Abstract
The diagnosis of lymphoma in pregnant patients poses a therapeutic challenge necessitating consideration of the developing fetus without compromise of therapy with curative potential for the mother. The decision to initiate therapy during pregnancy is heavily influenced by fetal, maternal and disease related factors, of which the most influential are the trimester at diagnosis, the stage and aggressiveness of the disease and the presence of life threatening symptoms. Recent data suggest that deferral of therapy until after the 1st trimester is desirable if it is perceived that postponement of therapy will not compromise maternal outcome. For some patients delay of therapy to the post-partum period is feasible.
Keywords: lymphoma, pregnancy, Hodgkin lymphoma, therapy
Introduction
Cancer diagnosed during pregnancy poses a medical dilemma, requiring careful consideration of risks and benefits of therapy in regards to maternal and fetal outcomes. One of 1000 pregnancies in the United States are affected by maternal cancer diagnosis [1]. Hematologic malignancies represent the second most common neoplasm diagnosed during pregnancy, after breast cancer, with Hodgkin lymphoma (HL) and non-Hodgkin lymphoma (NHL) representing roughly 6% and 5% of cancers diagnosed during pregnancy respectively [2, 3]. Given the infrequency of diagnosis, coupled with the heterogeneity of pathologic lymphoma subtypes, data regarding management and outcome are limited to case reports and retrospective cohort series. HL diagnosed outside of pregnancy has excellent outcomes [4]. Therefore, treatment strategies must strike a balance between administering curative therapy to the mother while limiting potential toxicity to the unborn fetus. These management and ethical challenges can be more arduous among patients with aggressive NHL, where treatment delay may be fatal in some cases. We review the current recommendations regarding diagnostic strategies, management and outcomes of pregnant women diagnosed with lymphoma.
Diagnosis
Pathologic examination of lymph node biopsy specimen(s) should be performed to make the diagnosis of HL or NHL. Serum studies like erythrocyte sedimentation rate (ESR) or lactate dehydrogenase (LDH) that can be prognostic for patients with HL and NHL respectively should be interpreted with caution because elevation of these markers can occur due to gestation and not necessarily disease related factors. Imaging plays a crucial role in diagnosis, staging and assessment of disease response to therapy. However, given the potential teratogenic, carcinogenic and in some cases fatal consequences of radiologic modalities that utilize ionizing radiation, the decision regarding utilization of various imaging approaches should not be taken lightly.
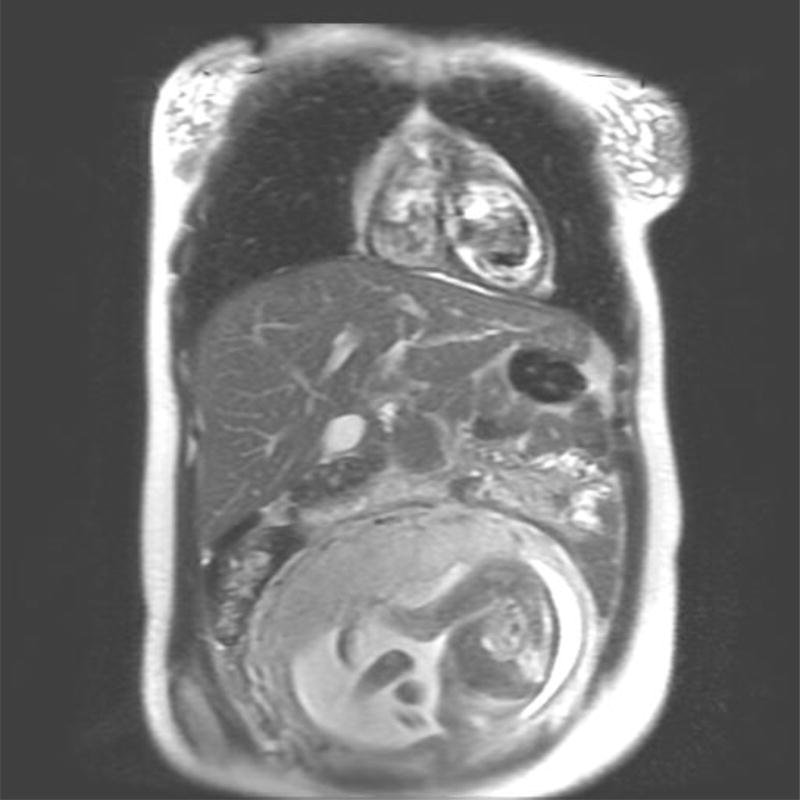
Imaging of the pregnant patient for diagnosis and response assessment poses a challenge to the multi-disciplinary team. Magnetic resonance imaging (MRI) is regarded as the modality of choice for acquisition of cross sectional imaging, as no studies to date have demonstrated adverse effects on the fetus from MRI (Figure 1) [5]. Gadolinium, which has been shown to have teratogenic effects in animal models, is not recommended for use during pregnancy [6]. Iodine based contrast should also be avoided during pregnancy. Computed tomography (CT), which employs ionizing radiation is discouraged, although it is possible to reduce abdominopelvic dose with various techniques including abdominal shielding and lower dose protocols for image acquisition [7].
