Abstract
Increasing temperature and CO2 concentrations can alter tritrophic interactions in ecosystems, but the impact of increasingly severe drought on such interactions is not well understood. We examined the response of a wheat-aphid-parasitoid system to variation in water-deficit stress levels. Our results showed that arid area clones of the aphid, Sitobion avenae (Fabricius), tended to have longer developmental times compared to semiarid and moist area clones, and the development of S. avenae clones tended to be slower with increasing levels of water-deficit. Body sizes of S. avenae clones from all areas decreased with increasing water-deficit levels, indicating their declining adaptation potential under drought. Compared to arid area clones, moist area clones of S. avenae had a higher frequency of backing under severe water stress only, but a higher frequency of kicking under well-watered conditions only, suggesting a water-deficit level dependent pattern of resistance against the parasitoid, Aphidius gifuensis (Ashmead). The number of S. avenae individuals attacked by the parasitoid in 10 min showed a tendency to decrease with increasing water-deficit levels. Clones of S. avenae tended to have lower parasitism rates under treatments with higher water-deficit levels. The development of the parasitoid tended to be slower under higher levels of water-deficit stress. Thus, the bottom-up effects of water-deficit stressed plants were negative on S. avenae. However, the top-down effects via parasitoids were compromised by water-deficit, which could favor the growth of aphid populations. Overall, the first trophic level under water-deficit stress was shown to have an indirect and negative impact on the third trophic level parasitoid, suggesting that parasitoids could be increasingly vulnerable in future warming scenarios.
Introduction
During the past 130 years, an approximate 0.85°C increase in the mean global surface temperature was recorded, and the warming extent could further intensify by 2.5–7.8°C before 2100 [1]. Meanwhile, an increase in extreme weather events (e.g., flooding, storms and drought) is predicted [1]. The warming trend is also evident in China, since significant rises in temperature (1.10C) and increasing numbers of drought spells have been observed in China over the past century of 1908–2007 [2]. Much of the climate change has been attributed to increased CO2 concentrations in the atmosphere [1]. The potential effects of increasing temperature and CO2 concentrations have been extensively explored, and both factors could have diverse effects (in terms of phenology, physiology, abundance and distribution) on plants, as well as on herbivores [3–4]. Increasing recent attention has been paid to tritrophic interactions in various ecosystems under elevated temperatures and CO2 levels [5–6]. For example, it was found that higher temperatures as a result of frequent occurrences of warm springs (a possible consequence of the climate change) could uncouple the phenological synchrony between Melitaea cinxia L. and its host plants, as well as between Cotesia melitaearum (Wilkinson) (a braconid parasitoid) and its host (M. cinxia) [7]. Bezemer et al. [8] presented a study of tritrophic interactions under elevated temperature and CO2 in a system consisting of four plants, the aphid Myzus persicae (Sulzer), and the parasitoid Aphidius matricariae (Haliday). In that study, the aphid’s abundances increased under both elevated CO2 and temperature, while its parasitism rates showed a tendency to rise with increasing temperatures [8]. Changes in tritrophic interactions may induce destabilization in the population dynamics of certain species by influencing their biology, ecology and evolution, which could ultimately lead towards their extinction [9–10].
Another significant indicator of the global climate change is changing dynamics of drought or water-deficit stress. It was predicted that drought could be more acute, prolonged and frequent in China for the period of 2020–2049 compared to the period of 1971–2000 [11]. Drought may compromise plant defensive strategies and make them more vulnerable to opportunistic herbivores [12]. Drought can also show variable direct impacts on herbivores depending on its mode (i.e., pulsed or continuous) and intensity [13–14]. Although the impact of drought on herbivores has been controversial [15–17], quite a few aphid species, such as the Russian wheat aphid [Diuraphis noxia (Kurdjumov)], corn leaf aphid [Rhopalosiphum maidis (Fitch)], greenbug [Schizaphis graminum (Rondani)], and cabbage aphid (Brevicoryne brassicae L.), manifested positive responses under water stressful conditions [18–21]. In addition, decreases in precipitation may affect parasitoids more than their host herbivores because of the cascading effects [22]. Thus, water-deficit stress can contribute to alteration in tritrophic interactions in agricultural ecosystems in the context of global warming [23–25]. However, studies in this respect have been rare and the mechanisms underlying the abovementioned interactions are not well understood.
In northwestern China, the warming trend is particularly evident [2], and this provides an excellent scenario for addressing the abovementioned issues. The wheat aphid, Sitobion avenae (Fabricius), has been shown to cause increasing damage to wheat production in this part of China [26]. This aphid has the ability to adapt rapidly to various unfavorable environments including water-deficit stress [27–32]. The endoparasitoid, Aphidius gifuensis (Ashmead), is one of the most important natural control agent for S. avenae on wheat [33–34]. So, we hypothesize that S. avenae clones from moist, semiarid and arid areas of northwestern China should have differential performance on water-deficit stressed host plants, and this in turn would affect the fitness of the parasitoid (A. gifuensis) under different water-stress treatments. The objectives of our study were to address the tritrophic interactions in the wheat-aphid-parasitoid system. Specifically, we aim to: 1) determine how water-stressed wheat plants affect the development and body size of S. avenae clones from moist, semiarid and arid areas; 2) examine how S. avenae clones of different areas respond to the attack of the parasitoid (A. gifuensis) under water-deficit conditions; and 3) evaluate the performance of A. gifuensis on all developmental stages of S. avenae under three water-stress treatments.
Materials and methods
Colony establishment
Following previous studies [26–27], the areas with a mean annual precipitation up to 200 mm or less, 200–400 mm, and 800 mm or more were considered to be arid, semiarid, and moist, respectively. Sitobion avenae sample collections were made at two locations in each area from April to June 2015 (Fig 1). At least 20 apterous adults were sampled per location. Individuals were collected from wheat plants with a minimum distance of 10 m in order to minimize the probability of sampling identical clones [32]. The aphid samples were genotyped using six microsatellite loci (Sa4Σ, Sm10, Sm12, Sm17, S5L and S17b) [28,35–36]. Six distinct genotypes from each source area were randomly selected for use in the following bioassays. All the selected genotypes were used to establish segregated stock colonies in the laboratory. These genotypes were reared on winter wheat seedlings (Triticum aestivum cv. Aikang 58) planted in plastic pots (7 cm in diameter). These pots contained a mixture of turfy soil, vermiculite, and perlite (4:3:1, v/v/v), and were well covered with transparent plastic tubes (7cm in diameter, 20 cm in height), which had a net on top (60 mesh) for ventilation. A small plastic pan was put at the base of each pot for providing suitable amount of water when needed.
Fig 1. Sampling locations of Sitobion avenae and Aphidius gifuensis (moist area: Longting Town of Yangxian Co., 33°12'43"N, 107°38'30"E; Jinshui Town of Yangxian Co., 33°16'20" N, 107°47'45" E; semiarid area: Yanlian Co., 35°41’29” N, 109°16’13” E; Yaozhou Co., 34° 53’38” N, 108°58’18” E; arid area: Shanglang Town of Minqin Co., 38°35'48" N, 103°06'17" E; Xiaotian Town of Minqin Co., 38°36'40" N, 103°07'18" E; A. gifuensis larvae sampled in Yangling, 34°16’56” N, 108°04’28” E).
