Abstract
Introduction
The success of a curative surgery for cancer is dependent on the complete removal of all cancer cells. Tumor visualization by the surgeon can be enhanced through fluorescent-antibody targeting. To further develop such technology, we selected humanized anti-carcinoembryonic antigen (CEA) conjugated to a near-infrared (NIR) dye to target orthotopically implanted human colon cancer in nude mice.
Materials & Methods
The HT-29 human colon cancer cell line was grown in culture and subcutaneously injected subcutaneously in mice. After 3 weeks of growth, tumors were resected and cut into 2 mm3 fragments that were sutured to the cecum of 5 additional nude mice for orthotopic implantation. The tumors were allowed to grow for 4 weeks at which point 3 had successful orthotopic tumor growth and were selected for injection of the humanized anti-CEA antibody conjugated to the NIR dye IRDye800CW (anti-CEA-IRDye800CW). The antibody-dye conjugate (75 μg) was administered via tail vein injection. Images were obtained with the Pearl Trilogy Small Animal Imaging System (LI-COR, Lincoln, NE) with both 700 nm and 800 nm channels and evaluated using Image Studio.
Results
Laparotomy was performed 24 hours after labeling the tumors. When imaged through the 800 nm channel, the tumors were observed to be strongly labeled with anti-CEA-IRDye800. At 48 hours laparotomy was repeated which again demonstrated strong labeling of the tumors through the 800 nm channel, but with a lower absolute intensity (in relative units), than at 24 hours for each of the 3 mice imaged.
Conclusion
Humanized anti-CEA-IRDye800CW can rapidly and effectively label CEA-expressing human colon cancer in an orthotopic nude mouse model. Given the ability of this technology to target and label tumors with great specificity, the anti-CEA-IRDye800CW is currently being developed for clinical use in fluorescence-guided surgery.
Keywords: Colon cancer, orthotopic, mouse models, CEA, fluorescence
INTRODUCTION
Fluorescence guided surgery (FGS) combines advanced imaging platforms with targeted fluorescent agents to enhance neoplasms and improve their intraoperative detection by the surgeon (1). One technique is to covalently bind organic dye to a monoclonal antibody targeting a known tumor-specific antigen (2). One such tumor biomarker that is being developed is anti-carcinoembryonic antigen (CEA) antibodies to target and label human colorectal cancer in nude mouse models (3–6). Mouse and chimeric (mouse/human) antibodies against CEA have been conjugated with fluorescent dye and are capable of enhancing visualization of submillimeter tumor deposits (5) and successful FGS (7, 8). This technique has been applied to human clinical trials to localize squamous cell carcinoma of the head and neck using a cetuximab-IRDye800CW conjugate (9).
In an effort to improve therapeutic efficacy in the clinic, Yazaki et al. humanized the murine anti-CEA T84.66 antibody with structurally similar “human” segments through a technique known as complementary determining region (CDR) grafting (10). This “humanized” antibody, which is currently in clinical trials, was selected for the current study to determine the extent of fluorophore labeling of human colon cancer in an orthotopic mouse model. IRDye800CW was selected as the fluorophore for this study due to its preliminary success in human use. Further, it has a similar excitation and emission profile to indocyanine green (ICG), for which numerous clinically available fluorescence imaging protocols and instrumentation already exist (11, 12).
MATERIALS AND METHODS
Cell Culture
The human colon cancer cell line HT-29 was grown in RPMI media (Gibco-BRL, Grand Island, NY) supplemented with 10% fetal calf serum (FCS; Hyclone, Logan, UT) and 1% penicillin/streptomycin (Gibco-BRL). The cells were cultured at 37°C in a 5% CO2 incubator.
