Abstract
Purpose of review
Cannabis use disorders (CUDs) are prevalent worldwide. Current epidemiological studies underscore differences in behaviors that contribute to cannabis use across cultures that can be leveraged towards prevention and treatment of CUDs. This review proposes a framework for understanding the effects of cross-cultural differences on psychological, neural, and genomic processes underlying CUDs that has the potential to inform global policies and impact global public health.
Recent findings
We found that cultural factors may influence (1) the willingness to acknowledge CUD-related symptoms among populations of different countries, and (2) neural responses related to the sense of self, perception, emotion, and attention. These findings leverage the potential effects of culture on neural mechanisms underlying CUDs.
Summary
As the number of individuals seeking treatment for CUDs increases globally, it is imperative to incorporate cultural considerations to better understand and serve differing populations and develop more targeted treatment strategies and interventions.
Keywords: cannabis use disorders, cultural neuroscience, global policy, public health, cross-cultural studies, neurocognitive deficits in cannabis use
Introduction
Cannabis is the most widely used illicit drug (although legality varies by jurisdiction) around the world with continued increase in recent years, particularly in North America and Western and Central Europe [1]. Although cannabis is often considered a relatively harmless drug, an increase in use has also been paralleled by more individuals seeking treatment for cannabis dependence in the Americas, Western and Central Europe, and Oceania [1]. Cannabis-use disorders (CUDs) are difficult to treat [2,3], and the lack of effective treatments may in part be due to a dearth of knowledge of the neural mechanisms underlying CUDs. Incorporating a neuroscience perspective and conducting studies with the goal of examining changes in the neural networks underlying CUDs may prove to be instrumental in developing effective treatments with greater success. Given the implications for global public health and the current legal climate in the United States, it is important to employ a cross-cultural investigation of neural mechanisms of CUDs. While the prevalence of CUDs is high globally, cannabis is arguably the least well-studied substance of abuse in the context of addiction, with a relatively limited number of neuroimaging studies on CUDs compared to other substance-use disorders. Environmental factors linked to cannabis use, including perceived risk of use, influence of peers, and social acceptance, may be culture-dependent and potentially impact differences in brain functionality in individuals with CUDs. The implications of CUDs and understanding the impairments resulting from changes in underlying neural networks are critical and exigent due to imminent changes in legislation.
Culture is a dynamic construct that encompasses the beliefs and practices of a particular group, influencing social perception and adherence of the group's members [4,5]. In mental health, the concept of culture has been deconstructed and examined through the lens of race and ethnicity, but is distinct from them [5]. Cultural neuroscience is a relatively new area of research that investigates the relationship between biological and cognitive mechanisms and culture. This multidisciplinary study of the effects of culture on neural responses, is based on the notion that both environmental and genetic factors influence structural and functional changes in the brain [6–8]. Cultural neuroscience provides a framework for the examination of structural and functional changes in the brain in this context. It posits that changes in the brain are likely a result of specific learning and experiential factors that may vary between cultures. The structural and functional changes, in turn, influence patterns of neural activations related to specific behaviors. As the brain continuously undergoes changes based on environmental experiences and influences, culture may contribute importantly to brain structure and function, including those related to psychopathologies, such as CUDs [9,10]. From a broader perspective, a cultural neuroscience approach has the potential to be beneficial not just for CUDs, but for mental disorders in general.
The aim of this review is to propose a framework for the need to better understand how cultural factors may influence neural mechanisms underlying CUDs. We will begin with exploring how culture and genomics may influence behavior and relate these interactions to structural and functional changes underlying differences in neural responses. We will describe both cultural differences as well as similarities with respect to CUDs and what is currently known about the effects of social and legal environments on cannabis use. While cultural differences in neural responses are a critical aspect of a cultural neuroscience approach, it is equally important to identify similarities between cultures for a thorough understanding of the intersection between culture and neuroscience with regards to CUDs. We will end with a rationale for investigating potential effects of culture on neural response in individuals with CUDs and why examining neural responses in individuals with CUDs within a cultural neuroscience context should lead to a more robust understanding of not only neural mechanisms of CUDs, but also implications for treatments.
Culture and genes have a cyclic effect on behavior
Culture is often considered an established set of traditions and customs that influence the population; however, culture is more accurately defined as a dynamic interaction of environmental, biological, and psychological factors that potentially influence social behavior and ultimately, construct and contribute to the presiding culture. Culture can therefore be characterized as cyclic in that it influences cognition and neurobiological development, but is also influenced, in turn, by these neurological processes [4]. Culture thus involves a relationship between social cognition and cultural norms, with both influencing each other.
In accordance with this dynamic and interactive characterization, the influence of genetics on this relationship should also be considered. Population genetics studies suggest that cultural customs affect genetic influences on behaviors [11–13] and that interactions between genetics and culture may have contributed importantly to evolutionary processes, such as in the development of handedness and mating preferences [14]. Lumsden & Wilson (1980) have proposed a model for the coevolution of genes and culture, in which both are created together through natural selection of environmentally adaptive genetic and cognitive traits [15]. For example, genetic variations that lead to more environmentally adept cognitive traits may proliferate in certain geographical locations over others. This is specifically illustrated in genetic variation in the gene encoding the serotonin transporter (SLC6A4) that has been related to many cognitive functions, including emotional regulation, reward processing, anxiety, and depression [16]. Genetic variations in this gene exist across cultural groups, where 80% of Japanese individuals carry the short allele (s/s or s/l), but only 40-45% of Europeans carry the same polymorphism [17]. This allele has been associated with increased anxiety-related traits [18] and is more commonly seen in cultures with collectivist principles than societies that hold individualistic attitudes [18]. From a co-evolutionary perspective, this allele may have risen as an anti-pathogenic trait, as it is highly prevalent in areas with greater risk of pathogen exposure. Collectivist cultures tend have an anti-pathogenic effect as well, suggesting a co-adaptive nature of biology and environment in cultural neuroscience [19].
Further support for this theory is seen in variations across different cultural groups in the gene encoding the dopamine D4 receptor (DRD4). Allelic variation (relating to a variable number tandem repeat) in this gene has been linked in candidate gene studies to substance-use disorders and sensation-seeking [20], although this relationship has not been observed in all studies [21]. The sensation-seeking-related allelic variant appears widespread in South American Indian populations with a frequency of 70-80%, but is extremely low in East Asian populations with a frequency of less than 1% [22]. Additional genetic variations are seen cross-culturally in the genes coding for aldehyde dehydrogenase (ALDH2) and alcohol dehydrogenase (ADH), enzymes that metabolize ethanol. The ADH2*2 allele is more frequently found in East Asian populations (∼70% frequency) [23], but rare in European and African populations [24]. This is also the case for the ALDH2*2 allele, with a frequency of about 40% in East Asian cultures and virtual nonexistence in European and African populations [24]. Furthermore, very few individuals of East Asian descent are found to carry the ALDH2*2 allele and no homozygous carriers have been reported in alcoholic samples [25,26], suggesting a strong protective role of ALDH2*2 against alcoholism. While some of these findings may represent the effects of limited admixture of genetic populations rather than cultural effects per se, an analogous investigation of gene variations like these across different cultures may provide insight into cannabis use and CUDs and the influences of culture on these phenomena.