Figure 1.
Non-contrasted MRI performed for disease staging in a 28 year old patient diagnosed with cHL.
A. Axial T1 Non-Contrasted image of the chest
B. Coronal T2 weighted image of the chest, abdomen and pelvis
The potential risks of fetal ionizing radiation exposure include risk of fetal death, induction of congenital malformations (including mental retardation) as well as carcinogenic potential for the unborn fetus[5]. There is no dose of ionizing radiation that is considered safe for a developing fetus, however at radiation exposures of less than 5 cGy, the fetus has a low risk of significant developmental abnormalities [8, 9]. Therefore any diagnostic study that is being considered in a pregnant patient should administer doses of less than 5 cGy to the fetus. Given these risks, there is concern regarding the use of CT as well as 18F fluorodeoxyglucose positron emission tomography (18F-FDG PET) imaging. In certain clinical scenarios however, the perceived diagnostic value of the study for the mother exceed potential risks to the fetus. Therefore each case should be considered on a case by case basis with input from the radiologist regarding the probable dose of radiation exposure to the fetus and associated predicted risks.
Therapy
Management of lymphoma during pregnancy should be conducted with a multi-disciplinary approach. A specialized maternal fetal medicine specialist should be an integral part of the management team. Input from a medical ethicist may also be appropriate in certain circumstances.
Fetal outcomes are largely influenced by the timing of therapy with regards to gestational age [10]. The vulnerability of the fetus at various periods throughout pregnancy are reflected in the events occurring in fetal development. Early gestation, also known as the preimplantation period that occurs up to 2 weeks after conception, is often referred to as the “all or nothing” stage because insults during this time result either in embryonic demise or normal development. In this period, the embryo has heightened radiation sensitivity that can be lethal, at reported threshold doses of 10 cGy [8]. Weeks 2–8 post conception represent the embryonic period (also known as organogenesis) when teratogenic potential is greatest due to critical organ development. In the last period, the fetal phase, from the end of embryonic stage to delivery, organ maturation and growth occurs. Teratogen exposure during this last phase is more likely to result in reduced fetal growth as opposed to structural abnormalities.
Classical HL (cHL), which is highly curable in non-pregnant patients, represents the most common lymphoma subtype diagnosed during pregnancy [2]. The management of cHL therapy during pregnancy is most impacted by gestational age. The decision to deliver antenatal therapy is heavily influenced by the stage of development of the fetus as well as the extent of disease present in the mother which will influence outcome if the decision is made to defer therapy. In general, initiation of treatment during the first trimester is avoided secondary to concerns of deleterious embryonic effects [11–14]. With close surveillance of slowly progressive cHL diagnosed during the first trimester, many patients can defer treatment until the 2nd trimester. Similarly for pregnant women diagnosed with indolent NHL, treatment can often be delayed, in many cases to the post-partum period. Patients with aggressive NHL as well as cHL patients with extensive disease burden pose an increased therapeutic challenge, especially when the diagnosis is made early in the pregnancy when systemic therapy would be expected to pose a serious threat to the developing fetus but significant delay of therapy could have fatal consequences for the mother [15].