Conjugation of Antibody to Fluorophore
The humanized monoclonal antibody hT84.55-M5A specific for CEA was conjugated with NHS-IRDye800CW (generous gift from LI-COR Biosciences, Lincoln, NE). Briefly, the antibody was combined with reconstituted reactive dye at a molar ratio of 10:1 (dye:antibody) in 0.1 M sodium bicarbonate and allowed to incubate at room temperature for 1 hour then overnight at 4°C. Excess dye was removed through an Amicon stirred cell concentrator (Millipore, Billerica, MA). The final concentration of antibody-dye conjugate was 6.6 mg/mL with an average of 1.6 dye molecules per IgG. The antibody-dye conjugate was stored in the 4°C refrigerator and was protected from light.
Animal Care
Athymic nu/nu nude mice (AntiCancer, Inc., San Diego, California), between 4 and 6 weeks of age were maintained in a barrier facility at AntiCancer, Inc., on high-efficiency particulate air-filtered racks. The animals were fed with autoclaved laboratory rodent diet (Teckland LM-485; Western Research Products, Orange, California). All surgical procedures and imaging were performed with the animals anesthetized by intramuscular injection of 0.02 mL of a solution of 50% ketamine, 38% xylazine, and 12% acepromazine maleate. All animal studies were conducted in accordance with the principles and procedures outlined in the NIH Guide for the Care and Use of Animals under PHS license number A3873-1.
Subcutaneous Injection of Tumor Cells
The HT-29 line of human colorectal cancer cells were harvested after 2 weeks of growth by trypsinization and washed with serum-free medium. 1×106 cells were combined with 100 μl Matrigel (Corning, Tewksbury, MA) and injected into the bilateral flanks of 5 athymic female nu/nu mice at 6 weeks of age. The tumors were allowed to grow until they reached a diameter of approximately 10 mm, which occurred after 3 weeks.
Passage and Orthotopic Implantation of HT-29 Tumor
Orthotopic human colon cancer xenografts were established in nude mice by suturing a small fragment of HT-29 tumor on the mesenteric border of the mouse cecum. To start, a 10 mm subcutaneous HT-29 tumor was resected and cut into 2 mm3 fragments. The fragments were then sutured to the cecum of 5 additional nude mice using 8-0 nylon sutures. The tumors were allowed to grow for 4 weeks at which point 3 had successful orthotopic tumor growth and were selected for fluorescence imaging. The remaining 2 mice did not survive the 4-week recovery period. Small tumor fragments were also implanted in the bilateral flanks of each mouse to track the growth of the tumors.
Antibody-Dye Conjugate Delivery
4 weeks after the orthotopic implantation of HT-29 tumor the animals were given a single intravenous dose of 100 μl of anti-CEA-IRDye800CW via tail vein injection. Each dose was 75 μg.
Mouse Imaging
Images were obtained with the Pearl Trilogy Small Animal Imaging System (LI-COR, Lincoln, NE) 24 and 48 hours post-injection (with laparotomy views) with both 700 nm and 800 nm channels. Images were evaluated using Image Studio. Signal from the 700 nm channel was displayed red and signal from the 800 nm channel was displayed as green.
RESULTS
Three mice with orthotopic HT-29 colon cancers were treated with 75 μg of anti-CEA-IRDye800CW. Images were then acquired under general anesthesia with the Pearl Trilogy Small Animal Imaging System (LI-COR, Lincoln, NE) at 24 and 48 hours post injection. The tumors were imaged directly after performing a midline laparotomy and delivering the cecum and neoplasm through the incision. At this time the tumor was strongly enhanced using the 800 nm channel (green) in all three animals (see Figure 1). Normal gut was visualized through the 700 nm channel (red), due to the signal emitted from plant chlorophylls within the mouse chow. There was no appreciable difference in enhancement between the three animals. At 48 hours laparotomy was repeated which again demonstrated strong labeling of the tumors through the 800 nm channel, but with a lower absolute intensity (in relative units), than at 24 hours for each of the 3 mice imaged.
Figure 1.