Genetic variance in the gene coding for the CB1 cannabinoid receptor (CNR1) and the enzyme that metabolizes the endocannabinoid anandamide, fatty acid amide hydrolase (FAAH), have been associated with CUDs [27–29]. The C allele of the rs2023239 variant has been linked to increased CB1 cannabinoid receptor (CB1R) binding in the nervous system [30], which may influence substance use and reward salience of substances of abuse [31]. Heavy cannabis users carrying the G allele of the same gene show an increased neural response to cannabis cues during withdrawal, suggesting stronger craving in these individuals [32]. Gene variations in FAAH may also influence cannabis dependence, specifically in relation to the rs324420 variant [33]. While the A/A genotype may be protective against CUDs [34], the C/C genotype is correlated with stronger neural responses to marijuana cue-induced craving and the risk alleles of CNR1 and FAAH may have additive effects on the underlying neural mechanisms of marijuana craving [32]. There may even be a genetic influence at each stage of cannabis use (transition from non-user to regular user to dependent user) as well as on any trajectory into other substance use (e.g., alcohol, tobacco, and other illicit drugs) [35]. While the extent to which the genes contribute to CUDs is still not fully understood, the potentially interactive nature of culture and genomics suggest the importance of a cross-cultural approach to investigating CUDs.
Effect of culture on neural response
Neural processes may also be influenced by environmental experiences, such that exposure to different practices and attitudes that define cultures may result in divergent neural responses across societies. Cultural neuroscience studies have reported experiential and cultural differences in brain structure and function. The London Taxi Cab Study compared taxi drivers in London that had expert knowledge of London's complex roadways to controls with equal driving experience and reported greater gray matter volume in the hippocampus in the taxi drivers [36]. In addition to this structural difference, the authors also found that the taxi drivers exhibited an impairment in learning new visuo-spatial information, thus exhibiting functional differences as well. In a meta-analysis of neural responses to writing systems across different Western alphabetic languages, Japanese Kana and Kanji, and Chinese characters, Bolger and colleagues found that the ventral occipito-temporal regions of the left hemisphere overlapped between the different writing systems, but regions in the right hemisphere were specifically associated with particular languages [37]. This result suggests that different writing systems, influenced by culture and other factors such as exposure and experience, exhibit both convergent and divergent neural responses. Studies have found differences in neural activations within Western alphabetic languages as well [38]. Similar structural and functional differences have been found in expert jugglers, pianists, musicians, dancers, and meditators [39–42].
Studies in object processing have reported that individuals from Western cultures exhibit an attentional preference for objects [43] and may more likely discount contextual information [44] compared to those from East Asian cultures; however, no cultural differences were found for the processing of background images [45]. This finding suggests differential attentional processes and underlying neural activations that may be influenced by culture [46]. While response to fear is largely automatic and is associated with activation in the amygdala, individuals viewing fearful faces belonging to their own cultural group had greater amygdala activation compared to viewing those from other cultural groups. These emotional processes have been found to vary between cultures [47]. The ability to infer the mental states of other individuals may also differ across cultures, where individuals extrapolating mental states of others of the same culture performed better than those from a different culture [48]. Differences in the neural correlates of inferring the mental states of others between cultures also may relate to empathy. A neuroimaging study examined neural activations of Koreans and Americans as they observed images of both Korean and American individuals in emotional pain [49]. The authors found cross-cultural differences in neural activations for empathy in response to images of individuals from the same culture compared to those from another culture. Korean participants exhibited greater activation compared to American participants bilaterally in the temporo-parietal junction, a region that has previously been associated with the inference of mental states. Similar cultural variances have been reported in moral decision-making in Koreans and Americans [50]. A study of the emotional processing of faces between European American and Chinese participants identified different neural correlates, in which European Americans exhibited greater activation in the ventral striatum and caudate when viewing excited faces as compared to Chinese participants [51].
Cross-cultural differences have also been reported in neural activations during self-awareness and the processing of self-relevant stimuli [52,53]. Zhu and colleagues found that while individuals from Chinese and Western cultures exhibited greater activation in the medial prefrontal cortex and anterior cingulate cortex in self-representation, Chinese individual also exhibited activations in the medial prefrontal cortex in representations of their mother, suggesting a representation of a interdependent self, compared to a more Western independent self [53].
Such cultural differences in neural responses underlying cognitive processes may also relate to psychopathologies [9,10]. If typical neural processes are influenced by culture, this change would be expected to transfer to disorders as well. In fact, studies have reported cultural differences in the manifestation of disorders, such as depression and schizophrenia [54–56] as well as in the stigmatization of individuals with psychopathologies [57].
Neurocognition in CUDs
In comparison to other substance-use disorders (e.g., alcohol- and tobacco-use disorders), relatively few studies involving individuals with CUDs (as opposed to chronic users) have been conducted and thus, less is known about the underlying neural mechanisms in CUDs. Chronic cannabis use has been linked to structural and functional neural changes linked to cognitive deficits and acute cannabis intoxication has been associated with functional changes [58–62] (see Figure 1B). Such cognitive deficits may be associated with a conversion of more voluntary cannabis-seeking behaviors to more compulsive habits and an impairment in cognitive control [63,64]. This process may lead to behaviors exhibited in CUDs wherein the individual is aware of the harmful consequences of use, but finds it difficult to exert restraint and cease use. Continued cannabis use may increase sensitization to cannabis-related cues [65], further promoting use and leading to dependence. The fronto-parietal and fronto-limbic circuits may underlie this progression of cannabis use to CUDs [66,67], with greater fronto-striatal functional connectivity found in cannabis-dependent individuals compared to non-dependent users [68]. Additionally, activations in the fronto-parietal network during an n-back working memory task have been associated with an increase in cannabis use [69]. These impairments in motivation and cognitive control suggest a role for CB1 cannabinoid receptors in these processes, consistent with their presence in high quantities in sensory and motor areas [33,70], with the highest densities in the basal ganglia, cerebellum, and hippocampus [71,72].
Figure 1. A) Proposed model of interactions between culture, neural mechanisms, diagnoses and treatments, and genetics in cannabis use disorders (CUDs) and B) neural networks that have been reported to be affected in CUDs.