Mummies of S. avenae that contained developing A. gifuensis larvae were sampled from local wheat fields in Yangling, Shaanxi Province (Fig 1) in June 2015. This location belongs to semiarid areas according to previous studies [26–27]. No particular permits have been required for insect collecting activities at all the sites mentioned above, and our target insects are not endangered or protected. Cultured stocks of S. avenae and A. gifuensis were kept and maintained in a climate room, controlled at a constant temperature of 21 ± 2°C, a relative humidity of 65 ± 5%, and a photoperiod of 16L: 8D, for 2 or 3 generations before experiments. This could minimize the confounding maternal effect of different source environments [31]. To obtain synchronous cohorts of all developmental stages (same age and size), apterous S. avenae adults from stock colonies were shifted to experimental seedlings, and the offspring produced in 6 h were then kept separately for later use in experiments.
Water level treatments
The experiments were carried out under continuous water-deficit stress. We established three treatments: well-watered, intermediately water-stressed, and severely water-stressed. These water treatments were conducted as detailed previously in [26]. Specifically, we applied 10, 7, and 5 ml of water every 3 d for wheat seedlings (T. aestivum cv. Aikang 58) with 35 g (dry weight) of growing media (i.e., the abovementioned mixture of turfy soil, vermiculite, and perlite) under well-watered, intermediately stressed, and severely stressed treatments, respectively. The resulting intermediate and severe water-deficit stress in the latter two treatments (according to [37]) was monitored by measuring soil water potential using a tensiometer (TEN30, Top Instrument, Hangzhou, China). The soil water potential in well-watered, intermediately stressed and severely stressed treatments was kept at a range of -0.02 to -0.01MPa, -0.035 to -0.02MPa and lower than -0.045MPa, respectively. Water potential was also measured in wheat leaves using the Chardakov method [38]. The corresponding water potential of wheat leaves in the three treatments was kept at a range of 0 to -0.2 Mpa, -0.2 to -0.6 MPa and -0.6 to -0.8 Mpa, respectively [27]. We kept the desirable water conditions in pots of wheat seedlings by measuring soil water potential and weighing the plants with the growing media twice a week.
Developmental time and body size of S. avenae clones
Data on developmental time and body size were collected as described previously in [31–32]. Briefly, 7–8 d old seedlings (the same wheat cultivar mentioned above) were inoculated with aphid individuals (one per plant) from rearing plants. Each seedling with a single aphid individual was covered with the abovementioned plastic tubes, and kept in a growth chamber with the following conditions: 20 ± 1°C (temperature), 65 ± 5% (relative humidity), and 16L: 8D (photoperiod). Six clones were randomly selected from aphid colonies of each area (i.e., arid, semi-arid and moist) for use in the bioassays, and the experiment was replicated three to four times for each clone. We monitored aphid individuals in the bioassays twice daily from birth until the emergence of adults, and recorded the timing of molts. Seven to nine days after the initiation of the experiment, newly emerged apterous adults of S. avenae clones from each area were used to measure the body length (μm) from the front of head to the end of abdomen (cornicles were excluded). The measurement was conducted under a dissecting microscope (Motic K-400L, Motic China Group Co. Ltd., Xiamen, China) using the software of Motic Images Advanced (version 3.2).
Preference of A. gifuensis
For this test, mated A. gifuensis females (1–2 d old) were transferred to pots of wheat seedlings. Each pot had a 7–8 d old wheat plant with well settled 20 aphids of each clone. A period of t10–15 min was needed for the aphids to settle on the plant. Each developmental stage of S. avenae was tested separately. We chose to use plastic pots containing wheat seedlings and aphid individuals for the experiment instead of petri dishes in order to mimic natural conditions. After its introduction into the cage, A. gifuensis was allowed to acclimate to the cage environment for 5 min. After that, we recorded the frequency of contacts between female A. gifuensis and the plant (i.e., the landing of A. gifuensis on the plant surface) for 10 min, and the number of aphid individuals attacked by A. gifuensis in the same time span. This experiment was replicated four times for each clone of the aphid.
Parasitoid attack and aphid resistance
Using similar settings in the abovementioned test, the number of attacks on single adult aphid (apterous) individuals by A. gifuensis in 30 min was recorded. In this test, one mated A. gifuensis female (1–2 d old) was released onto a 7–8 d old wheat seedling caged with one newly emerged apterous aphid adult, and allowed to acclimate for 5 min. The number of times for a female A. gifuensis’s probing with its ovipositor was considered as the number of attacks on the aphid. For the test on aphid resistance behaviors, the system was monitored for at least 10 min after allowing the parasitoid to acclimate for 5 min. It took the parasitoid at least 10 min to find the aphid on a wheat plant. Upon the encounter between them, the aphid chose to back, kick or drop from the plant. We then recorded the aphid resistance behavior for the first encounter between the parasitoid and the aphid. Both experiments were replicated four times for each aphid clone.
Parasitism
This experiment was designed to examine the potential parasitism by A. gifuensis against all aphid developmental stages of S. avenae from areas of different drought levels. To ensure the occurrence of mating, newly emerged pairs (1 male and 1 female) of A. gifuensis were transferred to a gelatin capsule with 10% honey solution, and kept there for 24 h [39]. On the other hand, 20 aphid nymphs or adults of the same age were introduced to the experimental wheat seedling (one or two-leaf plant) which was planted in a plastic pot as described above. Upon the settlement of aphid individuals on wheat seedlings, a mated pair of A. gifuensis was introduced into the plastic cage, and kept there for 6 h. After that, aphid individuals were maintained therein for up to 10–15 d (from the day of parasitoid exposure) under the same environmental conditions. They were examined carefully every 12 h from day 7, and mummified aphid individuals were isolated from plants and placed in petri dishes. Numbers of mummies and the dates of emergence were recorded. All aphid developmental stages were tested, and the test was replicated four times for each aphid clone.
Statistical analyses
Nested analyses of variance (nested ANOVAs) in SAS [40] were used to identify the effects of source areas, water treatments, and their interactions for the following parameters: the developmental time of 1st to 4th instar nymphs (DT1 to DT4) of S. avenae, the developmental time of the entire nymphal stage (DT5) for S. avenae, body size of S. avenae, developmental time of A. gifuensis, frequency of A. gifuensis attacks, number of S. avenae individuals attacked, and parasitism rates. If overall variation in ANOVAs was significant, mean separations were conducted using Tukey tests at α = 0.05. We used log-transformation of data when necessary to meet the requirements of normality and homoscedasticity in ANOVAs. Aphid resistance behaviors (i.e., backing, kicking and dropping), as well as the plant contact behavior of A. gifuensis, were analyzed with logistical regressions using the PROC LOGISTIC procedure in SAS [40].
Results
Developmental time and body size of S. avenae
Clones of S. avenae from arid areas tended to have longer developmental times compared to those from semiarid or moist areas (Table 1). Under the well-watered treatment, DT2 for arid area clones was longer than that for semiarid or moist area clones (F = 8.72; df = 2, 153; P < 0.001). Under well-watered and intermediately stressed conditions, arid area clones showed longer DT3 (F = 12.18; df = 2, 153; P < 0.001) and DT5 (F = 14.64; df = 2, 153; P < 0.001) than semiarid or moist area clones.