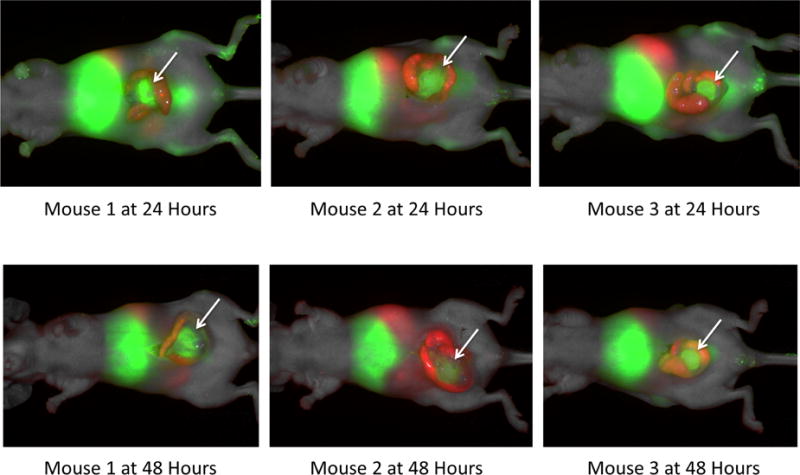
Anesthetized mice were imaged using the Li-Cor Pearl Trilogy Small Animal Imaging System pre-injection at 24 hours and 48 hours post-injection of the anti-CEA-IRDye800 conjugate. In this series laparotomy was performed prior to acquiring images. The colon cancer tumors are well enhanced with the targeted fluorophore (arrows). The images at 24 hours have stronger enhancement than at 48 hours. Red vs green
At 48 hours post injection the mice were sacrificed via tail vein injection of 0.1 mL of a solution of 50% ketamine, 38% xylazine, and 12% acepromazine maleate and a necropsy was performed. Each mouse was carefully dissected to remove the bilateral flank tumors, the colon tumor, bilateral kidneys, spleen, blood sample, and the liver and then carefully placed on a 6 well tissue culture plate (see Figure 2). Each plate was then imaged to determine the relative strength and accumulation of fluorescence signal by tissue type at 48 hours. Both the flank and orthotopic tumors retained their fluorescence signal. Considerable uptake was also appreciated in the liver and kidneys due to metabolism of the antibody-dye conjugate. There was no fluorescence signal in the blood samples at 48 hours and minimal signal in the spleens.
Figure 2.
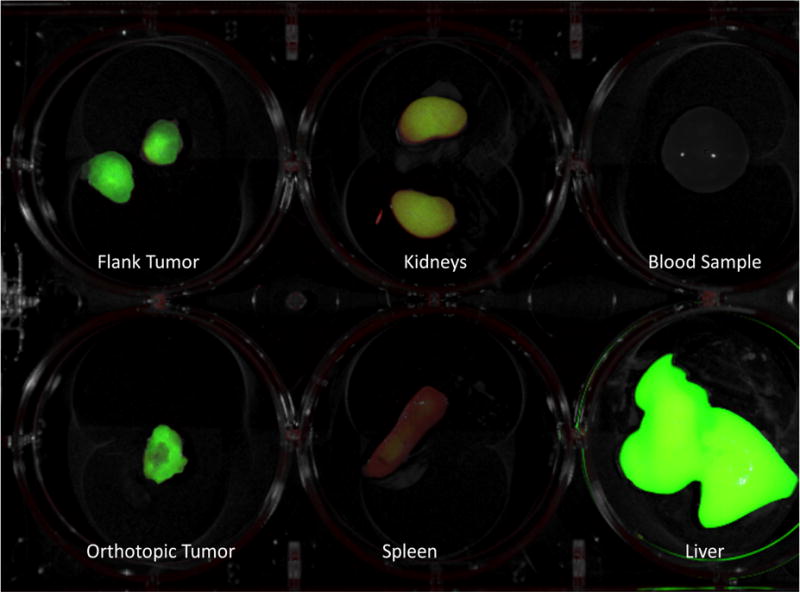
Following sacrifice, images were taken of the flank tumors, orthotopic tumors, kidneys, spleen, blood sample, and liver to determine dye accumulation at 48 hours. Notably, the tumors are well enhanced and there is no dye remaining in the blood due to the rapid clearance of the dye from the circulation.
