Abstract
The transition metal-catalyzed “cut and sew” transformation has recently emerged as a useful strategy for preparing complex molecular structures. After oxidative addition of a transition metal into a carbon–carbon bond, the resulting two carbon termini can be both functionalized in one step via the following migratory insertion and reductive elimination with unsaturated units, such as alkenes, alkynes, allenes, CO and polar multiple bonds. Three- or four-membered rings are often employed as reaction partners due to their high ring strains. The participation of non-strained structures generally relies on cleavage of a polar carbon–CN bond or assistance of a directing group.
Keywords: cut and sew, transition-metal catalysis, carbon–carbon activation, oxidative addition, reductive elimination

Introduction
The prosperous development of transition-metal (TM) catalysis has dramatically expanded chemists’ toolbox, enabling functionalization of many previously considered “inert” chemical bonds.1 The activation of carbon–carbon single bonds has recently drawn particular attentions,2 as one less reactive carbon–carbon bond can be converted into two more-reactive carbon–metal bonds leading to derivatization of each carbon terminus. Among various C–C activation-based transformations, the insertion of unsaturated moieties into a C–C single bond is of significant interest, because molecular complexity can be quickly built through uniting two fragments and reorganizing bond connections. This process typically involves TM insertion into a C–C bond via oxidative addition, a “cut” process, followed by migratory insertion into a π-unsaturated unit and reductive elimination, a “sew” process (Scheme 1). While there are a number of excellent review articles on C–C activation or cleavage,2 the main focus of this review is by no means to be comprehensive, but rather to provide a general overview of the development of such “cut and sew” transformations involving TM-catalyzed C–C activation. Formal “cut and sew” reactions, involving a β-carbon elimination process3 triggered by cyclometalation or nucleophilic addition or intramolecular migration, are also important developments in C–C activation, but not covered here due to space constraint.
Scheme 1.
“Cut and sew” transformations
As an overview, most “cut and sew” transformations via C–C activation utilize strained three/four-membered ring compounds as substrates, which is largely driven by strain relief when more stable four-/five-membered metallacycles are formed.4 For example, cyclopropane and cyclobutane5 derivatives have been frequently employed in a number of cycloadditions serving as C-3 and C-4 components, respectively. Substrates with less or no strain usually incorporate a directing group (DG) or take advantage of a more polar carbon–cyano (CN) bond. Regarding the unsaturate unit that can be “sewed” into a C–C bond, a large emphasis has been given to less polar alkene, alkyne and allene moieties. However, insertion of more polar π bonds, such as aldehydes and imines, has also been demonstrated recently. Apart from regular “cut and sew” processes, carbonylative and decarbonylative transformations that involve gain or loss of CO have also been developed and will be discussed in this article.
1. Three-membered rings
Cyclopropanes and their derivatives have been thoroughly explored as synthetically valuable building blocks.6 Driven by stain relief, these compounds generally undergo smooth ring opening with TMs. While direct “cut and sew” reactions with simple unactivated cyclopropanes remain challenging, vast success has been achieved with more reactive alkylidenecyclopropanes (ACPs), vinylcyclopropanes (VCP) or cyclopropanes with adjacent directing moieties.
1.1 ACP (alkylidenecyclopropane)
Cyclopropanes with exocyclic olefins, namely alkylidenecyclopropanes (ACPs), have been widely used in organic synthesis as C-3 building blocks.7 Early work of TM-catalyzed C–C activation of ACPs was reported by Noyori, Schuchardt, Trost and Tsuji.8 Based on different metal catalysts applied, two modes of activation with ACPs are possible: palladium and rhodium prefer to insert into the distal C3–C4 bond via oxidative addition, whereas ruthenium and nickel tend to cleave the proximal C2–C3 bond through two plausible intermediates 1 and 2 (Scheme 2). Intermediate 1 involves direct oxidative addition of a TM into the C2–C3 bond; in contrast, intermediate 2 involves cyclometalation between exocyclic methylene of ACPs, an unsaturated coupling partner, and a TM, followed by a β-carbon elimination process. On the other hand, when the distal C3–C4 is cleaved, the resulting intermediate can be considered as a metal–TMM (trimethylenemethane) species, rendering ACPs as a C–3 component in cycloaddition not only from C3–C4 but also from C1–C3 or C1–C4 positions. In this case, the mechanism may vary based on different substituents. For example, in 2001, Yamamoto reported a Pd-catalyzed heterocycle formation using ACPs and imines as coupling partners (Scheme 3a).9 In their proposed mechanism, palladium species cleaved the distal C3–C4 bond and led to the palladacyclobutane intermediate 3, where migratory insertion with the imines occurred at the C1 position to form the π-allyl species. After reductive elimination, pyrrolidine derivative 4 was afforded, with C1 and C3 incorporated in the five-membered ring (Scheme 3b). However, in one of their examples, a regioisomer from cycloaddition at C3 and C4 positions was observed. It was reasoned that due to the bulky tBu substituent in the imine substrate, the migratory insertion occurred at the less sterically hindered C3 position instead (Scheme 3c). While there are numerous transformations with ACPs, in this section only the reactions that clearly fall into the “cut and sew” portfolio are discussed.
Scheme 2.
Reaction patterns for ACPs
Scheme 3.
Substitution-controlled [3+2] cycloaddition of ACPs
a. Distal C–C Bond Cleavage
In 1988, Motherwell and coworkers studied intramolecular [3+2] cycloaddition of diphenylmethylenecyclopropanes with alkenes and alkynes (5 and 6, Scheme 4).10 With a palladium catalyst, the ACP moiety underwent a “cut and sew” transformation through cleavage of the distal C–C bond. The fused 5,5- and 5,6-bicycles were obtained in moderate yields. Shortly after, the same group expanded this method to methylenecyclopropanes (MCPs, 7).10b Additional phosphite ligand was employed to facilitate reductive elimination.
Scheme 4.
Pd-catalyzed intramolecular cycloaddition between ACPs and alkenes or alkynes
In 1991, when the Motherwell group explored the aforementioned “cut and sew” reaction in the absence of Thorpe–Ingold effect, heteroatom was found to assist the transformation by chelating to the metal catalyst (8 and 8’, Scheme 5).11 In sharp contrast to substrate 9 (R = H) that failed to provide any bicyclic products, substrate 10 (R = OBn) having a chelating oxygen atom afforded the desired adduct in a moderate yield.
Scheme 5.
Heteroatom-assisted intramolecular cycloaddition between simple MCPs and alkenes
The Lautens group later studied the stereochemistry of this reaction.12 When ACPs 11 containing C3 stereocenters were employed, retention of the stereo-information was obtained (Scheme 6). The acetylenic substituent has a marginal effect on the stereoselectivity.13 The reaction mechanism was proposed to initiate from the coordination of the alkyne to Pd(0), which is followed by distal C–C activation and alkyne migratory insertion (Scheme 7). A σ-π-allyl interconversion then occurs in intermediate 12 and leads to exchange of the two carbons supported by deuterium-labeling experiments. In 1996, the Lautens group further demonstrated that the intramolecular [3+2] cycloadditions with tethered alkenes were also stereospecific.14
Scheme 6.
Stereoselective intramolecular [3+2] reactions of ACPs with tethered alkynes
Scheme 7.
Proposed mechanism for the intramolecular [3+2] reactions of ACPs with tethered alkynes
b. Proximal C–C Bond Cleavage
In 2004, the Saito group reported that cyclopropylideneacetates 13 can couple with two equivalents of alkynes to provide cycloheptadienes using Ni(COD)2/PPh3 as a catalyst (Scheme 8a).15 In their following reports, a variety of internal and terminal alkynes bearing different electron properties were successfully coupled under similar reaction conditions.16 The replacement of alkyne moieties with tethered diynes17 or dienes18 were later realized, affording moderate to good yields and high regioselectivity (Scheme 8b).
Scheme 8.
Ni-catalyzed intermolecular cycloaddition with cyclopropylideneacetates
An interesting variation was reported when the [3+2+2] cycloaddition was investigated between 13 and two different alkynes (Scheme 9).19 Surprisingly, the cycloaddition proceeded in a high regioselective manner. In addition, the [3+2+2] cycloaddition also occurred smoothly with conjugated diynes20 or enynes21.
Scheme 9.
Ni-catalyzed intermolecular [3+2+2] cycloaddition with two different alkyne components
In 2010, López and Mascareńas extended the [3+2+2] cycloaddition reaction with tethered substrates (Scheme 10).22 The ACP-alkyne-tethered substrates 14 underwent [3+2+2] cyclization with electron deficient alkenes, while the use of unactivated alkenes resulted in recovery of the starting material. Deuterium-labeling experiments and DFT calculation studies both supported a pathway involving oxidative addition of Ni(0) into the proximal C–C bond (vide supra, intermediate 1, Scheme 2). In addition, substrates tethered with all the three components, ACP-alkyne-alkene (or another alkyne), can be employed, leading to efficient construction of 6,7,5- fused tricycles.23
Scheme 10.
Ni-catalyzed [3+2+2] cycloadditions between alkyne-tethered ACPs and alkenes
Similarly, an intramolecular cycloaddition between ACPs and alkynes was reported by the Zhang group in 2011, in which the proximal C–C bond was cleaved (Scheme 11).24 This transformation provides a unique approach to prepare cyclopenta[a]indene derivatives.
Scheme 11.
