Abstract
AIMS
To examine the effects of a different number of daily bladder squeezes on bladder dysfunction in mice with spinal cord injury (SCI).
METHODS
Spinal cord was transected at the Th8/9 in female C57BL/6N mice. Their bladders were manually squeezed to eliminate urine inside every day for 4 weeks. The mice were divided into three groups depending on the number of bladder squeezes; A: once daily, B: twice daily, C: three times daily. Four weeks after transection, single-filling cystometry were performed under an awake condition. NGF in the bladder mucosa and mRNA expression of P2X receptors and TRP channels in L6/S1 dorsal root ganglia (DRG) were measured.
RESULTS
Bladder weight in group C was less than that of group A. Bladder capacity, post-void residual, and the number of non-voiding contractions during the storage phase were significantly larger in group A compared to group B or C. The level of NGF in groups C were lower compared to group A or B. The expression of P2X3 and TRPA1 in groups B and C was decreased compared to group A. The expression of P2X2 was decreased in groups B compared to group A.
CONCLUSION
The post-injury bladder management after SCI with an increased number of daily bladder emptying improves the storage and voiding bladder dysfunction associated with the reduction of NGF in the bladder as well as P2X and TRP transcripts in lumbosacral DRG.
Keywords: management, mouse, neurogenic bladder, spinal cord injury
1 | INTRODUCTION
Spinal cord injury (SCI) eliminates the voluntary and supraspinal control of micturition, leading initially to areflexic bladder and urinary retention, and then to the development of neurogenic lower urinary tract dysfunction (LUTD) such as neurogenic detrusor overactivity (DO), inefficient voiding due to detrusor sphincter dyssynergia (DSD), and bladder hypertrophy. Levels of neurotrophins including nerve growth factor (NGF) in the bladder wall are increased and they are taken up by afferent nerves and transported to dorsal root ganglion (DRG) cells where it affects gene expression, leading to increased cell size, modulation of ion channels, and increased neuronal excitability.1–3 Hyperexcitability of bladder afferent pathways has been implicated as a pathophysiological basis of neurogenic DO and DSD after SCI.3,4 It has also been shown that ATP receptors such as P2X2/3 and TRP channels are involved in sensitization of C-fiber bladder afferent pathways after neural injury and tissue inflammation.3
Although many factors contribute to the emergence of LUTD after SCI, bladder overdistention and high storage pressure induced by functional bladder outlet obstruction due to DSD are considered to be involved in the progress of SCI-associated LUTD.3 The bladders of rats or mice with SCI are usually expressed manually two or three times daily to eliminate urine inside the bladder.5–7 In this study, we hypothesized that the post-injury bladder management that reduces bladder overdistention and high pressure storage after SCI could alter the severity of SCI-associated LUTD in the later phase. In addition, we have recently reported that mice with SCI that were treated with twice daily bladder squeezes exhibited DO and DSD that are considerably different from normal LUT function in spinal intact mice.5 We, therefore, examined how a different number of daily bladder squeezes (once to three times daily) affects storage and voiding dysfunction parameters during awake cystometry among SCI mice, and also compared bladder NGF levels and expression of TRP and P2X receptor transcripts in lumbosacral DRG of spinal intact and SCI mice.
2 | MATERIALS AND METHODS
All experimental protocols were conducted in accordance with NIH guidelines and approved by the University of Pittsburgh Institutional Animal Care and Use Committee.
2.1 | Animals
The spinal cord was transected at the level of T8–9 in adult female C57BL/6N mice (N = 30, body weight: 18–22 g). After spinal cord transection, their bladders were manually squeezed to eliminate the urine inside every day for 4 weeks until cystometric evaluation. The SCI mice were divided into three groups (n = 10 per group) depending on the number of bladder squeezes; A: once daily (only early morning around at 7:30 am), B: twice daily (early morning and late evening around at 10:30 pm), C: three times daily (early morning, afternoon around at 3:00 pm and late evening). Animals were housed in standard cages with wood-chip bedding and had free access to food and water. A 12-h light-dark cycle (lights on at 07:00 am) was used throughout. Spinal intact mice were used as controls for analyses of bladder NGF levels and expression of TRP and P2X receptor transcripts in L6/S1 DRG.