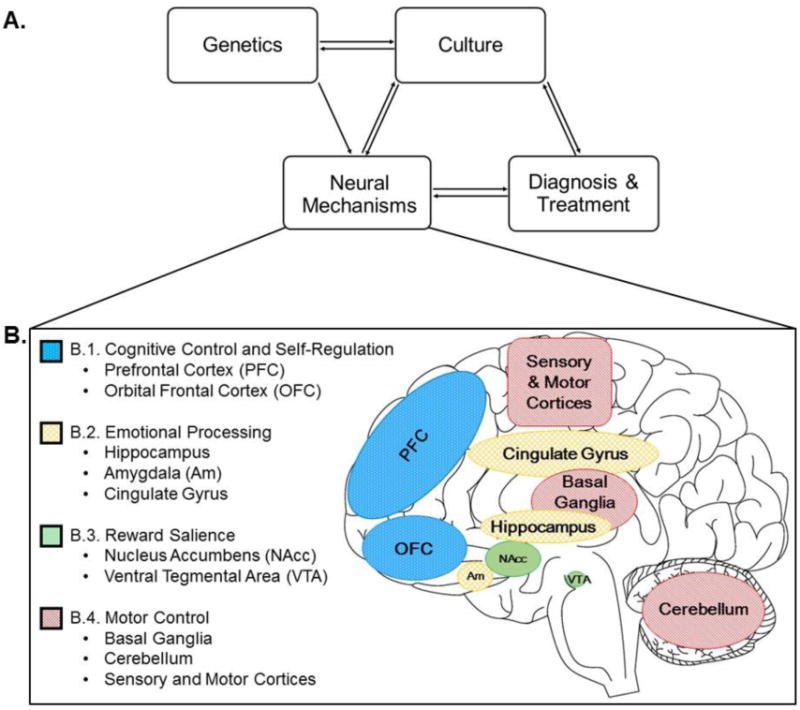
A) In this model, we propose that CUDs are affected by (1) culture, (2) underlying neural mechanisms, (3) diagnoses and treatments that have bi-directional influences on each other, and a partial bi-directional effect of (4) genetics. The cultural influences include social attitudes towards cannabis use, perceived risks of use, and legislation. These elements relate to diagnoses with cultural differences in endorsement of CUD criteria, likelihood of meeting criteria for dependence, treatment-seeking behaviors, and the psychosocial nature of treatments. Cultural factors may also affect neural processes and response to perception, emotion, attention, and self-representation, which may propagate to differences in the neural mechanisms related to CUDs. Neural processes, in turn also affect cultural perceptions. Lastly, genetic factors may influence, and may be influenced by, cultural factors, which in turn, may both influence neural networks. We hypothesize that the diverse nature of neural mechanisms of CUDs between cultures may affect the efficacy of diagnoses and treatments and these treatments in turn may affect underlying neural mechanisms. B) These neural circuits may have an overlapping influence from both cannabis use and culture. B.1) Processing self-relevant stimulus may be contributing factor to the transition from recreational use to compulsive use. The brain regions that regulate these higher-order, executive processes of self-awareness and self-regulation include the prefrontal cortex (PFC) and the orbitofrontal cortex (OFC). B.2) The cingulate gyrus, hippocampus, and amygdala (Am) are proposed to contribute to networks that process emotionally salient information. These areas have high CB1 cannabinoid receptor density and show structural differences in marijuana users compared to non-users. B.3) Reward processing is altered in CUDs. Regions that underlie processing of reward include the nucleus accumbens (NAcc) and the ventral tegmental area (VTA), which also have high CB1 cannabinoid receptor density. B.4) Lastly, motor control is impaired in acute cannabis intoxication. There exists high CB1 cannabinoid receptor density in the basal ganglia, cerebellum, and secondary sensory and motor cortices, regions implicated in motor processing. Culture may also affect these neurological processes, likely having an interactive effect with cannabis use.
In addition to impairments in cognitive control, individuals with CUDs may have altered processing of self-awareness and self-relevant stimuli that may underlie increased sensitivity and awareness of cannabis-related cues in the environment [73]. Such impairments in cognitive control and hypersensitivity to cannabis-related cues along with associated changes in activations and connectivity in relevant brain networks may represent suitable neural markers for severity of CUDs and be assessed longitudinally for understanding and tracking the progression of CUDs. Importantly, these cognitive processes may be differentially affected by culture and lead to differences in neural mechanisms. These differences in turn require determining whether potential neural markers of CUDs are universal or whether they should be personalized to the individual's cultural background. Given the dynamic and cyclic interaction of culture and neural response, examining longitudinal changes is important to more accurately understand such relationships. These processes may impact treatment strategies and contribute to suboptimal effectiveness of existing therapies, and thus they may link to global health disparities.
Cross-cultural differences related to cannabis use
Potential cultural differences in the manifestation of CUDs may be influenced by varying legal and social climates surrounding cannabis use. For example, cannabis users in Australia are more likely to meet DSM-IV criteria for dependence compared to those in the U.S. [74]. There are, however, key differences between the two countries as Australia promotes a harm-reduction philosophy and provides public healthcare [74], suggesting that both legal approaches to substance use and access to healthcare may influence treatment-seeking behaviors and diagnosis of CUDs.
Perception of risks of cannabis use may differ across cultures. There has been a significant decrease in the perceived risk of cannabis among 17-18 year-old high-school students in the U.S. in the past two decades, with 80% of students identifying cannabis use as risky in the 1990s and less than 40% doing so in 2014 [75]. This is in contrast with Europe where cannabis use has been consistently perceived as risky by 70-72% of high-school students in 2003, 2007, and 2011 [76]. The higher risk perception in Europe may contribute to the increasing number of individuals seeking treatment [1]. While perception of risk is a factor contributing to cannabis use, other potential influences include gender [77,78], sensation-seeking, influence of peers [79], poor performance in school [80], and low parental monitoring [1]. Importantly, as with risk perception, many of these factors exhibit cultural differences. For example, a study on twins in Finland found that males were more likely to use cannabis [77]; however, a study in the Netherlands did not find gender-related differences in use [78]. A Canadian study reported that males used cannabis at a younger age compared to females and female users reported poorer mental health compared to male users [81]. Studies have also reported that women in the U.S. scored higher on sensation-seeking measures compared to women in the U.K. [82,83].
Differences in laws regarding cannabis use may affect the number of treatment-seeking individuals. In the U.S., the number of arrests for cannabis possession corresponds with treatment statistics [1], suggesting that the criminal justice system contributes to the number of individuals in treatment. While this is also true for countries in Europe, there is a larger variation between nations with 3.9% of individuals in the Netherlands in treatment due to an arrest and 80.6% in Hungary [1]. As Hungary takes a strong legal stand against substance use, including cannabis [84], this difference is likely related to laws relating to cannabis use in Hungary and the Netherlands. Furthermore, current treatments are largely psychosocial in nature and thus, may be particularly sensitive to environmental and cultural influences [85]. The primary treatments that have been implemented include cognitive behavioral therapy and motivational enhancement therapy. While these therapies have enjoyed moderate success, they do not consistently incorporate cross-cultural differences, potentially compromising their effectiveness [86,87].
Effects of social and legal environment on CUDs
Legislation surrounding cannabis is widely debated and scrutinized around the world, but the impact of these laws on cannabis use and initiation are not well understood. While Australia, parts of Europe, and some states in the U.S. have decriminalized cannabis to varying degrees, empirical evidence examining the impacts of these laws remain limited [88]. Cross-cultural studies are particularly important in examining the effects of medicinal or recreational legalization through the comparison of countries with varying legislation on cannabis use; such studies should be extended to identify neural factors.