Ni-catalyzed cycloaddition of ACPs and alkynes via proximal C–C cleavage
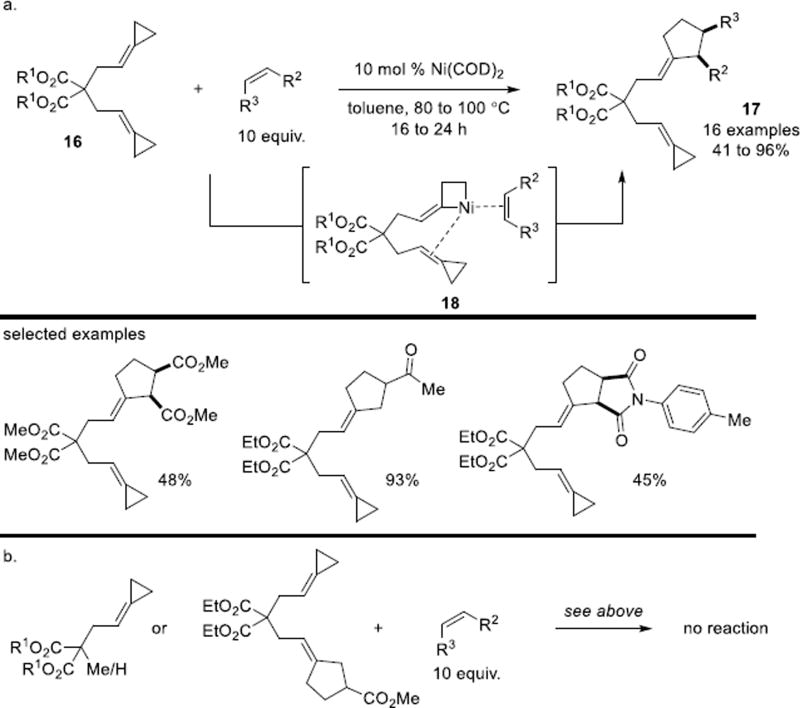
A Ni-catalyzed [3+2] cycloaddition between ACPs 16 and external olefins was reported by the Bhargava group in 2015.25 When bis(alkylidenecyclopropanes) were employed as substrates, a variety of electron-deficient olefins were coupled, providing the mono adduct in moderate to excellent yields (Scheme 12a). The afforded 2,3-disubstituted alkylidenecyclopentane 17 clearly indicated the proximal C–C bond cleavage rather than distal cleavage. The coordination with the alkenyl bond from the neighboring ACP moiety as shown in intermediate 18 was proposed to account for the reactivity. This hypothesis was supported by control experiments where substrates lacking the second tethered ACP resulted in no reaction (Scheme 12b).
Scheme 12.
Alkene-assisted intermolecular [3+2] cycloaddition of ACPs and alkenes
1.2 Simple cyclopropane
The first TM insertion into cyclopropane was reported by Tipper in 1955.26 Although different TMs have been extensively studied towards the activation of cyclopropane derivatives, their utilization in a “cut and sew” transformation was less-commonly seen in literature, majorly due to the facile β-hydrogen elimination side reaction from the metallacyclobutane complex.27 Narasaka and coworkers in 1999 reported the first rhodium-catalyzed carbonylative addition with a tethered cyclopropane and alkyne (Scheme 13).28 Though the presence of CO suppressed side-reactions, the reactions proceeded more slowly under increased CO pressure. The stereochemistry information in the starting material was delivered to the product and the cyclopropane cleavage occurred preferentially at the less hindered C–C bond. The proposed mechanism involved oxidative addition of Rh(I) into the cyclopropane C–C bond directed by the alkyne moiety (cf intermediate 19).
Scheme 13.
Rh-catalyzed intramolecular [3+2+1] cycloaddition of simple cyclopropanes with alkynes
In 2006, the Montgomery group discovered a Ni-catalyzed dimerization of cyclopropyl ketones (20, Scheme 14).29 Using Ni(COD)2 as a precatalyst and a N-heterocyclic carbene (NHC) ligand 21, trisubstituted cyclopentane derivatives with two carbonyls in trans-configuration were obtained in good yields and excellent diastereoselectivity. Regarding the reaction pathway, one cyclopropyl ketone was proposed to first undergo a Ni-catalyzed isomerization to conjugate enones, which then underwent a [3+2] cycloaddition with another equivalent of cyclopropyl ketone to afford the product. Based on this mechanistic hypothesis, external enones 22 were found to directly couple with cyclopropyl ketones. Good yields and excellent regioselectivity were obtained when the concentration of the enone were kept low via slow addition; titanium salts were added to accelerate the transformation. While cyclopropyl aldehydes failed to react, aldimines 23 were found to afford the desired [3+2] cycloadducts in good yields.30 In contrast, the two carbonyl groups in 24 were in cis-configuration, which was attributed to the ease of epimerizing the α-position of the aldehyde moiety.
Scheme 14.
Ni-catalyzed intermolecular [3+2] cycloaddition of acyl cyclopropanes with enones
The coupling of cyclopropyl ketones and alkynes was studied by the Ogoshi group in 2011.31 Me2AlCl was employed as a Lewis acid cocatalyst in addition to the Ni catalyst (Scheme 15). The mechanism was proposed to initiate from coordination of the alkyne and ketone moieties to Ni. Assisted by Me2AlCl, oxidative addition occurs to cleave the proximal C–C bond of the cyclopropyl group to form intermediate 25 where migratory insertion into alkynes leads to intermediate 26. Due to that 1,2-trans-disubstituted cyclopropanes afforded both 1,4-trans and 1,4-cis-products, an interconversion between intermediates 26 and 27 was proposed to occur prior to direct reductive elimination.
Scheme 15.
Ni-catalyzed intermolecular [3+2] cycloaddition of cyclopropyl ketones with alkynes
In 2013, the Bower group developed an intramolecular carbonylative “cut and sew” transformation of aminocyclopropanes with alkynes (28, Scheme 16).32 The choice of the amine protecting group is crucial as it needs to be able to coordinate with the metal and direct the C–C activation step while labile enough to be substituted by the alkyne for sequential migratory insertion. The urea group (R = NMe2) was found to be optimal to afford high reactivity when the substrate was treated with [Rh(COD)Cl]2 an electron-deficient mono-dentate phosphine ligand under 1 atmosphere of CO. For substituted cyclopropyl substrates, C–C bond cleavage occurred predominately at the less hindered side (e.g. 29 and 30, Scheme 16).
Scheme 16.
Urea-directed Rh-catalyzed intramolecular [3+2+1] cycloaddition of cyclopropyl amides with alkynes
The Bower group further extended the intramolecular [3+2+1] transformation to the coupling with alkenes (Scheme 17a).33 The carboxybenzyl (Cbz) group was found to be a more effective DG, and various trans-bicycles were provided. Substituted cyclopropanes were investigated where trans-1,2-disubstituted and trisubstituted cyclopropyl groups (31 and 32 respectively) can smoothly deliver the desired products in good yields. Moreover, compared with the previous conditions,32 the newly developed cationic rhodium condition was later proved to significantly increase the reactivity for the urea-directed intramolecular carbonylative [3+2+1] reactions with alkynes (Scheme 17b).34
Scheme 17.
Cationic rhodium-catalyzed for [3+2+1] cycloaddition of cyclopropyl amides
Recently, the same group successfully expanded the transformation to the use of aminomethylcyclopropanes 33 as substrates for construction of perhydroisoindoles 34 (Scheme 18).35 Similarly, the carbonyl unit in the nitrogen protecting group directed C–C activation to provide the six-membered ring intermediate 35, whereas due to the added methylene unit, the β-hydrogen elimination became the major side reaction. Compared with the cationic rhodium system, a neutral rhodium precursor efficiently suppressed the β-hydride elimination, presumably due to the lack of a vacant coordination site.
Scheme 18.
Directed intramolecular [3+2+1] cycloaddition of aminomethylcyclopropanes
1.3 Vinylcyclopropane (VCP)
Vinylcyclopropane is among the most extensively explored three-membered ring system.36 Typically, VCPs are classified based on their substitution positions. Terms such as 1-ene-/2-yne/β-yne-VCPs are often seen from literatures (Scheme 19). VCP has been frequently employed as a five-carbon component in cycloadditions, which involve cyclometalation then β-carbon elimination or oxidative addition then ring expansion (Scheme 20).37 On the other hand, VCPs that contain strong electron-withdrawing groups can be ionized by TMs (e.g. palladium) to generate a zwitterionic π-allyl intermediate, which can then couple with Michael acceptors (Scheme 21).38,39 Given that these transformations have been nicely reviewed previously and their mechanisms do not cleanly fall into the “cut and sew” scope, detailed discussions of these reactions will not be provided here. Some typical examples with VCPs as a three-carbon component are summarized.
Scheme 19.
General types of VCPs
Scheme 20.
Two mechanistic pathways for VCPs acting as a five-carbon component
Scheme 21.
VCPs serving as 1,3-dipoles
In 2008, Yu and co-workers reported the first Rh-catalyzed intramolecular cycloaddition of 2-ene-VCPs 36 with a tethered alkene moiety (Scheme 22).40 With a rhodium catalyst, a myriad of trans-1,2-disubstituted VCPs reacted to provide 5,5-bicycles in good yields. The use of a cationic rhodium catalyst, in situ generated from [Rh(CO)2Cl]2 and AgOTf, proved to give increased reactivity. The chirality information from the starting material can also be delivered to the bicyclic products (such as 37 and 38, Scheme 22). However, cis-1,2-disubstituted VCP 39 afforded [5+2] cycloadducts exclusively. The difference in reactivity between the trans- and cis-substrates was rationalized based on the distance of the two carbons that can undergo reductive elimination in these reactions.
Scheme 22.
Rh-catalyzed [3+2] cycloaddition of 2-ene-VCPs
Two years later, the same group achieved the first intramolecular [3+2] cycloaddition of 1-yne/ene -VCPs (Scheme 23).41 The desired bicyclic products were provided upon employment of a cationic rhodium/phosphine complex. Functional groups, such as esters and different heteroatom tethers, as well as 1-yne/allene-VCPs were well tolerated. The necessity of the vinyl group in the cyclopropane ring activation was supported by control experiments. The DFT studies suggested that the catalytic cycle starts from complexation of the rhodium catalyst to the vinyl group of the VCP, followed by insertion of Rh(I) into cyclopropane C–C bond to give π-allyl rhodium species 41 (Scheme 24). Subsequent migratory insertion into the tethered alkene/alkyne and reductive elimination provide the [3+2] cycloadduct.42
Scheme 23.
Rh-catalyzed [3+2] cycloaddition of 1-substituted-VCPs.
Scheme 24.
Reaction mechanism of the [3+2] cycloaddition of 1-substituted-VCPs
The asymmetric version of the reaction was also realized using in situ generated cationic rhodium in combination with chiral (R)-H8-BINAP ligand (Scheme 25).43 Further DFT studies suggested that the enantioselectivity was controlled by the alkyne-insertion step.
Scheme 25.
Asymmetric [3+2] cycloaddition of 1-substituted-VCPs
A novel [3+2+1] cycloaddition of 1-yne/ene-VCPs was also realized when the reactions were carried under a CO atmosphere (Scheme 26).44 Cyclohexanone/cyclohexenone products 42 bearing various functional groups were afforded in good yields. The reaction was proposed to follow the similar mechanism as the one described in Scheme 24. It is worthy to note that this [3+2+1] method has been effectively employed in the total synthesis of (±)-α-agarofuran and formal syntheses of gracilamine, (±)-galanthamine and (±)-lycoramine.45
Scheme 26.
Carbonylative [3+2+1] cycloadditions of 1-substituted-VCPs.
