Abstract
Dendritic cells (DCs) are highly specialized immune cells that capture antigens and then migrate to lymphoid tissue and present antigen to T cells. This critical function of DCs is well defined, and recent studies further demonstrate that DCs are also key regulators of several innate immune responses. Studies focused on the roles of DCs in the pathogenesis of common lung diseases, such as asthma, infection, and cancer, have traditionally driven our mechanistic understanding of pulmonary DC biology. The emerging development of novel DC reagents, techniques, and genetically modified animal models has provided abundant data revealing distinct populations of DCs in the lung, and allow us to examine mechanisms of DC development, migration, and function in pulmonary disease with unprecedented detail. This enhanced understanding of DCs permits the examination of the potential role of DCs in diseases with known or suspected immunological underpinnings. Recent advances in the study of rare lung diseases, including pulmonary Langerhans cell histiocytosis, sarcoidosis, hypersensitivity pneumonitis, and pulmonary fibrosis, reveal expanding potential pathogenic roles for DCs. Here, we provide a review of DC development, trafficking, and effector functions in the lung, and discuss how alterations in these DC pathways contribute to the pathogenesis of rare lung diseases.
Keywords: dendritic cells, histiocytosis, sarcoidosis, fibrosis, hypersensitivity pneumonitis
Dendritic Cells
Dendritic cells (DCs) were first described by Ralph Steinman and Zanvil Cohn in 1973 (1) as cells with branching, extended cellular processes that distinguish them from other adherent myeloid cells, such as monocytes and macrophages. DCs are distributed throughout the body and are the “professional” antigen-presenting cells of the immune system. Most notably, they are known to acquire antigen in tissues, migrate to draining lymph nodes (LNs), and initiate T cell–mediated immunity. Recently, our understanding of the origins, development, and effector functions of DCs has greatly expanded with the advent of DC-specific reagents and genetically engineered mice. These newly developed tools and their functions are summarized in Table 1 (2–9). Human DCs are notoriously difficult to isolate, maintain, and study. Therefore, most of our understanding of DC biology is derived from studies performed in mice. We focus on DC biology based on mouse DCs, but cite relevant human data to the extent that it is available.
Table 1.
Currently Available Advanced Tools in Dendritic Cell Area, Their Applications, and Beneficial Areas
| Tools | Application | Beneficial Area | Reference |
|---|---|---|---|
| Immortalized DC line | DC gene modification, in vitro responsiveness | DC activation and signaling | 2 |
| Multichannel flow cytometry and monoclonal antibodies | DC surface marker measurement, intracellular staining, DC tracking | DC biology and characterization | 3 |
| Reporter genes | DC trafficking | DC migration | 4, 5 |
| Magnetic bead–based DC isolation/fluorescence-activated cell sorting | DC isolation | DC biology | 6 |
| Cre-Loxp/Crispr-Cas gene modification | Mouse model development, induce specific mutations in DC | Disease models, define DC role in disease pathogenesis | 7 |
| RNAseq/epigenetic analyses | DC gene expression assay | Subpopulation delineation | 8, 9 |
Definition of abbreviations: Cas, CRISPR associated protein; Cre, Cre recombinase; Crispr, clustered regularly interspaced short palindromic repeats; DC, dendritic cell; Loxp, locus of X[cross]-over in P1; RNAseq, RNA sequencing.
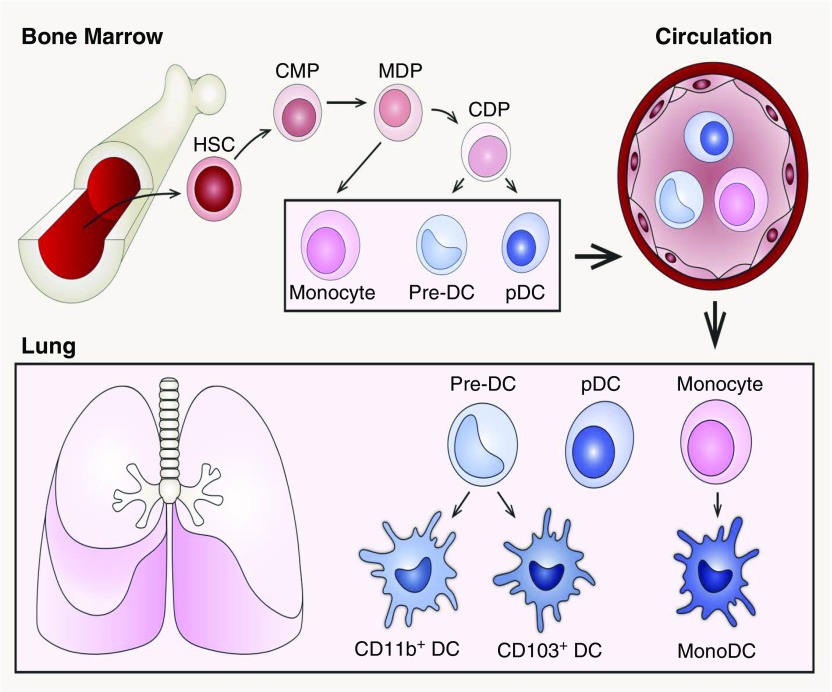
DCs originate in the bone marrow, circulate in the blood, and either enter the LNs via high endothelial venules (HEV) to give rise to lymphoid DCs or enter peripheral tissues to give rise to nonlymphoid DCs (10). Novel reporter mice reveal that DCs arise from monocyte–DC progenitors derived from hematopoietic stem cells. As illustrated in Figure 1, hematopoietic stem cells give rise to common myeloid progenitors, including a subset of FMS-related tyrosine kinase 3–expressing cells, which differentiate into more restricted macrophage and DC progenitors (MDPs) in the bone marrow. The FMS-related tyrosine kinase 3–expressing MDPs are the direct precursors to common DC progenitors, which give rise to precursor DCs (pre-DCs) and plasmacytoid DCs (pDCs) (11, 12). pre-DCs and pDCs leave the bone marrow and travel via the circulation to secondary lymphoid organs and nonlymphoid tissues. pre-DCs terminally differentiate into conventional DC (cDC) subsets in the periphery (13). Alternatively, MDPs give rise to common monocyte progenitors, which differentiate into circulating monocytes (14). Circulating Ly6C+ monocytes migrate into tissue or LNs via the HEV to either give rise to macrophages, if a niche is open, or differentiate into monocyte-derived DCs (moDCs) (15, 16). moDCs are defined by their expression of major histocompatibility complex class II and CD11c, and their DC functional properties, including antigen presentation (17).
Figure 1.
The origin and development of pulmonary dendritic cells (DCs) from hematopoietic stem cells (HSCs). In the bone marrow, HSCs give rise to common myeloid progenitors (CMPs), which differentiate into more restricted macrophage and DC progenitors (MDPs). MDPs give rise to common DC progenitors (CDPs), then to precursor DCs (pre-DCs) and plasmacytoid DCs (pDCs). Alternatively, MDPs can give rise to common monocyte progenitors, which then differentiate into monocytes in circulation. The pre-DCs, pDCs, and monocytes then leave the bone marrow and travel via the blood to secondary lymphoid organs and nonlymphoid tissues. After migrating into the lung, pre-DCs differentiate into CD103+ DC and CD11b+ DC subsets. Monocytes are recruited to the lungs under inflammatory stimuli and further differentiate into monocyte-derived DCs (monoDCs).
Pulmonary DC Populations
DCs are present throughout the epithelium and interstitium, where they are ideally positioned to monitor the luminal microenvironment. Flow cytometry is a powerful tool for studying the immune system, and DCs are most commonly distinguished from other cells by the expression of various combinations of surface markers. These markers include the presence and relative expression levels of adhesion molecules, chemokine and cytokine receptors, and Toll-like receptors (TLRs) as summarized in Table 2 (18–21). In mice, there are two major cDC subsets, CD103+ DCs and CD11b+ DCs, which are developmentally regulated by transcription factors basic leucine zipper transcription factor ATF-like 3 (Batf3) and interferon regulatory factor 4 (Irf4), respectively (22–25). CD103+ DCs and CD11b+ DCs have nonlymphoid and lymphoid counterparts based on function and origin. Nonlymphoid CD103+ DCs are thought to be the counterparts of lymphoid CD8+ DCs and nonlymphoid CD11b+ DCs of lymphoid CD8−CD11b+ DCs (15). Another major subset is pDCs, which are important in maintaining self-tolerance and are responsible for the production of large amounts of type I IFN-s (26, 27). In addition, the moDC population is a subset of DCs recruited from circulation into the lung in response to infection or injury (28).
Table 2.