Within the U.S., there have been reports of higher rates of use in states that have legalized marijuana for medical use [89,90]. However, these studies do not provide a causal link of legalization on increased use, as they do not internally compare results from states before and after medicinal legalization. In fact, some states may have started with higher rates of use [90], possibly due to more relaxed attitudes towards cannabis use that may have resulted in a stronger push for legalization than in other states.
The Netherlands has taken a more liberal stance on cannabis use since the 1960s, distinguishing it from “hard drugs” such as cocaine, heroin, ecstasy, and hallucinogenic mushrooms [88], and limiting prosecution of recreational possession, use, and sale in “coffee shops” [91]. Despite the leniency on criminalization, marijuana is still socially discouraged in the Netherlands [88] and use rates remain lower than in the U.S. (26% have used in their lifetime compared to 44% in the U.S.) [92,93]. Cross-cultural studies between the U.S. and the Netherlands have revealed similar trends in cannabis use, despite varying legal climates [94], questioning the efficacy of criminalization as a deterrent of use. Differences in reports of acknowledgement of DSM-IV criteria for CUDs across populations have been found such that users in the Netherlands are less likely than users in the U.S. to acknowledge DSM-IV criteria [95]. The findings suggest a potentially lower perceived risk of cannabis in Dutch users that may lower the likelihood of attempted abstinence. This difference relating to the acknowledgement of DSM-IV criteria may relate to environmental factors. For example, if the culture has a more lenient attitude towards cannabis use, the individual may be given a lower score, reflecting a more relaxed attitude. Conversely, a stringent atmosphere may contribute to a higher score in the same individual. Given these cultural effects on perceptions of cannabis use and influence on diagnoses, it is possible that legal climates may relate to neural responses in individuals with CUDs, although this possibility warrants more close and careful examination. Continued research on cross-cultural studies in countries with varying legislation around marijuana use will help inform policy makers and guide regulations on cannabis use in the future.
Potential cultural effects on neural responses in individuals with CUDs
While data suggest cross-cultural differences in the manifestations of CUDs, there are currently no published studies on cultural effects on neurocognitive or neural processes related to CUDs. Additionally, the existing literature on the neural mechanisms of CUDs is relatively sparse; however, we have extracted information from the literature to form hypotheses about potential effects of culture. As described above, studies report cross-cultural differences in neural responses during cognitive processes such as perception, self-awareness, emotion, and attention. Cultural differences have been reported in functional connectivity of the prefrontal cortex, suggesting that culture may in turn influence cognitive control. These processes are involved and altered in CUDs, suggesting differential changes in neural responses in individuals with CUDs which may differ in different cultures. Indeed, in a cross-cultural review of cue reactivity in nicotine and alcohol addiction, Lv and colleagues suggest that through emotional and attentional processes, culture may be related to neural processes underlying cue reactivity [96]. It is important to note that similarities across cultures have also been reported. For example, studies have found that the severity of problems related to cannabis use is associated with activity in the striatum and orbitofrontal cortex in response to cannabis cues in users in the U.S. [97] as well as in the Netherlands [98]. While these studies utilized different paradigms and thus cannot be directly compared per se, they suggest that specific neural responses may be independent of culture and underscore the importance of designing studies for direct comparisons between individuals from different cultures. Identifying neural mechanisms that are consistent across cultures is important to obtaining a cross-cultural perspective on CUDs. Additionally, there is a correlative link between cannabis use and psychotic illnesses [99], with cannabis users 2-3 times more likely to develop psychotic symptoms than non-users [100,101]. Factors such as age of first use, amount and frequency of use, family history of psychotic illness, and genetic variation all seem to influence this relationship, suggesting a gene-environment relationship that is likely to vary cross-culturally [101]. Understanding the cultural influences on CUDs also has the potential to lead to a more comprehensive understanding of neuroscience and mental health overall, as such a process may illuminate underlying factors of neurodevelopmental, mood and psychotic disorders, as well as substance use and dependence more broadly [102].
Thus, we propose that cross-cultural differences in the manifestation of CUDs may be driven by cultural differences that influence underlying neural mechanisms. These differences in the manifestations of CUDs in turn may influence diagnoses and treatments of CUDs; however, the presence or absence of a CUD diagnosis and psychosocial treatments may also influence neural mechanisms. We suggest that genes, culture, neural processes, and diagnostic and treatment strategies have a bidirectional influence on each other (see Figure 1A).
Cultural influences on neural activations and responses may lead to differences in encoding salient stimuli. Importantly, these differences require the development of both culture-specific and culture-independent neural markers and strategies to effectively treat individuals. The diagnosis of CUDs suggests a possible measurement bias between the U.S. and the Netherlands with cannabis users in the U.S. more likely to acknowledge criteria [95]. Diagnosing CUDs using a standardized assessment requires these differences to be considered and may have broad implications for global public health and policies.
There is a need for a direct comparison of neural mechanisms underlying CUDs in individuals from different countries. Of particular interest are countries with differing social and legal climates regarding cannabis use in order to gain an understanding of how changes in legalization may not only affect use and behavior, and but also the underlying neural mechanisms. These comparisons have the potential not only to fill important gaps in the scientific literature, but also to inform policies.
Conclusions
We have found that studies to date have focused on epidemiological differences between cannabis use in different countries. While these studies highlight differences in the characteristics of users, the question of whether the neural mechanisms of CUDs differ between individuals of different countries remains unanswered. Thus far, the potential influences of culture on psychopathologies are understudied and largely unknown. Targeted examinations of the effects of culture on differences in neural response in CUDs not only elucidate mechanisms of the disorder, but also have implications for policy, education, treatment, and prevention strategies. The interactions between culture, public policy, and cannabis use should be better understood in order to guide global public policies and improve global public health as well as mitigate population health disparities. Understanding cultural influences more broadly could result in more comprehensive approaches to CUDs that have greater applicability and effectiveness across different cultures.
Footnotes
Compliance with Ethical Standards: Conflict of Interest Shikha Prashad, Amber L. Milligan, Janna Cousijn, and Francesca M. Filbey declare that they have no conflicts of interest.
Human and Animal Rights and Informed Consent This article does not contain any studies with human or animal subjects performed by any of the authors.