In addition, the Yu group further found that, when the alkyne moiety of the substrate has a bulky substituent, carefully tuning the reaction conditions (e.g. solvent and CO pressure) led to selective formation of a distinct multifunctional angular tricyclic 5/5/6 skeleton (43–45, Scheme 27) through insertion of two CO units.46 This reaction can be considered as a formal [5+1]/[2+2+1] cycloaddition, though control experiments and DFT studies suggest a more complex mechanism involving sequential migratory insertion into alkyne, CO, alkene, and then CO after C–C cleavage. Interesting, when a longer tether was used, only the normal [3+2+1] product, e.g. 46, was observed.
Scheme 27.
Carbonylative [5 + 1]/[2 + 2 + 1] or [3+2+1] cycloadditions of 1-substituted-VCPs
In 2010, Yu and coworkers reported the first [3+2] cycloaddition with α-ene-VCPs (47, Scheme 28).47 Unlike β-ene-VCPs that tends to give [5+2] cycloaddition,48 a [3+2] reaction was observed exclusively with the α-ene-VCPs. Using a cationic Rh(I)-phosphine complex as the catalyst, 5,6- and 5,7-bicycles were formed in good yields and excellent diastereoselectivity. Tethered alkynes didn’t result in any desired [3+2] cycloaddition due to a rapid intramolecular cyclopropanation (48, Scheme 28).
Scheme 28.
[3+2] cycloaddition of α-substituted-VCPs
The Matsubara group in 2014 explored a Ni(0)-catalyzed intermolecular [3+2] cycloaddition between VCPs 49 and allenes (Scheme 29).49 The use of Ni(COD)2 and PMe3 proved to be an optimal combination. Allenes bearing a variety of functional groups were well tolerated. The reaction was proposed to go through oxidative addition of Ni(0) to cleave the cyclopropyl C–C bond, followed by allene migratory insertion and reductive elimination to afford the five-membered carbocycle.
Scheme 29.
Ni-catalyzed intermolecular [3+2] cycloaddition between VCPs and allenes
1.4 Miscellaneous
In addition to the rich transformations discussed above, “cut and sew” transformations with other three-membered rings are also known. Due to the high ring strain, cyclopropenes and cyclopropenones50 have also participated in various TM-catalyzed reactions.51 For example, in 1976 Baba and co-workers reported a cycloaddition of diphenylcyclopropenone 50 and N-sulfinylamine 51 with stoichiometric Ni(CO)4 (Scheme 30).52 The reaction was proposed to proceed through a six-membered metallacycle intermediate 52, in which an exchange of the S=O unit with CO from Ni(CO)4 occurs to provide the maleimide product.
Scheme 30.
Cyclopropenone-mediated cycloaddition
In 2006, an intramolecular [3+2] cycloaddition between cyclopropenones and alkynes was reported by Wender and coworkers, which offers a new way to prepare cyclopentadienones (Scheme 31). [RhCl(CO)2]2 was found to be an efficient precatalyst; a wide range of internal alkynes, such as aryl and alkyl-substituted alkynes, enynes, heteroaryl alkynes and benzynes, were well compatible. The cyclopentadienone products were afforded in good yields and excellent regioselectivity.53
Scheme 31.
Intermolecular [3+2] cycloaddition between cyclopropenones and alkynes
Recently, the Wang group realized a Rh-catalyzed intramolecular carbonylative cycloaddition between cyclopropenes and tethered alkenes or alkynes (53 and 54 respectively, Scheme 32).54 Under 1 atmosphere CO, a myriad of ene/yne-cyclopropene substrates underwent a [3+2+1] cycloaddition and provided the bicyclic scaffolds in good yields. For tethered alkene derivatives, all the cyclohexenone derivatives were obtained as single diastereomers. The mechanism was proposed to initiate from alkene or alkyne-directed cleavage of the cyclopropene C–C single bond, followed by subsequent carbonylation, migratory insertion and reductive elimination.
Scheme 32.
Intramolecular [3+2] cycloaddition of cyclopropenes
2. Four-membered rings
Four-membered ring compounds are another important class of substrates that has been profoundly explored for C–C activation. The “cut and sew” transformations with cyclobutanones, cyclobutenones, biphenylenes and their derivatives have been developed, which have led to a diverse range of polycyclic scaffolds.
2.1. Cyclobutanone
Early works on TM-catalyzed C–C activation of cyclobutanones were reported by Ito and Murakami where they enabled oxidative addition of rhodium into the C–C bond adjacent to the carbonyl group.2m,55 Ring-opening or decarbonylation products were obtained. These seminal examples paved the way for the development of “cut and sew” chemistry with cyclobutanones. In 2002 the same group reported a Rh(I)-catalyzed activation of styrene-tethered cyclobutanones to give bicyclo[3.2.1]octanes (Scheme 33).56 The mechanism was proposed to start with oxidative addition of Rh(I) into the cyclobutanone α-C–C bond followed by alkene migratory insertion and reductive elimination (Scheme 34).
Scheme 33.
Carboacylation with the styrene-tethered cyclobutanones
Scheme 34.
Proposed pathway for the Rh-catalyzed carboacylation of the styrene-tethered cyclobutanones
The asymmetric version of this transformation was later revealed by Cramer and coworkers in 2014 through a chiral rhodium-catalyzed enantiotopic C–C cleavage. Two sets of conditions were made available: first they developed a zwitterionic catalytic system and the reaction proceeded at lower reaction temperature with moderate enantioselectivity (Scheme 35a).57 Shortly after, they discovered that the use of DTBM-SEGPHOS (56) as the ligand can induce exceptionally high enantioselectivity (Scheme 35b). 58 Under the latter conditions, mono-, di- and tri-substituted alkenes can be used as the coupling partners.
Scheme 35.
Enantioselective carboacylation of the styrene-tethered cyclobutanones
One challenge to expand the scope of the “cut and sew” coupling between cyclobutanones and olefins is the competing decarbonylation: upon Rh(I) oxidative addition into cyclobutanone C–C bond if the olefin insertion is slow, the decarbonylation would dominate and lead to forming cyclopropane or propene (Scheme 36).55,56 To address this issue, Ko and Dong devised a strategy that uses 2-amino-3-picoline 57 as a cocatalyst (previously employed by Jun in activation of non-strained ketones)59 to in situ protect the cyclobutanone carbonyl group and simultaneously serve as a DG (Scheme 37a).60 The use of electron-deficient mono-dentate phosphine ligand P(3,5-C6H5(CF3)2)3 was also found critical for the success of the reaction. Under these conditions, a range of 6,6- and 5,6-fused bicyclic structures can be afforded. Mono-, and 1,1- and 1,2-disubstituted alkenes were used as coupling partners. Promising level of enantioselectivity was also achieved using a chiral phosphoramidite ligand (Scheme 37b).
Scheme 36.
Competing decarbonylation pathway
Scheme 37.
A temporary DG-based strategy for the “cut and sew” reaction with cyclobutanones and olefins
When allenes were used as the coupling partner for a “cut and sew” reaction with cyclobutanones, an unexpected [4+1] cycloaddition was observed by Zhou and Dong.61 Formally serving as a vinyl carbenoid equivalent, the central carbon of the tethered allene inserted into the cyclobutanone α-C–C bond providing a [4.2.1]-bicyclic skeleton 59 (Scheme 38). The corresponding [4+2] product was observed as a minor product (Scheme 39). The enantioselective variant was also realized when ligand 60b was employed. Both 1,3-di- and 1,1,3-tri-substituted allenes were competent for this transformation, while mono-substituted allenes tended to cause dimerization of the substrate. The proposed mechanism initiates from rhodium oxidative addition into the α-C–C bond of cyclobutanone derivatives, followed by allene migratory insertion (Scheme 39). The resulting rhodacycle 61 then undergoes β-hydrogen elimination/reinsertion to generate intermediate 62 or 63, where the following reductive elimination provides the [4+1] cycloadduct 59.
Scheme 38.
[4+1] cycloaddition of cyclobutanones and allenes
Scheme 39.
Proposed mechanism of the [4+1] cycloaddition of cyclobutanones and allenes
More recently, an intramolecular formal [4+2–1] cyclization between cyclobutanones and tethered olefins was realized by Dong and coworkers (Scheme 40).62 While decarbonylation of cyclobutanones to give cyclopropanes and the regular [4+2] process are highly competitive side reactions (vide supra, scheme 36), use of a bulky monodentate XPhos (2-Dicyclohexylphosphino-2′,4′,6′-triisopropylbiphenyl) ligand promoted an unusual [4+2–1] pathway. Control experiments indicated that the reaction is unlikely to go through a cyclopropane-mediated [3+2] pathway (from decarbonylation of the cyclobutanone) or decarbonylation of the [4+2] product; instead, the [4+2–1] reaction was proposed to arise from direct cyclobutanone C–C cleavage, CO extrusion, olefin migratory insertion, and reductive elimination. The synthetic utility of the method was exemplified in the synthesis of antifungal drug tolciclate (Scheme 41).
Scheme 40.
Rhodium-catalyzed [4+2-1] cycloaddition
Scheme 41.
Synthesis of tolciclate
In addition of using non-polar 2π coupling partners, Cramer and coworkers developed a novel carbonyl insertion variant to obtain bridged bicyclic lactones (65) in high enantioselectivity, a reaction analogous to the Tishchenko disproportionation (Scheme 42).63 The reaction was found to tolerate a number of cyclobutanone-substituted moieties as well as aldehyde and ketone insertion partners. Aldol condensation and cyclopropane formation from decarbonylation of cyclobutanones were observed as common side reactions. The proposed mechanism corresponds to the previous styrene insertion; formation of rhodacycle 66 leads to the subsequent insertion of the pendant carbonyl (Scheme 43).
Scheme 42.
Asymmetric carbonyl insertion to cyclobutanone
Scheme 43.
Proposed catalytic cycle of the Rh-catalyzed carbonyl-cyclobutanone coupling
2.2 Cyclobutenone and Cyclobutendione
Early work from the Liebeskind group shows that TMs, such as nickel, iron, rhodium and cobalt, can insert into cyclobutenedione 67 to form a five membered metallacycle 68, which can then couple with an alkyne to yield substituted quinones (Scheme 44).64 In attempts to expand this chemistry to cyclobutenones and benzocyclobutenones for syntheses of phenols and naphthols, the use of Rh(I) led to the isolation of a five-membered rhodacycle 69, which was inert with alkynes (Scheme 45).65 Later work from the same group found that the use of Co(I) instead was capable to induce subsequent coupling with alkynes.65b,66 A year later, the catalytic “cut and sew” transformation between cyclobutenones 70 and alkynes was realized with Ni(0), and the regioselectivity of the insertion product 71 can be controlled by using heteroatom-substituted alkynes (such as 72).65b,67 More recently, Auvinet and Harrity extended the scope of the nickel-catalyzed alkyne insertion to include alkynyl boronates (Scheme 46).68 The regioselectivity of the reaction is influenced by the substituents on the alkynyl boronates: forming regiosomer 73 is favored for alkynyl-bornoates with a sp3 substituent, whereas those with a sp2 substituent prefer to give regiosomer 74.