In the largest multicenter retrospective study of 90 patients with lymphoma (40 HL and 50 NHL), the authors observed that standard non-antimetabolite multi-agent chemotherapy administered in the 2nd and 3rd trimester resulted in expected maternal outcomes without increases in severe adverse fetal or perinatal events (Table 1) [13]. In this study by Evens et al, 6 patients elected to terminate pregnancy in order to facilitate immediate systemic therapy in the 1st trimester (n=5) or early 2nd trimester (n=1). Twenty eight patients diagnosed with lymphoma at a median of 30 weeks gestation deferred therapy until the post-partum period, while 56 women received antenatal treatment for lymphoma diagnosed at a median of 22 weeks. Among the 56 pregnant women who received chemotherapy, 66% (n=37) were treated in the 2nd trimester. The overall response rate (ORR) was 82% and the complete response rate was 64%. No patient received antenatal therapy during the first trimester. Interestingly, there were no differences in preterm or perinatal complications among women that received antenatal therapy compared to those where treatment was deferred. In addition, there were no statistically significant differences in treatment outcome based on administration of antenatal therapy versus deferral of treatment until the post-partum setting. For patients with HL, the 3 year progression free survival (PFS) and overall survival (OS) was 85% and 97% respectively. Patients with NHL had 3 year PFS and OS rates of 53% and 82%. The rate of miscarriage was low at 1.1%.
Table 1.
Summary of Case Series of Lymphoma Diagnosed During Pregnancy Since 2013
| Article | Sample | Treatment | Survival Outcomes |
Fetal Outcomes |
|---|---|---|---|---|
| Pinnix et al. (JAMA Oncol. 2016)[14] | N= 39 pregnant patients (31 HL; 8 NHL); n=32 (82%) had stage I – II disease | 24 patients received antenatal therapy (Chemotherapy and/or RT); 12 deferred therapy until after delivery; 3 electively terminated pregnancy to receive systemic therapy | 5-yr PFS: 74.7%; 5-yr OS: 82.4% (no difference in outcome based on timing of therapy) | 4 miscarriages in patients receiving antenatal therapy (2/4 in 1st trimester) |
| No difference in time to delivery between patients receiving antenatal vs post-natal treatment (p=0.21) | ||||
| No fetal abnormalities were observed. | ||||
|
| ||||
| Bachanova et al. (Curr Hematol Malig Rep. 2013)[28] | N= 18 pregnant patients with HL | 11 patients received post-natal treatment; 6 patients required vinblastine to control disease | 2/4 deaths were due to HL | No apparent abnormalities of children post delivery |
|
| ||||
| Evens et al. (J Clin Oncol 2013)[13] | N= 90 pregnant patients (40 HL; 50 NHL); n=54 (60%) had stage I – II disease | 56 received antenatal treatment (Chemotherapy and/or RT), with therapy initiated in the 2nd trimester in 37/56 patients (66%); 6 patients had termination of pregnancy. No patients treated during 1st trimester | 3-yr PFS and OS were 53% and 82% in NHL cases, and 85% and 97% in HL cases | Full term gestation in 56% of patients; 1 case of fetal demise |
| 8 deaths related to NHL | No differences in complications detected among patients who received antenatal vs deferred therapy | |||
| One case of microcephaly and one case of pelviactasis were detected in infants whose mother received antenatal treatment | ||||
NHL: Non-Hodgkin Lymphoma; HL: Hodgkin Lymphoma; PFS: progression-free survival; OS: Overall survival; RT: Radiation therapy
Another recent retrospective series examined a cohort of 39 pregnant women diagnosed with lymphoma, 31 with HL and 8 with NHL at MDACC [14]. Three patients underwent elective termination at diagnosis to facilitate emergent systemic therapy. Of the remaining 36 patients, 24 received therapy during pregnancy and 12 deferred treatment with no significant difference in maternal outcome according to receipt of antenatal therapy. While there was no statistically significant difference in the rate of miscarriage among patients that did and did not receive therapy during pregnancy, 4 of 24 patients who received antenatal therapy experienced a miscarriage compared to 0 of 12 patients who had treatment delayed until after delivery (p=0.3). The overall miscarriage rate in this study was 10% with 2 of 4 spontaneous fetal losses occurring among women treated with systemic therapy during the 1st trimester. This underscores the importance of delaying therapy until the 2nd trimester if it is clinically appropriate for the patient versus having very difficult discussions about potential termination of pregnancy if treatment deferral to 2nd trimester would result in very high risks of poor maternal outcomes as related to disease progression.