References
Papers of particular interest, published recently, have been highlighted as:
• Of importance
•• Of major importance
- 1.United Nations Office on Drugs and Crime. World Drug Report 2016. United Nations publication; 2016. Sales No. E.16.XI.7. [Google Scholar]
- 2.Danovitch I, Gorelick DA. State of the Art Treatments for Cannabis Dependence. Psychiatr Clin North Am. 2012;35:309–26. doi: 10.1016/j.psc.2012.03.003.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Walker DD, Stephens RS, Towe S, Banes K, Roffman R. Maintenance Check-ups Following Treatment for Cannabis Dependence. J Subst Abuse Treat. 2015;56:11–5. doi: 10.1016/j.jsat.2015.03.006.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Vogeley K, Roepstorff A. Contextualising culture and social cognition. Trends Cogn Sci. 2009;13:511–6. doi: 10.1016/j.tics.2009.09.006.. [DOI] [PubMed] [Google Scholar]
- 5.Barry DT, Beitel M. Cultural and ethnic considerations in young adult mental health. In: Grant JE, Potenza MN, editors. Oxf Handb Young Adult Ment Health. New York: Oxford University Press; 2010. [Google Scholar]
- 6.Chiao JY, Cheon BK, Pornpattananangkul N, Mrazek AJ, Blizinsky KD. Cultural Neuroscience. In: Gelfand MJ, Chiu CY, Hong YY, editors. Adv Cult Psychol. Vol. 4. New York: Oxford University Press; 2014. [Google Scholar]
- 7.Ambady N, Bharucha J. Culture and the brain. Curr Dir Psychol Sci. 2009;18:342–345. [Google Scholar]
- 8••.Hyde LW, Tompson S, Creswell JD, Falk EB. Cultural neuroscience: new directions as the field matures: What do cultural neuroscience findings mean? Cult Brain. 2015;3:75–92. doi: 10.1007/s40167-014-0024-6. This review paper examines the implications of cultural neuroscience studies and proposes a model for gene environment interactions that influences behavior. [DOI] [Google Scholar]
- 9.Choudhury S, Kirmayer LJ. Prog Brain Res. Vol. 178. Elsevier; 2009. Cultural neuroscience and psychopathology: prospects for cultural psychiatry; pp. 263–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Thakker J, Ward T, Strongman KT. Mental disorder and cross-cultural psychology: A constructivist perspective. Clin Psychol Rev. 1999;19:843–874. doi: 10.1016/s0272-7358(98)00077-4. [DOI] [PubMed] [Google Scholar]
- 11.Chudek M, Henrich J. Culture–gene coevolution, norm-psychology and the emergence of human prosociality. Trends Cogn Sci. 2011;15:218–26. doi: 10.1016/j.tics.2011.03.003. [DOI] [PubMed] [Google Scholar]
- 12.Feldman MW, Laland KN. Gene-culture coevolutionary theory. Trends Ecol Evol. 1996;11:453–7. doi: 10.1016/0169-5347(96)10052-5. [DOI] [PubMed] [Google Scholar]
- 13.Laland KN, Odling-Smee J, Myles S. How culture shaped the human genome: bringing genetics and the human sciences together. Nat Rev Genet. 2010;11:137–48. doi: 10.1038/nrg2734. [DOI] [PubMed] [Google Scholar]
- 14.Laland KN. Exploring gene-culture interactions: insights from handedness, sexual selection and niche-construction case studies. Philos Trans R Soc B Biol Sci. 2008;363:3577–89. doi: 10.1098/rstb.2008.0132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Lumsden CJ, Wilson EO. Translation of epigenetic rules of individual behavior into ethnographic patterns. Proc Natl Acad Sci. 1980;77:4382–4386. doi: 10.1073/pnas.77.7.4382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ketcherside A, Matthews I, Filbey F. The serotonin link between alcohol use and affective disorders. J Addict Prev. 2013;1 [PMC free article] [PubMed] [Google Scholar]
- 17.Nakamura T, Muramatsu T, Ono Y, Matsushita S, Higuchi S, Mizushima H, et al. Serotonin Transporter Gene Regulatory Region Polymorphism and Anxiety-Related Traits in the Japanese. Am J Med Genet Neuropsychiatr Genet. 1997;74:544–5. doi: 10.1002/(sici)1096-8628(19970919)74:5<544::aid-ajmg18>3.0.co;2-c. [DOI] [PubMed] [Google Scholar]
- 18.Sen S, Burmeister M, Ghosh D. Meta-analysis of the association between a serotonin transporter promoter polymorphism (5-HTTLPR) and anxiety-related personality traits. Am J Med Genet. 2004;127B:85–9. doi: 10.1002/ajmg.b.20158. [DOI] [PubMed] [Google Scholar]
- 19.Chiao JY, Blizinsky KD. Culture-gene coevolution of individualism-collectivism and the serotonin transporter gene. Proc R Soc B Biol Sci. 2010;277:529–37. doi: 10.1098/rspb.2009.1650. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Chen C, Burton M, Greenberger E, Dmitrieva J. Population migration and the variation of dopamine D4 receptor (DRD4) allele frequencies around. Issue Evol Hum Behav. 1999;20:5. [Google Scholar]
- 21.Gelernter J, Kranzler H, Coccaro E, Siever L, New A, Mulgrew CL. D4 dopamine-receptor (DRD4) alleles and novelty seeking in substance-dependent, personality-disorder, and control subjects. Am J Hum Genet. 1997;61:1144–1152. doi: 10.1086/301595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Chiao JY. Cultural Neuroscience: Visualizing Culture-Gene Influences on Brain Function. In: Decety J, Cacioppo JT, editors. Oxf Handb Soc Neurosci. Oxford University Press; 2011. [Google Scholar]
- 23.Chen CC, Lu RB, Chen YC, Wang MF, Chang YC, Li TK, et al. Interaction between the Functional Polymorphisms of the Alcohol- Metabolism Genes in Protection against Alcoholism. Am J Hum Genet. 1999;65:795–807. doi: 10.1086/302540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Hartz SM, Bierut LJ. Genetics of Addictions. Clin Lab Med. 2010;30:847–64. doi: 10.1016/j.cll.2010.07.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Thomasson HR, Edenberg HJ, Crabb DW, Mai XL, Jerome RE, Li TK, et al. Alcohol and aldehyde dehydrogenase genotypes and alcoholism in Chinese men. Am J Hum Genet. 1991;48:677. [PMC free article] [PubMed] [Google Scholar]
- 26.Higuchi S, Matsushita S, Imazeki H, Kinoshita T, Takagi S, Kono H. Aldehyde dehydrogenase genotypes in Japanese alcoholics. Lancet. 1994;343:741–2. doi: 10.1016/s0140-6736(94)91629-2. [DOI] [PubMed] [Google Scholar]
