Abstract
High glucose has been demonstrated to induce angiotensinogen (AGT) synthesis in the renal proximal tubular cells (RPTCs) of rats, which may further activate the intrarenal renin-angiotensin system (RAS) and contribute to diabetic nephropathy. This study aimed to investigate the effects of high glucose on AGT in the RPTCs of human origin and identify the glucose-responsive transcriptional factor(s) that bind(s) to the DNA sequences of AGT promoter in human RPTCs. Human kidney (HK)-2 cells were treated with normal glucose (5.5 mM) and high glucose (15.0 mM), respectively. Levels of AGT mRNA and AGT secretion of HK-2 cells were measured by real-time polymerase chain reaction (PCR) and enzyme-linked immunosorbent assay (ELISA), respectively. Consecutive 5’-end deletion mutant constructs and different site-directed mutagenesis products of human AGT promoter sequences were respectively transfected into HK-2 cells, followed by AGT promoter activity measurement through dual luciferase assay. High glucose significantly augmented the levels of AGT mRNA and AGT secretion of HK-2 cells, compared with normal glucose treatment. High glucose also significantly augmented AGT promoter activity in HK-2 cells transfected with the constructs of human AGT promoter sequences, compared with normal glucose treatment. Hepatocyte nuclear factor (HNF)-5 was found to be one of the glucose-responsive transcriptional factors of AGT in human RPTCs, since the mutation of its binding sites within AGT promoter sequences abolished the above effects of high glucose on AGT promoter activity as well as levels of AGT mRNA and its secretion. The present study has demonstrated, for the first time, that high glucose augments AGT in human RPTCs through HNF-5, which provides a potential therapeutic target for diabetic nephropathy.
Introduction
Intrarenal renin-angiotensin system (RAS) plays a crucial role in development of diabetic nephropathy (DN) [1], which is the major renal complication of diabetes mellitus [2] as well as the main cause of end-stage renal disease [3]. Previous studies reported that intrarenal angiotensinogen (AGT) mRNA and angiotensin (Ang) II levels were enhanced in type 2 diabetic rats [4, 5]. This could further lead to renal proximal tubular hypertrophy and interstitial fibrosis by stimulating reactive oxygen species and eventually result in diabetic nephropathy [6, 7]. Furthermore, by using human kidney biopsied samples, Kamiyama et al. [8] have demonstrated that AGT mRNA and protein levels in human renal cortex proximal tubules were significantly augmented in patients with type 2 diabetes.
In the kidney, AGT is the only known substrate of renin as well as the rate-limiting factor for RAS activity, which is mainly expressed in renal proximal tubular cells (RPTCs) [9]. Kobori et al. [10] have manifested that AGT synthesis in RPTCs regulates intrarenal RAS activity for its contribution to renal accumulation of Ang II in rats. Liu et al. [11] have demonstrated that high glucose treatment induced much higher levels of apoptosis and caspase-3 activity in rat RPTCs overexpressing AGT in comparison to control RPTCs in vitro, and induction of diabetes in transgenic mice that overexpressing AGT in RPTCs led to significant increases in apoptosis of RPTCs compared with diabetic nontransgenic littermates, moreover, the above effects were markedly attenuated by insulin and/or RAS blockers. Therefore, the level of AGT in RPTCs plays an important role in the pathogenesis of DN.
In the case of rats, it has been reported that high glucose stimulated AGT synthesis in RPTCs and a glucose-responsive element located in the DNA sequences of AGT promoter has been identified [5]. As to human, Acres et al. [12] have succeeded in determining the region of human AGT promoter sequences. However, it is still unknown whether high glucose directly activates AGT synthesis in human RPTCs. In addition, the existence of glucose-responsive transcriptional factor(s) that bind(s) to AGT promoter sequences has not been identified.
Therefore, the present study aimed to investigate the effects of high glucose on AGT in the RPTCs of human origin and identify the glucose-responsive transcriptional factor(s) that bind(s) to the DNA sequences of AGT promoter in human RPTCs.
Materials and methods
Cell culture and conditioned medium
Human kidney (HK)-2 cells, an immortalized human RPTC line, were kindly gifted by Dr. Masaomi Nangaku (Tokyo University, Tokyo, Japan). HK-2 cells were grown in Dulbecco's modified eagle medium (DMEM; Life Technologies, Carlsbad, USA, catalog#11885084) supplemented with 10% fetal bovine serum (Nichirei Biosciences, Tokyo, Japan, catalog#17012) and 2% penicillin-streptomycin (5,000 U/mL; Gibco, Carlsbad, USA, catalog#15070063). Cells were maintained at 37°C in a humidified incubator with 5% CO2/95% air. Based on the experiment demands, HK-2 cells were treated with medium with different glucose concentrations (5.5, 8, 12, 15, 20 and 25 mM) as described previously [13, 14].
Real-time PCR
HK-2 cells were lysed using ISOGEN (Nippon Gene, Toyama, Japan, catalog#319–90211) followed by RNA extraction with chloroform. RNA samples were reversely transcribed to cDNA using SuperScript III reverse transcriptase (Invitrogen, Carlsbad, USA, catalog#18080044). The cDNA served as the template for the following PCR reactions using specific human AGT primer sets (Table 1). Quantitative real-time PCR was performed using the real-time PCR system (Applied Biosystems, San Francisco, USA, catalog#7300), following manufacturers’ instructions. β-actin was used as an internal control.
Table 1. Nucleotide sequences of the primers used in this study.
|
PCR (5’– 3’) |
Forward primer | Reverse primer |
| Human AGT | aactggtgctgcaaggatct | tctctctcatccgcttcaag |
| HNF-5* | gcgactctcaccaaggtctc | ggatgcaggcattgaaagat |
|
Mutagenesis (5’– 3’) |
Forward primer | Reverse primer |
| HNF-5* | catcctgaaggcattttggtgtctttcaatctggct | agccagattgaaagacaccaaaatgccttcaggatg |
| MEF2* | cccacccctcagcggcatcgtgaccc | gggtcacgatgccgctgaggggtggg |
| CREB* | ctgcctcacccactggatcactggcttctg | cagaagccagtgatccagtgggtgaggcag |
|
DNA sequencing (5’– 3’) |
Primer sequence | |
| HNF-5* | gcgactctcaccaaggtctc | |
| MEF2* | gagcagctgaaggtcacaca | |
| CREB* | catctcctggcctcaaaaag | |
Asterisk (*) indicates binding sites of the transcriptional factor within human AGT promoter sequences.
ELISA
Secreted AGT in the culture medium of HK-2 cells was measured using the human angiotensinogen assay kit (Immuno-Biological Laboratories, Gunma, Japan, catalog#27412) according to the protocol provided by the manufacturer as well as the previous description [15]. AGT levels were normalized by total cellular protein in the dish.
Plasmid constructs
Seven consecutive 5’-end deletion mutant plasmid constructs of the region from −4358 to +122 (relative to the transcription start site of human AGT gene) around human AGT promoter sequences (AGT_−4,358/+122), which has been proved sufficient for the promoter activity in human RPTCs [12], were used as described in a previous study [12]. Mutations in binding sites of possible candidates of glucose-responsive transcriptional factor(s) were generated from intact human AGT_−4,358/+122 with the specific primer sets (Table 1) and the QuickChange site-directed mutagenesis kit (Agilent Technologies, California, USA, catalog#200519–5) according to manufacturer’s instructions. Whole sequences of all plasmid constructs were analyzed using the BigDye Terminator v3.1 cycle sequencing kit (Applied Biosystems, catalog#4336917), BigDye Terminator purification kit (Applied Biosystems, catalog#4376484) and genetic analyzer (Applied Biosystems, catalog#3130x1) based on the manuals provided by the manufacturers. Accuracy of the DNA sequences was confirmed as expected. The specific primers used for sequencing are listed in Table 1.
Plasmid transfection
HK-2 cells were initially seeded into 24-well plates. After cell confluence reached to about 60%, cells were serum-starved for 24 h before transfection. For plasmid transfection, cells in each well were transfected with 250 ng of plasmid construct and 20 ng pRL-TK renilla luciferase reporter vector plasmid (Promega, Wisconsin, USA, catalog#E2241), as an internal control for transfection efficiency, using the Lipofectamine LTX reagent (Thermo Fisher Scientific, Carlsbad, USA, catalog#15338030) according to the protocol provided by the manufacturer.