Table 1. Developmental times (SE) of four instars and the entire nymphal stage for Sitobion avenae clones from arid, semiarid and moist areas under three water treatments.
| Source | Treatment | DT1 | DT2 | DT3 | DT4 | DT5 |
|---|---|---|---|---|---|---|
| Arid area | Well-watered | 1.9AB (0.1) | 1.8AB (0.1) | 1.9ABC (0.2) | 2.1AB (0.1) | 7.8AB (0.2) |
| Intermediately stressed | 2.0AB (0.2) | 2.0A (0.2) | 2.1AB (0.2) | 2.1AB (0.1) | 8.1A (0.1) | |
| Severely stressed | 1.7B (0.1) | 2.1A (0.1) | 2.3A (0.2) | 2.0ABC (0.1) | 8.1A (0.1) | |
| Semiarid area | Well-watered | 2.0AB (0.1) | 1.3C (0.1) | 1.4D (0.1) | 2.3A (0.1) | 7.0C (0.2) |
| Intermediately stressed | 2.2AB (0.1) | 1.8AB (0.1) | 1.6CD (0.2) | 1.7C (0.1) | 7.3BC (0.2) | |
| Severely stressed | 1.9AB (0.2) | 1.8AB (0.1) | 2.1AB (0.1) | 1.9BC (0.2) | 7.7AB (0.2) | |
| Moist area | Well-watered | 2.0AB (0.1) | 1.3C (0.1) | 1.4D (0.1) | 2.1AB (0.1) | 6.8C (0.2) |
| Intermediately stressed | 1.8AB (0.1) | 1.8AB (0.1) | 1.4D (0.1) | 1.9BC (0.1) | 7.0C (0.2) | |
| Severely stressed | 2.3A (0.1) | 1.6BC (0.1) | 1.8BCD (0.1) | 2.2AB (0.1) | 7.9AB (0.3) |
Note: DT1 to DT4, developmental time of 1st to 4th instar; DT5, developmental time of the entire nymphal stage; n = 18; different letters after data within a column indicate significant differences among treatments at the P < 0.05 level, nested ANOVA followed by Tukey tests.
The developmental times for S. avenae clones tended to rise with increasing water-deficit levels in water treatments. This pattern was most evident in DT2 (F = 7.45; df = 2, 153; P < 0.001), DT3 (F = 9.02; df = 2, 153; P < 0.001) and DT5 (F = 9.35; df = 2, 153; P < 0.001) for semiarid area clones. This pattern was also identified in DT2 and DT5 for moist area clones. However, there were no significant differences in DT1-DT5 among water treatments for arid area clones.
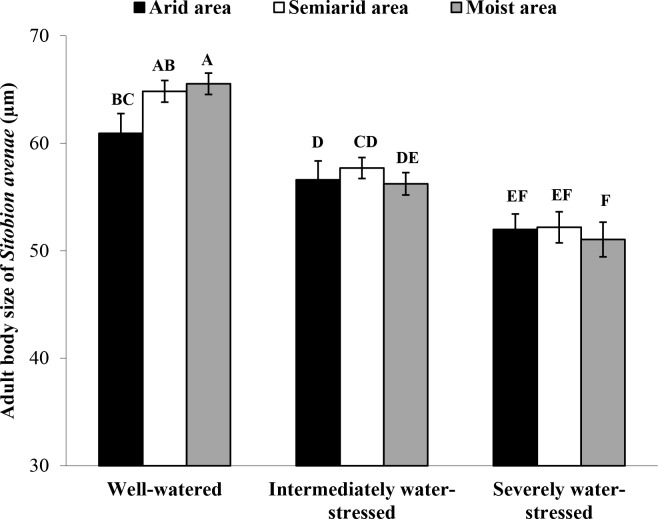
No significant differences in adult body size of S. avenae were identified among source areas under all water treatments but the well-watered treatment, where the body size of moist area clones was higher than that of arid area clones (Fig 2; F = 17.93; df = 2, 153; P < 0.001). For each source area, body size of S. avenae decreased with increasing levels of water-deficit in water treatments (F = 57.02; df = 2, 153; P < 0.001).
Fig 2. Comparison of body sizes of newly emerged adults of Sitobion avenae clones from arid, semiarid and moist areas under three water treatments (n = 18; different letters on bars indicate significant differences among treatments at the P < 0.05 level, nested ANOVA followed by Tukey tests).
Plant contact behavior of A. gifuensis
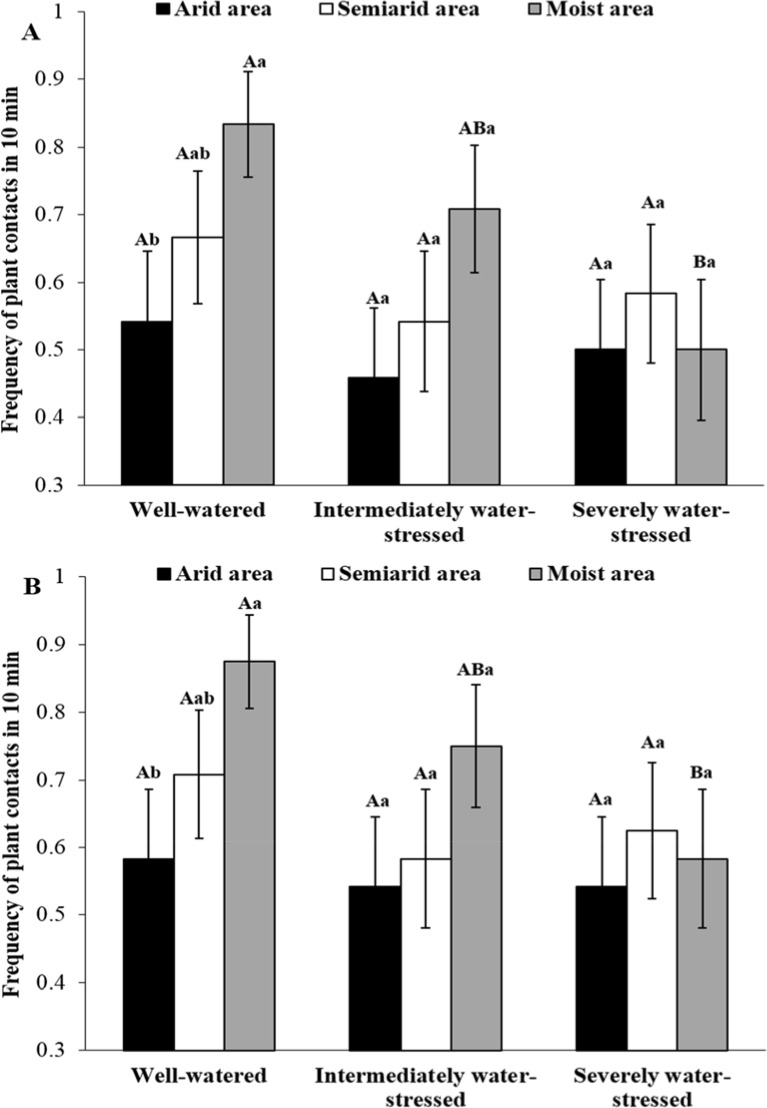
Under the well-watered treatment, moist area clones showed higher frequency of A. gifuensis plant contacts than arid area clones when using the 4th nymphal instar of S. avenae (Fig 3A; logistic regression: χ2 = 4.45, P < 0.05). Moist area clones had a higher frequency of A. gifuensis plant contacts under the well-watered treatment than under the severely stressed treatment (logistic regression: χ2 = 5.55, P < 0.05). In all the other cases, source area and water stress did not influence the frequency of plant contact behavior for the parasitoid. The same pattern in the A. gifuensis plant contact behavior was found when plants were inoculated with S. avenae adults instead of 4th instar nymphs (Fig 3B).
Fig 3.