- 27.Agrawal A, Wetherill L, Dick DM, Xuei X, Hinrichs A, Hesselbrock V, et al. Evidence for association between polymorphisms in the cannabinoid receptor 1 (CNR1) gene and cannabis dependence. Am J Med Genet B Neuropsychiatr Genet. 2009;150B:736–40. doi: 10.1002/ajmg.b.30881. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Haughey HM, Marshall E, Schacht JP, Louis A, Hutchison KE. Marijuana withdrawal and craving: influence of the cannabinoid receptor 1 (CNR1) and fatty acid amide hydrolase (FAAH) genes. Addiction. 2008;103:1678–86. doi: 10.1111/j.1360-0443.2008.02292.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Schacht JP, Hutchison KE, Filbey FM. Associations between cannabinoid receptor-1 (CNR1) variation and hippocampus and amygdala volumes in heavy cannabis users. Neuropsychopharmacology. 2012;37:2368–2376. doi: 10.1038/npp.2012.92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Hirvonen J, Zanotti-Fregonara P, George DT, Lyoo CH, Li CT, Hines CS, et al. Reduced cannabinoid CB1 receptor binding in alcohol dependence measured with positron emission tomography. Mol Psychiatry. 2013;18:916–21. doi: 10.1038/mp.2012.100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Hutchison KE, Haughey H, Niculescu M, Schacht J, Kaiser A, Stitzel J, et al. The incentive salience of alcohol: translating the effects of genetic variant in CNR1. Arch Gen Psychiatry. 2008;65:841–850. doi: 10.1001/archpsyc.65.7.841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Filbey FM, Schacht JP, Myers US, Chavez RS, Hutchison KE. Individual and additive effects of the CNR1 and FAAH genes on brain response to marijuana cues. Neuropsychopharmacology. 2010;35:967–975. doi: 10.1038/npp.2009.200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Parsons LH, Hurd YL. Endocannabinoid signalling in reward and addiction. Nat Rev Neurosci. 2015;16:579–94. doi: 10.1038/nrn4004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Tyndale RF, Payne JI, Gerber AL, Sipe JC. The fatty acid amide hydrolase C385A (P129T) missense variant in cannabis users: studies of drug use and dependence in Caucasians. Am J Med Genet B Neuropsychiatr Genet. 2007;144:660–666. doi: 10.1002/ajmg.b.30491. [DOI] [PubMed] [Google Scholar]
- 35.Agrawal A, Lynskey MT. The genetic epidemiology of cannabis use, abuse and dependence. Addiction. 2006;101:801–12. doi: 10.1111/j.1360-0443.2006.01399.x. [DOI] [PubMed] [Google Scholar]
- 36.Maguire EA, Woollett K, Spiers HJ. London taxi drivers and bus drivers: A structural MRI and neuropsychological analysis. Hippocampus. 2006;16:1091–101. doi: 10.1002/hipo.20233. [DOI] [PubMed] [Google Scholar]
- 37.Bolger DJ, Perfetti CA, Schneider W. Cross-cultural effect on the brain revisited: Universal structures plus writing system variation. Hum Brain Mapp. 2005;25:92–104. doi: 10.1002/hbm.20124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Paulesu E, McCrory E, Fazio F, Menoncello L, Brunswick N, Cappa SF, et al. A cultural effect on brain function. Nat Neurosci. 2000;3:91–96. doi: 10.1038/71163. [DOI] [PubMed] [Google Scholar]
- 39.Draganski B, Gaser C, Busch V, Schuierer G, Bogdahn U, May A. Neuroplasticity: changes in grey matter induced by training. Nature. 2004;427:311–312. doi: 10.1038/427311a. [DOI] [PubMed] [Google Scholar]
- 40.Kang DH, Jo HJ, Jung WH, Kim SH, Jung YH, Choi CH, et al. The effect of meditation on brain structure: cortical thickness mapping and diffusion tensor imaging. Soc Cogn Affect Neurosci. 2013;8:27–33. doi: 10.1093/scan/nss056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Bengtsson SL, Nagy Z, Skare S, Forsman L, Forssberg H, Ullén F. Extensive piano practicing has regionally specific effects on white matter development. Nat Neurosci. 2005;8:1148–50. doi: 10.1038/nn1516. [DOI] [PubMed] [Google Scholar]
- 42.Giacosa C, Karpati FJ, Foster NEV, Penhune VB, Hyde KL. Dance and music training have different effects on white matter diffusivity in sensorimotor pathways. NeuroImage. 2016;135:273–86. doi: 10.1016/j.neuroimage.2016.04.048. [DOI] [PubMed] [Google Scholar]
- 43.Ji LJ, Peng K, Nisbett RE. Culture, control, and perception of relationships in the environment. J Pers Soc Psychol. 2000;78:943. doi: 10.1037//0022-3514.78.5.943. [DOI] [PubMed] [Google Scholar]
- 44.Kitayama S, Duffy S, Kawamura T, Larsen JT. Perceiving an object and its context in different cultures A cultural look at new look. Psychol Sci. 2003;14:201–206. doi: 10.1111/1467-9280.02432. [DOI] [PubMed] [Google Scholar]
- 45.Goh JO, Chee MW, Tan JC, Venkatraman V, Hebrank A, Leshikar ED, et al. Age and culture modulate object processing and object—scene binding in the ventral visual area. Cogn Affect Behav Neurosci. 2007;7:44–52. doi: 10.3758/cabn.7.1.44. [DOI] [PubMed] [Google Scholar]
- 46.Hedden T, Ketay S, Aron A, Markus HR, Gabrieli JD. Cultural influences on neural substrates of attentional control. Psychol Sci. 2008;19:12–17. doi: 10.1111/j.1467-9280.2008.02038.x. [DOI] [PubMed] [Google Scholar]
- 47.Russell JA. Is there universal recognition of emotion from facial expression? A review of the cross-cultural studies Psychol Bull. 1994;115:102–41. doi: 10.1037/0033-2909.115.1.102. [DOI] [PubMed] [Google Scholar]
- 48.Adams RB, Jr, Rule NO, Franklin RG, Jr, Wang E, Stevenson MT, Yoshikawa S, et al. Cross-cultural reading the mind in the eyes: an fMRI investigation. J Cogn Neurosci. 2010;22:97–108. doi: 10.1162/jocn.2009.21187. [DOI] [PubMed] [Google Scholar]
- 49.Cheon BK, Im D, Harada T, Kim JS, Mathur VA, Scimeca JM, et al. Cultural influences on neural basis of intergroup empathy. NeuroImage. 2011;57:642–50. doi: 10.1016/j.neuroimage.2011.04.031. [DOI] [PubMed] [Google Scholar]
- 50.Han H, Glover GH, Jeong C. Cultural influences on the neural correlate of moral decision making processes. Behav Brain Res. 2014;259:215–28. doi: 10.1016/j.bbr.2013.11.012. [DOI] [PubMed] [Google Scholar]
- 51.Park B, Tsai JL, Chim L, Blevins E, Knutson B. Neural evidence for cultural differences in the valuation of positive facial expressions. Soc Cogn Affect Neurosci. 2016;11:243–52. doi: 10.1093/scan/nsv113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Han S, Gu X, Mao L, Ge J, Wang G, Ma Y. Neural substrates of self-referential processing in Chinese Buddhists. Soc Cogn Affect Neurosci. 2010;5:332–9. doi: 10.1093/scan/nsp027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Zhu Y, Zhang L, Fan J, Han S. Neural basis of cultural influence on self-representation. NeuroImage. 2007;34:1310–6. doi: 10.1016/j.neuroimage.2006.08.047. [DOI] [PubMed] [Google Scholar]
- 54.Draguns JG. Cultural influences upon psychopathology: Clinical and practical implications. J Soc Distress Homeless. 1995;4:79–103. [Google Scholar]