Dual luciferase assay
To measure human AGT promoter activity, dual luciferase assay was performed as described previously [16] using the Dual-luciferase reporter assay system kit (Promega, catalog#E1910) and microplate reader (Corona Electric, Wisconsin, USA, catalog#SH-9000), following the manuals from manufacturers. Data were normalized based on the value shown by the pRL-TK renilla luciferase activity.
Bioinformatics
DNA sequences of AGT promoter located on human chromosome 1 were obtained from the National Center for Biotechnology Information (accession#NG008836.1). The motif search by using GENETYX software (Informer Technologies, Roseau, Dominica) allowed us to screen all transcriptional factors that corresponding to the glucose-responsive region (-22 to -1,896) of human AGT promoter sequences. According to the GENETYX software, transcriptional factors with scores ≧ 20, indicating possible binding to the glucose-responsive region, were determined. The likelihood of binding increases as the score increases. Transcriptional factors with scores ≧ 50 have the highest likelihood of binding to the glucose -responsive region.
Chromatin immunoprecipitation
To measure the binding level of a transcriptional factor to human AGT promoter sequences, chromatin immunoprecipitation was performed, as described previously [17], using the EZ-CHIP chromatin immunoprecipitation kit (Merck Millipore, Darmstadt, Germany, catalog#17–371), and either HNF-5 (also called HNF-3 [18, 19]) monoclonal antibody (Santa Cruz, California, USA, catalog#sc-377033) or mouse IgG (Santa Cruz, catalog#sc-516176) as the negative control, according to the manufacturers’ instructions. Precipitated DNA was analyzed by PCR using the specific primer sets listed in Table 1.
Statistical analysis
Data are presented as mean ± SEM. Unpaired t test was used to compare values between two groups and one-way ANOVA followed by the Tukey’s test was used to compare values among more than two groups. P-value <0.05 was considered statistically significant. All statistical analyses were performed using Prism 5 software (GraphPad, California, USA).
Results
High glucose augmented levels of AGT mRNA and AGT secretion of HK-2 cells
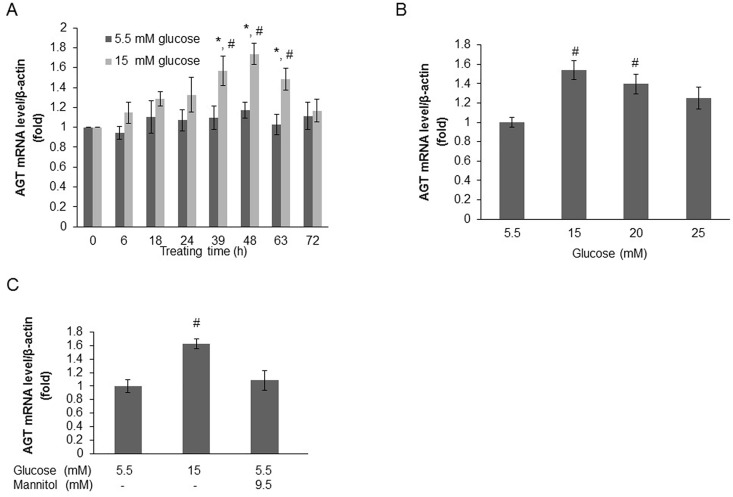
To detect the time-dependent effects of high glucose on AGT mRNA levels, HK-2 cells were treated with either normal glucose (5.5 mM) or high glucose (15.0 mM), and AGT mRNA levels were measured at different time points. Compared with normal glucose treatment, high glucose significantly augmented AGT mRNA levels from 39 h (1.10±0.12 vs. 1.57±0.15, relative ratios to the 0 h group; P<0.05) to 63 h (1.03±0.10 vs. 1.48±0.11, relative ratios to the 0 h group; P<0.05). Treatment for 48 h (1.17±0.08 vs. 1.74±0.11, relative ratios to the 0 h group; P<0.01) was the most effective (Fig 1A). To detect the dose-dependent effects of high glucose on AGT mRNA levels, HK-2 cells were treated with different concentrations of glucose for 48 h. Compared with normal glucose treatment, 15.0 mM or 20.0 mM glucose significantly augmented AGT mRNA levels (15 mM: 1.00±0.05 vs. 1.54±0.10, relative ratios to the normal glucose group; P<0.001; 20 mM: 1.00±0.05 vs. 1.40±0.01, relative ratios to the normal glucose group; P<0.01). However, high glucose of 25.0 mM did not augment AGT mRNA level (1.00±0.05 vs. 1.25±0.11, relative ratios to the normal glucose group; P = 0.069) (Fig 1B). In the additional experiment, we also found that 8 mM, the marginal level of postprandial blood glucose in healthy subjects, is the threshold glucose concentration that can stimulate increased AGT (S1A Fig). To exclude the influence of osmotic stress, mannitol was used to equalize the total osmotic stress, which could be induced by both glucose and mannitol, in each group. As expected, mannitol treatment for 48 h did not influence AGT mRNA levels in HK-2 cells (Fig 1C). Therefore, the effect of high glucose on AGT mRNA level was not due to osmotic stress. Taken together, these data suggested that high glucose directly augmented AGT mRNA levels of HK-2 cells in a time- and dose-dependent manner.
Fig 1. High glucose augments AGT mRNA level in HK-2 cells in a time- and dose-dependent manner.
(A) AGT mRNA levels measured at different time points in HK-2 cells respectively treated with normal glucose (5.5 mM) and high glucose (15.0 mM). Compared with normal glucose treatment, high glucose significantly augmented AGT mRNA level from 39 h to 63 h. (B) AGT mRNA levels measured in HK-2 cells respectively treated with different glucose concentrations for 48 h. Compared with normal glucose treatment, high glucose augmented AGT mRNA level with the most significant effects by 15.0 or 20.0 mM glucose. (C) The effect of high glucose on AGT mRNA level was not due to osmotic stress which was further balanced with mannitol treatment for 48 h. Data are expressed as relative values to the 0h group (A) or normal glucose group (B and C). Values are presented as mean ± SEM. *P<0.05 vs. 0h group with the same glucose concentration; #P<0.05 vs. normal glucose group with the same treating time. N = 3~6.
Previous in vivo studies conducted in our lab have shown that the augmentation of intrarenal AGT was inhibited by treatment with an Ang II receptor blocker (ARB) in diabetes [4, 8]. Additional experiments were performed to examine the effect of an ARB (valsartan) on high glucose-induced augmentation of AGT in HK2 cells. We used valsartan at a concentration of 10 μM, as previously described [20, 21]. However, high glucose-induced augmentation of AGT was not affected by treatment with valsartan (S1B Fig).
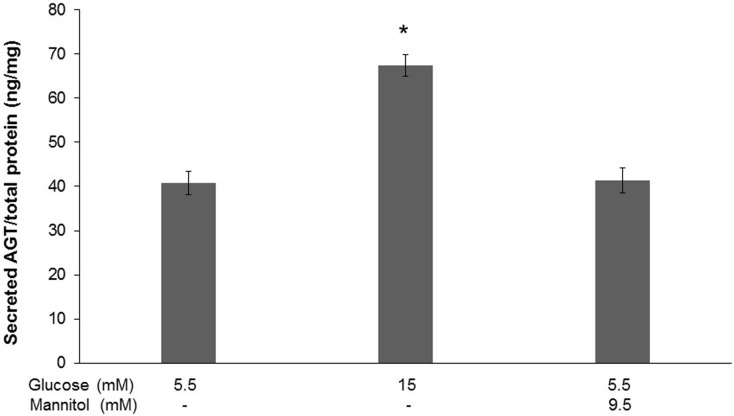
We further investigated the effects of high glucose on AGT secretion into the cell culture medium. Compared with normal glucose, high glucose (15.0 mM) treatment for 48 h significantly augmented secreted AGT levels (40.81±2.69 vs. 67.35±2.49 ng/mg total protein; P<0.0001), however, secretion of AGT was not affected by mannitol-induced osmotic stress (Fig 2).
Fig 2. High glucose augments AGT secretion of HK-2 cells.
Levels of secreted AGT in the culture medium of HK-2 cells respectively treated with normal glucose (5.5 mM), high glucose (15.0 mM) and normal glucose plus 9.5 mM mannitol. Compared with normal glucose treatment, high glucose significantly augmented AGT secretion into the medium, which was not affected by mannitol-induced osmotic stress. Data are expressed as relative values normalized by total cellular protein amount in the dish. Values are presented as mean ± SEM. *P<0.05 vs. normal glucose group. N = 3~6.