Radiation Therapy
The role of radiation therapy (RT) for non-pregnant lymphoma patients continues to evolve. The current approach with combined modality therapy for the non-pregnant patient involves consolidation RT after chemotherapy to improve local control and event free survival or for the palliation of symptomatic and often bulky masses [4, 16, 17]. Historical data has shown that RT can be delivered to pregnant patients with limited doses to the fetus when administering supra-diaphragmatic radiation with abdominopelvic shielding [18–20]. Woo et al reported on the outcomes of 16 patients that received mediastinal radiation during pregnancy with doses estimated to the mid fetus of 1.5 to 13.6 cGy. With a range of follow up of 1 to 31 years, no malformations on malignancies were detected in the children born to the patients in the study.
In both the Evens et al and Pinnix et al studies, a few patients were treated with antenatal RT. In the multicenter study by Evens et al, RT was given to 9 patients, mainly for supra-diaphragmatic lymphoma to doses of 25–30 Gy. Interestingly among the NHL cohort, patients that received antenatal RT had significantly inferior 3 year PFS of 0% (n=5) compared to patients that did not receive RT (65%, n=43, p=0.001). In the MDACC study, 4 patients received antenatal RT but it was not associated with inferior PFS (p=0.3) or OS (p=0.24).
Given the efficacy of systemic therapy, we do not advise routine utilization of RT during pregnancy. As combined modality therapy and not radiation alone is the standard of care for many lymphomas, there is no strong rationale for the pursuit of radiation as monotherapy during pregnancy [4]. Furthermore, even if the doses can be reduced via modern shielding methods, uncertainly exists regarding the long term effects on the fetus, most notably secondary childhood leukemias [10]. Given these considerations, we strongly recommend deferring RT until after delivery. When deemed to be critical for the mother however (superior vena cava syndrome or spinal cord compression) RT can be considered with extreme caution and diligence in shielding to reduce RT fetal exposures to as low as possible.
Targeted Therapy
The chimeric monoclonal antibody targeting CD20, rituximab, is indicated for many B cell hematologic malignancies. It is not approved for use during pregnancy, though use of rituximab during pregnancy has been identified in 231 cases in the rituximab global safety database[21]. Few congenital malformations were appreciated among 90 cases with known outcomes that resulted in live births. Four neonatal infections were reported. First trimester miscarriage did occur in 21% of women, which is higher than the rates of early pregnancy loss in the general population. It is important to note however that the study population included women that received Rituximab for many other indications distinct from lymphoma, including rheumatoid arthritis, systemic lupus erythematous and autoimmune hemolytic anemia. This population of patients with autoimmune disease likely have baseline elevated risks during pregnancy due to disease related factors as well as receipt of other potentially teratogenic medications. While rituximab is not approved for use during pregnancy, there are case reports of successful treatment of lymphoma with rituximab in pregnant patients with good fetal outcomes and thus the lack of formal approval needs to weighed against the potential benefit particularly for aggressive B-cell NHLs [22, 23]. For newer targeted therapies including brentuximab vedotin and others, administration during pregnancy is not recommended given lack of fetus safety data for this therapy available to date.
Pediatric Outcomes
It has been consistently demonstrated that pre-term delivery, as opposed to fetal chemotherapy exposure, is the greatest predictor of neurocognitive deficiency [3, 11, 24] of children born to mothers that received cancer therapy during pregnancy. Amant and co-authors performed a large multi-center case controlled study of 129 children born to mothers diagnosed with cancer during pregnancy matched to a control cohort group of children of mothers without cancer [3]. Inferior cognitive outcome was associated with premature gestational age at birth and was independent of cancer therapy administered during pregnancy. In this study 16% of children in the study cohort were born to mothers diagnosed with a hematologic malignancy (NHL, n=6, 4.8%; HL, n=8, 6.4%). Chemotherapy exposure occurred in 74.4% of children in the cohort (n=96) and 8.5% were exposed to radiation therapy (n=11). It should be noted that all chemotherapy was given in the 2nd or 3rd trimester which likely contributed to the good fetal outcomes.