- 55.Draguns JG, Tanaka-Matsumi J. Assessment of psychopathology across and within cultures: issues and findings. Behav Res Ther. 2003;41:755–76. doi: 10.1016/S0005-7967(02)00190-0. [DOI] [PubMed] [Google Scholar]
- 56.Kleinman A. Culture and depression. N Engl J Med. 2004;351:951–3. doi: 10.1056/NEJMp048078. [DOI] [PubMed] [Google Scholar]
- 57.Krendl AC. An fMRI investigation of the effects of culture on evaluations of stigmatized individuals. NeuroImage. 2016;124:336–49. doi: 10.1016/j.neuroimage.2015.08.030. [DOI] [PubMed] [Google Scholar]
- 58.Volkow ND, Baler RD, Compton WM, Weiss SRB. Adverse health effects of marijuana use. N Engl J Med. 2014;370:2219–27. doi: 10.1056/NEJMra1402309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Batalla A, Bhattacharyya S, Yücel M, Fusar-Poli P, Crippa JA, Nogué S, et al. Structural and Functional Imaging Studies in Chronic Cannabis Users: A Systematic Review of Adolescent and Adult Findings. PLoS ONE. 2013;8:e55821. doi: 10.1371/journal.pone.0055821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60••.Curran HV, Freeman TP, Mokrysz C, Lewis DA, Morgan CJA, Parsons LH. Keep off the grass? Cannabis, cognition and addiction. Nat Rev Neurosci. 2016;17:293–306. doi: 10.1038/nrn.2016.28. This review summarizes recent research on CUDs and cannabis-induced neurocognitive deficits as well as factors that may influence the trajectory of these problems. [DOI] [PubMed] [Google Scholar]
- 61.Prashad S, Filbey FM. Cognitive motor deficits in cannabis users. Curr Opin Behav Sci. 2017;13:1–7. doi: 10.1016/j.cobeha.2016.07.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62••.Lorenzetti V, Batalla A, Cousijn J. Cannabis Use Disorders and Altered Brain Morphology: Where Is the Evidence? Curr Addict Rep. 2016;3:189–98. doi: 10.1007/s40429-016-0102-2. This review examines the literature on CUDs, describes differences between dependent users and controls in brain morphology, and highlights the deficiency of studies investigating individuals with CUDs. [DOI] [Google Scholar]
- 63.Everitt BJ, Robbins TW. Drug Addiction: Updating Actions to Habits to Compulsions Ten Years On. Annu Rev Psychol. 2016;67:23–50. doi: 10.1146/annurev-psych-122414-033457. [DOI] [PubMed] [Google Scholar]
- 64.Harding IH, Solowij N, Harrison BJ, Takagi M, Lorenzetti V, Lubman DI, et al. Functional connectivity in brain networks underlying cognitive control in chronic cannabis users. Neuropsychopharmacology. 2012;37:1923–1933. doi: 10.1038/npp.2012.39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Filbey FM, DeWitt SJ. Cannabis cue-elicited craving and the reward neurocircuitry. Prog europsychopharmacol Biol Psychiatry. 2012;38:30–35. doi: 10.1016/j.pnpbp.2011.11.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Cousijn J. Embracing comorbidity: a way toward understanding the role of motivational and control processes in cannabis use disorders. Front Psychol. 2015;6 doi: 10.3389/fpsyg.2015.00677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Cousijn J, van Benthem P, van der Schee E, Spijkerman R. Motivational and control mechanisms underlying adolescent cannabis use disorders: A prospective study. Dev Cogn Neurosci. 2015;16:36–45. doi: 10.1016/j.dcn.2015.04.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68•.Filbey FM, Dunlop J. Differential reward network functional connectivity in cannabis dependent and non-dependent users. Drug Alcohol Depend. 2014;140:101–11. doi: 10.1016/j.drugalcdep.2014.04.002. This paper is novel in that it reports differences in functional connectivity during cue-elicited craving between cannabis users that have CUDs and those that do not. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Cousijn J, Wiers RW, Ridderinkhof KR, van den Brink W, Veltman DJ, Goudriaan AE. Effect of baseline cannabis use and working-memory network function on changes in cannabis use in heavy cannabis users: A prospective fMRI study: Working-Memory Network Function and Cannabis Use. Hum Brain Mapp. 2014;35:2470–82. doi: 10.1002/hbm.22342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Mechoulam R, Parker LA. The Endocannabinoid System and the Brain. Annu Rev Psychol. 2013;64:21–47. doi: 10.1146/annurev-psych-113011-143739. [DOI] [PubMed] [Google Scholar]
- 71.Fernández-Ruiz J, González S. Cannabinoid control of motor function at the basal ganglia. Berlin Heidelberg: Springer; 2005. [DOI] [PubMed] [Google Scholar]
- 72.Romero J, Lastres-Becker I, de Miguel R, Berrendero F, Ramos JA, Fernández-Ruiz J. The endogenous cannabinoid system and the basal ganglia: Biochemical, pharmacological, and therapeutic aspects. Pharmacol Ther. 2002;95:137–52. doi: 10.1016/s0163-7258(02)00253-x. [DOI] [PubMed] [Google Scholar]
- 73.DeWitt SJ, Ketcherside A, McQueeny TM, Dunlop JP, Filbey FM. The hyper-sentient addict: an exteroception model of addiction. Am J Drug Alcohol Abuse. 2015;41:374–81. doi: 10.3109/00952990.2015.1049701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Teesson M, Baillie A, Lynskey M, Manor B, Degenhardt L. Substance use, dependence and treatment seeking in the United States and Australia: A cross-national comparison. Drug Alcohol Depend. 2006;81:149–55. doi: 10.1016/j.drugalcdep.2005.06.007. [DOI] [PubMed] [Google Scholar]
- 75.Johnston LD, O'Malley PM, Bachman JG, Schulenberg JE, Miech RA. Ann Arbor. Michigan: Institute for Social Research, University of Michigan; 2015. Monitoring the Future national survey results on drug use, 1975–2014: Volume 2, College students and adults ages 19–55. [Google Scholar]
- 76.Kraus L, Guttormsson U, Leifman H, Arpa S, Molinaro S, Monshouwer K, et al. ESPAD Report 2015 Results from the European School Survey Project on Alcohol and Other Drugs. Luxembourg: European Monitoring Centre for Drugs and Drug Addiction; 2015. [Google Scholar]
- 77.Korhonen T, Huizink A, Dick D, Pulkkinen L, Rose R, Kaprio J. Role of individual, peer and family factors in the use of cannabis and other illicit drugs: A longitudinal analysis among Finnish adolescent twins. Drug Alcohol Depend. 2008;97:33–43. doi: 10.1016/j.drugalcdep.2008.03.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Monshouwer K, Van Dorsselaer S, Verdurmen J, TER BOGTT, De Graaf RON, Vollebergh W. Cannabis use and mental health in secondary school children. Br J Psychiatry. 2006;188:148–153. doi: 10.1192/bjp.188.2.148. [DOI] [PubMed] [Google Scholar]