High glucose augmented AGT promoter activity through glucose-responsive region (-22 to -1,896) of human AGT promoter sequences
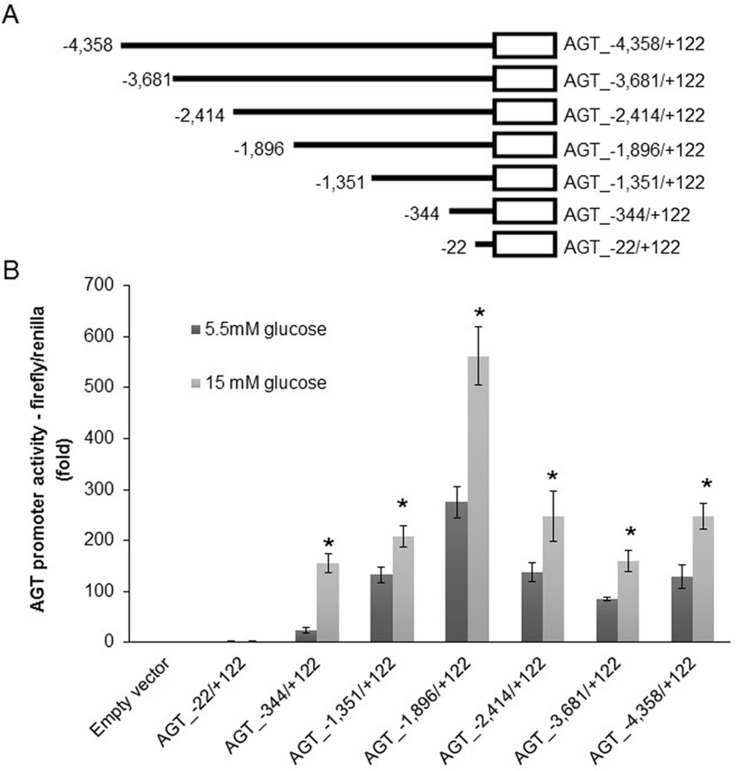
Seven consecutive 5’-end deletion mutant plasmid constructs of the DNA sequence region from −4358 to +122 of human AGT promoter (AGT_−4,358/+122), which has been proved sufficient for the promoter activity in human RPTCs [12], were employed (Fig 3A). Equal amounts of each construct as well as empty vector were respectively transfected into HK-2 cells, followed by the measurement of AGT promoter activity through dual luciferase assay. Compared with normal glucose (5.5 mM), high glucose (15.0 mM) treatment for 48 h significantly augmented AGT promoter activity of HK-2 cells transfected with the constructs with 5’-ends from -344 to -4,358 (hAGT_-344/+122: 23±5 vs. 155±18, P<0.0001; hAGT_-1,351/+122: 133±15 vs. 242±21, P<0.01; hAGT_-1,896/+122: 275±31 vs. 562±57, P<0.01; hAGT_-2,414/+122: 138±18 vs. 274±50, P<0.05; hAGT_-3,681/+122: 85±4 vs. 160±21, P<0.01; hAGT_-4,358/+122: 130±23 vs. 248±25, P<0.01; relative ratios to empty vector transfection group under the same glucose concentration) (Fig 3B). Additionally, within the high glucose treatment groups, consecutive increases of AGT promoter activity were observed in HK-2 cells harboring constructs with 5’-ends from -344 to -1,896, while followed by marked decreases of AGT promoter activity in HK-2 cells harboring constructs with 5’-ends from -2,414 to -4,358 (Fig 3B). These data suggested that binding sites of glucose-responsive transcriptional factor(s) may lie in the promoter region from -22 to -1,896, which was regarded as glucose-responsive region.
Fig 3. High glucose augments AGT promoter activity of HK-2 cells transfected with the promoter sequences constructs.
(A) Consecutive 5’-end deletion mutant constructs of the DNA sequences of human AGT promoter used in this study. Horizontal line represents DNA sequences of the relevant deletion mutant construct. Open box represents the luciferase reporter. (B) Effects of high glucose on AGT promoter activity of HK-2 cells respectively transfected the constructs with 5’-ends from -22 to -4,358. Compared with normal glucose (5.5 mM) treatment, high glucose (15.0 mM) significantly augmented AGT promoter activity of HK-2 cells respectively transfected with the constructs with 5’-ends from -344 to -4,358. Additionally, within high glucose treatment groups, consecutive increase of AGT promoter activity was observed in HK-2 cells harboring constructs with 5’-ends from -344 to -1,896, while followed by marked decrease of AGT promoter activity in HK-2 cells harboring constructs with 5’-ends from -2,414 to -4,358. Data are expressed as relative values to the empty vector transfection group under the same glucose concentration. Values are presented as mean ± SEM. *P<0.05 vs. normal glucose group with the same construct. N = 3~6.
Possible glucose-responsive transcriptional factor candidates
GENETYX software was used to screen all the corresponding transcriptional factors of the glucose-responsive region (-22 to -1,896) in human AGT promoter sequences. Among the transcriptional factors that might bind to the glucose-responsive region (score ≧20) (Tables 2–4), three ones were found to be both glucose-responsive and closely associated with human AGT regulation as described below. The hepatocyte nuclear factor (HNF) family including HNF-5 (site position: -634 to -639; score >50, Table 2) is extensively involved in AGT regulation and/or high glucose effects [22, 23]. A previous study reported that glucosamine can stimulate AGT mRNA expression by activating cAMP-responsive element-binding protein (CREB) (site position: -837 to -841; score>40, Table 3) in rat RPTCs [24]. Additionally, Feng et al. [25] reported that AGT mRNA expression was significantly higher in the hearts of diabetic rats compared with healthy rats, which is in association with increased myocyte enhancer factor (MEF)2 (site position: -32 to -41; score>20, Table 4). Based on above information, we determined HNF-5, CREB and MEF2 as glucose-responsive transcriptional factor candidates of the AGT promoter in human RPTCs.
Table 2. Corresponding transcriptional factors of human AGT promoter sequence region -344 to -1896 (score ≧ 50).
| Score | Position | Motifs | Factors | Consensus sequence (5’–3’) |
Sequence start site |
|---|---|---|---|---|---|
| 50.89 | -1818 to -1846 | CAP-SITE | unknown | cacttt | -1833 |
| IE1.2 | unknown | ctttcc | -1831 | ||
| 50.3 | -1772 to -1815 | NF-E1_CS1 | GATA-1 | attatctt | -1801 |
| GAL4-GAL1-1.6 | GAL4 | ttatc | -1800 | ||
| NF-E1.5 | GATA-1 | tatctt | -1799 | ||
| H2A_CONSERVED _US |
unknown | tcattc | -1775 | ||
| 52.07 | -1374 to -1406 | C/EBP-TTRS3 | C/EBP | tcttactc | -1382 |
| C/EBP_CS2 | C/EBP | tcttactc | -1382 | ||
| GCN4-HIS3.4 | GCN4 | ttactc | -1380 | ||
| GAMMA-IRE_CS | unknown | ctgtagcc | -1375 | ||
| 53.25 | -1130 to -1190 | HINF-A_RS | HiNF-A | atttcagaattt | -1163 |
| BHLH_CS | multiple | catgtg | -1144 | ||
| 52.07 | -621 to -654 | HNF-5_CS | HNF-5 | gtttgt | -634 |
| CAP-SITE | unknown | caatct | -621 | ||
| 58.58 | -602 to -622 | LBP-1_RS | LBP-1 | tctgg | -618 |
| LBP-1_CS | LBP-1 | tctgg | -618 | ||
| 51.48 | -518 to -566 | BHLH_CS | multiple | cacttg | -549 |
| SV40.11 | unknown | cccag | -530 |
Table 4. Corresponding transcriptional factors of human AGT promoter sequence region -22 to -344 (score ≧ 20).