Cell Surface Phenotype of Human and Mouse Dendritic Cells
| Species | Subpopulation | Subsets | Cell Surface Marker | Residence in Lung under Steady State | Toll-Like Receptors | CCRs on Immature DCs | CCRs on Mature DCs |
|---|---|---|---|---|---|---|---|
| Mouse | cDC | CD11b+ DC | CD11c+, CD8α−, CD11b hi, CD103−, MHC class II+, Langerin− | Yes | 1, 2, 4, 6, 7, 8, 9, 13 | CCR1, CCR2, CCR4, CCR5, CXCR3, CXCR4 | CCR7, CXCR4 |
| CD103+ DC | CD11c+, CD8α−, CD11b lo/−, CD103+, MHC class II+, Langerin+ | Yes | 2, 3, 4, 6, 9, 11, 12, 13 | ||||
| pDC | pDC | CD11c int, CD11b lo/−, B220+, Ly6c+, MHC class II−/lo, SiglecH+ | Yes | 7, 9, 12 | |||
| moDC | moDC | CD11c+, CD64+, CD11b hi, CD103−, MHC class II+, Langerin−, Ly6C+ | No | 3, 7, 8 | |||
| Human | cDC | CD1c+ DC | CD1c+, CD11b+, CD11c+, CD13+, HLA-DR+, BDCA-1+ | Yes | 1, 2, 3, 4, 5, 6, 8, 10 | CCR1, CCR2, CCR4, CCR5, CCR6, CXCR1, CXCR4 | CCR7, CXCR4 |
| CD141+ DC | CD141+, XCR1+, CLEC9A+, BDCA-3+ | Yes | 2, 3, 8 | ||||
| pDC | pDC | CD11c dim, BDCA2+, BDCA4+, CD123+, l-selectin+, IL-3R+, HLA-DR−/lo | Yes | 7, 9 | |||
| moDC | moDC | CD14+, HLA-DR+, CD1c−, CD141−, DC-SIGN+, CD163+ | No | 3, 7, 8 |
Definition of abbreviations: CCR, chemokine receptor; cDC, conventional DC; CXCR, C-X-C chemokine receptor; DC, dendritic cell; HLA-DR, human leukocyte antigen-antigen D related; MHC, major histocompatibility complex; moDC, monocyte-derived DC; pDC, plasmacytoid DC.
Compared with mouse DCs, there is no clear consensus regarding the classification of human pulmonary DC subsets. Human DCs and mouse DCs share basic similarities, and can be divided into cDC and pDC populations (29, 30). Genomic and functional studies indicate human epithelial-associated DCs can also be divided into four major subpopulations, which are: pDCs, CD1c+ DCs, CD141+ DCs, and moDCs (10, 31). The specific surface markers that facilitate discrimination between these subpopulations are detailed in Table 2. However, this classification has been challenged by a recent study that identified five different DC subsets in human lung based on differential expression of Langerin, CD1c, and CD14 (8). Clearly, it is important to obtain and collate additional transcriptional and functional data to definitively classify human pulmonary DCs and other mononuclear phagocytes.
DC Localization and Migration
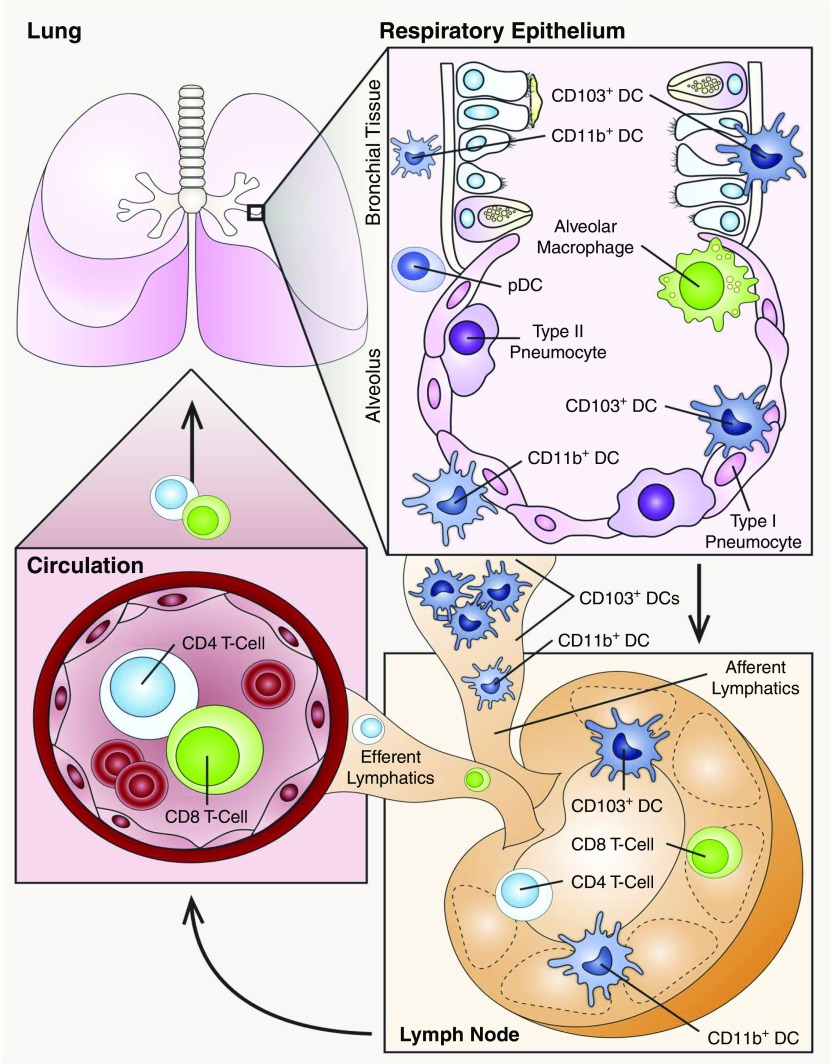
In mice, all DC subtypes are found in the lung under steady-state conditions (32). However, subsets of lung DCs are differentially distributed. CD103+ DCs are closely associated with the respiratory epithelium and extend processes into the airway lumen, whereas CD11b+ DCs mainly reside beneath the airway basement membrane (Figure 2) (33–35). Under steady-state conditions, a limited number of DCs reside in lung tissue and patrol the environment, whereas circulating pre-DCs migrate into the lung at a constant rate to replenish the resident DC pool, as it turns over every 10–14 days (13, 36, 37). In mice, DCs constitute up to 0.5–2% of total lung leukocytes at steady state (6). After exposure to antigens, pathogens, or proinflammatory cytokines, different subsets of lung DCs are rapidly recruited to the lung, and comprise up to 5% of total lung leukocytes (18). However, the number of DCs in the lung is difficult to estimate, given their relatively short lifespan and their migratory behavior. For example, after DCs encounter antigens, they become activated, deploy innate immune defenses, and migrate to draining mediastinal LNs, where they initiate adaptive immune responses and facilitate peripheral tolerance (38). Moreover, during inflammation, monocytes are rapidly recruited into the lung, differentiate into moDCs, and participate in the inflammatory response by secreting cytokines and chemokines, which, in turn, recruit and activate additional leukocytes (Figure 2) (28, 39).
Figure 2.
Distribution and migration of pulmonary DCs. Subsets of DCs are widely distributed throughout the lung, and segregate into unique niches that vary by lung compartment. In the upper respiratory tract, CD103+ DCs mainly reside in the mucosal wall and extend processes into the airway lumen in between epithelial cells. CD11b+ DCs and pDCs are mainly located underneath the basement membrane. After maturation, both CD103+ DCs and CD11b+ DCs can migrate to lymph nodes (LNs) via lymphatics and present antigens to T cells. CD103+ DCs are the migratory subsets that populate LNs.
DC migration is tightly regulated by specific chemokines and adhesion molecules under both physiological and inflammatory conditions (32). DC migration from the blood into the inflamed tissues is controlled by several chemokine receptors (CCRs), including CCR1, CCR2, CCR5, and CCR6 (40–42). CCR6 on immature DCs and its ligand, CCL20, is the most important chemokine axis for recruitment of DCs to the lung from circulation (32, 43). Lung epithelial cells express a basal level of CCL20 to maintain DC homeostasis. However, CCL20 expression is rapidly induced after encountering pathogenic and environmental stimuli (43). Lung DC recruitment is also mediated by additional CCRs, such as CCR2, as well as other factors that facilitate DC migration, including e- and p-selectins, adhesion molecule L1, and integrins CD11b/CD18 (44–47).
DC trafficking from the lung to the draining LNs rapidly increases during inflammation and infection (48, 49). Lymphatic channels provide important routes for cell migration and are critical for DC trafficking between peripheral tissues and LNs (50). In the lung, DCs first migrate to bronchopulmonary LNs through the lymphatics, then drain into the mediastinal trunk (Figure 2) (51). Egress of DCs from lung to draining LNs is tightly regulated (32). After pathogenic or environmental activation, DCs undergo maturation, decrease CCR6 expression, and increase CCR7 expression (32, 52). CCR7 is the CCR that drives DC migration along gradients toward higher concentrations of ligands, CCL19 and CCL21, produced in the lymphatics and draining LNs (53, 54). CCL19 is primarily secreted by stromal cells and mature DCs in the T cell zone of LNs (55), whereas CCL21 is produced constitutively by stromal cells and endothelial cells of lymphatic vessels and of HEVs (56). DC egress can also be influenced by other mediators, such as prostaglandin E2 and sphingosine-1-phosphate (57, 58). Knowledge of how human antigen-laden DCs migrate from lung to peripheral LNs is limited. However, like mouse DCs, human DCs decrease CCR6 expression and increase CCR7 expression after maturation allowing migration toward CCR7 ligands (59).