In a recent Swedish nationwide cohort study of women diagnosed with cancer during (n=984 births) or shortly after pregnancy (n=2723 births) between 1973 and 2012, maternal cancer diagnosed during pregnancy was associated with increased risk for stillbirth, preterm birth and neonatal mortality [25]. This study included women diagnosed with many different types of cancers, including hematopoietic cancers in 7.6% of those diagnosed during pregnancy and 9.4% of those diagnosed shortly after pregnancy. Interestingly, the authors demonstrated that the increased risk of preterm birth among children born to mothers with cancer was mainly due to iatrogenic preterm birth, not spontaneous. Furthermore, the elevated risk of neonatal mortality in this study was primarily secondary to preterm birth, which explained 89% of the increased risk. This underscores the importance of decision making regarding the timing of delivery, as shortened gestation has potential adverse consequences for the fetus.
Pregnancy after Lymphoma
After completion of therapy for lymphoma, there are concerns from female patients and oncologists that pregnancy may increase the risk of relapse. In a recent Swedish healthcare registry study, pregnancy associated relapse was evaluated among 449 women treated for HL between 1992 and 2009 [26]. Among this cohort, 32% of women became pregnant in the follow up period (n=144). Relapses occurred in 47 women, but only one patient was diagnosed with relapse in the setting of pregnancy. The authors did not identify an increased risk of relapse among their cohort of patients treated for HL that became pregnant in the follow up period. They did identify that the greatest risk of relapse in this patient population overall was within the first 2–3 years after diagnosis and therefore it is suggested that if possible women delay pregnancy until after the first 2 years of therapy completion.
Newer technology with noninvasive prenatal testing (NIPT) that utilizes genome sequencing of cell-free fetal DNA that is present in maternal plasma, may be helpful for diagnosis of cancer in asymptomatic pregnant patients. A study published in JAMA Oncology in 2015 reported outcomes of NIPT performed prospectively in 4000 pregnancies. NIPT led to the diagnosis of cancer in 3 women with genome profiles identified that were distinct from the fetus and the mother [27]. Neoplasm was suspected in these three patients and ultimately MRI and biopsy confirmed the diagnosis of ovarian cancer, follicular lymphoma and HL. This technology could have implications for early detection of tumors in women contemplating pregnancy in the post therapy period.
Conclusions
The decision to initiate therapy during pregnancy is heavily influenced by the fetal, maternal and disease related factors, of which the most influential are the trimester at diagnosis, the stage and aggressiveness of the disease and the presence of life threatening symptoms. Deferral of therapy until after delivery is often feasible for women diagnosed with lymphoma during the 3rd trimester and can be contemplated for women diagnosed during the second trimester, if it is perceived that delay of therapy will not compromise the maternal outcome. Based on recent data we conclude that:
Multi-agent (non-antimetabolite) systemic therapy administered beyond the 1st trimester appears safe for the mother and fetus with expected maternal disease control and fetal developmental and cognitive outcomes.
Premature gestational age at the time of delivery and not antenatal chemotherapy exposure is the strongest predictor of inferior outcomes for children born to mothers diagnosed with cancer during pregnancy. This likely only applies to mothers treated after completion of the 1st trimester. Efforts to delay delivery are important if this will not have a perceived adverse effect on maternal outcome.
Multi-disciplinary management is essential with consistent care provided by a maternal fetal medicine specialist.
Footnotes
Conflict of Interest
Chelsea C Pinnix, Therese Y. Andraos, Sarah Milgrom and, Michelle A. Fanale each declare no potential conflicts of interest.
Human and Animal Rights and Informed Consent
This article does not contain any studies with human or animal subjects performed by any of the authors.