| Score | Position | Motifs | Factors | Consensus sequence (5’–3’) |
Sequence start site |
|---|---|---|---|---|---|
| 21.89 to 35.50 | -329 to -361 | ER_HALF-SITE | ER | ggtca | -337 |
| SP1_CS4 | Sp1 | ggggctggg | -344 | ||
| 26.4 | -254 to -299 | LVC-MO-MULV | LVc | cctgc | -255 |
| LF-A1_RSLF-A1 | LF-A1 | tggccc | -266 | ||
| H-APF-1 _RSH-APF-1 |
H-APF-1 | ctgggaa | -276 | ||
| 20.71 | -204 to -234 | LVC_RS | LVc | cctgc | -224 |
| 24.26 | -124 to -168 | LBP-1_CS/RS | LBP-1 | tctgg | -124 |
| GR-MT-IIA GR | GR | tgtcct | -130 | ||
| BHLH_CS | multiple | catctg | -134 | ||
| TGGCA-BP_RS | TGGCA-BP | tggca | -137 | ||
| 26.63 | -90 to -123 | P7II_CS | P7II | gtaaccctc | -94 |
| IBP-1_CS2 | IBP-1 | aagtga | -101 | ||
| BHLH_CS | multiple | caagtg | -102 | ||
| LBP-1_RS | LBP-1 | tctgg | -108 | ||
| LBP-1_CS | LBP-1 | tctgg | -108 | ||
| CAP-SITE | unknown | cagcct | -118 | ||
| 24.26 | -22 to -86 | TATA-BOX-CS | TFIID | tataaat | -31 |
| TFIID/TBF-RS | TFIID/TBF | tataaa | -31 | ||
| B-FACTOR-HSP70 | B-factor | tataaata | -31 | ||
| TFIID/TBP-H2B1 | TFIID/TBP | tataaatag | -31 | ||
| HIS3-TR-TATA | TFIID | tataaa | -31 | ||
| TATA-BOX.2 | TFIID | tataaa | -31 | ||
| MEF2_CS | MEF2 | ctataaatag | -32 | ||
| CTCF_RS | CTCF | ccctc | -39 | ||
| AP-2_CS6 | AP-2 | ccccaccc | -45 | ||
| AP-2_CS4 | AP-2 | tccccacccc | -46 |
Table 3. Corresponding transcriptional factors of human AGT promoter sequence region -344 to -1896 (50 > score ≧ 40).
| Score | Position | Motifs | Factors | Consensus sequence (5’–3’) |
Sequence start site |
|---|---|---|---|---|---|
| 42.01 ~ 50.89 | -1772 ~ -1846 | C-MYB_CS | c-Myb | cagttg | -1810 |
| BHLH_CS | multiple | cagttg | -1810 | ||
| NF-E1_CS1 | GATA-1 | attatctt | -1801 | ||
| GAL4-GAL1-1.6 | GAL4 | ttatc | -1800 | ||
| NF-E1.5 | GATA-1 | tatctt | -1799 | ||
| 49.11 | -1672 ~ -1710 | BHLH_CS | multiple | catatg | -1695 |
| 40.24 ~ 52.07 | -1334 ~ -1444 | C-MYC_RS1 | c-Myc | tctctta | -1435 |
| C/EBP-TTRS3 | C/EBP | tcttactc | -1382 | ||
| C/EBP_CS2 | C/EBP | tcttactc | -1382 | ||
| GCN4-HIS3.4 | GCN4 | ttactc | -1380 | ||
| 43.79 | -1274 ~ -1320 | TCF-1_CS | TCF-1 | aaaag | -1309 |
| E2A_CS | E2A | acagatg | -1294 | ||
| BHLH_CS | multiple | cagatg | -1293 | ||
| T-AG-SV40.2 | T-Ag | gaggc | -1286 | ||
| 42.60 ~ 47.34 | -1178 ~ -1256 | TCF-1_CS | TCF-1 | caaag | -1251, -1183 |
| 40.24 ~ 53.25 | -1095 ~ -1175 | MFA2.1 | MAT-alpha-2 | atgtattt | -1167 |
| HINF-A_RS | HiNF-A | atttcagaattt | -1163 | ||
| BHLH_CS | multiple | catgtg | -1144 | ||
| TCF-1_CS | TCF-1 | aaaag | -1097 | ||
| 43.2 | -1048 ~ -1075 | AP1-IL2 | AP-1 | ttcagtcagt | -1068 |
| GCN4-HIS4.3 | GCN4 | cagtca | -1066 | ||
| 42.01 ~ 45.56 | -706 ~ -1007 | MCBF_RS | MCBF | cattcct | -994 |
| LF-A1_RS | LF-A1 | tgaacc | -945 | ||
| LVC_RS | LVc | cctgc | -941, -853 | ||
| LVC-MO-MULV | LVc | cctgc | -941, -853 | ||
| MFA2.1 | MAT-alpha-2 | atgtattt | -887 | ||
| CRE.1 | CREB | cgtca | -837 | ||
| BHLH_CS | multiple | cacttg/catgtg | -834, -716 | ||
| TCF-1_CS | TCF-1 | aaaag | -767 | ||
| 41.42 | -671 ~ -699 | TCF-1_CS | TCF-1 | cacag | -678 |
| 40.24 ~ 58.58 | -566 ~ -634 | LBP-1_RS | LBP-1 | tctgg/actgg | -618, -575 |
| LBP-1_CS | LBP-1 | tctgg/actgg | -618, -575 | ||
| TCF-1_CS | TCF-1 | aacag | -593 | ||
| HSTF_CS2 | HSTF | ctggaaacttccag | -574 | ||
| HSTF_CS1 | HSTF | ctggaaacttccag | -574 | ||
| H-APF-1_RS | H-APF-1 | ctggaaa | -574 | ||
| HSE_CS_INVERTED _REPEAT |
HSTF | ctggaaacttccag | -574 | ||
| HSTF_CS3 | HSTF | ggaaacttccagaag | -572 |
Mutation in HNF-5 binding sites reduced the effects of high glucose on human AGT promoter activity as well as AGT mRNA and its secretion levels
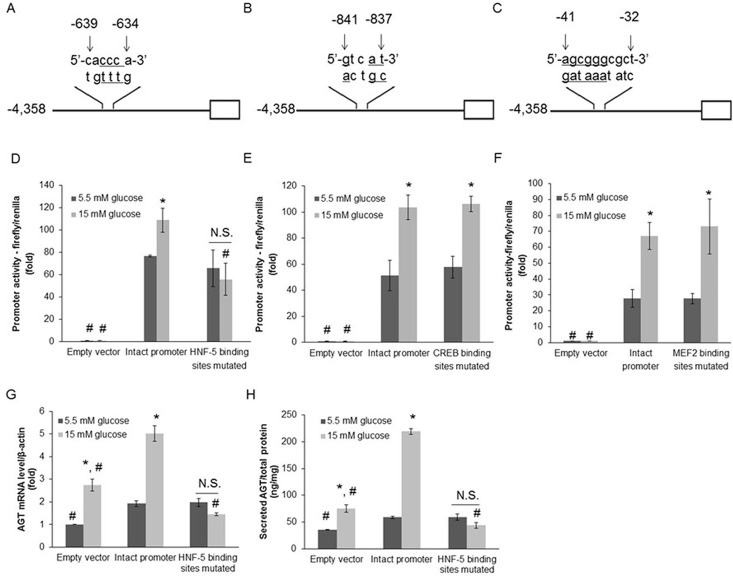
Constructs with respective mutations of HNF-5 (Fig 4A), CREB (Fig 4B) or MEF2 (Fig 4C) binding sites in human AGT promoter sequences (AGT_-4,358/+122) were prepared. Equal amounts of empty vector, vector harboring either intact human AGT_-4,358/+122 or binding sites mutated human AGT_-4,358/+122 were respectively transfected into HK-2 cells, followed by AGT promoter activity measurement. Compared with normal glucose (5.5 mM), high glucose (15 mM) treatment for 48 h significantly augmented AGT promoter activity of HK-2 cells with intact human AGT_-4,358/+122 transfection (77±1 vs. 109±11, relative ratios to empty vector transfection group under the same glucose concentration; P<0.05) (Fig 4D). However, similar effect was not observed in HK-2 cells with mutated HNF-5 binding sites (66±17 vs. 56±14, relative ratios to empty vector transfection group under the same glucose concentration; P = 0.68) (Fig 4D). Different from the mutation of HNF-5 binding sites, mutations in the binding sites of CREB (58±8 vs. 106±6, relative ratios to empty vector transfection group under the same glucose concentration; P<0.01) and MEF-2 (28±3 vs. 73±17, relative ratios to empty vector transfection group under the same glucose concentration; P<0.05) had no effect on the response of AGT promoter activity to high glucose treatment (Fig 4E and 4F). Next, we investigated whether mutation in HNF-5 binding sites could attenuate AGT mRNA expression and its secretion under high glucose condition. In general, compared with groups transfected with the empty vector, those transfected with intact human AGT_-4,358/+122 showed much higher AGT mRNA and protein levels, especially under the high glucose condition (Fig 4G and 4H). These results suggest that the plasmid DNA could be integrated into the genome of HK-2 cells after transfection, moreover, the integrated promoter sequences even occupied a considerable proportion and were able to control AGT mRNA and protein expression together with the native AGT promoter sequences in HK-2 cells. Compared with normal glucose (5.5 mM), high glucose (15 mM) treatment for 48 h significantly augmented AGT mRNA levels of HK-2 cells transfected with either empty vector (1±0.01 vs. 2.74±0.27, relative ratios to the empty vector transfection group with normal glucose; P<0.001) or intact human AGT_-4,358/+122 transfection (1.93±0.13 vs. 5.02±0.35, relative ratios to the empty vector transfection group with normal glucose; P<0.001) (Fig 4G). However, this effect was not observed in HK-2 cells transfected with HNF-5 binding sites mutated AGT_-4,358/+122 (1.97±0.17 vs. 1.44±0.06, relative ratios to the empty vector transfection group with normal glucose; P = 0.10) (Fig 4G). Similar data were observed for AGT secretion levels. Compared with normal glucose (5.5 mM), high glucose (15 mM) treatment for 48 h significantly augmented AGT protein levels in the medium of HK-2 cells transfected with either empty vector (35.38±0.83 vs. 75.23±6.54 ng/mg total protein; P<0.0001) or intact human AGT_-4,358/+122 transfection (58.82±2.29 vs. 219.37±5.17 ng/mg total protein; P<0.0001) (Fig 4H). However, these effects were not observed in HK-2 cells transfected with HNF-5 binding sites mutated AGT_-4,358/+122 (59.12±5.49 vs. 43.72±5.65 ng/mg total protein; P = 0.12) (Fig 4H).