DC Function in Pulmonary Disease
Much of our knowledge of DC function in pulmonary disease derives from the study of infectious diseases and common lung diseases. We know that DC function is tissue, pathogen, and context specific. For example, during viral infection, DCs endocytose viral antigens and migrate to the mediastinal LNs, where they activate antigen-specific naive and memory T cells (60). Mouse studies reveal that these T cells undergo multiple rounds of proliferation and migrate out of the LN into peripheral tissues, where they interact with newly recruited DCs that present viral antigens within the infected tissue. In infected tissue, cytotoxic effector T cells orchestrate the direct killing of infected cells expressing viral antigens on the cell surface and contribute to the production of proinflammatory cytokines that help in the resolution of infection (61, 62). DCs respond differently in the context of Mycobacterium tuberculosis lung infections. During M. tuberculosis infection, DCs phagocytose the pathogen, leading to the production of high levels of inflammatory cytokines, including IL-6, TNF-α, IL-12, IL-1α, and IL-1β. These cytokines stimulate additional leukocytes and contribute to granuloma formation as a mechanism to control pathogen growth and distribution (63). The unique roles of DCs in asthma further exemplify the complex and plastic functional properties of DCs in lung diseases. In patients with asthma and mouse models of allergic asthma, multiple DC populations are increased in the lung that secrete lymphocyte activating cytokines, which are associated with disease severity (64). In asthma, otherwise harmless antigens modify airway epithelial barrier function and activate epithelial cells in a manner that leads to DC activation and the initiation of an allergic T cell response. For example, house dust mite feces contain allergens with proteolytic activity that costimulate TLRs on resident lung cells. These events lead to production of epithelial chemokines, including CCL20, which recruit lung DCs. Furthermore, the proteolytic activity of the allergens can promote the production of cytokines that drive immune responses in DCs (64–66).
DC Function in Rare Lung Disease
Pulmonary Langerhans Cell Histiocytosis
Pulmonary Langerhans cell histiocytosis (PLCH) is a rare interstitial lung disease characterized by the accumulation of Langerin-positive DCs, bronchiolocentric nodule formation, and cystic remodeling of the lung (67). PLCH is usually a single-system disorder, with pulmonary impairment ranging from asymptomatic disease to life-threatening respiratory failure (68). PLCH occurs almost exclusively in active and former smokers (67) with the crude prevalence estimated at 0.27 and 0.07 per 100,000 in males and females, respectively (68, 69). Historically, PLCH was considered an idiopathic reactive disease, because of large numbers of inflammatory cells found around pulmonary lesions and the presence of high levels of inflammatory cytokines (70). However, recent genetic analyses indicate that PLCH is more accurately defined as an inflammatory neoplastic disorder. This classification is based on studies that demonstrate more than 50% of patients with PLCH have an acquired, activating mutation in the proto-oncogene rapidly accelerated fibrosarcoma B (BRAF) within the DC lineage that results in constitutive activation of the mitogen-activated protein kinase (MAPK) pathway (71, 72). The most common mutation identified is BRAF V600E, but recent studies revealed mutations in other signaling proteins in the MAPK pathway (73, 74). Interestingly, the DCs in PLCH lesions express high levels of DC maturation markers, which may contribute to the local “cytokine storm” that drives nodule formation and/or cystic remodeling (75). DCs exhibit decreased expression of CCR6 and increased CCR7 expression after maturation, which promotes DC migration toward draining LNs (32). However, Fleming and colleagues (76) showed that DCs within lesions express both CCR6 and CCR7 in the pediatric form of systemic Langerhans cell histiocytosis. Therefore, it is possible that altered regulation of CCR6 and CCR7 contributes to the increased accumulation and activation of DCs in PLCH. Alternatively, the aberrant accumulation of DCs in the lung may be a consequence of enhanced DC proliferation or viability. Indeed, MAPK pathway activation, especially BRAFV600E, has been known to be involved in increased cell proliferation and/or decreased cell apoptosis in melanoma and thyroid cancer (77, 78).
In addition to genetic mutations in the MAPK pathway, cigarette smoke is believed to be a key complementary factor in PLCH pathogenesis. Smoking is known to increase the expression of several proinflammatory mediators in the lung, such as TNF-α, granulocyte/macrophage colony–stimulating factor, transforming growth factor-β, and CCL20, which are involved in DC differentiation and function (79). The effects of smoking on DC function have been investigated in other smoking-related diseases, such as chronic obstructive pulmonary disease (COPD). Overall, the number and maturation state of pulmonary DCs is increased in COPD. However, regional lung differences in patients with COPD are present, such as increased numbers of immature DCs in small airways and decreased total numbers of DCs in large airways (80–82). Cigarette smoke increases CCL20 and recruits DCs into the lung in patients with COPD and lung cancer (83–85). In addition, DCs from patients with COPD and mice exposed to cigarette smoke exhibit enhanced survival (86), and studies from smokers and mouse models indicate that multiple cytokines and chemokines that affect DC maturation, activation, and migration are elevated after smoking (87, 88). The facts that the majority of patients with PLCH are current smokers and that the disease sometimes regresses with smoking cessation suggest that the pulmonary microenvironment created by cigarette smoke is required for disease expression and persistence. However, the specific nature of the alterations in the pulmonary microenvironment responsible for recruiting and retaining DCs are unknown.
Sarcoidosis
Sarcoidosis is a systemic granulomatous disease of unknown etiology that causes inflammation and tissue damage in multiple organs, most commonly the lung. The incidence of sarcoidosis in the United States is approximately 10.9 and 35.5 per 100,000 persons of European and African descent, respectively (89, 90). Although the etiology is unknown, increasing evidence suggests that sarcoidosis is an antigen-driven autoimmune disease (91, 92). It is characterized by Th1 T cell polarization and exaggerated immune responses against unknown causative antigens (93–95). Although most research has focused on alveolar macrophages as the primary pathogenic antigen-presenting cells in sarcoidosis, more recent evidence indicates that DCs play a causative role. There is an increased number of DCs in the draining LNs of patients with sarcoidosis and, unlike other inflammatory lung diseases, there is an increased number of immature DCs in the lung tissue, especially the alveolar space (94). Furthermore, there is a twofold increase in cDCs in bronchoalveolar lavage (BAL) from patients with sarcoidosis, which express lower levels of the maturation marker, CD83, and the costimulatory molecule, CD86 (96). Pulmonary DCs from patients with sarcoidosis demonstrate a reduced capacity to induce T cell proliferation ex vivo compared with DCs isolated from healthy lungs, suggesting that the immature surface marker phenotype correlates with an observed reduction in DC function (97). IFN-γ is one of the most important cytokines that induce DC maturation and cytokine production, including the major Th1 polarizing cytokine, IL-12. Interestingly, IFN-γ and IL-12 levels are elevated in sarcoidosis BAL fluid, suggesting a defect in the process of IFN-γ–dependent DC maturation (98, 99). Furthermore, moDCs isolated from patients with sarcoidosis display normal maturation markers, but have an enhanced ability to induce TNF-α expression when cocultured with allogeneic naive CD4+ T cells (93). The role of TNF-α in sarcoidosis pathogenesis and prognosis has been well studied. Although the role of DCs remains to be defined, the high abundance of TNF-α receptor on DCs suggests that DCs may be important regulators of TNF-α–mediated disease progression. Similar to another DC-involved autoimmune disease, psoriasis, TNF-α inhibitors have been clinically tested in patients with sarcoidosis with some promising preliminary outcomes in selected patient subsets (100, 101). Due to undeveloped techniques and knowledge, most earlier studies did not differentiate macrophages from DCs in BAL fluid, and additional studies are necessary to define distinct roles in sarcoidosis. Taken together, these data clearly demonstrate dysfunction within the DC population in sarcoidosis, and the enrichment of DCs in the lung and draining LNs suggests that abnormal DC tracking contributes to pathogenesis. As increasing evidence emerges, it is possible that DC-targeted therapy may become a promising clinical option.