- 79.Pinchevsky GM, Arria AM, Caldeira KM, Garnier-Dykstra LM, Vincent KB, O'Grady KE. Marijuana Exposure Opportunity and Initiation during College: Parent and Peer Influences. Prev Sci. 2012;13:43–54. doi: 10.1007/s11121-011-0243-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Townsend L, Flisher AJ, King G. A Systematic Review of the Relationship between High School Dropout and Substance Use. Clin Child Fam Psychol Rev. 2007;10:295–317. doi: 10.1007/s10567-007-0023-7. [DOI] [PubMed] [Google Scholar]
- 81.Tu AW, Ratner PA, Johnson JL. Gender Differences in the Correlates of Adolescents' Cannabis Use. Subst Use Misuse. 2008;43:1438–63. doi: 10.1080/10826080802238140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Zuckerman M, Eysenck SB, Eysenck HJ. Sensation seeking in England and America: cross-cultural, age, and sex comparisons. J Consult Clin Psychol. 1978;46:139. doi: 10.1037//0022-006x.46.1.139. [DOI] [PubMed] [Google Scholar]
- 83.Zuckerman M. Sensation Seeking (Psychology Revivals): Beyond the Optimal Level of Arousal. Psychology Press; 2014. [Google Scholar]
- 84.Racz J, Melles K, Marvanykovi F, Lencse M, Petke Z. Communicating the principle of “treatment instead of punishment” in Hungary on the basis of an examination of the patients at a drug outpatient clinic. Drugs Educ Prev Policy. 2011;18:207–18. doi: 10.3109/09687637.2010.507558. [DOI] [Google Scholar]
- 85.Ramesh D, Haney M. Treatment of Cannabis Use Disorders. In: el-Guebaly N, Carrà G, Galanter M, editors. Textb Addict Treat Int Perspect. Milano: Springer Milan; 2015. pp. 367–80. [Google Scholar]
- 86.Hays PA. Multicultural applications of cognitive-behavior therapy. Prof Psychol Res Pract. 1995;26:309. [Google Scholar]
- 87.Horrell SCV. Effectiveness of cognitive-behavioral therapy with adult ethnic minority clients: A review. Prof Psychol Res Pract. 2008;39:160–8. doi: 10.1037/0735-7028.39.2.160. [DOI] [Google Scholar]
- 88•.Ammerman S, Ryan S, Adelman WP. The Impact of Marijuana Policies on Youth: Clinical, Research, and Legal Update. Pediatrics. 2015;135:e769–85. doi: 10.1542/peds.2014-4147. This is a thorough review that covers varying policy on legalization of marijuana and the societal impact of these policies, particularly on adolescents. [DOI] [PubMed] [Google Scholar]
- 89.Cerdá M, Wall M, Keyes KM, Galea S, Hasin D. Medical marijuana laws in 50 states: Investigating the relationship between state legalization of medical marijuana and marijuana use, abuse and dependence. Drug Alcohol Depend. 2012;120:22–7. doi: 10.1016/j.drugalcdep.2011.06.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Hasin DS, Wall M, Keyes KM, Cerdá M, Schulenberg J, O'Malley PM, et al. Medical marijuana laws and adolescent marijuana use in the USA from 1991 to 2014: results from annual, repeated cross-sectional surveys. Lancet Psychiatry. 2015;2:601–8. doi: 10.1016/S2215-0366(15)00217-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Spapens T, Müller T, van de Bunt H. The Dutch Drug Policy from a Regulatory Perspective. Eur J Crim Policy Res. 2015;21:191–205. doi: 10.1007/s10610-014-9249-3. [DOI] [Google Scholar]
- 92.Smith K, Strashny A. Characteristics of Criminal Justice System Referrals Discharged from Substance Abuse Treatment and Facilities with Specially Designed Criminal Justice Programs. Rockville, MD: Substance Abuse and Mental Health Services Administration, Center for Behavioral Health Statistics and Quality; 2016. [PubMed] [Google Scholar]
- 93.van Laar MW, Cruts AAN, van Ooyen-Houben MMJ, Meijer RF, Croes EA, Brunt T, et al. Netherlands National Drug Monitor NDM Annual Report 2010. Netherlands: Netherlands Ministry of Justice; 2010. [Google Scholar]
- 94.Reinarman C, Cohen PDA, Kaal HL. The Limited Relevance of Drug Policy: Cannabis in Amsterdam and in San Francisco. Am J Public Health. 2004;94:836–42. doi: 10.2105/ajph.94.5.836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Delforterie M, Creemers H, Agrawal A, Lynskey M, Jak S, van der Ende J, et al. Functioning of Cannabis Abuse and Dependence Criteria Across Two Different Countries: The United States and The Netherlands. Subst Use Misuse. 2015;50:242–50. doi: 10.3109/10826084.2014.952445. [DOI] [PubMed] [Google Scholar]
- 96••.Lv W, Wu Q, Liu X, Chen Y, Song H, Yang L, et al. Cue Reactivity in Nicotine and Alcohol Addiction: A Cross-Cultural View. Front Psychol. 2016;7 doi: 10.3389/fpsyg.2016.01335. This is review summarizes fMRI and EEG studies that investigate cue-reactivity in alcohol and nicotine users employing a cross-cultural perspective. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Filbey FM, Schacht JP, Myers US, Chavez RS, Hutchison KE. Marijuana craving in the brain. Proc Natl Acad Sci. 2009;106:13016–13021. doi: 10.1073/pnas.0903863106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Cousijn J, Goudriaan AE, Ridderinkhof KR, van den Brink W, Veltman DJ, Wiers RW. Neural responses associated with cue-reactivity in frequent cannabis users: Brain activity to cannabis cues. Addict Biol. 2013;18:570–80. doi: 10.1111/j.1369-1600.2011.00417.x. [DOI] [PubMed] [Google Scholar]
- 99.Henquet C, Di Forti M, Morrison P, Kuepper R, Murray RM. Gene-Environment Interplay Between Cannabis and Psychosis. Schizophr Bull. 2008;34:1111–21. doi: 10.1093/schbul/sbn108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Moore THM, Zammit S, Lingford-Hughes A, Barnes TRE, Jones PB, Burke M, et al. Cannabis use and risk of psychotic or affective mental health outcomes: A systematic review. Lancet. 2007;370:319–28. doi: 10.1016/S0140-6736(07)61162-3. [DOI] [PubMed] [Google Scholar]
- 101.Nesvåg R, Reichborn-Kjennerud T, Gillespie NA, Knudsen GP, Bramness JG, Kendler KS, et al. Genetic and Environmental Contributions to the Association Between Cannabis Use and Psychotic-Like Experiences in Young Adult Twins. Schizophr Bull. 2016:sbw101. doi: 10.1093/schbul/sbw101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.van der Pol P, Liebregts N, de Graaf R, ten Have M, Korf DJ, van den Brink W, et al. Mental health differences between frequent cannabis users with and without dependence and the general population: (Dependent) heavy cannabis use and mental health. Addiction. 2013;108:1459–69. doi: 10.1111/add.12196. [DOI] [PubMed] [Google Scholar]