Scheme 44.
Quinone synthesis from C–C activation of cyclobutenediones
Scheme 45.
Phenol synthesis from C–C activation of cyclobutenones
Scheme 46.
Alkynyl boronate insertion into cyclobutenones
In addition to coupling with alkynes, Kondo, Mitsudo and coworkers successfully expanded the substrate scope to electron-deficient olefins, norbornene, and ethylene using rhodium and ruthenium catalysts. First, Ru3(CO)12 was discovered to induce a decarbonylative coupling of cyclobutenediones 75 with norbornene to yield cyclopentenone 76 (Scheme 47, path a).69 When higher carbon monoxide pressure was applied, direct insertion to give hydroquinone product 77 was observed (Scheme 47, path b). The proposed mechanism is similar to the aforementioned alkyne insertion into cyclobutenones (vide supra, Scheme 45).
Scheme 47.
Norbornene insertion into cyclobutenediones
Later, cyclobutenone activation by [Rh(CO)2Cl]2 was found to result in either direct norbornene insertion or decarbonylative insertion (Scheme 48, paths a and b respectively); similar to their previous work, the carbon monoxide pressure was the key factor to control the reaction selectivity.70 A notable side reaction was cyclobutenone dimerization (Scheme 49). The mechanism was proposed to involve Rh(I) insertion into cyclobutenone 78 to give metallacycle 79. Migratory insertion with the olefin gives fused ring 80, which can undergo decarbonylation under argon atmosphere followed by reductive elimination to give cyclopentene 81, or direct reductive elimination under carbon monoxide atmosphere to provide cyclohexenone 82 (Scheme 50). The work was later expanded to insertion of electron-deficient alkenes to yield 2-substituted phenol derivatives (83, Scheme 51).71
Scheme 48.
Rh-catalyzed norbornene-cyclobutenone coupling
Scheme 49.
Cyclobutenone dimerization
Scheme 50.
Proposed reaction pathway of the norbornene-cyclobutenone coupling
Scheme 51.
Coupling of electron-deficient olefins with cyclobutenones
An intramolecular decarbonylative coupling between cyclobutenediones and olefins were reported by Yamamoto and coworkers (Scheme 52).72 Derived from squaric acid, substrate 84 underwent C–C cleavage between the two carbonyls, followed by decarbonylation and alkene insertion, to afford a range of fused cyclopentenone bicycles 85, which is catalyzed by Wilkinson’s complex or in situ generated Wilkinson’s complex.
Scheme 52.
Intramolecular decarbonylative alkene insertion with cyclobutenediones
The Dong group has been focusing on developing intramolecular “cut and sew” transformations with benzocyclobutenones. While cleavage of the benzocyclobutenone C1–C8 bond is kinetically favored, the work by Xu and Dong showed that benzocyclobutenone substrate 86 can undergo cleavage of the proximal C1–C2 bond using Rh(I) as the catalyst (Scheme 53).73 Mono-, di-, and tri-substituted alkenes can be coupled, leading to various benzo-fused tricyclic scaffolds 87 with high functional group tolerance. The use of ZnCl2 as a Lewis acid cocatalyst was found to be critical for more challenging substrates, such as those with aryl olefins and longer tethers (Scheme 54). Control experiments showed that no desired insertion product was obtained in the absence of the rhodium catalyst either in the presence or absence of ZnCl2. A more detailed DFT studies suggest a stepwise C1–C2 activation pathway involving cleavage of the C1–C8 bond followed by a decarbonylative CO migration (Scheme 55).74
Scheme 53.
Selective proximal C1–C2 cleavage of benzocyclobutenones and tethered olefin insertion
Scheme 54.
Effect of ZnCl2 on challenging olefin substrates
Scheme 55.
Indirect proximal C1–C2 activation through decarbonylative CO migration
Stimulated by forming chiral all-carbon quaternary centers, in 2012 the Dong group developed an enantioselective version of the transformation using [Rh(COD)Cl]2 and DTBM-SEGPHOS (56) as the metal-ligand combination (Scheme 56).75 High enantioselectivity was obtained at relatively high reaction temperature. A reductive dearomatization protocol was developed to access saturated fused chiral “half-cage”-like compounds 88 (Scheme 57). Selective hydrogenation from the convex face afforded saturated fused compounds as single diastereomers.
Scheme 56.
Enantioselective “cut and sew” transformation with benzocyclobutenones
Scheme 57.
Reductive dearomatization of the tricyclic products
To enable insertion of sterically hindered alkenes, a more reactive catalyst system was discovered by Xu and Dong using [Rh(CO)2Cl]2 and P(C6F5)3 (Scheme 58a). The increased π-acidity of the metal center enhanced its binding affinity with the alkene moiety. A number of tri-substituted alkene substrates, non-reactive under the previous conditions, were efficiently coupled with benzocyclobutenones (such as 89). Using this new protocol, they accomplished the first synthesis of the proposed structure of cycloinumakiol 90 and its C5 epimer 91 in a concise fashion (Scheme 58b).76
Scheme 58.
Coupling of trisubstituted olefins and its application in the total synthesis of cycloinumakiol
It is not surprising that alkynes can also undergo the intramolecular “cut and sew” reaction with benzocyclobutenones (Scheme 59). Various fused β-naphthols 93 were obtained via isomerization of the initially formed enones 92. The addition of ZnCl2 co-catalyst was again found to assist forming larger rings (such as 94, Scheme 60). A decarbonylative alkyne-insertion pathway was achieved by using bulky bidentate ligand DTBM-SEGPHOS in addition to refluxing in xylenes under argon atmosphere.77 This decarbonylative “cut and sew” reaction offers a unique approach to access fused indene rings (95).
Scheme 59.
Alkyne insertion into benzocyclobutenones
Scheme 60.
A divergent approach to access fused naphthols and indenes
Apart from alkene and alkyne insertions, in 2015 the Dong group developed an enantioselective “cut and sew” reaction with more polar C=N bonds. The intramolecular carboacylation of oxime ethers (96) with benzocyclobutenones provides an efficient entry to fused tetrahydroisoquinoline rings (Scheme 61).78 High levels of enantioselectivity were maintained even using substrates as a mixture of E/Z oxime isomers. Subsequent derivatization of the lactams was possible through N-arylation and alkylation upon cleavage of the N–OMe bond (not shown). Saturated tricyclic scaffold 97 was also accessed by hydrogenation of the arene (Scheme 62).
Scheme 61.
Enantioselective C=N bond insertion into benzocyclobutenones
Scheme 62.
Diastereoselective hydrogenation of the fused scaffold
Martin and coworkers realized a highly regioselective intermolecular coupling between benzocyclobutenones and 1,3-dienes or diphenylacetylenes (Scheme 63a).79 Ni(COD)2 and tri(4-trifluoromethylphenyl)phosphine were found to be most efficient to catalyze insertion of 1,3-dienes to give [4+4] adducts. When PPh3 was used as ligand, the coupling with diphenylacetylene smoothly occurred to give [4+2] products. In all cases, exclusive C1–C2 was observed. While a cyclometalation/β-carbon elimination sequence was proposed in the original work, a recent DFT study supports an oxidative addition-initiated pathway (Scheme 63b).80
Scheme 63.
Intermolecular coupling of benzocyclobutenones with 1,3-dienes and acetylenes
Recently, Matsuda and coworkers reported an intermolecular coupling between methylidenecyclobutenes (98) and alkynes via a pyridine-directed C–C cleavage (Scheme 64).81 Poly-substituted benzenes were obtained as the final product. For unsymmetrical cyclobutene 99, olefin isomerization (via 100) prior to the C–C bond cleavage was proposed to explain the observed two regioisomers 101 and 102.
Scheme 64.
Rh-catalyzed coupling between methylidenecyclobutenes and alkynes
2.3 Biphenylene
Although TM-mediated activation of biphenylenes has been extensively studied since 1960s, early work mainly focused on either using stoichiometric metals82 or catalytic cleavage followed by hydrogenation83 or dimerization84. The first catalytic “cut and sew” reaction with biphenylenes was reported by Jones and coworkers in 1999, where a (dppe)Ni(alkyne) metal complex 103 was found to allow the cycloaddition with acetylene derivatives to generate phenanthrene 104 (Scheme 65a).85 Under nearly 0.6 mol % O2 atomosphere, the phosphine ligand of 103 is first oxidized to generate the active nickel species, which enables oxidative cyclization with the biphenylenes to form Ni complex 105. Subsequent migratory insertion and reductive elimination provide the phenanthrene products. Using different Ni precatalysts, C-1 unit insertions were realized with CO and isocyanides, affording fluorenones 106 and fluorine imines 107. Later, a similar transformation was also achieved by rhodium catalysts (Scheme 65b).86
Scheme 65.
Ni-catalyzed insertion of alkynes, CO and isocyanides with biphenylenes
To circumvent the use of O2 to generate the active catalyst, Jones and coworkers prepared a new nickel complex 108 containing a P,N-ligand, which has a higher catalytic performance towards the alkyne-insertion reaction (Scheme 66).87 In their design, the dissociation of the more labile nitrogen ligand resulted in an open coordination site on the nickel, thus providing a higher reactivity. The combination of a nickel catalyst with a NHC ligand in this transformation was investigated later by the Radius group.88
Scheme 66.
Using a Ni catalyst with a P,N-ligand
Later in 2008, the Shibata group reported an asymmetric version of this transformation for generating phenanthrene derivatives with axial chirality (109, Scheme 67). Employing an iridium catalyst with chiral (S, S)-Me-BPE ligand 110, a variety of arylacetylenes were afforded in good yields and moderate to high enantiomeric exces.89
Scheme 67.
Formation of axial chirality via the “cut and sew” reaction with biphenylenes
Using biphenylene-mediated “cut and sew” reactions to form heteroarenes has also been realized (Scheme 68). The Kotora group reported a rhodium-catalyzed biphenylene-nitrile coupling. Bidentate phosphine ligand was used to give the desired phenanthridines 111 in decent yields.90
Scheme 68.