Hypersensitivity Pneumonitis
Hypersensitivity pneumonitis (HP) is an interstitial lung disease commonly characterized by granulomatous inflammation with prominent infiltration of lymphocytes, macrophages, and fibroblasts around indigestible antigens or particles. A large variety of antigen and/or particle exposures can result in HP. Based on the rate of progression, HP can be divided into acute, subacute, and chronic forms, although clinical presentations may overlap (102). HP pathogenesis is complex, but a consensus understanding of HP involves pre-existing genetic susceptibilities and/or environmental factors, followed by exposure to specific antigens. It has been suggested that acute HP is mediated by immune complex formation, whereas enhanced Th1 responses are responsible for the subacute and chronic forms (102). Studies also implicate cytotoxic delayed hypersensitivity in the observed abundance of Th1/Th17 cytokines and mechanisms of tissue injury (103). In addition, type III and type IV hypersensitivity have been noted to result in fewer granulomas, but more extensive fibrotic remodeling in some patients (104). Given the importance of DCs in maintaining lung immune homeostasis, the development of granuloma formation, and mediating hypersensitivity reactions, a causative role in the development and progression of HP is certain. Human data are limited, although there is a fivefold increase in the number of DCs in the lungs of patients with chronic HP compared with healthy control subjects (105). Much of our current understanding of HP pathogenesis is derived from animal models. An acute HP mouse model indicates that increased numbers of mature DCs persist in the lungs of Saccharopolyspora rectivirgula–challenged animals, and that these DCs play a causative role in granuloma formation via increased production of proinflammatory chemokines (106). Indeed, mice deficient in IFN-γ do not develop granulomatous lesions after exposure to S. rectivirgula (107). Furthermore, depletion of CD34, a cell surface receptor involved in DC migration and activation, impairs DC migration from lung to peripheral LN and renders mice resistant to the development of acute HP (108). The DCs from CD34-deficient mice show impaired cytokine secretion and fail to appropriately deliver antigen to T cells in the acute HP model. Mouse studies also reveal that DCs are critical to the development of HP pathology, as depletion of the pathogen recognition receptor, TLR9, renders DCs unable to respond to antigens. The end result is restricted Th1 cytokine and chemokine release, and attenuated lung pathology (109). Similar to patients with HP, there are increased numbers of mature DCs in the lungs of the acute HP mouse model (106). The apoptosis of granulocytes and nonhematopoietic cells are thought to contribute to HP pathogenesis by activating DCs to increase proinflammatory chemokine production (110). The local enrichment of these chemokines recruits other inflammatory cells to the lung, amplifying HP pathological changes. Together, the evidence suggests that DC maturation, migration, and interaction with other cells are critical to the pathogenesis of HP. Unfortunately, neither the human studies nor mouse models have identified specific populations of DCs involved in the pathogenesis of HP. Identifying these populations or other mechanistic details will facilitate future targeted approaches to reduce the immune-mediated components of HP pathology.
Idiopathic Interstitial Pneumonias
Idiopathic interstitial pneumonias (IIPs) are a cluster of diseases sharing similar clinical and radiologic presentations. Based on histological features, IIPs are further divided into eight different subsets (111). The etiopathogenesis of all types of IIPs is poorly understood. In this review, we focus on idiopathic pulmonary fibrosis (IPF) and nonspecific interstitial pneumonia (NSIP), the most common members of this family of scarring lung diseases. NSIP is an important IIP with a clinical presentation that is similar to IPF, but is associated with a better prognosis. NSIP is the second most common cause of IIP, and is commonly associated with autoimmune connective tissue diseases (112). Little is known about the pathogenesis of NSIP, except that there is a robust accumulation of DCs in the lungs of patients with NSIP, which are in close proximity to CD8+ and CD4+ lymphocytes (113). In contrast, there are several lines of research that strongly implicate DC dysfunction in the pathogenesis of IPF, which is one of the most lethal fibrotic lung diseases (114, 115). Lung biopsy of patients with IPF reveals usual interstitial pneumonia, characterized by patchy, spatially, and temporally heterogeneous areas of early and completed fibrosis in the lung periphery and lymphatic structures. The lungs of patients with IPF are heavily infiltrated by immature DCs in areas of epithelial hyperplasia and fibrosis, whereas mature DCs, characterized by the expression of maturation markers, like CD40, CD86, and CD80, accumulate in well organized, lymph node–like structures (116). This finding is supported by other studies demonstrating increased numbers of immature DCs in the lavage fluid of patients with IPF (117). Moreover, fibroblasts and epithelial cells in patients with IPF express high levels of CCL19, which can lead to increased DC recruitment from the circulation (118, 119). Along these lines, Freynet and colleagues (120) have shown that coculture of DCs with lung fibroblasts from patients with IPF diminishes the expression of DC activation markers. Together, these findings suggest that pulmonary fibroblasts may influence IPF progression by maintaining populations of immature DCs, which are unable to suppress ongoing inflammation.
The most common mouse model for IPF involves the administration of bleomycin to induce acute or subacute fibrosis in the lung. In this model, there is a significant accumulation of DCs in the lung after bleomycin challenge. However, unlike human IPF, the mouse DCs express high levels of maturation markers, including CD40, CD86, and CD83, and exist in close proximity to memory T cells (121). These findings are consistent with observations in other pulmonary diseases, which have revealed that maturation of lung DCs is followed by a secondary activation of memory T cells that may perpetuate chronic inflammation. Interestingly, experiments using antibodies to block DC maturation or deplete mature DC CCRs attenuate the pathological hallmarks of pulmonary fibrosis in this model (121, 122). Unfortunately, the discrepancy of DC phenotypes between mouse models and patients with IPF suggest that the bleomycin-induced model does not accurately reflect the immune alterations observed in human disease. Mouse models that more authentically recapitulate the immune dysfunction observed in IPF are needed to further our understanding of IPF pathogenesis and the possible involvement of DC dysfunction.
Conclusions
The recent development of DC-specific reagents and informative mouse models reveal new insights into the development, maintenance, and function of pulmonary DC populations. These advances have furthered our understanding of the unique roles of DCs in controlling tissue and immune homeostasis and in the initiation and progression of common and rare pulmonary diseases. Our future challenge is to develop high-fidelity animal models of rare lung disorders using newly available tools to study the contribution of DC development, trafficking, and lung-specific effector functions to disease pathogenesis. In addition, we need to conduct focused translational studies that use new DC-specific reagents to determine the causal relationship between specific alterations in DC function and pulmonary pathology. These efforts promise to reveal novel insights into rare diseases, inform our understanding of common lung diseases, and lead to the development of new treatment strategies.
Footnotes
This work was supported in part by the support from National Institutes of Health grants HL119538 (M.T.B.), HL115334 (C.J.), and U54HL127672 (F.X.M.), and Veteran’s Administration grant I01BX002347 (M.T.B.).
Author Contributions: Conception and design—M.T.B. and F.X.M.; drafting the manuscript for important intellectual content—H.L., C.J., A.R.O., R.L.N., N.G., F.X.M., and M.T.B.
Originally Published in Press as DOI: 10.1165/rcmb.2017-0051PS on June 6, 2017
Author disclosures are available with the text of this article at www.atsjournals.org.