DISCUSSION
The purpose of this experiment was to label colon cancer by means of a clinically relevant humanized anti-CEA antibody conjugated with a near infrared 800 nm fluorophore. By selecting IRDye800CW as the fluorophore, we are able to use the ICG-based imaging instrument that is rapidly expanding in clinical use (13). Thus, our successful labeling colon cancer with these two components serves as a bridge from our preclinical work with tumor labeling to practical surgical applications clinically for CEA-expressing cancers.
One limitation of the anti-CEA-IRDye800CW is the rapid clearance from circulation due to the pharmacokinetics of the conjugate. The IRDye800CW is a hydrophobic molecule that is fully cleared from the blood stream by approximately 24 hours, compared to the monoclonal antibody alone with clearance that approaches 72 hours (14). This results in greater accumulation in the liver and the kidneys and allows for fewer passages through the tumor circulation and thus less tumor enhancement. In this proof of principle study, we used NHS-IR800 to conjugate to surface primary amines on the anti-CEA antibody where the hydrophobic dye is exposed. In a previous study we noted that PEGylated dyes have less accumulation in the liver and as such are better suited for imaging liver metastasis (15).
We selected HT-29 as the cell line for our study because it is known to express CEA and it reliably grows in orthotopic colon cancer models in nude mice when tumor fragments are sutured to the mesenteric border of the mouse cecum (6). We have previously demonstrated that serosal transplantation of intact colon tumor tissue in nude mice (surgical orthotopic implantation (SOI)) allows a clinical pattern of metastases to occur (16, 17) which seems to be a general phenomenon for other tumor types (18–20).
We have previously shown NIR dye can penetrate through a layer of skin and body wall in a murine model which suggests that intraluminal tumors would still be clearly visible when imaged through the colonic wall (21). We previously developed an orthotopic mouse model with HT-29 human colorectal tumors growing on the mucosa of the descending colon to better resemble the clinical disease (22). Fluorescence was observed along the entire descending colon after intracolonical administration of lectin-immobilized fluorescent nanospheres from the anus (22). In the present study, tumors grew to be 4–7 mm in diameter at the time of imaging. We have previously demonstrated the ability to identify sub-millimeter tumor deposits during fluorescence-guided surgery (FGS) and even single cancer cells with proper magnification, which could lead to more rapid identification of lymph node metastasis with greater accuracy (23).
The authors group has previously shown in an orthotopic model of colon cancer that fluorescence-guided surgery (FGS) improves outcome in colon cancer compared to bright light surgery by increasing the percentage of R0 resections, decreasing recurrence, and increasing survival (5, 8).The present study showed further increases in the benefits of FGS for colon cancer should be possible because of increases in tumor visibility afforded by the NIR-conjugated humanized antibody.
Our study utilizes a nude mouse model that lacks an immune system. In future studies, we plan to use humanized NSG mice implanted with patient derived orthotopic xenografts (PDOX) colon cancers. NSG mice are engrafted with human immune cells. This will allow evaluation of patient tumors in an immunocompetent model.
In conclusion, increasing the surgeon’s ability to detect cancer during the operation has the dual benefit of identification of disease that can be fully resected and recognizing locally advanced disease that cannot. FGS has the potential to advance our ability to recognize where an individual’s disease actually is rather than relying on our understanding of where it most likely should be. With applications in staging laparoscopy, tumor localization, real-time assessment of margins during FGS, and surveillance for local recurrence, fluorescence tumor labeling will become an essential tool for improving outcomes in surgical oncology.