Coupling of biphenylenes with nitriles to form phenanthridines
3. Less strained compounds
Clearly, the “cut and sew” transformations with small ring systems are largely driven by strain release. However, significant progress has also been achieved with C–C activation of less strained compounds. The current strategy primarily relies on activation of a polar C–CN bond or employing a DG.
3.1 Activation of C–CN bond
C–CN bonds generally possess significantly higher bond dissociation energy (>100 kcal/mol) than C–C single bonds (around 85 kcal/mol). However, due to their strong electron-withdrawing nature and high binding affinity with TM of the CN moiety, the polar C–CN bonds are prone to undergo oxidative addition with TMs.91 Hence, catalytic C–CN bond activation is feasible and has been extensively studied. In particular, carbocyanation of an unsaturated π moiety via C–CN bond activation provides a unique approach to prepare nitrile compounds.92 Since the early work of intermolecular coupling between arylnitriles and internal acetylenes by Hiyama and Nakao in 2004,93 a variety of nitrile derivatives, such as allylnitriles,94 carbonocyanidates,95 carbamoyl cyanides,96 alkynylnitriles, and alkenylnitriles were demonstrated to be well compatible for such a “cut and sew” transformation. In addition, the carbocyanation reaction also proceeded in an intramolecular fashion,93b,96a–b,97 where use of chiral ligands provided the cyclization products with high enantioselectivities.96c,98 Acyl nitriles99 and α-iminonitriles100 have also been employed as the coupling partners, leading to more functionalized nitrile products. Importantly, Lewis acids, such as BPh3, AlMe3 and AlMe2Cl, were demonstrated in 2007 by Hiyama and Nakao to greatly increase the reactivity of the carbocyanation reactions,101 presumably through coordination with the cyano group to facilitate the oxidative addition step. Similarly, the incorporation of a Lewis acid co-catalyst also enabled reactions with challenging substrates such as alkenylnitriles, alkynylnitriles101,102 and alkylnitriles101, 103.
Generally, the reaction initiates from the complexation between a TM and a nitrile substrate, followed by oxidative addition into the C–CN bond (Scheme 69). The resulting alkyl/aryl/acyl–metal bond then undergoes migratory insertion into the unsaturated unit, such as alkenes, alkynes and allenes,104 followed by subsequent C–CN bond-forming reductive elimination to furnish the “cut and sew” product 114. Given that this area has recently been extensively reviewed by Nakao92b–c, 105, Chatani106, and others92a,d,f,107, detailed discussions will not be included in this perspective.
Scheme 69.
General mechanism for the TM-catalyzed “cut and sew” reactions via C–CN bond activation
3.2 Use of a Directing group
One important strategy to activate unstrained C–C bonds is employing an intramolecular coordinating group to deliver a TM to insert into a particular C–C bond.108 Such a directing strategy generally lowers the kinetic barrier for the oxidative addition step, and often forms a five-membered metallacycle as the key intermediate. In 1981, Suggs and Cox reported the first directed C–C activation of a linear ketone using a quinoline moiety as the DG.109 However, the corresponding “cut and sew” transformation with the 8-acylquinoline system did not appear until the Douglas’ work in 2009.110 Using alkene-tethered substrate 115, they achieved an intramolecular carboacylation via directed C–C cleavage of the acyl–aryl bond (Scheme 70). A variety of 1,1-disubstituted alkenes were used, providing products 116 bearing quaternary carbon centers in good yields, while mono-substituted alkenes gave low yields likely caused by a β-hydrogen elimination pathway. Mechanistic studies by Johnson and coworkers111 revealed that for substrates with a less-hindered alkene, the oxidative addition is the rate-determining step in this transformation; whereas larger substituents on the alkene or substrates with longer tethers decelerated the migratory insertion step, rendering it the rate-determining step (Scheme 71).
Scheme 70.
8-Quinoline-directed intramolecular carboacylation of tethered alkenes
Scheme 71.
Proposed reaction mechanism for the 8-quinoline-directed intramolecular carboacylation of tethered alkenes
Shortly after, the transformation was extended to an intermolecular carboacylation with norbornene derivatives (Scheme 72).112 To minimize the undesired C–H activation pathway, the Douglas group employed a cationic rhodium precatalyst with a triflate counterion in a more polar solvent such as THF. This condition favors the “cut and sew” transformation providing the insertion products in moderate yields.
Scheme 72.
Directed intermolecular carboacylation of norbornene derivatives
In 2015, Zeng and Dong reported a directed C–C activation of isatin derivatives (117),113 in which one equivalent of CO was removed from the substrate followed by alkyne insertion to provide various 2-quinolinones (Scheme 73). The overall reaction is considered as a [5+2–1] transformation. To circumvent the undesired ortho C–H activation, 3-methylpyridyl group was found to be effective by controlling the orientation of the metal. Alkynes bearing a myriad of functional groups, such as unprotected alcohols, esters and ketones were tolerated. Unsymmetrical internal alkynes provide high regioselectivity with larger substituents at the 4-position (e.g. 118). Terminal alkynes, though tolerated, gave moderate yields and regioselectivity (e.g. 119). Mechanistic exploration shows that the Rh insertion into the C–C bond can occur at room temperature; however, migratory insertion of alkynes did not happen below 130 °C.
Scheme 73.
Directed selective C–C activation of isatins followed by decarbonylative coupling with alkynes
Very recently, the Dong group developed an intermolecular isatin/isocyanates coupling reaction through a double decarbonylation pathway (Scheme 74).114 Use of electron-deficient ligands such as AsPh3 proved crucial to provide the benzimidazolidinone products 120 in excellent yields. A range of functional groups were found compatible. Use of in situ generated isocyanates from the corresponding acyl azides was also efficient (such as in products 121 and 122). Interestingly, an isotope labelling study using the 13C-labeled isocyanate revealed both the carbonyl groups from the isatin starting material were removed during the reaction (Scheme 75).
Scheme 74.
C–C activation of isatins followed by decarbonylation and coupling with isocyanates
Scheme 75.
Proposed mechanism for the double-decarbonylative coupling reaction
Conclusion and Outlook
Taking advantage of C–C activation as a unique mode of reactivity, a large variety of “cut and sew” transformations has been developed. These reactions either provide unusual bond disconnection strategies to access known systems, or afford novel scaffolds that are challenging to be prepared using conventional approaches. These transformations are typically pH and redox neutral, and highly atom economical. Consequently, the functional group tolerance is generally excellent. It is expected that with a better understanding of the mechanism of the TM-mediated C–C activation processes, more efficient catalyst systems (e.g. catalysts with high TONs and TOFs) and milder reaction conditions, such as lower reaction temperature, will be developed in the future. While the majority of current methods employ strained substrates to gain thermodynamic driving forces, participation of less strained systems recently started to grow. However, general “cut and sew” transformations with less or non-stained substrates are rare and typically require use of a permeant directing moiety, except the C–CN bond activation methods. It is envisioned that more synthetically useful transformations or new C–C activation modes with less strained systems might become an interesting research direction in the near future.