Mean frequencies (/10 min) of Aphidius gifuensis contacts with plants carrying 4th intar (A) and adult (B) individuals of Sitobion avenae under three water treatments (n = 24; different uppercase and lowercase letters on bars indicate significant differences among water treatments within an area and among areas within a water treatment, respectively; logistic regression, P < 0.05).
Number of A. gifuensis attacks and aphid individuals attacked
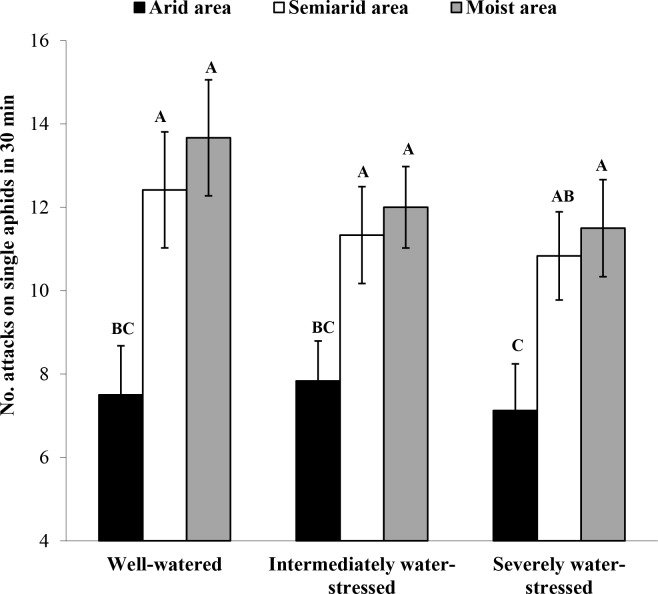
The frequency of A. gifuensis attacks (i.e., probes /30 min) on arid area clones of S. avenae was significantly lower than that on semiarid or moist area clones under all the three water treatments (Fig 4; F = 15.14; df = 2, 207; P < 0.001). There were no significant differences among the three water treatments in the number of A. gifuensis attacks on S. avenae clones from any area.
Fig 4. Number of Aphidius gifuensis attacks in 30 min on apterous adults of Sitobion avenae clones from arid, semiarid and moist areas under three water treatments (n = 24; different letters on bars indicate significant differences among treatments at the P < 0.05 level, nested ANOVA followed by Tukey tests).
No significant differences in the numbers of S. avenae individuals attacked by A. gifuensis in 10 min were found among source areas or among water treatments except 3rd nymphal instar to the adult stage of moist area clones, in which the number of attacks was higher under well-watered conditions compared to severe water stress, with intermediate values for intermediate water stress (Table 2; 3rd instar: F = 3.80; df = 2, 207; P < 0.05; 4th instar: F = 5.47; df = 2, 207; P < 0.01; adult: F = 7.07; df = 2, 207; P < 0.01).
Table 2. Numbers (SE) of aphid individuals attacked in 10 minutes by Aphidius gifuensis for different developmental stages of Sitobion avenae under three water treatments.
| Source | Treatment | 1st instar | 2nd instar | 3rd instar | 4th instar | Adult |
|---|---|---|---|---|---|---|
| Arid area | Well-watered | 1.2A (0.3) | 1.6A (0.4) | 1.7ABC (0.4) | 2.0BCD (0.4) | 2.3BCD (0.5) |
| Intermediately stressed | 1.0A (0.3) | 1.1A (0.4) | 1.5BC (0.4) | 1.5D (0.4) | 1.6D (0.3) | |
| Severely stressed | 1.1A (0.3) | 1.5A (0.4) | 1.4BC (0.4) | 1.7CD (0.4) | 1.8CD (0.4) | |
| Semiarid area | Well-watered | 1.4A (0.3) | 1.7A (0.4) | 2.4AB (0.4) | 2.8ABC (0.4) | 3.4AB (0.5) |
| Intermediately stressed | 1.0A (0.3) | 1.4A (0.4) | 1.5BC (0.4) | 1.8CD (0.4) | 2.0CD (0.4) | |
| Severely stressed | 1.2A (0.3) | 1.5A (0.4) | 2.1ABC (0.4) | 2.3BCD (0.4) | 2.4BCD (0.4) | |
| Moist area | Well-watered | 1.1A (0.3) | 1.9A (0.4) | 2.7A (0.4) | 3.7A (0.4) | 4.2A (0.5) |
| Intermediately stressed | 1.0A (0.3) | 1.4A (0.4) | 1.4BC (0.4) | 3.0AB (0.5) | 2.9BC (0.4) | |
| Severely stressed | 0.8A (0.3) | 0.9A (0.3) | 1.2C (0.4) | 1.8CD (0.4) | 2.3BCD (0.4) |
Note: n = 24; different letters after data within a column indicate significant differences among treatments at the P < 0.05 level, nested ANOVA followed by Tukey tests.
Resistance behaviors of S. avenae confronting a parasitoid
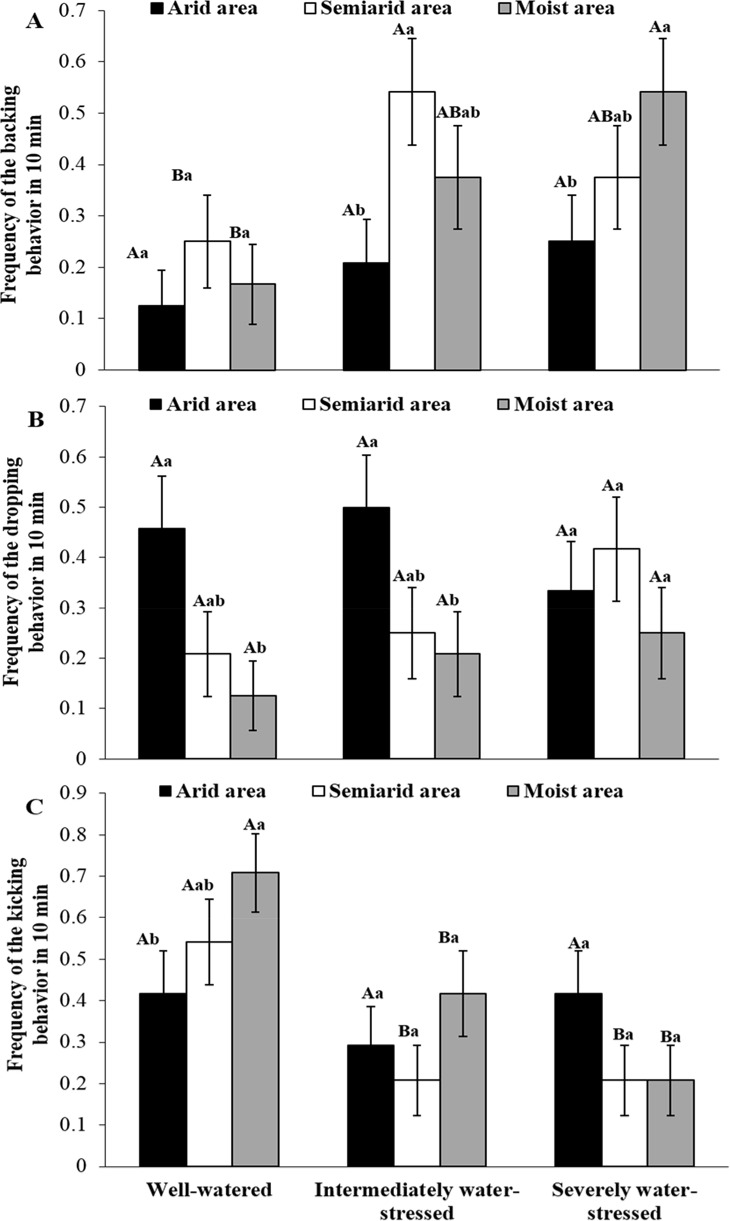
The frequency of backing for arid area clones of S. avenae was lower than that for semiarid area clones under the intermediately stressed treatment (Fig 5; logistic regression: χ2 = 5.37, P < 0.05), and it was also lower than that for moist area clones under the severely stressed treatment (logistic regression: χ2 = 4.11, P < 0.05). Semiarid (intermediate water stress, logistic regression: χ2 = 4.11, P < 0.05) and moist (severe water stress, logistic regression: χ2 = 6.75, P < 0.01) area clones backed more often under water-stressed conditions than under well-watered conditions.