2.2 | Cystometric analysis
Four weeks after spinal cord transection, single-filling cystometrograms (CMGs) were recorded under an awake condition. A lower midline abdominal incision was performed and a PE-50 tube (Clay-Adams, Parsippany, NJ) with the end flared by heat was inserted into the bladder from the bladder dome as a cystostomy catheter under 1.5–2.0% isoflurane anesthesia. The midline abdominal incision was then closed. After recovery from anesthesia, the animals were gently restrained in a cage (Economy holder 15–30 g, Torrington, CT) and the cystostomy catheter was connected via a three-way stopcock to a pressure transducer and to a syringe pump. After the animals were recovered from anesthesia completely, CMGs were recorded by filling the bladder with saline (0.01 mL/min) to observe rhythmic and stable bladder contractions for at least 180 min. Then, the bladder was emptied by drainage through the bladder catheter, and saline was infused again to collect the data. We evaluated the number of non-voiding contractions (NVCs; more than 8 cmH2O increases in intravesical pressure above the baseline) during the storage phase, threshold pressure (TP; bladder pressure at the start of detrusor contraction for micturition), maximal micturition pressure (MP), and post-void residual urine (PVR) withdrawn through the bladder catheter by gravity right after voiding. Bladder capacity was calculated from the infusion rate (0.01 mL/min) multiplied by the time to void (minutes) after starting saline infusion, and voided volume (VV) was calculated by subtracting PVR from the calculated bladder capacity. We also calculated % voiding efficiency (VV/bladder capacity × 100). These parameters were evaluated in two serial single-filling CMGs by measuring PVR after each void and then averaged for statistical analyses. These cystometric parameters were compared among three different groups of SCI mice to evaluate the effects of a different number of bladder squeezes on SCI-associated LUTD.
The bladder and DRGs at the level of L6 and S1 were harvested from each SCI mouse after cystometric evaluation and also from normal (spinal intact) mice (n = 7). The whole bladder above the ureteral orifices was dissected out under anesthesia. The removed bladder was opened and separated into mucosal and muscle layers under a microscope after weighing. Four L6 and S1 DRGs from each animal were grouped together for the following analyses. These specimens were immediately frozen in liquid nitrogen and stored at −80°C until further processing.
2.3 | NGF assay (bladder mucosa)
The NGF protein levels in the bladder mucosa were measured by ELISA method according to the manufacturer's instructions using a Mouse NGF/NGF Beta™ ELISA Kit (Boster Co., Ltd., Pleasanton, CA). After the amount of NGF in each bladder mucosa specimen was evaluated, the concentration was calculated as the NGF amount divided by the total protein concentration (µg/mg protein).
2.4 | Real-time RT-PCR (DRG)
Total RNA was isolated using TRIzol reagent (Invitrogen, Carlsbad, CA), according to the manufacturer's instruction. All samples were treated with DNase (Promega, Madison, WI) to prevent the contamination of genomic DNA followed by cleaning up with an RNeasy mini kit (Qiagen, Valencia, CA) or TRIzol reagent. RNA was quantified by a spectrophotometer (Biochrom, Cambridge, UK). One microgram total RNA from DRG tissues was reverse-transcribed using Thermoscript with oligo (dT) primers (Invitrogen) according to the manufacturer's instruction. Real-time PCR was performed in 96-well reaction plates using an MX3000P system (Stratagene, La Jolla, CA). The reaction mixture contained 1 µL diluted cDNA, 25 µL SYBR Green PCR Master Mix (Qiagen). and 0.3M primer pair in the total volume of 25 µL. Details of primers are listed in Supplemental Table S1. These primers were tested in silico for specificity against sequences for mus musculus using BLAST software (National Center for Biotechnology Information, Bethesda, MD) and synthesized by Integrated DNA Technologies (Coralville, IA). The cycle conditions comprised 15-min polymerase activation, 40 cycles with denaturation at 94°C for 30 s, annealing at 55°C for 30 s, and extension at 72°C for 30 s. Fivefold dilution series of cDNA were used to establish standard curves. The ratio of each marker to GAPDH mRNA was used for statistical analyses. Thereafter, the fold increase of each marker expression in SCI groups compared to spinal intact mouse group was calculated using the average values of each group. The GAPDH transcript levels were similar among the different groups.
2.5 | Statistics analysis
Results were presented as mean ± SEM. For statistical comparison of cystometric parameters among SCI groups (A–C), one-way ANOVA with Dunnett post hoc test was used. For statistical analysis of tissue molecular expression, Student's t-test was first used for comparison between the normal spinal intact group and each of SCI groups (A–C), and then one-way ANOVA with Dunnett post hoc test was used for the comparison among SCI groups. P < 0.05 was considered to indicate a statistical significance.