Fig 4. Mutation in HNF-5 binding sites reduces the effects of high glucose on AGT promoter activity as well as AGT mRNA and secretion levels.
(A, B and C) Constructs with respective mutation of HNF-5 (A), CREB (B) or MEF2 (C) binding sites in main human AGT promoter sequences (AGT_-4,358/+122). Within binding sites, the underlined base pairs were substituted by mutagenesis. Horizontal line represents human AGT_-4,358/+122 with relevant mutation. Open box represents the luciferase reporter. (D, E and F) Effects of high glucose treatment on AGT promoter activity in HK-2 cells, which were transfected with either intact or relevant binding sites mutated construct of human AGT_-4,358/+122. Compared with normal glucose (5.5 mM) treatment, high glucose (15.0 mM) significantly augmented AGT promoter activity of HK-2 cells transfected with the intact construct of human AGT_-4,358/+122 (D, E and F). Furthermore, the above effect was abolished in HK-2 cells with mutated HNF-5 binding sites (D), while not affected by the binding sites mutation of CREB (E) or MEF2 (F). g: guanine; a: adenine; c: cytosine; t: thymine. Data are expressed as relative values to the empty vector transfection group under the same glucose concentration. (G) Mutation in HNF-5 binding sites attenuated the effects of high glucose on AGT mRNA level. Data are expressed as relative values to the empty vector transfection group with normal glucose. (H) Mutation in HNF-5 binding sites attenuated the effects of high glucose on AGT secretion level. Values are presented as mean ± SEM. *P<0.05 vs. normal glucose (5.5 mM) group with the same construct; #P<0.05 vs. intact human AGT_-4,358/+122 transfection group under the same glucose concentration; N.S.: no significant difference. N = 3~6.
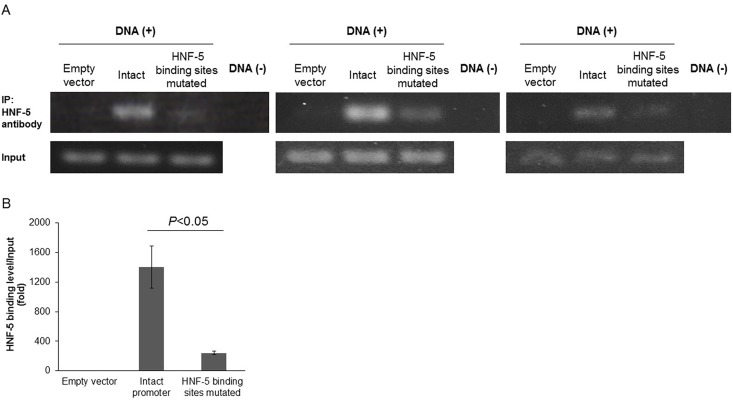
Binding level of HNF-5 to AGT promoter sequences was reduced by mutation in its binding sites and elevated by high glucose treatment
Equal amounts of empty vector, vector harboring intact human AGT_-4,358/+122 and vector harboring HNF-5 binding sites mutated human AGT_-4,358/+122 was transfected into HK-2 cells, respectively. Then the binding levels of HNF-5 protein to human AGT promoter sequences were detected by chromatin immunoprecipitation. As shown in at least 3 independent experiments, compared with the intact human AGT_-4,358/+122 transfection groups, HK-2 cells with HNF-5 binding sites mutation showed significantly attenuated HNF-5 binding levels. This was detected through both band intensities after PCR (Fig 5A) and quantitative real-time PCR (1402±287 vs. 240±23, relative ratios to the empty vector transfection group; P<0.05) (Fig 5B). These data confirm that mutation in HNF-5 binding sites reduced the binding level of HNF-5 to AGT promoter sequences. Additionally, we also measured the binding level of HNF-5 to the native genome of HK-2 cells without plasmid transfection. HNF-5 binding level was very low under normal glucose (5.5 mM), however, high glucose (15.0 mM) significantly augmented HNF-5 binding level. This was detected through both band intensities after PCR (S2A Fig) and quantitative real-time PCR (1±0.01 vs. 20.52±2.64, relative ratios to the normal glucose group; P<0.05) (S2B Fig).
Fig 5. Binding level of HNF-5 to AGT promoter sequences is reduced by mutation in its binding sites.
(A and B) Levels of DNA immuno-precipitated by HNF-5 protein from HK-2 cells respectively transfected with empty vector, intact and HNF-5 binding sites mutated main human AGT promoter sequences (AGT_-4,358/+122), detected through band intensities after PCR (A) and quantitative real-time PCR (B). Compared with the group with intact human AGT_-4,358/+122, the binding level of HNF-5 protein to human AGT_-4,358/+122 significantly decreased in HK-2 cells with HNF-5 binding sites mutation. Data of quantitative real-time PCR are expressed as relative values to the empty vector transfection group. DNA (−) indicates the absence of DNA, IP: immuno-precipitation. Values are presented as mean ± SEM. N = 3~6.
Discussion
Three novel findings were uncovered in the present study. First, high glucose augments the levels of AGT mRNA and AGT secretion of human RPTCs. Second, high glucose augments AGT promoter activity in human RPTCs and the glucose-responsive region within human AGT promoter sequences is from -22 to -1,896. Third, HNF-5 is one of the glucose-responsive transcriptional factors which bind to the glucose-responsive region of AGT promoter sequences and mutation of its binding sites may abolish the high glucose effects on AGT in human RPTCs
Intrarenal AGT is primarily generated in RPTCs and secreted into tubular fluid [10, 26]. Visavadiya et al. [27] have shown that high glucose can increase the expression levels of the RAS components including renin, angiotensin-converting enzyme as well as Ang II levels in HK-2 cells. In the present study, we aim to determine the detailed molecular mechanism responsible for high glucose-induced proximal tubular augmentation of AGT levels. We found that high glucose significantly augmented AGT mRNA levels in human RPTCs with the most obvious effects by 48 h- treatment with 15 or 20 mM glucose, which is similar to the blood glucose level of diabetic patients. Consistent findings were previously discovered in the case of rats, it is reported that high glucose stimulated AGT gene expression in rat RPTCs, which subsequently resulted in cell hypertrophy [13, 14]; On the other hand, stable transfection of antisense rat AGT cDNA into rat RPTCs effectively inhibited AGT expression and prevented high glucose-induced cell hypertrophy [28]. However, high glucose-induced augmentation of AGT in HK-2 cells was not affected by treatment with valsartan. We have no good explanation as to why ARB does not inhibit high glucose-induced augmentation of AGT in vitro, however, it is probably due to the low renin activity and AGT utilizing efficiency in HK-2 cells.