References
- 1.Steinman RM, Cohn ZA. Identification of a novel cell type in peripheral lymphoid organs of mice. I. Morphology, quantitation, tissue distribution. J Exp Med. 1973;137:1142–1162. doi: 10.1084/jem.137.5.1142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Fuertes Marraco SA, Grosjean F, Duval A, Rosa M, Lavanchy C, Ashok D, Haller S, Otten LA, Steiner QG, Descombes P, et al. Novel murine dendritic cell lines: a powerful auxiliary tool for dendritic cell research. Front Immunol. 2012;3:331. doi: 10.3389/fimmu.2012.00331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Misharin AV, Morales-Nebreda L, Mutlu GM, Budinger GR, Perlman H. Flow cytometric analysis of macrophages and dendritic cell subsets in the mouse lung. Am J Respir Cell Mol Biol. 2013;49:503–510. doi: 10.1165/rcmb.2013-0086MA. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Kim HS, Woo J, Lee JH, Joo HJ, Choi Y, Kim H, Moon WK, Kim SJ. In vivo tracking of dendritic cell using MRI reporter gene, Ferritin. PLoS One. 2015;10:e0125291. doi: 10.1371/journal.pone.0125291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Lee HW, Yoon SY, Singh TD, Choi YJ, Lee HJ, Park JY, Jeong SY, Lee S-W, Ha J-H, Ahn B-C, et al. Tracking of dendritic cell migration into lymph nodes using molecular imaging with sodium iodide symporter and enhanced firefly luciferase genes. Sci Rep. 2015;5:9865. doi: 10.1038/srep09865. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Lancelin W, Guerrero-Plata A. Isolation of mouse lung dendritic cells. J Vis Exp. 2011;(57):pii:3563. doi: 10.3791/3563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Rocha-Martins M, Cavalheiro GR, Matos-Rodrigues GE, Martins RA. From gene targeting to genome editing: transgenic animals applications and beyond. An Acad Bras Cienc. 2015;87(2) suppl:1323–1348. doi: 10.1590/0001-3765201520140710. [DOI] [PubMed] [Google Scholar]
- 8.Patel VI, Booth JL, Duggan ES, Cate S, White VL, Hutchings D, Kovats S, Burian DM, Dozmorov M, Metcalf JP. Transcriptional classification and functional characterization of human airway macrophage and dendritic cell subsets. J Immunol. 2017;198:1183–1201. doi: 10.4049/jimmunol.1600777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Breton G, Zheng S, Valieris R, Tojal da Silva I, Satija R, Nussenzweig MC. Human dendritic cells (DCs) are derived from distinct circulating precursors that are precommitted to become CD1c+ or CD141+ DCs. J Exp Med. 2016;213:2861–2870. doi: 10.1084/jem.20161135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Collin M, McGovern N, Haniffa M. Human dendritic cell subsets. Immunology. 2013;140:22–30. doi: 10.1111/imm.12117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Liu K, Nussenzweig MC. Origin and development of dendritic cells. Immunol Rev. 2010;234:45–54. doi: 10.1111/j.0105-2896.2009.00879.x. [DOI] [PubMed] [Google Scholar]
- 12.Belz GT, Nutt SL. Transcriptional programming of the dendritic cell network. Nat Rev Immunol. 2012;12:101–113. doi: 10.1038/nri3149. [DOI] [PubMed] [Google Scholar]
- 13.Liu K, Victora GD, Schwickert TA, Guermonprez P, Meredith MM, Yao K, Chu FF, Randolph GJ, Rudensky AY, Nussenzweig M. In vivo analysis of dendritic cell development and homeostasis. Science. 2009;324:392–397. doi: 10.1126/science.1170540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hettinger J, Richards DM, Hansson J, Barra MM, Joschko AC, Krijgsveld J, Feuerer M. Origin of monocytes and macrophages in a committed progenitor. Nat Immunol. 2013;14:821–830. doi: 10.1038/ni.2638. [DOI] [PubMed] [Google Scholar]
- 15.Helft J, Ginhoux F, Bogunovic M, Merad M. Origin and functional heterogeneity of non-lymphoid tissue dendritic cells in mice. Immunol Rev. 2010;234:55–75. doi: 10.1111/j.0105-2896.2009.00885.x. [DOI] [PubMed] [Google Scholar]
- 16.Jakubzick C, Gautier EL, Gibbings SL, Sojka DK, Schlitzer A, Johnson TE, Ivanov S, Duan Q, Bala S, Condon T, et al. Minimal differentiation of classical monocytes as they survey steady-state tissues and transport antigen to lymph nodes. Immunity. 2013;39:599–610. doi: 10.1016/j.immuni.2013.08.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Larson SR, Atif SM, Gibbings SL, Thomas SM, Prabagar MG, Danhorn T, Leach SM, Henson PM, Jakubzick CV. Ly6C(+) monocyte efferocytosis and cross-presentation of cell-associated antigens. Cell Death Differ. 2016;23:997–1003. doi: 10.1038/cdd.2016.24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Condon TV, Sawyer RT, Fenton MJ, Riches DWH. Lung dendritic cells at the innate-adaptive immune interface. J Leukoc Biol. 2011;90:883–895. doi: 10.1189/jlb.0311134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Banchereau J, Briere F, Caux C, Davoust J, Lebecque S, Liu YJ, Pulendran B, Palucka K. Immunobiology of dendritic cells. Annu Rev Immunol. 2000;18:767–811. doi: 10.1146/annurev.immunol.18.1.767. [DOI] [PubMed] [Google Scholar]
- 20.Cravens PD, Lipsky PE. Dendritic cells, chemokine receptors and autoimmune inflammatory diseases. Immunol Cell Biol. 2002;80:497–505. doi: 10.1046/j.1440-1711.2002.01118.x. [DOI] [PubMed] [Google Scholar]
- 21.Worbs T, Hammerschmidt SI, Förster R. Dendritic cell migration in health and disease. Nat Rev Immunol. 2017;17:30–48. doi: 10.1038/nri.2016.116. [DOI] [PubMed] [Google Scholar]
- 22.Plantinga M, Hammad H, Lambrecht BN. Origin and functional specializations of DC subsets in the lung. Eur J Immunol. 2010;40:2112–2118. doi: 10.1002/eji.201040562. [DOI] [PubMed] [Google Scholar]
- 23.Kim TS, Braciale TJ. Respiratory dendritic cell subsets differ in their capacity to support the induction of virus-specific cytotoxic CD8+ T cell responses. PLoS One. 2009;4:e4204. doi: 10.1371/journal.pone.0004204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Hildner K, Edelson BT, Purtha WE, Diamond M, Matsushita H, Kohyama M, Calderon B, Schraml BU, Unanue ER, Diamond MS, et al. Batf3 deficiency reveals a critical role for CD8α+ dendritic cells in cytotoxic T cell immunity. Science. 2008;322:1097–1100. doi: 10.1126/science.1164206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Tamura T, Tailor P, Yamaoka K, Kong HJ, Tsujimura H, O’Shea JJ, Singh H, Ozato K. IFN regulatory factor-4 and -8 govern dendritic cell subset development and their functional diversity. J Immunol. 2005;174:2573–2581. doi: 10.4049/jimmunol.174.5.2573. [DOI] [PubMed] [Google Scholar]
- 26.Grouard G, Rissoan M-C, Filgueira L, Durand I, Banchereau J, Liu Y-J. The enigmatic plasmacytoid T cells develop into dendritic cells with interleukin (IL)-3 and CD40-ligand. J Exp Med. 1997;185:1101–1111. doi: 10.1084/jem.185.6.1101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Colonna M, Trinchieri G, Liu Y-J. Plasmacytoid dendritic cells in immunity. Nat Immunol. 2004;5:1219–1226. doi: 10.1038/ni1141. [DOI] [PubMed] [Google Scholar]
- 28.Lin KL, Suzuki Y, Nakano H, Ramsburg E, Gunn MD. CCR2+ monocyte–derived dendritic cells and exudate macrophages produce influenza-induced pulmonary immune pathology and mortality. J Immunol. 2008;180:2562–2572. doi: 10.4049/jimmunol.180.4.2562. [DOI] [PubMed] [Google Scholar]
- 29.Shortman K, Liu YJ. Mouse and human dendritic cell subtypes. Nat Rev Immunol. 2002;2:151–161. doi: 10.1038/nri746. [DOI] [PubMed] [Google Scholar]
- 30.Segura E, Amigorena S. Inflammatory dendritic cells in mice and humans. Trends Immunol. 2013;34:440–445. doi: 10.1016/j.it.2013.06.001. [DOI] [PubMed] [Google Scholar]
- 31.Desch AN, Gibbings SL, Goyal R, Kolde R, Bednarek J, Bruno T, Slansky JE, Jacobelli J, Mason R, Ito Y, et al. Flow cytometric analysis of mononuclear phagocytes in nondiseased human lung and lung-draining lymph nodes. Am J Respir Crit Care Med. 2016;193:614–626. doi: 10.1164/rccm.201507-1376OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Hammad H, Lambrecht BN. Lung dendritic cell migration. Adv Immunol. 2007;93:265–278. doi: 10.1016/S0065-2776(06)93007-7. [DOI] [PubMed] [Google Scholar]