Fig 5.
Mean frequencies of behavioral resistance against Aphidius gifuensis for adults of Sitobion avenae clones from arid, semiarid and moist areas under three water treatments (A, backing; B, dropping; C, kicking; n = 24; different uppercase and lowercase letters on bars indicate significant differences among water treatments within an area and among areas within a water treatment, respectively; logistic regression, P < 0.05).
Arid area clones dropped more often than moist area clones under all water treatments (well-watered: logistic regression, χ2 = 5.46, P < 0.05; intermediate stress: logistic regression, χ2 = 4.25, P < 0.05) except severe water stress for which S. avenae clones of the three areas showed similar frequency of dropping. No significant differences in frequency of dropping for S. avenae clones from any area were found among water treatments.
Under the well-watered treatment, arid area clones of S. avenae had a lower frequency of kicking than moist area clones (logistic regression: χ2 = 4.01, P < 0.05). Semiarid area clones had a higher frequency of kicking under the well-watered treatment than under the other two water treatments (intermediately stressed: logistic regression, χ2 = 5.37, P < 0.05; severely stressed: logistic regression, χ2 = 5.36, P < 0.05). Moist area clones were also found to have a higher frequency of kicking under well-watered conditions than under the other two water treatments (intermediately stressed: logistic regression, χ2 = 4.01, P < 0.05; severely stressed: logistic regression, χ2 = 10.87, P < 0.001).
Overall, under well-watered conditions, the percentage of backing, dropping and kicking for S. avenae clones (three areas combined) was 18.1%, 26.4% and 55.5%, respectively. The respective percentage of backing, dropping and kicking was 37.5%, 31.9% and 30.6% under intermediate water stress, and it was 38.9%, 33.3% and 27.8% under severe water stress.
Parasitism
Arid area clones tended to have lower parasitism rates in comparison to S. avenae clones of the other two areas. Under all three water treatments, 1st instar nymphs of arid area clones showed a lower parasitism rate than those of semiarid or moist area clones (Table 3; F = 59.06; df = 2, 207; P < 0.001); a similar pattern was found for 2nd instar nymphs (F = 17.10; df = 2, 207; P < 0.001); for 3rd instar nymphs, arid area clones showed a lower parasitism rate than moist area clones under well-watered or severely stressed conditions (F = 8.13; df = 2, 207; P < 0.001). Adults of S. avenae from areas of higher drought levels tended to have lower parasitism rates under all three water treatments (F = 31.19; df = 2, 207; P < 0.001).
Table 3. Parasitism rates (SE) of Aphidius gifuensis on all developmental stages of Sitobion avenae clones from arid, semiarid and moist areas under three water treatments.
| Source | Treatment | 1st instar | 2nd instar | 3rd instar | 4th instar | Adult |
|---|---|---|---|---|---|---|
| Arid area | Well-watered | 46.5CD (0.6) | 62.2CD (0.8) | 64.1CD (1.2) | 46.2BC (0.8) | 42.2B (0.7) |
| Intermediately stressed | 43.2E (0.7) | 59.2E (0.8) | 64.2BCD (0.7) | 42.9D (0.6) | 35.8D (0.7) | |
| Severely stressed | 39.4F (0.7) | 55.0F (1.2) | 56.8F (1.6) | 39.6E (1.1) | 31.9E (1.2) | |
| Semiarid area | Well-watered | 51.6A (0.6) | 65.5AB (1.0) | 63.9CD (1.0) | 47.6AB (0.9) | 45.5A (1.1) |
| Intermediately stressed | 47.9BC (0.8) | 63.7BC (0.7) | 67.9A (0.7) | 48.1AB (0.7) | 39.6BC (0.8) | |
| Severely stressed | 44.8DE (0.9) | 58.0EF (1.4) | 59.5EF (1.3) | 41.7DE (1.1) | 37.3CD (1.1) | |
| Moist area | Well-watered | 53.1A (0.7) | 66.8A (0.8) | 67.2AB (0.9) | 49.5A (1.0) | 46.0A (1.0) |
| Intermediately stressed | 49.0B (0.8) | 63.9BC (0.8) | 66.5ABC (0.7) | 47.9AB (0.8) | 41.7B (0.7) | |
| Severely stressed | 46.2CD (1.0) | 59.9DE (1.5) | 62.0DE (1.3) | 43.4CD (1.1) | 40.6B (1.2) |
Note: n = 24; different letters after data within a column indicate significant differences among treatments at the P < 0.05 level, nested ANOVA followed by Tukey tests.
The parasitism rate of S. avenae clones showed a tendency to decrease with increasing water-deficit extents in water treatments. The parasitism rate of 1st instar nymphs for S. avenae clones from any area decreased significantly with increasing water-deficit levels (F = 63.25; df = 2, 207; P < 0.001); third instar nymphs of arid area clones had a lower parasitism rate under severely stressed conditions than under well-watered or intermediately stressed conditions (F = 33.86; df = 2, 207; P < 0.001); the parasitism rate of 4th instar nymphs of arid area clones declined significantly with increasing water-deficit levels (F = 37.06; df = 2, 207; P < 0.001); and S. avenae adults of any area tended to have lower parasitism rates under higher levels of water-deficit (F = 52.64; df = 2, 207; P < 0.001).
Developmental time of A. gifuensis
Under well-watered conditions, the developmental time of A. gifuensis on arid area clones of S. avenae was longer than that on semiarid or moist area clones for any developmental stage of the aphid (Table 4; 1st instar: F = 33.60; df = 2, 207; P < 0.001; 2nd instar: F = 38.58; df = 2, 207; P < 0.001; 3rd instar: F = 36.58; df = 2, 207; P < 0.001; 4th instar: F = 27.51; df = 2, 207; P < 0.001; adult: F = 32.61; df = 2, 207; P < 0.001). Under intermediately stressed conditions, the developmental time of A. gifuensis on S. avenae of all stages increased with increasing levels of water-deficit in source areas. A similar pattern was found under severely stressed conditions.