Acknowledgments
We thank NIGMS (R01GM109054) and the Welch Foundation (F1781) for research grants. G.D. is a Searle Scholar and Sloan Fellow. T. T. thanks Ito Foundation for International Education Exchange fellowship.
References
- 1.Murakami M, Ito Y. In: Activation of Unreactive Bonds and Organic Synthesis. Topics in Organometallic Chemistry. Murai S, editor. Vol. 3. Springer; Berlin: 1999. pp. 97–129. [Google Scholar]
- 2.(a) Dong G, editor. C-C Bond Activation. Topics in Current Chemistry. Vol. 346. Springer; Berlin: 2014. [DOI] [PubMed] [Google Scholar]; (b) Souillart L, Cramer N. Chem. Rev. 2015;115:9410–9464. doi: 10.1021/acs.chemrev.5b00138. [DOI] [PubMed] [Google Scholar]; (c) Rybtchinski B, Milstein D. Angew. Chem. Int. Ed. 1999;38:870–883. doi: 10.1002/(SICI)1521-3773(19990401)38:7<870::AID-ANIE870>3.0.CO;2-3. [DOI] [PubMed] [Google Scholar]; (d) Perthuisot C, Edelbach BL, Zubris DL, Simhai N, Iverson CN, Müller C, Satoh T, Jones WD. J. Mol. Catal. A: Chem. 2002;189:157–168. [Google Scholar]; (e) Ruhland K. Eur. J. Org. Chem. 2012;2012:2683–2706. [Google Scholar]; (f) Chen F, Wang T, Jiao N. Chem. Rev. 2014;114:8613–8661. doi: 10.1021/cr400628s. [DOI] [PubMed] [Google Scholar]; (g) Dermenci A, Coe JW, Dong G. Org. Chem. Front. 2014;1:567–581. doi: 10.1039/c4qo00053f. [DOI] [PMC free article] [PubMed] [Google Scholar]; (h) Kondo T, Mitsudo T-a. Chem. Lett. 2005;34:1462–1467. [Google Scholar]; (i) Teruyuki K, Take-aki M. Chem. Lett. 2005;34:1462–1467. [Google Scholar]; (j) Jones WD. Nature. 1993;364:676–677. [Google Scholar]; (k) Murakami M, Matsuda T. Chem. Commun. 2011;47:1100–1105. doi: 10.1039/c0cc02566f. [DOI] [PubMed] [Google Scholar]; (l) Jun C-H. Chem. Soc. Rev. 2004;33:610–618. doi: 10.1039/b308864m. [DOI] [PubMed] [Google Scholar]; (m) Murakami M, Amii H, Ito Y. Nature. 1994;370:540–541. [Google Scholar]
- 3.(a) Tsuji J, editor. Palladium in Organic Synthesis. Topics in Organometallic Chemistry. Vol. 14 Springer; Berlin: 2005. [Google Scholar]; (b) Cramer N, Seiser T. Synlett. 2011;2011:449–460. [Google Scholar]
- 4.(a) Kondo T. Eur. J. Org. Chem. 2016;2016:1232–1242. [Google Scholar]; (b) Chen P-H, Dong G. Chem. Eur. J. 2016;22:18290–18315. doi: 10.1002/chem.201603382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.(a) Bellus D, Ernst B. Angew. Chem. Int. Ed. 1988;27:797–827. [Google Scholar]; (b) Namyslo JC, Kaufmann DE. Chem. Rev. 2003;103:1485–1538. doi: 10.1021/cr010010y. [DOI] [PubMed] [Google Scholar]; (c) Sadana AK, Saini RK, Billups WE. Chem. Rev. 2003;103:1539–1602. doi: 10.1021/cr010022j. [DOI] [PubMed] [Google Scholar]; (d) Seiser T, Saget T, Tran DN, Cramer N. Angew. Chem. Int. Ed. 2011;50:7740–7752. doi: 10.1002/anie.201101053. [DOI] [PubMed] [Google Scholar]; (e) Belluš D, Ernst B. Angew. Chem. Int. Ed. 1988;27:797–827. [Google Scholar]; (f) Flores-Gaspar A, Martin R. Synthesis. 2013;45:563–580. [Google Scholar]
- 6.(a) Nikolaev A, Orellana A. Synthesis. 2016;48:1741–1768. [Google Scholar]; (b) Rassadin VA, Six Y. Tetrahedron. 2016;72:4701–4757. [Google Scholar]
- 7.(a) Nakamura I, Yamamoto Y. Adv. Synth. Catal. 2002;344:111–129. [Google Scholar]; (b) Brandi A, Cicchi S, Cordero FM, Goti A. Chem. Rev. 2003;103:1213–1270. doi: 10.1021/cr010005u. [DOI] [PubMed] [Google Scholar]; (c) Brandi A, Cicchi S, Cordero FM, Goti A. Chem. Rev. 2014;114:7317–7420. doi: 10.1021/cr400686j. [DOI] [PubMed] [Google Scholar]; (d) Pellissier H. Tetrahedron. 2014;70:4991–5031. [Google Scholar]; (e) Yu L, Liu M, Chen F, Xu Q. Org. Biomol. Chem. 2015;13:8379–8392. doi: 10.1039/c5ob00868a. [DOI] [PubMed] [Google Scholar]; (f) Binger P, Büch HM. In: Small Ring Compounds in Organic Synthesis II. Topics in Current Chemistry. Meijere de A, editor. Vol. 135. Springer; Berlin: 1987. pp. 77–151. [Google Scholar]
- 8.(a) Noyori R, Kumagai Y, Umeda I, Takaya H. J. Am. Chem. Soc. 1972;94:4018–4020. doi: 10.1021/ja00766a065. [DOI] [PubMed] [Google Scholar]; (b) Noyori R, Odagi T, Takaya H. J. Am. Chem. Soc. 1970;92:5780–5781. [Google Scholar]; (c) Binger P, Schuchardt U. Angew. Chem. Int. Ed. 1977;16:249–250. [Google Scholar]; (d) Trost BM, Chan DMT. J. Am. Chem. Soc. 1983;105:2315–2325. [Google Scholar]; (e) Trost BM, Chan DMT. J. Am. Chem. Soc. 1983;105:2326–2335. [Google Scholar]; (f) Shimizu I, Ohashi Y, Tsuji J. Tetrahedron Lett. 1984;25:5183–5186. [Google Scholar]
- 9.Oh BH, Nakamura I, Saito S, Yamamoto Y. Tetrahedron Lett. 2001;42:6203–6205. [Google Scholar]
- 10.(a) Lewis RT, Motherwell WB, Shipman M. J. Chem. Soc., Chem. Commun. 1988:948–950. [Google Scholar]; (b) Antony Bapuji S, Motherwell WB, Shipman M. Tetrahedron Lett. 1989;30:7107–7110. [Google Scholar]
- 11.Motherwell WB, Shipman M. Tetrahedron Lett. 1991;32:1103–1106. [Google Scholar]
- 12.(a) Lautens M, Ren Y, Delanghe PHM. J. Am. Chem. Soc. 1994;116:8821–8822. [Google Scholar]; (b) Lautens M, Ren Y, Delanghe P, Chiu P, Ma S, Colucci J. Can. J. Chem. 1995;73:1251–1257. [Google Scholar]
- 13.Lautens M, Ren Y. J. Am. Chem. Soc. 1996;118:9597–9605. [Google Scholar]
- 14.Lautens M, Ren Y. J. Am. Chem. Soc. 1996;118:10668–10669. [Google Scholar]
- 15.Saito S, Masuda M, Komagawa S. J. Am. Chem. Soc. 2004;126:10540–10541. doi: 10.1021/ja0494306. [DOI] [PubMed] [Google Scholar]
- 16.Saito S, Komagawa S, Azumaya I, Masuda M. J. Org. Chem. 2007;72:9114–9120. doi: 10.1021/jo7014714. [DOI] [PubMed] [Google Scholar]
- 17.Maeda K, Saito S. Tetrahedron Lett. 2007;48:3173–3176. [Google Scholar]
- 18.Saito S, Takeuchi K. Tetrahedron Lett. 2007;48:595–598. [Google Scholar]
- 19.Komagawa S, Saito S. Angew. Chem. Int. Ed. 2006;45:2446–2449. doi: 10.1002/anie.200504050. [DOI] [PubMed] [Google Scholar]
- 20.Yamasaki R, Sotome I, Komagawa S, Azumaya I, Masu H, Saito S. Tetrahedron Lett. 2009;50:1143–1145. [Google Scholar]
- 21.Komagawa S, Takeuchi K, Sotome I, Azumaya I, Masu H, Yamasaki R, Saito S. J. Org. Chem. 2009;74:3323–3329. doi: 10.1021/jo900189g. [DOI] [PubMed] [Google Scholar]
- 22.Saya L, Bhargava G, Navarro MA, Gulías M, López F, Fernández I, Castedo L, Mascareñas JL. Angew. Chem. Int. Ed. 2010;49:9886–9890. doi: 10.1002/anie.201004438. [DOI] [PubMed] [Google Scholar]
- 23.Saya L, Fernández I, López F, Mascareñas JL. Org. Lett. 2014;16:5008–5011. doi: 10.1021/ol502288x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Yao B, Li Y, Liang Z, Zhang Y. Org. Lett. 2011;13:640–643. doi: 10.1021/ol1028628. [DOI] [PubMed] [Google Scholar]
- 25.Kuila B, Mahajan D, Singh P, Bhargava G. Tetrahedron Lett. 2015;56:1307–1311. [Google Scholar]
- 26.Tipper CFH. J. Chem. Soc. 1955:2045–2046. [Google Scholar]
- 27.(a) Rubin M, Rubina M, Gevorgyan V. Chem. Rev. 2007;107:3117–3179. doi: 10.1021/cr050988l. [DOI] [PubMed] [Google Scholar]; (b) Shaw MH, Bower JF. Chem. Commun. 2016;52:10817–10829. doi: 10.1039/c6cc04359c. and references therein. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Koga Y, Narasaka K. Chem. Lett. 1999;28:705–706. [Google Scholar]
- 29.(a) Liu L, Montgomery J. J. Am. Chem. Soc. 2006;128:5348–5349. doi: 10.1021/ja0602187. [DOI] [PubMed] [Google Scholar]; (b) Lloyd-Jones GC. Angew. Chem. Int. Ed. 2006;45:6788–6790. doi: 10.1002/anie.200602629. [DOI] [PubMed] [Google Scholar]; (c) Ogoshi S, Nagata M, Kurosawa H. J. Am. Chem. Soc. 2006;128:5350–5351. doi: 10.1021/ja060220y. [DOI] [PubMed] [Google Scholar]
- 30.Liu L, Montgomery J. Org. Lett. 2007;9:3885–3887. doi: 10.1021/ol071376l. [DOI] [PubMed] [Google Scholar]
- 31.Tamaki T, Ohashi M, Ogoshi S. Angew. Chem. Int. Ed. 2011;50:12067–12070. doi: 10.1002/anie.201106174. [DOI] [PubMed] [Google Scholar]
- 32.Shaw MH, Melikhova EY, Kloer DP, Whittingham WG, Bower JF. J. Am. Chem. Soc. 2013;135:4992–4995. doi: 10.1021/ja401936c. [DOI] [PubMed] [Google Scholar]
- 33.Shaw MH, McCreanor NG, Whittingham WG, Bower JF. J. Am. Chem. Soc. 2015;137:463–468. doi: 10.1021/ja511335v. [DOI] [PubMed] [Google Scholar]