- 33.Kim TH, Lee HK. Differential roles of lung dendritic cell subsets against respiratory virus infection. Immune Netw. 2014;14:128–137. doi: 10.4110/in.2014.14.3.128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Beaty SR, Rose CE, Jr, Sung SS. Diverse and potent chemokine production by lung CD11bhigh dendritic cells in homeostasis and in allergic lung inflammation. J Immunol. 2007;178:1882–1895. doi: 10.4049/jimmunol.178.3.1882. [DOI] [PubMed] [Google Scholar]
- 35.Shortman K, Naik SH. Steady-state and inflammatory dendritic-cell development. Nat Rev Immunol. 2007;7:19–30. doi: 10.1038/nri1996. [DOI] [PubMed] [Google Scholar]
- 36.Liu K, Waskow C, Liu X, Yao K, Hoh J, Nussenzweig M. Origin of dendritic cells in peripheral lymphoid organs of mice. Nat Immunol. 2007;8:578–583. doi: 10.1038/ni1462. [DOI] [PubMed] [Google Scholar]
- 37.Holt PG, Haining S, Nelson DJ, Sedgwick JD. Origin and steady-state turnover of class II MHC-bearing dendritic cells in the epithelium of the conducting airways. J Immunol. 1994;153:256–261. [PubMed] [Google Scholar]
- 38.Cook PC, MacDonald AS. Dendritic cells in lung immunopathology. Semin Immunopathol. 2016;38:449–460. doi: 10.1007/s00281-016-0571-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Manh TP, Alexandre Y, Baranek T, Crozat K, Dalod M. Plasmacytoid, conventional, and monocyte-derived dendritic cells undergo a profound and convergent genetic reprogramming during their maturation. Eur J Immunol. 2013;43:1706–1715. doi: 10.1002/eji.201243106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Geissmann F, Jung S, Littman DR. Blood monocytes consist of two principal subsets with distinct migratory properties. Immunity. 2003;19:71–82. doi: 10.1016/s1074-7613(03)00174-2. [DOI] [PubMed] [Google Scholar]
- 41.Tacke F, Alvarez D, Kaplan TJ, Jakubzick C, Spanbroek R, Llodra J, Garin A, Liu J, Mack M, van Rooijen N, et al. Monocyte subsets differentially employ CCR2, CCR5, and CX3CR1 to accumulate within atherosclerotic plaques. J Clin Invest. 2007;117:185–194. doi: 10.1172/JCI28549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Molon B, Gri G, Bettella M, Gómez-Moutón C, Lanzavecchia A, Martínez-A C, Mañes S, Viola A. T cell costimulation by chemokine receptors. Nat Immunol. 2005;6:465–471. doi: 10.1038/ni1191. [DOI] [PubMed] [Google Scholar]
- 43.Ito T, Carson WF, IV, Cavassani KA, Connett JM, Kunkel SL. CCR6 as a mediator of immunity in the lung and gut. Exp Cell Res. 2011;317:613–619. doi: 10.1016/j.yexcr.2010.12.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Pendl GG, Robert C, Steinert M, Thanos R, Eytner R, Borges E, Wild MK, Lowe JB, Fuhlbrigge RC, Kupper TS, et al. Immature mouse dendritic cells enter inflamed tissue, a process that requires e- and P-selectin, but not p-selectin glycoprotein ligand 1. Blood. 2002;99:946–956. doi: 10.1182/blood.v99.3.946. [DOI] [PubMed] [Google Scholar]
- 45.Schneeberger EE, Vu Q, LeBlanc BW, Doerschuk CM. The accumulation of dendritic cells in the lung is impaired in CD18−/− but not in ICAM-1−/− mutant mice. J Immunol. 2000;164:2472–2478. doi: 10.4049/jimmunol.164.5.2472. [DOI] [PubMed] [Google Scholar]
- 46.Maddaluno L, Verbrugge SE, Martinoli C, Matteoli G, Chiavelli A, Zeng Y, Williams ED, Rescigno M, Cavallaro U. The adhesion molecule L1 regulates transendothelial migration and trafficking of dendritic cells. J Exp Med. 2009;206:623–635. doi: 10.1084/jem.20081211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Robays LJ, Maes T, Lebecque S, Lira SA, Kuziel WA, Brusselle GG, Joos GF, Vermaelen KV. Chemokine receptor CCR2 but not CCR5 or CCR6 mediates the increase in pulmonary dendritic cells during allergic airway inflammation. J Immunol. 2007;178:5305–5311. doi: 10.4049/jimmunol.178.8.5305. [DOI] [PubMed] [Google Scholar]
- 48.Vermaelen K, Pauwels R. Accelerated airway dendritic cell maturation, trafficking, and elimination in a mouse model of asthma. Am J Respir Cell Mol Biol. 2003;29:405–409. doi: 10.1165/rcmb.2003-0008OC. [DOI] [PubMed] [Google Scholar]
- 49.Grayson MH, Ramos MS, Rohlfing MM, Kitchens R, Wang HD, Gould A, Agapov E, Holtzman MJ. Controls for lung dendritic cell maturation and migration during respiratory viral infection. J Immunol. 2007;179:1438–1448. doi: 10.4049/jimmunol.179.3.1438. [DOI] [PubMed] [Google Scholar]
- 50.Swartz MA, Hubbell JA, Reddy ST. Lymphatic drainage function and its immunological implications: from dendritic cell homing to vaccine design. Semin Immunol. 2008;20:147–156. doi: 10.1016/j.smim.2007.11.007. [DOI] [PubMed] [Google Scholar]
- 51.Schraufnagel DE. Lung lymphatic anatomy and correlates. Pathophysiology. 2010;17:337–343. doi: 10.1016/j.pathophys.2009.10.008. [DOI] [PubMed] [Google Scholar]
- 52.Nakano H, Burgents JE, Nakano K, Whitehead GS, Cheong C, Bortner CD, Cook DN. Migratory properties of pulmonary dendritic cells are determined by their developmental lineage. Mucosal Immunol. 2013;6:678–691. doi: 10.1038/mi.2012.106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Dieu MC, Vanbervliet B, Vicari A, Bridon JM, Oldham E, Aït-Yahia S, Brière F, Zlotnik A, Lebecque S, Caux C. Selective recruitment of immature and mature dendritic cells by distinct chemokines expressed in different anatomic sites. J Exp Med. 1998;188:373–386. doi: 10.1084/jem.188.2.373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Ricart BG, John B, Lee D, Hunter CA, Hammer DA. Dendritic cells distinguish individual chemokine signals through CCR7 and CXCR4. J Immunol. 2011;186:53–61. doi: 10.4049/jimmunol.1002358. [DOI] [PubMed] [Google Scholar]
- 55.Willimann K, Legler DF, Loetscher M, Roos RS, Delgado MB, Clark-Lewis I, Baggiolini M, Moser B. The chemokine SLC is expressed in T cell areas of lymph nodes and mucosal lymphoid tissues and attracts activated T cells via CCR7. Eur J Immunol. 1998;28:2025–2034. doi: 10.1002/(SICI)1521-4141(199806)28:06<2025::AID-IMMU2025>3.0.CO;2-C. [DOI] [PubMed] [Google Scholar]
- 56.Gunn MD, Tangemann K, Tam C, Cyster JG, Rosen SD, Williams LT. A chemokine expressed in lymphoid high endothelial venules promotes the adhesion and chemotaxis of naive T lymphocytes. Proc Natl Acad Sci USA. 1998;95:258–263. doi: 10.1073/pnas.95.1.258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Yen JH, Khayrullina T, Ganea D. PGE2-induced metalloproteinase-9 is essential for dendritic cell migration. Blood. 2008;111:260–270. doi: 10.1182/blood-2007-05-090613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Mandala S, Hajdu R, Bergstrom J, Quackenbush E, Xie J, Milligan J, Thornton R, Shei GJ, Card D, Keohane C, et al. Alteration of lymphocyte trafficking by sphingosine-1-phosphate receptor agonists. Science. 2002;296:346–349. doi: 10.1126/science.1070238. [DOI] [PubMed] [Google Scholar]
- 59.Clatworthy MR, Aronin CE, Mathews RJ, Morgan NY, Smith KG, Germain RN. Immune complexes stimulate CCR7-dependent dendritic cell migration to lymph nodes. Nat Med. 2014;20:1458–1463. doi: 10.1038/nm.3709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Belz G, Mount A, Masson F.Dendritic cells in viral infections Dendritic cells Lombardi G, Riffo-Vasquez Y.New York: Springer; 200951–77. [DOI] [PubMed] [Google Scholar]
- 61.Neyt K, Lambrecht BN. The role of lung dendritic cell subsets in immunity to respiratory viruses. Immunol Rev. 2013;255:57–67. doi: 10.1111/imr.12100. [DOI] [PubMed] [Google Scholar]
- 62.Yoo J-K, Kim TS, Hufford MM, Braciale TJ.Viral infection of the lung: host response and sequelae J Allergy Clin Immunol 20131321263–1276.[Quiz, 1277.] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Mihret A. The role of dendritic cells in Mycobacterium tuberculosis infection. Virulence. 2012;3:654–659. doi: 10.4161/viru.22586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Gill MA. The role of dendritic cells in asthma. J Allergy Clin Immunol. 2012;129:889–901. doi: 10.1016/j.jaci.2012.02.028. [DOI] [PubMed] [Google Scholar]