Table 4. Comparison of developmental times (SE) of Aphidius gifuensis on all developmental stages of Sitobion avenae clones from arid, semiarid and moist areas under three water treatments.
| Source | Treatment | 1st instar | 2nd instar | 3rd instar | 4th instar | Adult |
|---|---|---|---|---|---|---|
| Arid area | Well-watered | 10.9BC (0.2) | 11.1B (0.2) | 11.3B (0.2) | 11.4B (0.2) | 12.3BC (0.1) |
| Intermediately stressed | 11.4A (0.2) | 11.9A (0.2) | 12.0A (0.2) | 11.5B (0.2) | 12.7B (0.2) | |
| Severely stressed | 11.6A (0.1) | 11.7A (0.1) | 11.9A (0.1) | 12.0A (0.1) | 13.2A (0.1) | |
| Semiarid area | Well-watered | 10.2DE (0.1) | 10.4C (0.1) | 10.6C (0.1) | 10.8C (0.1) | 11.8DE (0.1) |
| Intermediately stressed | 10.6CD (0.1) | 11.0B (0.2) | 11.3B (0.2) | 10.9C (0.2) | 12.0CD (0.1) | |
| Severely stressed | 11.2AB (0.1) | 11.6A (0.1) | 11.6AB (0.1) | 11.7AB (0.1) | 12.4B (0.2) | |
| Moist area | Well-watered | 10.1E (0.2) | 10.2C (0.1) | 10.4C (0.1) | 10.6CD (0.1) | 11.4EF (0.2) |
| Intermediately stressed | 10.0E (0.2) | 10.4C (0.2) | 10.5C (0.1) | 10.2D (0.2) | 11.3F (0.2) | |
| Severely stressed | 10.9BC (0.1) | 11.0B (0.2) | 11.3B (0.1) | 11.5B (0.1) | 12.5B (0.1) |
Note: n = 24; different letters after data within a column indicate significant differences among treatments at the P < 0.05 level, nested ANOVA followed by Tukey tests.
When using 1st to 3rd instar nymphs of S. avenae (1st instar: F = 25.54; df = 2, 207; P < 0.001; 2nd instar: F = 27.21; df = 2, 207; P < 0.001; 3rd instar: F = 27.00; df = 2, 207; P < 0.001), the developmental rate of A. gifuensis was higher under well-watered conditions than under intermediately or severely stressed conditions. For 4th instar nymphs (F = 34.78; df = 2, 207; P < 0.001) and adults (F = 28.61; df = 2, 207; P < 0.001) of S. avenae clones from arid areas, the developmental time of A. gifuensis was longer under severely stressed conditions than under well-watered or intermediately stressed conditions. A similar pattern was found for semiarid or moist area clones. The parasitoid’s development on 2nd and 3rd instar nymphs of semiarid area clones was slower under the intermediately stressed treatment than under the well-watered treatment.
Discussion
In the context of global warming, increasing intensity and frequency of drought events can have significant impacts on herbivores like aphids in agricultural systems. In our study, the developmental time of S. avenae clones from source areas (i.e., moist, semiarid and arid) tended to be prolonged on wheat plants under increasing levels of water-deficit (i.e., well-watered, intermediately stressed, and severely stressed). Arid area clones of S. avenae had smaller body sizes than moist area clones under well-watered conditions, and S. avenae clones from all areas had declining body sizes on wheat plants growing under increasing water-deficit levels. However, it has been reported that levels of proteins and amino acids can be increased in leaf tissues of plants under drought, which may result in faster development and bigger body size of aphids [13,41]. One explanation for our results is that drought can enhance mesophyll or phloem resistance (probably due to changes in phloem sap viscosity and solute concentrations), and thus increase the difficulty for aphids to get substantial amount of nutrients [42–43]. In addition, alterations in the water potential of host plants under drought may undermine the ability of aphids to consume xylem sap [44]. These changes of 1st trophic level could have caused the abovementioned bottom-up effects on the 2nd trophic level aphid. Our previous studies showed that S. avenae clones from arid areas had a relatively low fecundity, and that their adaptation potential was positively correlated with their body size [26–27]. Thus, S. avenae clones under drought can have not only lower fitness, but also lower adaptation potential, showing negative effects of water-deficit stressed plants on this aphid.
The survival and fitness of S. avenae under drought can also be affected by top-down effects of natural enemies. In our study, in terms of its plant contact behavior, the parasitoid (A. gifuensis) preferred S. avenae clones of moist areas over those of arid areas under all water treatments except severe water stress. Similarly, the number of attacks (/30 min) on moist area aphid clones by A. gifuensis was much higher than that on arid area clones under all three water treatments. Since semiochemicals from aphids and their habitats can provide cues for parasitoids to locate target prey [45–46], certain cues from moist area clones of S. avenae might be more concentrated (or attractive) for the parasitoid (A. gefuensis) than those from arid area clones. In this study, the parasitoid also preferred to attack S. avenae clones under well-watered conditions over those under water-deficit stress. This suggests that plants carrying S. avenae individuals may emit more attractive semiochemicals under well-watered conditions than those under water-deficit conditions [47]. Subsequently, arid area clones of S. avenae showed lower parasitism rates by A. gifuensis than moist area clones, and the parasitism rate for any developmental stage of S. avenae clones from different areas tended to decrease with increasing water-deficit extents in water treatments. An alternative explanation for lower parasitism of S. avenae clones under water-deficit conditions is that A. gifuensis can prefer to choose larger prey individuals in order to maximize its fitness. This makes sense since large prey can represent quality hosts (larger body size means more nutrition and space) for the parasitoid [48–49]. In addition, lower parasitism rates of A. gifuensis under drought can be related to aphid resistance behaviors (e.g., kicking, backing and dropping). In this study, relative to arid area clones, moist area clones of S. avenae had a higher frequency of backing under severely stressed conditions only, but a higher frequency of kicking under well-watered conditions only. The water-deficit level dependent pattern of S. avenae’s resistance against parasitoids could be important for the survival of this aphid under different drought conditions. Endosymbionts (e.g., Hamiltonella defensa) could also be important in successful defense against A. gifuensis for S. avenae clones under drought [50]. Further studies are needed to determine how endosymbionts of this aphid can contribute to the abovementioned differential performances of S. avenae clones confronting the parasitoid.
In addition to the abovementioned factors on the aphid side, the top-down effects of A. gifuensis under drought are highly dependent on the factors of the parasitoid side (e.g., development). In this study, the development of A. gifuensis on arid area clones of S. avenae (all developmental stages) was slower than that on moist area clones under all water treatments. Similarly, under intermediate water stress, A. gifuensis’s developmental rates on S. avenae of all stages decreased with increasing levels of water-deficit in source areas (i.e., moist, semiarid and arid). The developmental time of the parasitoid on all stages of S. avenae clones also tended to increase with increasing levels of water-deficit in water treatments. Such results suggest that the fitness of A. gifuensis could be decreased when preying on S. avenae clones of any origin under water-deficit conditions. This could further compromise its preying effects on S. avenae under increasing drought conditions. Therefore, the top-down effects of natural enemies (e.g., A. gifuensis), but not the bottom-up effects of the water-stressed first trophic level, can partly explain the phenomenon that increasing events of S. avenae outbreaks on cereal crops coincide with the climate change trend in drought-inflicted northwestern China [51–52].
Overall, our results are consistent with the finding that drought conditions (or water-deficit stress) have significant impacts on insect pests and their natural enemies [5,53]. It is evident from our study that the development of S. avenae can be slowed and its body size can be reduced under drought, showing negative bottom-up effects of water-deficit stressed plants. However, the performance of the parasitoid (A. gifuensis) is also negatively affected by water-deficit stress, suggesting that the top-town effects via parasitoids can be compromised by drought. Thus, the water-deficit-stressed first trophic level can have indirect and negative impacts on the performance of the third trophic level parasitoids. This suggests that parasitoids can be more sensitive to future warming scenarios than other trophic levels [5,53], and that their vulnerability can be potentially increased in future warming scenarios. The rapid adaptation of aphids to water-deficit stress, as well as wide-spread occurrences of their local adaptation, have been demonstrated in some studies [26,28–29,54–55]. This could push parasitoids of aphids to an increasingly vulnerable status in the face of the climate change through their interactions shown in this study. In order to enhance our understanding of how water-deficit stress affects tritrophic (e.g., plant-insect pest-natural enemy) interactions, further studies should be conducted to examine the ecological and evolutionary consequences in field conditions as well as the underlying mechanisms from a perspective of molecular ecology.