Additionally, we also found that high glucose significantly augmented AGT secretion from human RPTCs. AGT secreted from proximal tubules is regarded as the main source of urinary AGT [29]. Moreover, urinary AGT level reflects the activity of intrarenal RAS [30, 31] and tubular injury [32] in patients with type 2 diabetes. Elevation of urinary AGT has also been confirmed to be related to not only development but also progression of DN based on studies in patients with DN [31, 33], as well as mouse [34, 35] and rat [6, 36] models of diabetes. Therefore, finding in the present study suggests that high glucose-induced AGT secretion from RPTCs may play an important role in the pathogenesis of DN.
Previous studies have demonstrated that proximal promoter elements of human AGT genes from −4358 to +122 are sufficient to measure AGT promoter activity in human RPTCs [12, 37]. Furthermore, the importance of proximal regulatory elements to human AGT regulation has been confirmed by analyses with deletion mutants of AGT promoter sequences [12, 37]. Based on the previous study [12], we also employed the region from -4358 to +122 within human AGT promoter sequences and further identified its glucose-responsive region in human RPTCs. We found that high glucose treatment significantly augmented AGT promoter activity in HK-2 cells respectively transfected with the deletion mutant constructs with 5’-ends from -344 to -4,358. These data indicated that high glucose activates AGT by increasing AGT promoter activity in human RPTCs. Furthermore, within the high glucose treatment groups, consecutive increases of AGT promoter activity were observed in HK-2 cells harboring constructs with 5’-ends from -344 to -1,896, while followed by the marked decreases of AGT promoter activity in HK-2 cells harboring constructs with 5’-ends from -2,414 to -4,358. These data indicated that the glucose-responsive region, where glucose-responsive transcriptional factor(s) bind(s), should be from -22 to -1,896 of human AGT promoter sequences.
Among all the transcriptional factors that possibly bind to the glucose-responsive region of human AGT promoter sequences (score ≧ 20), regulatory effect on AGT promoter activity in human RPTCs was observed in HNF-5. Soon after HNF-5 was initially designated [38], Nitsch et al. [18] demonstrated that HNF-5 is identical to HNF-3, because the HNF-3 binding sites within gene promoter sequences, identified by them, coincides with those of HNF-5. In the following literatures, both names were kept and applied to refer to the same factor [19]. HK-2 cells transfected with the HNF-5 binding sites mutated human AGT_-4,358/+122 did not show any increase in AGT promoter activity as well as AGT mRNA and its secretion levels under high glucose treatment. In other words, mutation of HNF-5 binding sites in human AGT promoter sequences abolished the high glucose effects on AGT in human RPTCs. Moreover, we also confirmed that binding level of HNF-5 to AGT promoter sequences was reduced by mutation in its binding sites and elevated by high glucose treatment. All of the above results uncovered the molecular mechanism that in human RPTCs, through HNF-5, high glucose enhances the activity of the AGT gene promoter, thus increasing the levels of transcription and secretion of AGT. As a result, the levels of AGT mRNA and AGT secretion were augmented (Fig 6). It has been well known that the HNF family is extensively involved in human AGT regulation, for example, HNF-1α [39] and HNF-4 [23] can bind to the AGT promoter sequences and activate AGT expression in human hepatoma cells. In the present study, we demonstrated, for the first time, that HNF-5 is one of the glucose-responsive transcriptional factors that bind to the AGT promoter sequences in human RPTCs. As far as we know, HNF-5 also plays a role as a glucocorticoid-responsive transcriptional factor in hepatoma cells [40]. However, in hepatoma cells, glucose-induced AGT augmentation is mediated predominantly through HNF-1α- and HNF-4-dependent pathways [23, 39]. Further studies are needed to determine the possible role of HNF-5 in AGT production in other organs.
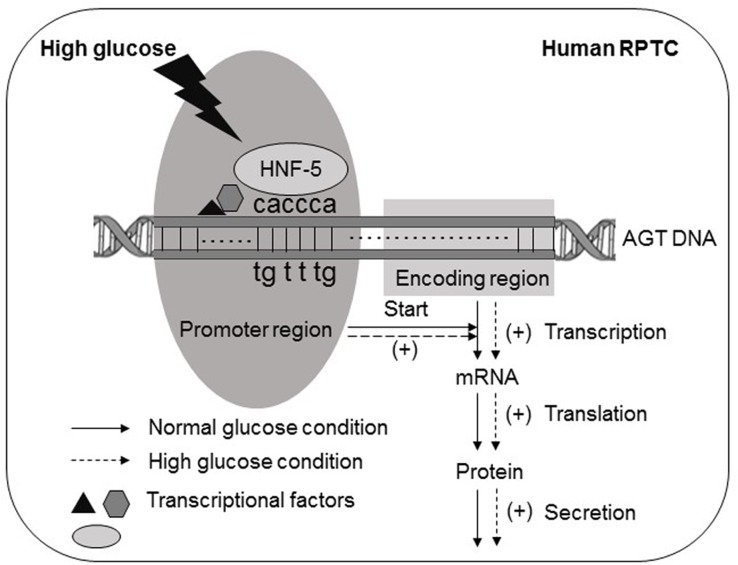
Fig 6. Schematic diagram summarizing the role of HNF-5 in AGT augmentation by high glucose in human RPTCs.
In human RPTCs, through HNF-5, high glucose enhances the activity of AGT gene promoter, thus increases the levels of transcription, translation and secretion of AGT, in result, the levels of AGT mRNA and AGT secretion were augmented. g: guanine; a: adenine; c: cytosine; t: thymine.
As shown in Fig 4E and 4F, mutation of CREB or MEF2 binding sites within AGT promoter sequences did not abolish the high glucose-induced enhancement of AGT promoter activity in HK-2 cells. These data suggest that CREB and MEF2 does not contribute to augmenting AGT expression in RPTCs under high glucose condition. Using GENETYX software and published data, candidates for glucose-responsive transcriptional factors were selected based on their likelihood of binding to human AGT promoter sequences. There may be other glucose-responsive transcriptional factors that work on AGT promoter, but we omitted them due to the lack of published supporting evidence for their potential involvement in high glucose-induced effects and/or AGT regulation. HNF-5 is located from -634 to -639. However, as shown in Fig 3B, AGT promoter activity apparently stepped-up from AGT_-1,351/+122 to AGT -1,896/+122, suggesting that the sequences between -1,351 and -1,896 may play an important role in amplifying AGT induction under high glucose condition. Therefore, other glucose-responsive transcriptional factors that bind to this region should be investigated further.
In conclusion, the present study has demonstrated, for the first time, that high glucose augments AGT in human RPTCs through HNF-5, a glucose-responsive transcriptional factor that functions on the AGT gene promoter, which provides a potential therapeutic target for DN in humans.
Supporting information
(A) AGT mRNA levels measured in HK-2 cells respectively treated with different glucose concentrations for 48 h. Compared with normal glucose (5.5 mM) treatment, high glucose augments AGT mRNA level, with the threshold effective concentration of 8 mM. (B) The effect of high glucose on AGT mRNA level could not be affected by valsartan. There is no difference between AGT mRNA levels in HK-2 cells respectively treated with either high glucose (15 mM) or high glucose plus 10 μM valsartan for 48 h. Data are expressed as relative values to the corresponding normal glucose group. Values are presented as mean ± SEM. #P<0.05, ##P<0.01, ###P<0.001 vs. normal glucose group. N.S.: no significant difference. N = 3~6.
(TIF)
(A and B) Levels of DNA immuno-precipitated by HNF-5 protein from native genome of HK-2 cells respectively treated with normal (5.5 mM) and high (15 mM) glucose, detected through band intensities after PCR (A) and quantitative real-time PCR (B). HK-2 cells were without plasmid transfection. Compared with normal glucose treatment, high glucose significantly augmented HNF-5 binding levels. Data of quantitative real-time PCR are expressed as relative values to the normal glucose group. DNA (−) indicates the absence of DNA, IP: immuno-precipitation, NG: normal glucose; HG: high glucose. Values are presented as mean ± SEM. N = 3~6.
