Lynch KG, Langleben DD. Content matters: Neuroimaging investigation of brain and behavioral impact of televised anti-tobacco public service announcements. Journal of Neuroscience. 2013;33:7420–7427. doi: 10.1523/JNEUROSCI.3840-12.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Webb TL, Sheeran P. Does changing behavioral intentions engender behavior change? A meta-analysis of the experimental evidence. Psychological Bulletin. 2006;132:249–268. doi: 10.1037/0033-2909.132.2.249. [DOI] [PubMed] [Google Scholar]
- Weber R, Westcott-Baker A, Anderson G. A multilevel analysis of antimarijuana public service announcement effectiveness. Communication Monographs. 2013;80:302–330. doi: 10.1080/03637751.2013.788254. [DOI] [Google Scholar]
- Whitfield-Gabrieli S, Moran JM, Nieto-Castañón A, Triantafyllou C, Saxe R, Gabrieli JDE. Associations and dissociations between default and self-reference networks in the human brain. NeuroImage. 2011;55(1):225–232. doi: 10.1016/j.neuroimage.2010.11.048. [DOI] [PubMed] [Google Scholar]
- Wong NCH, Cappella JN. Antismoking threat and efficacy appeals: Effects on smoking cessation intentions for smokers with low and high readiness to quit. Journal of Applied Communication Research. 2009;37(1):1–20. doi: 10.1080/00909880802593928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wright AJ, French DP, Weinman J, Marteau TM. Can genetic risk information enhance motivation for smoking cessation? An analogue study. Health Psychology. 2006;25:740–752. doi: 10.1037/0278-6133.25.6.740. [DOI] [PubMed] [Google Scholar]
- Zaki J, Schirmer J, Mitchell JP. Social influence modulates the neural computation of value. Psychological Science. 2011;22:894–900. doi: 10.1177/0956797611411057. [DOI] [PubMed] [Google Scholar]