Acknowledgments
We would like to thank P. Dai (Northwest A&F University, China) for laboratory and field assistance. This research was supported by the National Natural Science Foundation of China (No. 31572002), and a grant from Northwest A&F University (No. QN2011059).
Data Availability
All relevant data are within the paper.
Funding Statement
This work was supported by the National Natural Science Foundation of China (No. 31572002), received by D.L. (https://isisn.nsfc.gov.cn), also supported by a grant from Northwest A&F University (No. QN2011059), received by D.L. (http://www.nwsuaf.edu.cn). The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1.IPCC. Climate Change 2014: Synthesis Report. Contribution of Working Groups I, II and III to the Fifth Assessment Report of the Intergovernmental Panel on Climate Change [Core Writing Team, R.K. Pachauri and L.A. Meyer (eds.)]. IPCC, Geneva, Switzerland. 2014; p1-20.
- 2.Anonymous. Information office of the state council of the People’s Republic of China: China’s policies and actions for addressing climate change. 2008. (http://www.gov.cn/english/2008-10/29/content_1134544.htm) (accessed Jul. 27, 2017).
- 3.Ainsworth EA, Rogers A. The response of photosynthesis and stomatal conductance to rising CO2: mechanisms and environmental interactions. Plant Cell Environ. 2007; 30: 258–270. doi: 10.1111/j.1365-3040.2007.01641.x [DOI] [PubMed] [Google Scholar]
- 4.Murray TJ, Ellsworth DS, Tissue DT, Riegler M. Interactive direct and plant-mediated effects of elevated atmospheric CO2 and temperature on a eucalypt-feeding insect herbivore. Glob Chang Biol. 2013; 19: 1407–1416. doi: 10.1111/gcb.12142 [DOI] [PubMed] [Google Scholar]
- 5.Romo CM, Tylianakis JM. Elevated temperature and drought interact to reduce parasitoid effectiveness in suppressing hosts. PLOS ONE. 2013; 8: e58136 doi: 10.1371/journal.pone.0058136 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Munir S, Dosdall LM, O'Donovan JT, Keddie A. Diadegma insulare development is altered by Plutella xylostella reared on water-stressed host plants. J Appl Entomol. 2016; 140: 364–375. [Google Scholar]
- 7.Van Nouhuys S, Lei G. Parasitoid-host metapopulation dynamics: the causes and consequences of phenological asynchrony. J Anim Ecol. 2004; 73: 526–535. [Google Scholar]
- 8.Bezemer TM, Jones TH, Knight KJ. Long-term effects of elevated CO2 and temperature on populations of the peach potato aphid Myzus persicae and its parasitoid Aphidius matricariae. Oecologia. 1998; 116: 128–135. doi: 10.1007/s004420050571 [DOI] [PubMed] [Google Scholar]
- 9.Walther G-R, Post E, Convey P, Menzel A, Parmesan C, Beebee TJC, et al. Ecological responses to recent climate change. Nature. 2002; 416: 389–395. doi: 10.1038/416389a [DOI] [PubMed] [Google Scholar]
- 10.Parmesan C. Ecological and evolutionary responses to recent climate change. Annu Rev Ecol Evol Syst. 2006; 37: 637–669. [Google Scholar]
- 11.Leng G, Tang Q, Rayburg S. Climate change impacts on meteorological, agricultural and hydrological droughts in China. Global Planet Change. 2015; 126: 23–34. [Google Scholar]
- 12.Cannon RJC. The implications of predicted climate change for insect pests in the UK, with emphasis on non-indigenous species. Global Change Biol. 1998; 4: 785–796. [Google Scholar]
- 13.Huberty AF, Denno RF. Plant water stress and its consequences for herbivorous insects: A new synthesis. Ecology. 2004; 85: 1383–1398. [Google Scholar]
- 14.Mody K, Eichenberger D, Dorn S. Stress magnitude matters: different intensities of pulsed water stress produce non-monotonic resistance responses of host plants to insect herbivores. Ecol Entomol. 2009; 34:133–143. [Google Scholar]
- 15.Gutbrodt B, Mody K, Dorn S. Drought changes plant chemistry and causes contrasting responses in lepidopteran herbivores. Oikos. 2011; 120: 1732–1740. [Google Scholar]
- 16.Han P, Lavoir A-V, Le Bot J, Amiens-Desneux E, Desneux N. Nitrogen and water availability to tomato plants triggers bottom-up effects on the leafminer Tuta absoluta. Sci Rep. 2014; 4: 4455 doi: 10.1038/srep04455 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Han P, Desneux N, Michel T, Le Bot J, Seassau A, Wajnberg E, et al. Does plant cultivar difference modify the bottom-up effects of resource limitation on plant-insect herbivore interactions? J Chem Ecol. 2016; 42: 1293–1303. doi: 10.1007/s10886-016-0795-7 [DOI] [PubMed] [Google Scholar]
- 18.Archer TI, Bynum ED Jr, Onken AB, Wendt CW. Influence of water and nitrogen-fertilizer on biology of the Russian wheat aphid (Homoptera: Aphididae) on wheat. Crop Prot. 1995; 14: 165–169. [Google Scholar]
- 19.Li H, Payne WA, Michels GJ, Rush CM. Reducing plant abiotic and biotic stress: Drought and attacks of greenbugs, corn leaf aphids and virus disease in dryland sorghum. Environ Exp Bot. 2008; 63: 305–316. [Google Scholar]
- 20.Dorschner KW, Johnson RC, Eikenbary RD, Ryan JD. Insect-plant interactions: greenbug (Homoptera: Aphididae) disrupt acclimation of winter wheat to drought stress. Environ Entomol. 1986; 15: 118–121. [Google Scholar]
- 21.Burgess AJ, Warrington S, Allen-Williams L. Cabbage aphid (Brevicoryne brassicae L.) performance on oilseed rape (Brassica napus L.) experiencing water deficiency: roles of temperature and food quality. Acta Hortic (ISHS). 1996; 407: 499–506. [Google Scholar]
- 22.Coley PD. Possible effects of climate change on plant / herbivore interactions in moist tropical forests. Climatic Change. 1998; 39: 455–472. [Google Scholar]
- 23.Olson DM, Cortesero AM, Rains GC, Potter T, Lewis WJ. (2009). Nitrogen and water affect direct and indirect plant systemic induced defense in cotton. Biological Control, 49(3), 239–244. [Google Scholar]
- 24.Han P, Dong Y, Lavoir A-V, Adamowicz S, Bearez P, Wajnberg E, et al. Effect of plant nitrogen and water status on the foraging behavior and fitness of an omnivorous arthropod. Ecol. Evol. 2015; 5: 5468–5477. doi: 10.1002/ece3.1788 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Han P, Bearez P, Adamowicz S, Lavoir A-V, Amiens-Desneux E, Desneux N. Nitrogen and water limitations in tomato plants trigger negative bottom-up effects on the omnivorous predator Macrolophus pygmaeus. J Pest Sci. 2015; 88: 685–691. [Google Scholar]
- 26.Dai P, Liu D-G, Shi X-Q. Impacts of water deficiency on life history of Sitobion avenae clones from semi-arid and moist areas. J Econ Entomol. 2015; 108: 2250–2258. doi: 10.1093/jee/tov210 [DOI] [PubMed] [Google Scholar]