(TIF)
Data Availability
All relevant data are within the paper and its Supporting Information files.
Funding Statement
This study was supported by the Grants-in-Aid for Scientific Research from Japanese Society for the Promotion of Science (KAKENHI) (26460343) and the Hoansha Foundation to Akira Nishiyama. https://www.jsps.go.jp/english/e-grants/ The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1.Yacoub R, Campbell KN. Inhibition of RAS in diabetic nephropathy. International journal of nephrology and renovascular disease. 2015;8:29–40. Epub 2015/05/01. doi: 10.2147/IJNRD.S37893 ; PubMed Central PMCID: PMC4403683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Tervaert TW, Mooyaart AL, Amann K, Cohen AH, Cook HT, Drachenberg CB, et al. Pathologic classification of diabetic nephropathy. Journal of the American Society of Nephrology: JASN. 2010;21(4):556–63. Epub 2010/02/20. doi: 10.1681/ASN.2010010010 . [DOI] [PubMed] [Google Scholar]
- 3.Tang SC, Lai KN. The pathogenic role of the renal proximal tubular cell in diabetic nephropathy. Nephrology, dialysis, transplantation: official publication of the European Dialysis and Transplant Association—European Renal Association. 2012;27(8):3049–56. Epub 2012/06/27. doi: 10.1093/ndt/gfs260 . [DOI] [PubMed] [Google Scholar]
- 4.Nagai Y, Yao L, Kobori H, Miyata K, Ozawa Y, Miyatake A, et al. Temporary angiotensin II blockade at the prediabetic stage attenuates the development of renal injury in type 2 diabetic rats. Journal of the American Society of Nephrology: JASN. 2005;16(3):703–11. Epub 2005/01/14. doi: 10.1681/ASN.2004080649 ; PubMed Central PMCID: PMC2572705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Choi KC, Kim NH, An MR, Kang DG, Kim SW, Lee J. Alterations of intrarenal renin-angiotensin and nitric oxide systems in streptozotocin-induced diabetic rats. Kidney international Supplement. 1997;60:S23–7. Epub 1997/09/01. . [PubMed] [Google Scholar]
- 6.Miyata K, Ohashi N, Suzaki Y, Katsurada A, Kobori H. Sequential activation of the reactive oxygen species/angiotensinogen/renin-angiotensin system axis in renal injury of type 2 diabetic rats. Clinical and experimental pharmacology & physiology. 2008;35(8):922–7. Epub 2008/04/24. doi: 10.1111/j.1440-1681.2008.04938.x ; PubMed Central PMCID: PMC2575127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ziyadeh FN, Snipes ER, Watanabe M, Alvarez RJ, Goldfarb S, Haverty TP. High glucose induces cell hypertrophy and stimulates collagen gene transcription in proximal tubule. The American journal of physiology. 1990;259(4 Pt 2):F704–14. Epub 1990/10/01. . [DOI] [PubMed] [Google Scholar]
- 8.Kamiyama M, Garner MK, Farragut KM, Sofue T, Hara T, Morikawa T, et al. Detailed localization of augmented angiotensinogen mRNA and protein in proximal tubule segments of diabetic kidneys in rats and humans. International journal of biological sciences. 2014;10(5):530–42. Epub 2014/06/10. doi: 10.7150/ijbs.8450 ; PubMed Central PMCID: PMC4046880. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Hsieh TJ, Fustier P, Wei CC, Zhang SL, Filep JG, Tang SS, et al. Reactive oxygen species blockade and action of insulin on expression of angiotensinogen gene in proximal tubular cells. The Journal of endocrinology. 2004;183(3):535–50. Epub 2004/12/14. doi: 10.1677/joe.1.05871 . [DOI] [PubMed] [Google Scholar]
- 10.Kobori H, Harrison-Bernard LM, Navar LG. Expression of angiotensinogen mRNA and protein in angiotensin II-dependent hypertension. Journal of the American Society of Nephrology: JASN. 2001;12(3):431–9. Epub 2001/02/22. ; PubMed Central PMCID: PMC2573050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Liu F, Brezniceanu ML, Wei CC, Chenier I, Sachetelli S, Zhang SL, et al. Overexpression of angiotensinogen increases tubular apoptosis in diabetes. Journal of the American Society of Nephrology: JASN. 2008;19(2):269–80. Epub 2007/12/07. doi: 10.1681/ASN.2007010074 ; PubMed Central PMCID: PMC2396745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Acres OW, Satou R, Navar LG, Kobori H. Contribution of a nuclear factor-kappaB binding site to human angiotensinogen promoter activity in renal proximal tubular cells. Hypertension. 2011;57(3):608–13. Epub 2011/02/02. doi: 10.1161/HYPERTENSIONAHA.110.165464 ; PubMed Central PMCID: PMC3051182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Zhang SL, Filep JG, Hohman TC, Tang SS, Ingelfinger JR, Chan JS. Molecular mechanisms of glucose action on angiotensinogen gene expression in rat proximal tubular cells. Kidney international. 1999;55(2):454–64. Epub 1999/02/13. doi: 10.1046/j.1523-1755.1999.00271.x . [DOI] [PubMed] [Google Scholar]
- 14.Zhang SL, Tang SS, Chen X, Filep JG, Ingelfinger JR, Chan JS. High levels of glucose stimulate angiotensinogen gene expression via the P38 mitogen-activated protein kinase pathway in rat kidney proximal tubular cells. Endocrinology. 2000;141(12):4637–46. Epub 2000/12/07. doi: 10.1210/endo.141.12.7844 . [DOI] [PubMed] [Google Scholar]
- 15.Katsurada A, Hagiwara Y, Miyashita K, Satou R, Miyata K, Ohashi N, et al. Novel sandwich ELISA for human angiotensinogen. American journal of physiology Renal physiology. 2007;293(3):F956–60. Epub 2007/06/08. doi: 10.1152/ajprenal.00090.2007 ; PubMed Central PMCID: PMC2094097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kobori H, Hayashi M, Saruta T. Thyroid Hormone Stimulates Renin Gene Expression Through the Thyroid Hormone Response Element. Hypertension. 2001;37(1):99–104. Epub 2001/02/24. ; PubMed Central PMCID: PMC2573046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Tachibana K, Kobayashi Y, Tanaka T, Tagami M, Sugiyama A, Katayama T, et al. Gene expression profiling of potential peroxisome proliferator-activated receptor (PPAR) target genes in human hepatoblastoma cell lines inducibly expressing different PPAR isoforms. Nuclear receptor. 2005;3:3 Epub 2005/10/04. doi: 10.1186/1478-1336-3-3 ; PubMed Central PMCID: PMC1262764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Nitsch D, Boshart M, Schutz G. Activation of the tyrosine aminotransferase gene is dependent on synergy between liver-specific and hormone-responsive elements. Proceedings of the National Academy of Sciences of the United States of America. 1993;90(12):5479–83. Epub 1993/06/15. ; PubMed Central PMCID: PMC46744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Lemaigre FP, Durviaux SM, Truong O, Lannoy VJ, Hsuan JJ, Rousseau GG. Hepatocyte nuclear factor 6, a transcription factor that contains a novel type of homeodomain and a single cut domain. Proceedings of the National Academy of Sciences of the United States of America. 1996;93(18):9460–4. Epub 1996/09/03. ; PubMed Central PMCID: PMC38450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Xiong XL, Jia RH, Yang DP, Ding GH. Irbesartan attenuates contrast media-induced NRK-52E cells apoptosis. Pharmacological research. 2006;54(4):253–60. Epub 2006/07/11. doi: 10.1016/j.phrs.2006.05.005 . [DOI] [PubMed] [Google Scholar]
- 21.Peng PA, Wang L, Ma Q, Xin Y, Zhang O, Han HY, et al. Valsartan protects HK-2 cells from contrast media-induced apoptosis by inhibiting endoplasmic reticulum stress. Cell biology international. 2015;39(12):1408–17. Epub 2015/08/08. doi: 10.1002/cbin.10521 . [DOI] [PubMed] [Google Scholar]
- 22.Bilman V, Mares-Guia L, Nadu AP, Bader M, Campagnole-Santos MJ, Santos RA, et al. Decreased hepatic gluconeogenesis in transgenic rats with increased circulating angiotensin-(1–7). Peptides. 2012;37(2):247–51. Epub 2012/08/21. doi: 10.1016/j.peptides.2012.08.002 . [DOI] [PubMed] [Google Scholar]