- 65.van Helden MJ, Lambrecht BN. Dendritic cells in asthma. Curr Opin Immunol. 2013;25:745–754. doi: 10.1016/j.coi.2013.10.002. [DOI] [PubMed] [Google Scholar]
- 66.Nathan AT, Peterson EA, Chakir J, Wills-Karp M. Innate immune responses of airway epithelium to house dust mite are mediated through β-glucan–dependent pathways. J Allergy Clin Immunol. 2009;123:612–618. doi: 10.1016/j.jaci.2008.12.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Vassallo R, Ryu JH, Colby TV, Hartman T, Limper AH. Pulmonary Langerhans’-cell histiocytosis. N Engl J Med. 2000;342:1969–1978. doi: 10.1056/NEJM200006293422607. [DOI] [PubMed] [Google Scholar]
- 68.Vassallo R, Ryu JH, Schroeder DR, Decker PA, Limper AH. Clinical outcomes of pulmonary Langerhans’-cell histiocytosis in adults. N Engl J Med. 2002;346:484–490. doi: 10.1056/NEJMoa012087. [DOI] [PubMed] [Google Scholar]
- 69.Aricò M, Girschikofsky M, Généreau T, Klersy C, McClain K, Grois N, Emile JF, Lukina E, De Juli E, Danesino C. Langerhans cell histiocytosis in adults: report from the International Registry of the Histiocyte Society. Eur J Cancer. 2003;39:2341–2348. doi: 10.1016/s0959-8049(03)00672-5. [DOI] [PubMed] [Google Scholar]
- 70.Elia D, Torre O, Cassandro R, Caminati A, Harari S. Pulmonary Langerhans cell histiocytosis: a comprehensive analysis of 40 patients and literature review. Eur J Intern Med. 2015;26:351–356. doi: 10.1016/j.ejim.2015.04.001. [DOI] [PubMed] [Google Scholar]
- 71.Badalian-Very G, Vergilio JA, Degar BA, MacConaill LE, Brandner B, Calicchio ML, Kuo FC, Ligon AH, Stevenson KE, Kehoe SM, et al. Recurrent BRAF mutations in Langerhans cell histiocytosis. Blood. 2010;116:1919–1923. doi: 10.1182/blood-2010-04-279083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Kamionek M, Ahmadi Moghaddam P, Sakhdari A, Kovach AE, Welch M, Meng X, Dresser K, Tomaszewicz K, Cosar EF, Mark EJ, et al. Mutually exclusive extracellular signal–regulated kinase pathway mutations are present in different stages of multi-focal pulmonary Langerhans cell histiocytosis supporting clonal nature of the disease. Histopathology. 2016;69:499–509. doi: 10.1111/his.12955. [DOI] [PubMed] [Google Scholar]
- 73.Chakraborty R, Hampton OA, Shen X, Simko SJ, Shih A, Abhyankar H, Lim KP, Covington KR, Trevino L, Dewal N, et al. Mutually exclusive recurrent somatic mutations in MAP2K1 and BRAF support a central role for ERK activation in LCH pathogenesis. Blood. 2014;124:3007–3015. doi: 10.1182/blood-2014-05-577825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Mourah S, How-Kit A, Meignin V, Gossot D, Lorillon G, Bugnet E, Mauger F, Lebbe C, Chevret S, Tost J, et al. Recurrent NRAS mutations in pulmonary Langerhans cell histiocytosis. Eur Respir J. 2016;47:1785–1796. doi: 10.1183/13993003.01677-2015. [DOI] [PubMed] [Google Scholar]
- 75.Tazi A, Moreau J, Bergeron A, Dominique S, Hance AJ, Soler P. Evidence that Langerhans cells in adult pulmonary Langerhans cell histiocytosis are mature dendritic cells: importance of the cytokine microenvironment. J Immunol. 1999;163:3511–3515. [PubMed] [Google Scholar]
- 76.Fleming MD, Pinkus JL, Fournier MV, Alexander SW, Tam C, Loda M, Sallan SE, Nichols KE, Carpentieri DF, Pinkus GS, et al. Coincident expression of the chemokine receptors CCR6 and CCR7 by pathologic Langerhans cells in Langerhans cell histiocytosis. Blood. 2003;101:2473–2475. doi: 10.1182/blood.V101.7.2473. [DOI] [PubMed] [Google Scholar]
- 77.Cantwell-Dorris ER, O’Leary JJ, Sheils OM. BRAFV600E: implications for carcinogenesis and molecular therapy. Mol Cancer Ther. 2011;10:385–394. doi: 10.1158/1535-7163.MCT-10-0799. [DOI] [PubMed] [Google Scholar]
- 78.Jinih M, Foley N, Osho O, Houlihan L, Toor AA, Khan JZ, Achakzai AA, Redmond HP. BRAFV600E mutation as a predictor of thyroid malignancy in indeterminate nodules: a systematic review and meta-analysis. Eur J Surg Oncol. 2017;43:1219–1227. doi: 10.1016/j.ejso.2016.11.003. [DOI] [PubMed] [Google Scholar]
- 79.Bracke KR, D’hulst AI, Maes T, Moerloose KB, Demedts IK, Lebecque S, Joos GF, Brusselle GG. Cigarette smoke–induced pulmonary inflammation and emphysema are attenuated in CCR6-deficient mice. J Immunol. 2006;177:4350–4359. doi: 10.4049/jimmunol.177.7.4350. [DOI] [PubMed] [Google Scholar]
- 80.Van Pottelberge GR, Bracke KR, Joos GF, Brusselle GG. The role of dendritic cells in the pathogenesis of COPD: liaison officers in the front line. COPD. 2009;6:284–290. doi: 10.1080/15412550903049124. [DOI] [PubMed] [Google Scholar]
- 81.Tsoumakidou M, Demedts IK, Brusselle GG, Jeffery PK. Dendritic cells in chronic obstructive pulmonary disease: new players in an old game. Am J Respir Crit Care Med. 2008;177:1180–1186. doi: 10.1164/rccm.200711-1727PP. [DOI] [PubMed] [Google Scholar]
- 82.Givi ME, Redegeld FA, Folkerts G, Mortaz E. Dendritic cells in pathogenesis of COPD. Curr Pharm Des. 2012;18:2329–2335. doi: 10.2174/138161212800166068. [DOI] [PubMed] [Google Scholar]
- 83.Wang B, Shi L, Sun X, Wang L, Wang X, Chen C. Production of CCL20 from lung cancer cells induces the cell migration and proliferation through PI3K pathway. J Cell Mol Med. 2016;20:920–929. doi: 10.1111/jcmm.12781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Wang GZ, Cheng X, Li XC, Liu YQ, Wang XQ, Shi X, Wang ZY, Guo YQ, Wen ZS, Huang YC, et al. Tobacco smoke induces production of chemokine CCL20 to promote lung cancer. Cancer Lett. 2015;363:60–70. doi: 10.1016/j.canlet.2015.04.005. [DOI] [PubMed] [Google Scholar]
- 85.Demedts IK, Bracke KR, Van Pottelberge G, Testelmans D, Verleden GM, Vermassen FE, Joos GF, Brusselle GG. Accumulation of dendritic cells and increased CCL20 levels in the airways of patients with chronic obstructive pulmonary disease. Am J Respir Crit Care Med. 2007;175:998–1005. doi: 10.1164/rccm.200608-1113OC. [DOI] [PubMed] [Google Scholar]
- 86.Vassallo R, Walters PR, Lamont J, Kottom TJ, Yi ES, Limper AH. Cigarette smoke promotes dendritic cell accumulation in COPD; a Lung Tissue Research Consortium study. Respir Res. 2010;11:45. doi: 10.1186/1465-9921-11-45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Suri HS, Yi ES, Nowakowski GS, Vassallo R. Pulmonary langerhans cell histiocytosis. Orphanet J Rare Dis. 2012;7:16. doi: 10.1186/1750-1172-7-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Givi ME, Peck MJ, Boon L, Mortaz E. The role of dendritic cells in the pathogenesis of cigarette smoke–induced emphysema in mice. Eur J Pharmacol. 2013;721:259–266. doi: 10.1016/j.ejphar.2013.09.027. [DOI] [PubMed] [Google Scholar]
- 89.Sawahata M, Sugiyama Y. An epidemiological perspective of the pathology and etiology of sarcoidosis. Sarcoidosis Vasc Diffuse Lung Dis. 2016;33:112–116. [PubMed] [Google Scholar]
- 90.Ungprasert P, Crowson CS, Matteson EL. Seasonal variation in incidence of sarcoidosis: a population-based study, 1976–2013. Thorax. 2016;12:1164–1166. doi: 10.1136/thoraxjnl-2016-209032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Forman JD, Klein JT, Silver RF, Liu MC, Greenlee BM, Moller DR. Selective activation and accumulation of oligoclonal V β–specific T cells in active pulmonary sarcoidosis. J Clin Invest. 1994;94:1533–1542. doi: 10.1172/JCI117494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Baughman RP, Culver DA, Judson MA. A concise review of pulmonary sarcoidosis. Am J Respir Crit Care Med. 2011;183:573–581. doi: 10.1164/rccm.201006-0865CI. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Ten Berge B, Kleinjan A, Muskens F, Hammad H, Hoogsteden HC, Hendriks RW, Lambrecht BN, Van den Blink B. Evidence for local dendritic cell activation in pulmonary sarcoidosis. Respir Res. 2012;13:33. doi: 10.1186/1465-9921-13-33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Ota M, Amakawa R, Uehira K, Ito T, Yagi Y, Oshiro A, Date Y, Oyaizu H, Shigeki T, Ozaki Y, et al. Involvement of dendritic cells in sarcoidosis. Thorax. 2004;59:408–413. doi: 10.1136/thx.2003.006049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Zaba LC, Smith GP, Sanchez M, Prystowsky SD. Dendritic cells in the pathogenesis of sarcoidosis. Am J Respir Cell Mol Biol. 2010;42:32–39. doi: 10.1165/rcmb.2009-0033TR. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Lommatzsch M, Bratke K, Bier A, Julius P, Kuepper M, Luttmann W, Virchow JC. Airway dendritic cell phenotypes in inflammatory diseases of the human lung. Eur Respir J. 2007;30:878–886. doi: 10.1183/09031936.00036307. [DOI] [PubMed] [Google Scholar]