References
- 1.Pavlidis NA. Coexistence of pregnancy and malignancy. Oncologist. 2002;7:279–287. [PubMed] [Google Scholar]
- 2.Van Calsteren K, Heyns L, De Smet F, et al. Cancer During Pregnancy: An Analysis of 215 Patients Emphasizing the Obstetrical and the Neonatal Outcomes. Journal of Clinical Oncology. 2010;28:683–689. doi: 10.1200/JCO.2009.23.2801. [DOI] [PubMed] [Google Scholar]
- 3.Amant F, Vandenbroucke T, Verheecke M, et al. Pediatric Outcome after Maternal Cancer Diagnosed during Pregnancy. N Engl J Med. 2015;373:1824–1834. doi: 10.1056/NEJMoa1508913. [DOI] [PubMed] [Google Scholar]
- 4.Engert A, Plutschow A, Eich HT, et al. Reduced treatment intensity in patients with early-stage Hodgkin's lymphoma. N Engl J Med. 2010;363:640–652. doi: 10.1056/NEJMoa1000067. [DOI] [PubMed] [Google Scholar]
- 5.Woitek R, Prayer D, Hojreh A, Helbich T. Radiological staging in pregnant patients with cancer. ESMO Open. 2016;1:e000017. doi: 10.1136/esmoopen-2015-000017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Okuda Y, Sagami F, Tirone P, et al. [Reproductive and developmental toxicity study of gadobenate dimeglumine formulation (E7155) (3)--Study of embryo-fetal toxicity in rabbits by intravenous administration] J Toxicol Sci. 1999;24(Suppl 1):79–87. doi: 10.2131/jts.24.supplementi_79. [DOI] [PubMed] [Google Scholar]
- 7.Kennedy EV, Iball GR, Brettle DS. Investigation into the effects of lead shielding for fetal dose reduction in CT pulmonary angiography. Br J Radiol. 2007;80:631–638. doi: 10.1259/bjr/31771954. [DOI] [PubMed] [Google Scholar]
- 8*.Rimawi BH, Green V, Lindsay M. Fetal Implications of Diagnostic Radiation Exposure During Pregnancy: Evidence-based Recommendations. Clin Obstet Gynecol. 2016;59:412–418. doi: 10.1097/GRF.0000000000000187. Evidence based recommendations for diagnostic radiographic studies during pregnancy with concise review of radiation doses associated with fetal risk as well as radiation doses from standard radiological studies. [DOI] [PubMed] [Google Scholar]
- 9.Brent RL. The effect of embryonic and fetal exposure to x-ray, microwaves, and ultrasound: counseling the pregnant and nonpregnant patient about these risks. Semin Oncol. 1989;16:347–368. [PubMed] [Google Scholar]
- 10.Martin DD. Review of radiation therapy in the pregnant cancer patient. Clin Obstet Gynecol. 2011;54:591–601. doi: 10.1097/GRF.0b013e318236e935. [DOI] [PubMed] [Google Scholar]
- 11.Loibl S, Han SN, von Minckwitz G, et al. Treatment of breast cancer during pregnancy: an observational study. Lancet Oncol. 2012;13:887–896. doi: 10.1016/S1470-2045(12)70261-9. [DOI] [PubMed] [Google Scholar]
- 12.Abdel-Hady el S, Hemida RA, Gamal A, et al. Cancer during pregnancy: perinatal outcome after in utero exposure to chemotherapy. Arch Gynecol Obstet. 2012;286:283–286. doi: 10.1007/s00404-012-2287-5. [DOI] [PubMed] [Google Scholar]
- 13**.Evens AM, Advani R, Press OW, et al. Lymphoma occurring during pregnancy: antenatal therapy, complications, and maternal survival in a multicenter analysis. J Clin Oncol. 2013;31:4132–4139. doi: 10.1200/JCO.2013.49.8220. Largest multi-institutional study of 90 patients with lymphoma diagnosed during pregnancy. There was no significant difference in maternal or fetal complications between the women that received antenatal therapy as compared to those who had treatment deferred to the post-partum period. No patient received treatment during the first trimester of pregnancy. [DOI] [PubMed] [Google Scholar]