- 27.Dai P, Li S-R, Liu D-G, Ahmed SS, Shang Z-M, Shi X-Q. Life-history responses of insect pests to water-deficit stress: a case study on the aphid Sitobion avenae. BMC Ecol. 2017; under review. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Huang X-L, Liu D-G, Wang D, Shi X-Q, Simon J-C. Molecular and quantitative genetic differentiation in Sitobion avenae populations from both sides of the Qinling Mountains. PLOS ONE. 2015; 10: e0122343 doi: 10.1371/journal.pone.0122343 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Huang X-L, Liu D-G, Gao S-X, Chen H. Differential performance of Sitobion avenae populations from both sides of the Qinling Mountains under common garden conditions. Environ Entomol. 2013; 42: 1174–1183. doi: 10.1603/EN13132 [DOI] [PubMed] [Google Scholar]
- 30.Dai X-J, Gao S-X, Liu D- G. Genetic basis and selection for life-history trait plasticity on alternative host plants for the cereal aphid Sitobion avenae. PLOS ONE. 2014; 9: e106179 doi: 10.1371/journal.pone.0106179 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Gao S- X, Liu D- G, Chen H, Meng X- X. Fitness traits and underlying genetic variation related to host plant specialization in the aphid Sitobion avenae. Insect Sci. 2014; 21: 352–362. doi: 10.1111/1744-7917.12085 [DOI] [PubMed] [Google Scholar]
- 32.Gao S-X, Liu D-G. Differential performance of Sitobion avenae clones from wheat and barley with implications for its management through alternative cultural practices. J Econ Entomol. 2013; 106: 1294–1301. [DOI] [PubMed] [Google Scholar]
- 33.Yang S, Wei J, Yang S, Kuang R. Current status and future trends of augmentative release of Aphidius gifuensis for control of Myzus persicae in China's Yunnan Province. J Entomol Res Soc. 2011; 13: 87–99. [Google Scholar]
- 34.Yang S, Xu R, Yang S-Y, Kuang R-P. Olfactory responses of Aphidius gifuensis to odors of host plants and aphid-plant complexes. Insect Sci. 2009; 16: 503–510. [Google Scholar]
- 35.Simon J-C, Baumann S, Sunnucks P, Hebert PDN, Pierre J-S, Le Gallic J-F, et al. Reproductive mode and population genetic structure of the cereal aphid Sitobion avenae studied using phenotypic and microsatellite markers. Mol Ecol. 1999; 8: 531–545. [DOI] [PubMed] [Google Scholar]
- 36.Wilson AC, Massonnet B, Simon JC, Prunier-Leterme N, Dolatti L, Llewellyn KS, et al. Cross-species amplification of microsatellite loci in aphids: assessment and application. Mol Ecol Notes. 2004; 4: 104–109. [Google Scholar]
- 37.Hsiao TC. Plant responses to water stress. Ann Rev Plant Physiol. 1973; 24: 519–570. [Google Scholar]
- 38.Vickers L. Aphid responses to drought: a combined physiological and transcriptomic approach. Dissertation, University of Birmingham, Birmingham, United Kingdom; 2011.
- 39.Pan M-Z, Liu T- X. Suitability of three aphid species for Aphidius gifuensis, (Hymenoptera: Braconidae): Parasitoid performance varies with hosts of origin. Biol Control. 2014; 69: 90–96. [Google Scholar]
- 40.SAS. SAS System, version 9.1.3, SAS Institute Inc., Cary, NC, USA; 2008.
- 41.Johnson SN, Staley JT, Mcleod FAL, Hartley SE. Plant-mediated effects of soil invertebrates and summer drought on above-ground multitrophic interactions. J Ecol. 2011; 99: 57–65. [Google Scholar]
- 42.Guo H, Sun Y, Peng X, Wang Q, Harris M, Ge F. Up-regulation of abscisic acid signaling pathway facilitates aphid xylem absorption and osmoregulation under drought stress. J Exp Bot. 2015; 32: 365–368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Garg BK, Kathju S, Burman U. Influence of Water Stress on Water Relations, Photosynthetic Parameters and Nitrogen Metabolism of Moth Bean Genotypes. Biol Plantarum. 2001; 44: 289–292. [Google Scholar]
- 44.Pompon J, Dan Q, Goyer C, Giordanengo P, Pelletier Y. A phloem-sap feeder mixes phloem and xylem sap to regulate osmotic potential. J Insect Physiol. 2011; 57: 1317–1322. doi: 10.1016/j.jinsphys.2011.06.007 [DOI] [PubMed] [Google Scholar]
- 45.Vinson SB. Parasitoid-host relationship In: Chemical Ecology of Insects (eds Bell W.J. and Cardé R.T.), Chapman and Hall, London, 1984, pp. 205–233. [Google Scholar]
- 46.Wickremasinghe MGV, Emden HFV. Reactions of adult female parasitoids, particularly Aphidius rhopalosiphi, to volatile chemical cues from the host plants of their aphid prey. Physiol Entomol. 1992; 17: 297–304. [Google Scholar]
- 47.Becker C, Desneux N, Monticelli L, Fernandez X, Michel T, Lavoir A-V. Effects of abiotic factors on HIPV-mediated interactions between plants and parasitoids. Biomed Res Int. 2015; 2015: 342982 doi: 10.1155/2015/342982 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Godfray HCJ. Parasitoids: Behavioural and Evolutionary Ecology. Princeton University Press, Princeton, 1994, NJ., p. 473. [Google Scholar]
- 49.Harvey JA. Factors affecting the evolution of development strategies in parasitoid wasps: the importance of functional constraints and incorporating complexity. Entomol Exp Appl. 2005; 117: 1–13. [Google Scholar]
- 50.Oliver KM, Degnan PH, Burke GR, Moran NA. Facultative symbionts in aphids and the horizontal transfer of ecologically important traits. Ann Rev Entomol. 2010; 55: 247–266. [DOI] [PubMed] [Google Scholar]
- 51.Deng Z-Y, Wang Q, Zhang Q, Qing J-Z, Yang Q- G, Yuan Z-P, et al. Impact of climate warming and drying on food crops in northern China and the countermeasures. Acta Ecol Sin. 2010; 30: 6278–6288. [Google Scholar]
- 52.Cao Y-Z, Li K-B, Yin J, Zhang K-C. Occurring dynamics and sustainable management strategies and practices of wheat major insect pests. China Plant Prot. 2006; 26: 11–14. [Google Scholar]
- 53.Voigt W, Perner J, Davis AJ, Eggers T, Schumacher J, Bahrmann R, et al. Trophic levels are differentially sensitive to climate. Ecology. 2003; 84: 2444–2453. [Google Scholar]
- 54.Dai P, Shi X-Q, Liu D-G, Ge Z-H, Wang D, Dai X-J, et al. Life-history trait plasticity and its relationships with plant adaptation and insect fitness: a case study on the aphid Sitobion avenae. Sci Rep. 2016; 6: 29974 doi: 10.1038/srep29974 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Wang D, Shi X-Q, Dai P, Liu D-G, Dai X-J, Shang Z-M, et al. Comparison of fitness traits and their plasticity on multiple plants for Sitobion avenae infected and cured of a secondary endosymbiont. Sci Rep. 2016; 6: 23177 doi: 10.1038/srep23177 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
All relevant data are within the paper.