Acknowledgments
Work supported in part by grants from the National Cancer Institute CA142669 and CA132971 (to M.B. and AntiCancer, Inc).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Author’s contributions: Conception and design: JCD, TM, PJYRMH, MB; analysis and interpretation: JCD, RMH, MB; data collection: JCD, TM, RMH, MB; writing the article: JCD; critical revision of the article: RMH, MB; obtaining funding: RMH, MB.
Author’s disclosures: RMH is a non-salaried affiliate of AntiCancer, Inc.
Presented at the 2017 12th Annual Academic Surgical Congress, February 3–5, 2017.
References
- 1.DeLong JC, Hoffman RM, Bouvet M. Current status and future perspectives of fluorescence-guided surgery for cancer. Expert Rev Anticancer Ther. 2016;16:71–81. doi: 10.1586/14737140.2016.1121109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bouvet M, Hoffman RM. Glowing tumors make for better detection and resection. Sci Transl Med. 2011;3:110fs110. doi: 10.1126/scitranslmed.3003375. [DOI] [PubMed] [Google Scholar]
- 3.Hiroshima Y, Lwin TM, Murakami T, Mawy AA, Kuniya T, et al. Effective fluorescence-guided surgery of liver metastasis using a fluorescent anti-CEA antibody. J Surg Oncol. 2016 doi: 10.1002/jso.24462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Hiroshima Y, Maawy A, Metildi CA, Zhang Y, Uehara F, et al. Successful fluorescence-guided surgery on human colon cancer patient-derived orthotopic xenograft mouse models using a fluorophore-conjugated anti-CEA antibody and a portable imaging system. J Laparoendosc Adv Surg Tech A. 2014;24:241–247. doi: 10.1089/lap.2013.0418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Metildi CA, Kaushal S, Luiken GA, Talamini MA, Hoffman RM, et al. Fluorescently labeled chimeric anti-CEA antibody improves detection and resection of human colon cancer in a patient-derived orthotopic xenograft (PDOX) nude mouse model. J Surg Oncol. 2014;109:451–458. doi: 10.1002/jso.23507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kaushal S, McElroy MK, Luiken GA, Talamini MA, Moossa AR, et al. Fluorophore-conjugated anti-CEA antibody for the intraoperative imaging of pancreatic and colorectal cancer. J Gastrointest Surg. 2008;12:1938–1950. doi: 10.1007/s11605-008-0581-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Metildi CA, Kaushal S, Pu M, Messer KA, Luiken GA, et al. Fluorescence-guided surgery with a fluorophore-conjugated antibody to carcinoembryonic antigen (CEA), that highlights the tumor, improves surgical resection and increases survival in orthotopic mouse models of human pancreatic cancer. Ann Surg Oncol. 2014;21:1405–1411. doi: 10.1245/s10434-014-3495-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Metildi CA, Kaushal S, Snyder CS, Hoffman RM, Bouvet M. Fluorescence-guided surgery of human colon cancer increases complete resection resulting in cures in an orthotopic nude mouse model. J Surg Res. 2013;179:87–93. doi: 10.1016/j.jss.2012.08.052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Rosenthal EL, Warram JM, de Boer E, Chung TK, Korb ML, et al. Safety and Tumor Specificity of Cetuximab-IRDye800 for Surgical Navigation in Head and Neck Cancer. Clin Cancer Res. 2015;21:3658–3666. doi: 10.1158/1078-0432.CCR-14-3284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Yazaki PJ, Sherman MA, Shively JE, Ikle D, Williams LE, et al. Humanization of the anti-CEA T84.66 antibody based on crystal structure data. Protein Eng Des Sel. 2004;17:481–489. doi: 10.1093/protein/gzh056. [DOI] [PubMed] [Google Scholar]