- 97.Spiteri MA, Clarke SW, Poulter LW. Phenotypic and functional changes in alveolar macrophages contribute to the pathogenesis of pulmonary sarcoidosis. Clin Exp Immunol. 1988;74:359–364. [PMC free article] [PubMed] [Google Scholar]
- 98.Moller DR, Forman JD, Liu MC, Noble PW, Greenlee BM, Vyas P, Holden DA, Forrester JM, Lazarus A, Wysocka M, et al. Enhanced expression of IL-12 associated with Th1 cytokine profiles in active pulmonary sarcoidosis. J Immunol. 1996;156:4952–4960. [PubMed] [Google Scholar]
- 99.Wahlström J, Berlin M, Sköld CM, Wigzell H, Eklund A, Grunewald J. Phenotypic analysis of lymphocytes and monocytes/macrophages in peripheral blood and bronchoalveolar lavage fluid from patients with pulmonary sarcoidosis. Thorax. 1999;54:339–346. doi: 10.1136/thx.54.4.339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Ziegenhagen MW, Müller-Quernheim J. The cytokine network in sarcoidosis and its clinical relevance. J Intern Med. 2003;253:18–30. doi: 10.1046/j.1365-2796.2003.01074.x. [DOI] [PubMed] [Google Scholar]
- 101.Doty JD, Mazur JE, Judson MA. Treatment of sarcoidosis with infliximab. Chest. 2005;127:1064–1071. doi: 10.1378/chest.127.3.1064. [DOI] [PubMed] [Google Scholar]
- 102.Selman M, Pardo A, King TE., Jr Hypersensitivity pneumonitis: insights in diagnosis and pathobiology. Am J Respir Crit Care Med. 2012;186:314–324. doi: 10.1164/rccm.201203-0513CI. [DOI] [PubMed] [Google Scholar]
- 103.Agache IO, Rogozea L. Management of hypersensivity pneumonitis. Clin Transl Allergy. 2013;3:5. doi: 10.1186/2045-7022-3-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Myers JL. Hypersensitivity pneumonia: the role of lung biopsy in diagnosis and management. Mod Pathol. 2012;25:S58–S67. doi: 10.1038/modpathol.2011.152. [DOI] [PubMed] [Google Scholar]
- 105.Greer AM, Matthay MA, Kukreja J, Bhakta NR, Nguyen CP, Wolters PJ, Woodruff PG, Fahy JV, Shin JS. Accumulation of BDCA1+ dendritic cells in interstitial fibrotic lung diseases and Th2-high asthma. PLoS One. 2014;9:e99084. doi: 10.1371/journal.pone.0099084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Girard M, Israël-Assayag E, Cormier Y. Mature CD11c(+) cells are enhanced in hypersensitivity pneumonitis. Eur Respir J. 2009;34:749–756. doi: 10.1183/09031936.00140908. [DOI] [PubMed] [Google Scholar]
- 107.Gudmundsson G, Hunninghake GW. Interferon-γ is necessary for the expression of hypersensitivity pneumonitis. J Clin Invest. 1997;99:2386–2390. doi: 10.1172/JCI119420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Blanchet MR, Bennett JL, Gold MJ, Levantini E, Tenen DG, Girard M, Cormier Y, McNagny KM. CD34 is required for dendritic cell trafficking and pathology in murine hypersensitivity pneumonitis. Am J Respir Crit Care Med. 2011;184:687–698. doi: 10.1164/rccm.201011-1764OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Daito H, Kikuchi T, Sakakibara T, Gomi K, Damayanti T, Zaini J, Tode N, Kanehira M, Koyama S, Fujimura S, et al. Mycobacterial hypersensitivity pneumonitis requires TLR9-MyD88 in lung CD11b+ CD11c+ cells. Eur Respir J. 2011;38:688–701. doi: 10.1183/09031936.00177110. [DOI] [PubMed] [Google Scholar]
- 110.Hwang SJ, Kim HS, Chung DH. Fas/Fas ligand-mediated apoptosis promotes hypersensitivity pneumonitis in mice by enhancing maturation of dendritic cells. Am J Respir Crit Care Med. 2010;181:1250–1261. doi: 10.1164/rccm.200909-1337OC. [DOI] [PubMed] [Google Scholar]
- 111.Travis WD, Costabel U, Hansell DM, King TE, Jr, Lynch DA, Nicholson AG, Ryerson CJ, Ryu JH, Selman M, Wells AU, et al. ATS/ERS Committee on Idiopathic Interstitial Pneumonias. An official American Thoracic Society/European Respiratory Society statement: Update of the international multidisciplinary classification of the idiopathic interstitial pneumonias. Am J Respir Crit Care Med. 2013;188:733–748. doi: 10.1164/rccm.201308-1483ST. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Collard HR, King TE., Jr Demystifying idiopathic interstitial pneumonia. Arch Intern Med. 2003;163:17–29. doi: 10.1001/archinte.163.1.17. [DOI] [PubMed] [Google Scholar]
- 113.Shimizu S, Yoshinouchi T, Ohtsuki Y, Fujita J, Sugiura Y, Banno S, Yamadori I, Eimoto T, Ueda R. The appearance of S-100 protein-positive dendritic cells and the distribution of lymphocyte subsets in idiopathic nonspecific interstitial pneumonia. Respir Med. 2002;96:770–776. doi: 10.1053/rmed.2002.1345. [DOI] [PubMed] [Google Scholar]
- 114.Raghu G, Collard HR, Egan JJ, Martinez FJ, Behr J, Brown KK, Colby TV, Cordier JF, Flaherty KR, Lasky JA, et al. ATS/ERS/JRS/ALAT Committee on Idiopathic Pulmonary Fibrosis. An official ATS/ERS/JRS/ALAT statement: idiopathic pulmonary fibrosis: evidence-based guidelines for diagnosis and management. Am J Respir Crit Care Med. 2011;183:788–824. doi: 10.1164/rccm.2009-040GL. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Ley B, Collard HR. Epidemiology of idiopathic pulmonary fibrosis. Clin Epidemiol. 2013;5:483–492. doi: 10.2147/CLEP.S54815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Marchal-Sommé J, Uzunhan Y, Marchand-Adam S, Valeyre D, Soumelis V, Crestani B, Soler P. Cutting edge: nonproliferating mature immune cells form a novel type of organized lymphoid structure in idiopathic pulmonary fibrosis. J Immunol. 2006;176:5735–5739. doi: 10.4049/jimmunol.176.10.5735. [DOI] [PubMed] [Google Scholar]
- 117.Tsoumakidou M, Karagiannis KP, Bouloukaki I, Zakynthinos S, Tzanakis N, Siafakas NM. Increased bronchoalveolar lavage fluid CD1c expressing dendritic cells in idiopathic pulmonary fibrosis. Respiration. 2009;78:446–452. doi: 10.1159/000226244. [DOI] [PubMed] [Google Scholar]
- 118.Marchal-Sommé J, Uzunhan Y, Marchand-Adam S, Kambouchner M, Valeyre D, Crestani B, Soler P. Dendritic cells accumulate in human fibrotic interstitial lung disease. Am J Respir Crit Care Med. 2007;176:1007–1014. doi: 10.1164/rccm.200609-1347OC. [DOI] [PubMed] [Google Scholar]
- 119.Nuovo GJ, Hagood JS, Magro CM, Chin N, Kapil R, Davis L, Marsh CB, Folcik VA. The distribution of immunomodulatory cells in the lungs of patients with idiopathic pulmonary fibrosis. Mod Pathol. 2012;25:416–433. doi: 10.1038/modpathol.2011.166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Freynet O, Marchal-Sommé J, Jean-Louis F, Mailleux A, Crestani B, Soler P, Michel L. Human lung fibroblasts may modulate dendritic cell phenotype and function: results from a pilot in vitro study. Respir Res. 2016;17:36. doi: 10.1186/s12931-016-0345-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Bantsimba-Malanda C, Marchal-Sommé J, Goven D, Freynet O, Michel L, Crestani B, Soler P. A role for dendritic cells in bleomycin-induced pulmonary fibrosis in mice? Am J Respir Crit Care Med. 2010;182:385–395. doi: 10.1164/rccm.200907-1164OC. [DOI] [PubMed] [Google Scholar]
- 122.Trujillo G, Hartigan AJ, Hogaboam CM. T regulatory cells and attenuated bleomycin-induced fibrosis in lungs of CCR7−/− mice. Fibrogenesis Tissue Repair. 2010;3:18. doi: 10.1186/1755-1536-3-18. [DOI] [PMC free article] [PubMed] [Google Scholar]