- 14**.Pinnix CC, Osborne EM, Chihara D, et al. Maternal and Fetal Outcomes After Therapy for Hodgkin or Non-Hodgkin Lymphoma Diagnosed During Pregnancy. JAMA Oncol. 2016;2:1065–1069. doi: 10.1001/jamaoncol.2016.1396. Single institutional study of 39 patients diagnosed with lymphoma while pregnant at MDACC. There was no statistically significant difference in maternal outcome according to receipt of antenatal therapy. Four miscarriages occurred, all among patients treated during pregnancy, with 2 of the 4 occurring in patients that receive 1st trimester therapy. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15*.Avivi I, Farbstein D, Brenner B, Horowitz NA. Non-Hodgkin lymphomas in pregnancy: tackling therapeutic quandaries. Blood Rev. 2014;28:213–220. doi: 10.1016/j.blre.2014.06.004. Case based review of the diagnostic and therapeutic challenges of NHL diagnosed during pregnancy. [DOI] [PubMed] [Google Scholar]
- 16.Hodgson DC, Mikhaeel NG. Consolidative Radiation in DLBCL: Evidence-Based Recommendations. Curr Oncol Rep. 2015;17:49. doi: 10.1007/s11912-015-0472-y. [DOI] [PubMed] [Google Scholar]
- 17.Ng AK, Dabaja BS, Hoppe RT, et al. Re-Examining the Role of Radiation Therapy for Diffuse Large B-Cell Lymphoma in the Modern Era. J Clin Oncol. 2016;34:1443–1447. doi: 10.1200/JCO.2015.64.9418. [DOI] [PubMed] [Google Scholar]
- 18.Cygler J, Ding GX, Kendal W, Cross P. Fetal dose for a patient undergoing mantle field irradiation for Hodgkin's disease. Med Dosim. 1997;22:135–137. doi: 10.1016/s0958-3947(97)00011-3. [DOI] [PubMed] [Google Scholar]
- 19.Mazonakis M, Varveris H, Fasoulaki M, Damilakis J. Radiotherapy of Hodgkin's disease in early pregnancy: embryo dose measurements. Radiother Oncol. 2003;66:333–339. doi: 10.1016/s0167-8140(02)00329-8. [DOI] [PubMed] [Google Scholar]
- 20.Woo SY, Fuller LM, Cundiff JH, et al. Radiotherapy during pregnancy for clinical stages IA-IIA Hodgkin's disease. Int J Radiat Oncol Biol Phys. 1992;23:407–412. doi: 10.1016/0360-3016(92)90761-6. [DOI] [PubMed] [Google Scholar]
- 21.Chakravarty EF, Murray ER, Kelman A, Farmer P. Pregnancy outcomes after maternal exposure to rituximab. Blood. 2011;117:1499–1506. doi: 10.1182/blood-2010-07-295444. [DOI] [PubMed] [Google Scholar]
- 22.Mandal PK, Dolai TK, Bagchi B, et al. B cell suppression in newborn following treatment of pregnant diffuse large B-cell lymphoma patient with rituximab containing regimen. Indian J Pediatr. 2014;81:1092–1094. doi: 10.1007/s12098-013-1336-9. [DOI] [PubMed] [Google Scholar]
- 23.Burnette BL, Jentoft MA, Porrata LF, et al. Single-agent rituximab for primary CNS lymphoma during pregnancy as a bridge to definitive management. J Clin Oncol. 2014;32:e14–17. doi: 10.1200/JCO.2012.47.6994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24**.Amant F, Van Calsteren K, Halaska MJ, et al. Long-term cognitive and cardiac outcomes after prenatal exposure to chemotherapy in children aged 18 months or older: an observational study. Lancet Oncol. 2012;13:256–264. doi: 10.1016/S1470-2045(11)70363-1. Multicenter case-control study of 129 children born to mothers diagnosed with cancer during pregnancy matched to children of mothers without cancer. Gestational age at the time of delivery (i.e. prematurity) was associated with inferior cognitive outcome in both groups. There was no adverse effect of antenatal therapy detected in this study. [DOI] [PubMed] [Google Scholar]
- 25.Donghao Lu JFL, Karin E. Smedby, Katja Fall, Unner Valdimarsdottir, Sven Cnattingius and Fang Fand. Maternal Cancer During Pregnancy and Risks of Stillbirth and Infant Mortality. Journal of Clinical Oncology. 2017 doi: 10.1200/JCO.2016.69.9439. published online. [DOI] [PubMed] [Google Scholar]
- 26.Weibull CE, Eloranta S, Smedby KE, et al. Pregnancy and the Risk of Relapse in Patients Diagnosed With Hodgkin Lymphoma. J Clin Oncol. 2016;34:337–344. doi: 10.1200/JCO.2015.63.3446. [DOI] [PubMed] [Google Scholar]
- 27.Amant F, Verheecke M, Wlodarska I, et al. Presymptomatic Identification of Cancers in Pregnant Women During Noninvasive Prenatal Testing. JAMA Oncol. 2015;1:814–819. doi: 10.1001/jamaoncol.2015.1883. [DOI] [PubMed] [Google Scholar]
- 28.Bachanova V, Connors JM. Hodgkin lymphoma in pregnancy. Curr Hematol Malig Rep. 2013;8:211–217. doi: 10.1007/s11899-013-0163-4. [DOI] [PMC free article] [PubMed] [Google Scholar]