- 23.Yanai K, Hirota K, Taniguchi-Yanai K, Shigematsu Y, Shimamoto Y, Saito T, et al. Regulated expression of human angiotensinogen gene by hepatocyte nuclear factor 4 and chicken ovalbumin upstream promoter-transcription factor. The Journal of biological chemistry. 1999;274(49):34605–12. Epub 1999/11/27. . [DOI] [PubMed] [Google Scholar]
- 24.Hsieh TJ, Fustier P, Zhang SL, Filep JG, Tang SS, Ingelfinger JR, et al. High glucose stimulates angiotensinogen gene expression and cell hypertrophy via activation of the hexosamine biosynthesis pathway in rat kidney proximal tubular cells. Endocrinology. 2003;144(10):4338–49. Epub 2003/09/10. doi: 10.1210/en.2003-0220 . [DOI] [PubMed] [Google Scholar]
- 25.Feng B, Chen S, Chiu J, George B, Chakrabarti S. Regulation of cardiomyocyte hypertrophy in diabetes at the transcriptional level. American journal of physiology Endocrinology and metabolism. 2008;294(6):E1119–26. Epub 2008/04/17. doi: 10.1152/ajpendo.00029.2008 . [DOI] [PubMed] [Google Scholar]
- 26.Castrop H, Hocherl K, Kurtz A, Schweda F, Todorov V, Wagner C. Physiology of kidney renin. Physiological reviews. 2010;90(2):607–73. Epub 2010/04/16. doi: 10.1152/physrev.00011.2009 . [DOI] [PubMed] [Google Scholar]
- 27.Visavadiya NP, Li Y, Wang S. High glucose upregulates upstream stimulatory factor 2 in human renal proximal tubular cells through angiotensin II-dependent activation of CREB. Nephron Experimental nephrology. 2011;117(3):e62–70. Epub 2010/09/04. doi: 10.1159/000320594 ; PubMed Central PMCID: PMC2948662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Zhang SL, To C, Chen X, Filep JG, Tang SS, Ingelfinger JR, et al. Effect of renin-angiotensin system blockade on the expression of the angiotensinogen gene and induction of hypertrophy in rat kidney proximal tubular cells. Experimental nephrology. 2001;9(2):109–17. Epub 2001/01/11. 52601.. [DOI] [PubMed] [Google Scholar]
- 29.Kobori H, Alper AB Jr., Shenava R, Katsurada A, Saito T, Ohashi N, et al. Urinary angiotensinogen as a novel biomarker of the intrarenal renin-angiotensin system status in hypertensive patients. Hypertension. 2009;53(2):344–50. Epub 2008/12/17. doi: 10.1161/HYPERTENSIONAHA.108.123802 ; PubMed Central PMCID: PMC2658771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Ogawa S, Kobori H, Ohashi N, Urushihara M, Nishiyama A, Mori T, et al. Angiotensin II Type 1 Receptor Blockers Reduce Urinary Angiotensinogen Excretion and the Levels of Urinary Markers of Oxidative Stress and Inflammation in Patients with Type 2 Diabetic Nephropathy. Biomarker insights. 2009;4:97–102. Epub 2009/08/05. ; PubMed Central PMCID: PMC2716676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Saito T, Urushihara M, Kotani Y, Kagami S, Kobori H. Increased urinary angiotensinogen is precedent to increased urinary albumin in patients with type 1 diabetes. The American journal of the medical sciences. 2009;338(6):478–80. Epub 2009/11/04. doi: 10.1097/MAJ.0b013e3181b90c25 ; PubMed Central PMCID: PMC2795783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Terami T, Wada J, Inoue K, Nakatsuka A, Ogawa D, Teshigawara S, et al. Urinary angiotensinogen is a marker for tubular injuries in patients with type 2 diabetes. International journal of nephrology and renovascular disease. 2013;6:233–40. Epub 2013/11/01. doi: 10.2147/IJNRD.S51829 ; PubMed Central PMCID: PMC3808213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Kobori H, Kamiyama M, Harrison-Bernard LM, Navar LG. Cardinal role of the intrarenal renin-angiotensin system in the pathogenesis of diabetic nephropathy. Journal of investigative medicine: the official publication of the American Federation for Clinical Research. 2013;61(2):256–64. Epub 2012/12/26. doi: 10.231/JIM.0b013e31827c28bb ; PubMed Central PMCID: PMC3554867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Park S, Bivona BJ, Kobori H, Seth DM, Chappell MC, Lazartigues E, et al. Major role for ACE-independent intrarenal ANG II formation in type II diabetes. American journal of physiology Renal physiology. 2010;298(1):F37–48. Epub 2009/10/23. doi: 10.1152/ajprenal.00519.2009 ; PubMed Central PMCID: PMC2806123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Kamiyama M, Zsombok A, Kobori H. Urinary angiotensinogen as a novel early biomarker of intrarenal renin-angiotensin system activation in experimental type 1 diabetes. Journal of pharmacological sciences. 2012;119(4):314–23. Epub 2012/08/02. ; PubMed Central PMCID: PMC3443320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Suzaki Y, Ozawa Y, Kobori H. Intrarenal oxidative stress and augmented angiotensinogen are precedent to renal injury in Zucker diabetic fatty rats. International journal of biological sciences. 2007;3(1):40–6. Epub 2007/01/04. ; PubMed Central PMCID: PMC1657083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Fukamizu A, Takahashi S, Seo MS, Tada M, Tanimoto K, Uehara S, et al. Structure and expression of the human angiotensinogen gene. Identification of a unique and highly active promoter. The Journal of biological chemistry. 1990;265(13):7576–82. Epub 1990/05/05. . [PubMed] [Google Scholar]
- 38.Rigaud G, Roux J, Pictet R, Grange T. In vivo footprinting of rat TAT gene: dynamic interplay between the glucocorticoid receptor and a liver-specific factor. Cell. 1991;67(5):977–86. Epub 1991/11/29. . [DOI] [PubMed] [Google Scholar]
- 39.Jain S, Li Y, Patil S, Kumar A. HNF-1alpha plays an important role in IL-6-induced expression of the human angiotensinogen gene. American journal of physiology Cell physiology. 2007;293(1):C401–10. Epub 2007/05/04. doi: 10.1152/ajpcell.00433.2006 . [DOI] [PubMed] [Google Scholar]
- 40.Grange T, Roux J, Rigaud G, Pictet R. Cell-type specific activity of two glucocorticoid responsive units of rat tyrosine aminotransferase gene is associated with multiple binding sites for C/EBP and a novel liver-specific nuclear factor. Nucleic acids research. 1991;19(1):131–9. Epub 1991/01/11. ; PubMed Central PMCID: PMC333543. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
(A) AGT mRNA levels measured in HK-2 cells respectively treated with different glucose concentrations for 48 h. Compared with normal glucose (5.5 mM) treatment, high glucose augments AGT mRNA level, with the threshold effective concentration of 8 mM. (B) The effect of high glucose on AGT mRNA level could not be affected by valsartan. There is no difference between AGT mRNA levels in HK-2 cells respectively treated with either high glucose (15 mM) or high glucose plus 10 μM valsartan for 48 h. Data are expressed as relative values to the corresponding normal glucose group. Values are presented as mean ± SEM. #P<0.05, ##P<0.01, ###P<0.001 vs. normal glucose group. N.S.: no significant difference. N = 3~6.
(TIF)
(A and B) Levels of DNA immuno-precipitated by HNF-5 protein from native genome of HK-2 cells respectively treated with normal (5.5 mM) and high (15 mM) glucose, detected through band intensities after PCR (A) and quantitative real-time PCR (B). HK-2 cells were without plasmid transfection. Compared with normal glucose treatment, high glucose significantly augmented HNF-5 binding levels. Data of quantitative real-time PCR are expressed as relative values to the normal glucose group. DNA (−) indicates the absence of DNA, IP: immuno-precipitation, NG: normal glucose; HG: high glucose. Values are presented as mean ± SEM. N = 3~6.
(TIF)
Data Availability Statement
All relevant data are within the paper and its Supporting Information files.