- 34.Shaw MH, Whittingham WG, Bower JF. Tetrahedron. 2016;72:2731–2741. [Google Scholar]
- 35.Wang G-W, McCreanor NG, Shaw MH, Whittingham WG, Bower JF. J. Am. Chem. Soc. 2016;138:13501–13504. doi: 10.1021/jacs.6b08608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Wang Y, Yu Z-X. Acc. Chem. Res. 2015;48:2288–2296. doi: 10.1021/acs.accounts.5b00037. [DOI] [PubMed] [Google Scholar]
- 37.(a) Wender Paul A, Bi FC, Gamber Gabriel G, Gosselin F, Hubbard Robert D, Scanio Marc JC, Sun R, Williams Travis J, Zhang L. Pure Appl. Chem. 2002;74:25–31. [Google Scholar]; (b) Butenschön H. Angew. Chem. Int. Ed. 2008;47:5287–5290. doi: 10.1002/anie.200801738. [DOI] [PubMed] [Google Scholar]; (c) Jiao L, Yu Z-X. J. Org. Chem. 2013;78:6842–6848. doi: 10.1021/jo400609w. [DOI] [PubMed] [Google Scholar]; (d) Ylijoki KEO, Stryker JM. Chem. Rev. 2013;113:2244–2266. doi: 10.1021/cr300087g. [DOI] [PubMed] [Google Scholar]; (e) Pellissier H. Adv. Synth. Catal. 2011;353:189–218. [Google Scholar]; (f) Wender PA, Gamber GG, Williams TJ. In: Modern Rhodium-Catalyzed Organic Reactions. Evans PA, editor. Wiley-VCH Verlag GmbH & Co; KGaA: 2005. pp. 263–299. [Google Scholar]
- 38.Shimizu I, Ohashi Y, Tsuji J. Tetrahedron Lett. 1985;26:3825–3828. [Google Scholar]
- 39.(a) Goldberg AFG, Stoltz BM. Org. Lett. 2011;13:4474–4476. doi: 10.1021/ol2017615. [DOI] [PMC free article] [PubMed] [Google Scholar]; (b) Trost BM, Morris PJ. Angew. Chem. Int. Ed. 2011;50:6167–6170. doi: 10.1002/anie.201101684. [DOI] [PMC free article] [PubMed] [Google Scholar]; (c) Hiroi K, Yamada A. Tetrahedron: Asymmetry. 2000;11:1835–1841. [Google Scholar]; (d) Mei L-y, Wei Y, Xu Q, Shi M. Organometallics. 2012;31:7591–7599. [Google Scholar]; (e) Trost BM, Morris PJ, Sprague SJ. J. Am. Chem. Soc. 2012;134:17823–17831. doi: 10.1021/ja309003x. [DOI] [PMC free article] [PubMed] [Google Scholar]; (f) Halskov KS, Næsborg L, Tur F, Jørgensen KA. Org. Lett. 2016;18:2220–2223. doi: 10.1021/acs.orglett.6b00852. [DOI] [PubMed] [Google Scholar]
- 40.Jiao L, Ye S, Yu Z-X. J. Am. Chem. Soc. 2008;130:7178–7179. doi: 10.1021/ja8008715. [DOI] [PubMed] [Google Scholar]
- 41.Jiao L, Lin M, Yu Z-X. Chem. Commun. 2010;46:1059–1061. doi: 10.1039/b922417c. [DOI] [PubMed] [Google Scholar]
- 42.Jiao L, Lin M, Yu Z-X. J. Am. Chem. Soc. 2011;133:447–461. doi: 10.1021/ja107396t. [DOI] [PubMed] [Google Scholar]
- 43.Lin M, Kang G-Y, Guo Y-A, Yu Z-X. J. Am. Chem. Soc. 2012;134:398–405. doi: 10.1021/ja2082119. [DOI] [PubMed] [Google Scholar]
- 44.Jiao L, Lin M, Zhuo L-G, Yu Z-X. Org. Lett. 2010;12:2528–2531. doi: 10.1021/ol100625e. [DOI] [PubMed] [Google Scholar]
- 45.(a) Feng Y, Yu Z-X. J. Org. Chem. 2015;80:1952–1956. doi: 10.1021/jo502604p. [DOI] [PubMed] [Google Scholar]; (b) Bose S, Yang J, Yu Z-X. J. Org. Chem. 2016;81:6757–6765. doi: 10.1021/acs.joc.6b00608. [DOI] [PubMed] [Google Scholar]
- 46.Lin M, Li F, Jiao L, Yu Z-X. J. Am. Chem. Soc. 2011;133:1690–1693. doi: 10.1021/ja110039h. [DOI] [PubMed] [Google Scholar]
- 47.Li Q, Jiang G-J, Jiao L, Yu Z-X. Org. Lett. 2010;12:1332–1335. doi: 10.1021/ol100237h. [DOI] [PubMed] [Google Scholar]
- 48.(a) Wender PA, Husfeld CO, Langkopf E, Love JA, Pleuss N. Tetrahedron. 1998;54:7203–7220. [Google Scholar]; (b) Wender PA, Haustedt LO, Lim J, Love JA, Williams TJ, Yoon J-Y. J. Am. Chem. Soc. 2006;128:6302–6303. doi: 10.1021/ja058590u. [DOI] [PubMed] [Google Scholar]; (c) Wang Y, Wang J, Su J, Huang F, Jiao L, Liang Y, Yang D, Zhang S, Wender PA, Yu Z-X. J. Am. Chem. Soc. 2007;129:10060–10061. doi: 10.1021/ja072505w. [DOI] [PubMed] [Google Scholar]; (d) Fan X, Tang M-X, Zhuo L-G, Tu YQ, Yu Z-X. Tetrahedron Lett. 2009;50:155–157. [Google Scholar]; (e) Fan X, Zhuo L-G, Tu YQ, Yu Z-X. Tetrahedron. 2009;65:4709–4713. [Google Scholar]; (f) Liang Y, Jiang X, Yu Z-X. Chem. Commun. 2011;47:6659–6661. doi: 10.1039/c1cc11005e. [DOI] [PubMed] [Google Scholar]
- 49.Tombe R, Iwamoto T, Kurahashi T, Matsubara S. Synlett. 2014;25:2281–2284. [Google Scholar]
- 50.(a) Johnson WTG, Borden WT. J. Am. Chem. Soc. 1997;119:5930–5933. [Google Scholar]; (b) Potts KT, Baum JS. Chem. Rev. 1974;74:189–213. [Google Scholar]
- 51.(a) Krebs AW. Angew. Chem. 1965;77:10–22. [Google Scholar]; (b) Eicher T, Weber JL. Cyclic Compounds. Springer Berlin Heidelberg; Berlin, Heidelberg: 1975. Structure reactivity of cyclopropenones and triafulvenes; pp. 1–109. [DOI] [PubMed] [Google Scholar]
- 52.Baba A, Ohshiro Y, Agawa T. Chem. Lett. 1976;5:11–12. [Google Scholar]
- 53.Wender PA, Paxton TJ, Williams TJ. J. Am. Chem. Soc. 2006;128:14814–14815. doi: 10.1021/ja065868p. [DOI] [PubMed] [Google Scholar]
- 54.Li C, Zhang H, Feng J, Zhang Y, Wang J. Org. Lett. 2010;12:3082–3085. doi: 10.1021/ol101091r. [DOI] [PubMed] [Google Scholar]
- 55.(a) Murakami M, Amii H, Shigeto K, Ito Y. J. Am. Chem. Soc. 1996;118:8285–8290. [Google Scholar]; (b) Murakami M, Takahashi K, Amii H, Ito Y. J. Am. Chem. Soc. 1997;119:9307–9308. [Google Scholar]; (c) Murakami M, Itahashi T, Amii H, Takahashi K, Ito Y. J. Am. Chem. Soc. 1998;120:9949–9950. [Google Scholar]
- 56.Murakami M, Itahashi T, Ito Y. J. Am. Chem. Soc. 2002;124:13976–13977. doi: 10.1021/ja021062n. [DOI] [PubMed] [Google Scholar]
- 57.Parker E, Cramer N. Organometallics. 2014;33:780–787. [Google Scholar]
- 58.Souillart L, Parker E, Cramer N. Angew. Chem. Int. Ed. 2014;53:3001–3005. doi: 10.1002/anie.201311009. [DOI] [PubMed] [Google Scholar]
- 59.(a) Lee H, Jun C-H. J. Am. Chem. Soc. 1999;121:880–881. [Google Scholar]; (b) Park YJ, Park J-W, Jun C-H. Acc. Chem. Res. 2008;41:222–234. doi: 10.1021/ar700133y. [DOI] [PubMed] [Google Scholar]; (c) Jun C-H, Lee H, Lim S-G. J. Am. Chem. Soc. 2001;123:751–752. doi: 10.1021/ja0033537. [DOI] [PubMed] [Google Scholar]
- 60.Ko HM, Dong G. Nat. Chem. 2014;6:739–744. doi: 10.1038/nchem.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Zhou X, Dong G. J. Am. Chem. Soc. 2015;137:13715–13721. doi: 10.1021/jacs.5b09799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Zhou X, Ko HM, Dong G. Angew. Chem. Int. Ed. 2016;55:13867–13871. doi: 10.1002/anie.201608158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Souillart L, Cramer N. Angew. Chem. Int. Ed. 2014;53:9640–9644. doi: 10.1002/anie.201405834. [DOI] [PubMed] [Google Scholar]
- 64.(a) Liebeskind LS. Tetrahedron. 1989;45:3053–3060. [Google Scholar]; (b) Liebeskind LS, Baysdon SL, South MS, Iyer S, Leeds JP. Tetrahedron. 1985;41:5839–5853. [Google Scholar]; (c) South MS, Liebeskind LS. J. Am. Chem. Soc. 1984;106:4181–4185. [Google Scholar]; (d) Liebeskind LS, Leeds JP, Baysdon SL, Iyer S. J. Am. Chem. Soc. 1984;106:6451–6453. [Google Scholar]
- 65.(a) Huffman MA, Liebeskind LS, Pennington WT. Organometallics. 1990;9:2194–2196. [Google Scholar]; (b) Huffman MA, Liebeskind LS, Pennington WT. Organometallics. 1992;11:255–266. [Google Scholar]
- 66.Huffman MA, Liebeskind LS. J. Am. Chem. Soc. 1990;112:8617–8618. [Google Scholar]
- 67.Huffman MA, Liebeskind LS. J. Am. Chem. Soc. 1991;113:2771–2772. [Google Scholar]
- 68.Auvinet A-L, Harrity JPA. Angew. Chem. Int. Ed. 2011;50:2769–2772. doi: 10.1002/anie.201007598. [DOI] [PubMed] [Google Scholar]
- 69.Kondo T, Nakamura A, Okada T, Suzuki N, Wada K, Mitsudo T-a. J. Am. Chem. Soc. 2000;122:6319–6320. [Google Scholar]
- 70.Kondo T, Taguchi Y, Kaneko Y, Niimi M, Mitsudo T-a. Angew. Chem. Int. Ed. 2004;43:5369–5372. doi: 10.1002/anie.200461002. [DOI] [PubMed] [Google Scholar]
- 71.Kondo T, Niimi M, Nomura M, Wada K, Mitsudo T-a. Tetrahedron Lett. 2007;48:2837–2839. [Google Scholar]
- 72.Yamamoto Y, Kuwabara S, Hayashi H, Nishiyama H. Adv. Synth. Catal. 2006;348:2493–2500. [Google Scholar]
- 73.Xu T, Dong G. Angew. Chem. Int. Ed. 2012;51:7567–7571. doi: 10.1002/anie.201202771. [DOI] [PubMed] [Google Scholar]
- 74.Lu G, Fang C, Xu T, Dong G, Liu P. J. Am. Chem. Soc. 2015;137:8274–8283. doi: 10.1021/jacs.5b04691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Xu T, Ko HM, Savage NA, Dong G. J. Am. Chem. Soc. 2012;134:20005–20008. doi: 10.1021/ja309978c. [DOI] [PubMed] [Google Scholar]