- 11.Boni L, David G, Mangano A, Dionigi G, Rausei S, et al. Clinical applications of indocyanine green (ICG) enhanced fluorescence in laparoscopic surgery. Surg Endosc. 2015;29:2046–2055. doi: 10.1007/s00464-014-3895-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Namikawa T, Sato T, Hanazaki K. Recent advances in near-infrared fluorescence-guided imaging surgery using indocyanine green. Surg Today. 2015;45:1467–1474. doi: 10.1007/s00595-015-1158-7. [DOI] [PubMed] [Google Scholar]
- 13.AV DS, Lin H, Henderson ER, Samkoe KS, Pogue BW. Review of fluorescence guided surgery systems: identification of key performance capabilities beyond indocyanine green imaging. J Biomed Opt. 2016;21:80901. doi: 10.1117/1.JBO.21.8.080901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Yazaki PJ, Lee B, Channappa D, Cheung CW, Crow D, et al. A series of anti-CEA/anti-DOTA bispecific antibody formats evaluated for pre-targeting: comparison of tumor uptake and blood clearance. Protein Eng Des Sel. 2013;26:187–193. doi: 10.1093/protein/gzs096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Maawy AA, Hiroshima Y, Zhang Y, Luiken GA, Hoffman RM, et al. Polyethylene glycol (PEG) linked to near infrared (NIR) dyes conjugated to chimeric anti-carcinoembryonic antigen (CEA) antibody enhances imaging of liver metastases in a nude-mouse model of human colon cancer. PLoS One. 2014;9:e97965. doi: 10.1371/journal.pone.0097965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Fu X, Herrera H, Kubota T, Hoffman RM. Extensive liver metastasis from human colon cancer in nude and scid mice after orthotopic onplantation of histologically-intact human colon carcinoma tissue. Anticancer Res. 1992;12:1395–1397. [PubMed] [Google Scholar]
- 17.XY Fu, Besterman JM, Monosov A, Hoffman RM. Models of human metastatic colon cancer in nude mice orthotopically constructed by using histologically intact patient specimens. Proc Natl Acad Sci U S A. 1991;88:9345–9349. doi: 10.1073/pnas.88.20.9345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.An Z, Jiang P, Wang X, Moossa AR, Hoffman RM. Development of a high metastatic orthotopic model of human renal cell carcinoma in nude mice: benefits of fragment implantation compared to cell-suspension injection. Clin Exp Metastasis. 1999;17:265–270. doi: 10.1023/a:1006654600095. [DOI] [PubMed] [Google Scholar]
- 19.Fu X, Guadagni F, Hoffman RM. A metastatic nude-mouse model of human pancreatic cancer constructed orthotopically with histologically intact patient specimens. Proc Natl Acad Sci U S A. 1992;89:5645–5649. doi: 10.1073/pnas.89.12.5645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Furukawa T, Fu X, Kubota T, Watanabe M, Kitajima M, et al. Nude mouse metastatic models of human stomach cancer constructed using orthotopic implantation of histologically intact tissue. Cancer Res. 1993;53:1204–1208. [PubMed] [Google Scholar]
- 21.Maawy AA, Hiroshima Y, Kaushal S, Luiken GA, Hoffman RM, et al. Comparison of a chimeric anti-carcinoembryonic antigen antibody conjugated with visible or near-infrared fluorescent dyes for imaging pancreatic cancer in orthotopic nude mouse models. J Biomed Opt. 2013;18:126016. doi: 10.1117/1.JBO.18.12.126016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kitamura T, Sakuma S, Shimosato M, Higashino H, Masaoka Y, et al. Specificity of lectin-immobilized fluorescent nanospheres for colorectal tumors in a mouse model which better resembles the clinical disease. Contrast Media Mol Imaging. 2015;10:135–143. doi: 10.1002/cmmi.1609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Hoffman RM, Yang M. Subcellular imaging in the live mouse. Nat Protoc. 2006;1:775–782. doi: 10.1038/nprot.2006.109. [DOI] [PubMed] [Google Scholar]