- 76.Xu T, Dong G. Angew. Chem. Int. Ed. 2014;53:10733–10736. doi: 10.1002/anie.201404802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Chen P-H, Xu T, Dong G. Angew. Chem. Int. Ed. 2014;53:1674–1678. doi: 10.1002/anie.201310100. [DOI] [PubMed] [Google Scholar]
- 78.Deng L, Xu T, Li H, Dong G. J. Am. Chem. Soc. 2016;138:369–374. doi: 10.1021/jacs.5b11120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Juliá-Hernández F, Ziadi A, Nishimura A, Martin R. Angew. Chem. Int. Ed. 2015;54:9537–9541. doi: 10.1002/anie.201503461. [DOI] [PubMed] [Google Scholar]
- 80.Yang S, Xu Y, Li J. Org. Lett. 2016;18:6244–6247. doi: 10.1021/acs.orglett.6b02943. [DOI] [PubMed] [Google Scholar]
- 81.Matsuda T, Matsumoto T. Org. Biomol. Chem. 2016;14:5023–5027. doi: 10.1039/c6ob00734a. [DOI] [PubMed] [Google Scholar]
- 82.(a) Eisch JJ, Piotrowski AM, Han KI, Kruger C, Tsay YH. Organometallics. 1985;4:224–231. [Google Scholar]; (b) Lu Z, Jun C-H, de Gala SR, Sigalas M, Eisenstein O, Crabtree RH. J. Chem. Soc., Chem. Commun. 1993:1877–1880. [Google Scholar]; (c) Perthuisot C, Edelbach BL, Zubris DL, Jones WD. Organometallics. 1997;16:2016–2023. [Google Scholar]; (d) Edelbach BL, Lachicotte RJ, Jones WD. J. Am. Chem. Soc. 1998;120:2843–2853. [Google Scholar]; (e) Simhai N, Iverson CN, Edelbach BL, Jones WD. Organometallics. 2001;20:2759–2766. [Google Scholar]
- 83.(a) Edelbach BL, Vicic DA, Lachicotte RJ, Jones WD. Organometallics. 1998;17:4784–4794. [Google Scholar]; (b) Wick DD, Jones WD. Inorg. Chim. Acta. 2009;362:4416–4421. [Google Scholar]
- 84.Schwager H, Spyroudis S, Vollhardt KPC. J. Organometallic Chem. 1990;382:191–200. [Google Scholar]
- 85.Edelbach BL, Lachicotte RJ, Jones WD. Organometallics. 1999;18:4040–4049. [Google Scholar]
- 86.Iverson CN, Jones WD. Organometallics. 2001;20:5745–5750. [Google Scholar]
- 87.Müller C, Lachicotte RJ, Jones WD. Organometallics. 2002;21:1975–1981. [Google Scholar]
- 88.Schaub T, Backes M, Radius U. Organometallics. 2006;25:4196–4206. [Google Scholar]
- 89.Shibata T, Nishizawa G, Endo K. Synlett. 2008;2008:765–768. [Google Scholar]
- 90.(a) Korotvička A, Císařová I, Roithová J, Kotora M. Chem. Eur. J. 2012;18:4200–4207. doi: 10.1002/chem.201103888. [DOI] [PubMed] [Google Scholar]; (b) Korotvička A, Frejka D, Hampejsová Z, Císařová I, Kotora M. Synthesis. 2016;48:987–996. [Google Scholar]
- 91.Jones WD. In: C-C Bond Activation. Topics in Current Chemistry. Dong G, editor. Vol. 346. Springer; Berlin: 2014. pp. 1–31. [DOI] [PubMed] [Google Scholar]
- 92.(a) Wang R, Falck JR. RSC Adv. 2014;4:1062–1066. doi: 10.1039/C3RA45178J. [DOI] [PMC free article] [PubMed] [Google Scholar]; (b) Nakao Y, Hiyama T. Pure Appl. Chem. 2008;80:1097–1107. [Google Scholar]; (c) Nakao Y. Bull. Chem. Soc. Jpn. 2012;85:731–745. [Google Scholar]; (d) Nájera C, Sansano JM. Angew. Chem. Int. Ed. 2009;48:2452–2456. doi: 10.1002/anie.200805601. [DOI] [PubMed] [Google Scholar]; (e) Wang R, Falck JR. Cat. Rev. 2014;56:288–331. doi: 10.1080/01614940.2014.920178. [DOI] [PMC free article] [PubMed] [Google Scholar]; (f) Wen Q, Lu P, Wang Y. RSC Adv. 2014;4:47806–47826. [Google Scholar]
- 93.(a) Nakao Y, Oda S, Hiyama T. J. Am. Chem. Soc. 2004;126:13904–13905. doi: 10.1021/ja0448723. [DOI] [PubMed] [Google Scholar]; (b) Nakao Y, Oda S, Yada A, Hiyama T. Tetrahedron. 2006;62:7567–7576. [Google Scholar]
- 94.(a) Nakao Y, Yukawa T, Hirata Y, Oda S, Satoh J, Hiyama T. J. Am. Chem. Soc. 2006;128:7116–7117. doi: 10.1021/ja060519g. [DOI] [PubMed] [Google Scholar]; (b) Hirata Y, Yukawa T, Kashihara N, Nakao Y, Hiyama T. J. Am. Chem. Soc. 2009;131:10964–10973. doi: 10.1021/ja901374v. [DOI] [PubMed] [Google Scholar]
- 95.(a) Nishihara Y, Inoue Y, Itazaki M, Takagi K. Org. Lett. 2005;7:2639–2641. doi: 10.1021/ol050749k. [DOI] [PubMed] [Google Scholar]; (b) Nishihara Y, Inoue Y, Izawa S, Miyasaka M, Tanemura K, Nakajima K, Takagi K. Tetrahedron. 2006;62:9872–9882. [Google Scholar]; (c) Nakao Y, Hirata Y, Hiyama T. J. Am. Chem. Soc. 2006;128:7420–7421. doi: 10.1021/ja0606834. [DOI] [PubMed] [Google Scholar]; (d) Hirata Y, Inui T, Nakao Y, Hiyama T. J. Am. Chem. Soc. 2009;131:6624–6631. doi: 10.1021/ja9010282. [DOI] [PubMed] [Google Scholar]
- 96.(a) Kobayashi Y, Kamisaki H, Yanada R, Takemoto Y. Org. Lett. 2006;8:2711–2713. doi: 10.1021/ol060733+. [DOI] [PubMed] [Google Scholar]; (b) Rondla NR, Levi SM, Ryss JM, Vanden Berg RA, Douglas CJ. Org. Lett. 2011;13:1940–1943. doi: 10.1021/ol200274h. [DOI] [PubMed] [Google Scholar]; (c) Yasui Y, Kamisaki H, Takemoto Y. Org. Lett. 2008;10:3303–3306. doi: 10.1021/ol801168j. [DOI] [PubMed] [Google Scholar]; (d) Yasui Y, Kamisaki H, Ishida T, Takemoto Y. Tetrahedron. 2010;66:1980–1989. [Google Scholar]
- 97.Nakao Y, Ebata S, Yada A, Hiyama T, Ikawa M, Ogoshi S. J. Am. Chem. Soc. 2008;130:12874–12875. doi: 10.1021/ja805088r. [DOI] [PubMed] [Google Scholar]
- 98.Watson MP, Jacobsen EN. J. Am. Chem. Soc. 2008;130:12594–12595. doi: 10.1021/ja805094j. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Murahashi S, Naota T, Nakajima N. J. Org. Chem. 1986;51:898–901. [Google Scholar]
- 100.Rondla NR, Ogilvie JM, Pan Z, Douglas CJ. Chem. Commun. 2014;50:8974–8977. doi: 10.1039/c4cc04068f. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Nakao Y, Yada A, Ebata S, Hiyama T. J. Am. Chem. Soc. 2007;129:2428–2429. doi: 10.1021/ja067364x. [DOI] [PubMed] [Google Scholar]
- 102.(a) Hirata Y, Tanaka M, Yada A, Nakao Y, Hiyama T. Tetrahedron. 2009;65:5037–5050. [Google Scholar]; (b) Nakao Y, Hirata Y, Tanaka M, Hiyama T. Angew. Chem. Int. Ed. 2008;47:385–387. doi: 10.1002/anie.200704095. [DOI] [PubMed] [Google Scholar]
- 103.(a) Akira Y, Tomoya Y, Hiroaki I, Yoshiaki N, Tamejiro H. Bull. Chem. Soc. Jpn. 2010;83:619–634. [Google Scholar]; (b) Yada A, Yukawa T, Nakao Y, Hiyama T. Chem. Commun. 2009:3931–3933. doi: 10.1039/b907290j. [DOI] [PubMed] [Google Scholar]; (c) Nakao Y, Yada A, Hiyama T. J. Am. Chem. Soc. 2010;132:10024–10026. doi: 10.1021/ja1017078. [DOI] [PubMed] [Google Scholar]; (d) Hirata Y, Yada A, Morita E, Nakao Y, Hiyama T, Ohashi M, Ogoshi S. J. Am. Chem. Soc. 2010;132:10070–10077. doi: 10.1021/ja102346v. [DOI] [PubMed] [Google Scholar]
- 104.Ishitsuka T, Okuda Y, Szilagyi RK, Mori S, Nishihara Y. Dalton Trans. 2016;45:7786–7793. doi: 10.1039/c6dt00341a. [DOI] [PubMed] [Google Scholar]
- 105.Nakao Y. In: C-C Bond Activation. Topics in Current Chemistry. Dong G, editor. Vol. 346. Springer; Berlin: 2014. pp. 33–58. [DOI] [PubMed] [Google Scholar]
- 106.Tobisub M, Chatani N. Chem. Soc. Rev. 2008;37:300–307. doi: 10.1039/b702940n. [DOI] [PubMed] [Google Scholar]
- 107.Kou X, Fan J, Tong X, Shen Z. Chin. J. Org. Chem. 2013;33:1407–1422. [Google Scholar]
- 108.Jun C-H, Park J-W. In: Directed Metallation. Topics in Organometallic Chemistry. Chatani N, editor. Vol. 24. Springer; Berlin: 2007. pp. 117–143. [Google Scholar]
- 109.Suggs JW, Cox SD. J. Organomet. Chem. 1981;221:199–201. [Google Scholar]
- 110.Dreis AM, Douglas CJ. J. Am. Chem. Soc. 2009;131:412–413. doi: 10.1021/ja8066308. [DOI] [PubMed] [Google Scholar]
- 111.(a) Rathbun CM, Johnson JB. J. Am. Chem. Soc. 2011;133:2031–2033. doi: 10.1021/ja109686v. [DOI] [PubMed] [Google Scholar]; (b) Lutz JP, Rathbun CM, Stevenson SM, Powell BM, Boman TS, Baxter CE, Zona JM, Johnson JB. J. Am. Chem. Soc. 2012;134:715–722. doi: 10.1021/ja210307s. [DOI] [PubMed] [Google Scholar]
- 112.Wentzel MT, Reddy VJ, Hyster TK, Douglas CJ. Angew. Chem. Int. Ed. 2009;48:6121–6123. doi: 10.1002/anie.200902215. [DOI] [PubMed] [Google Scholar]
- 113.Zeng R, Dong G. J. Am. Chem. Soc. 2015;137:1408–1411. doi: 10.1021/ja512306a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Zeng R, Chen P-H, Dong G. ACS Cat. 2016;6:969–973. doi: 10.1021/acscatal.5b02532. [DOI] [PMC free article] [PubMed] [Google Scholar]