3 | RESULTS
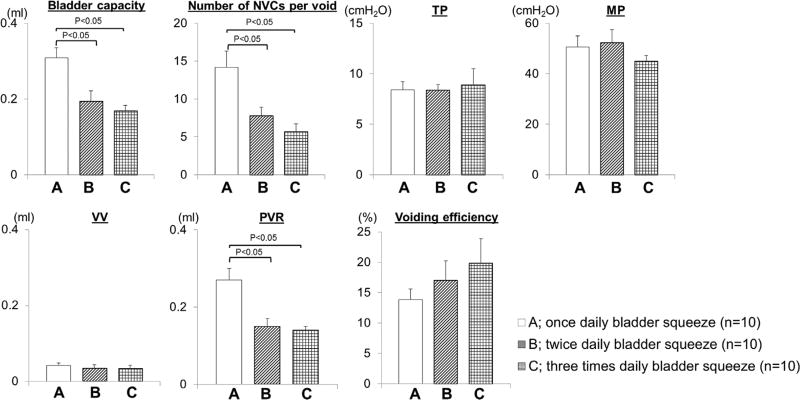
The results of cystometric evaluation are shown in Fig. 1. Bladder capacity, PVR and the number of NVCs during storage were significantly larger in group A (bladder squeezed once daily) compared to group B or C. There were no differences in these parameters between groups B and C. MP, TP, VV, or voiding efficiency were not significantly different among three groups.
FIGURE 1.
Bladder weight and urodynamic evaluation in three groups of SCI mice. The bladder weight of group C was lower than that of group A. Compared to group A, bladder capacity, the number of NVCs and PVR were significantly less in group B and C using one-way ANOVA with Dunnett's post hoc test
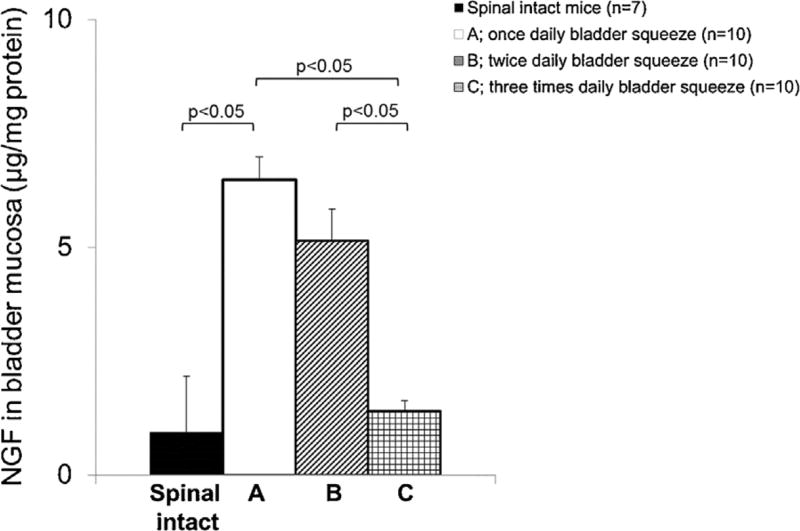
At 4 weeks after SCI, the bladder weight of group C was significantly smaller than that of group A. Levels of NGF protein in the bladder mucosa of SCI mice were significantly higher compared to normal spinal intact mice (Fig. 2) whereas the level of NGF in group C was significantly lower compared to group A or B.
FIGURE 2.
NGF protein concentrations in the bladder mucosa from normal spinal intact and SCI mice. In SCI mice, bladder mucosal NGF was significantly increased compared to normal spinal intact mice, using Student t-test. The level of NGF in groups C were lower compared to group A or B using one-way ANOVA with Dunnett's post hoc test
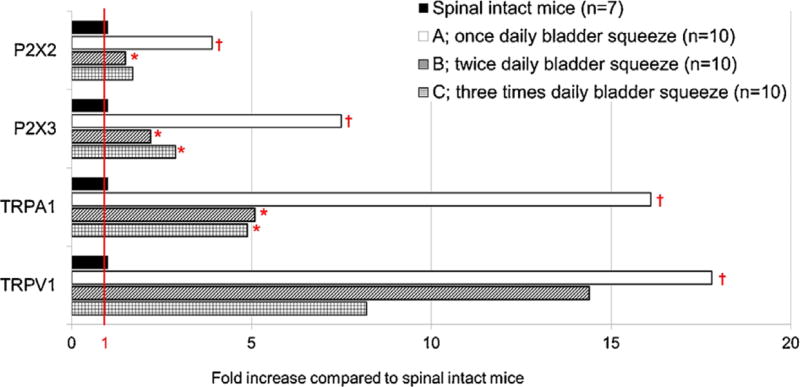
The expression of mRNA (Fig. 3) of P2X2, P2X3, TRPA1, and TRPV1 was increased in all three groups of SCI mice compared to spinal intact mice. However, the expression of P2X3 and TRPA1 in groups B and C were significantly decreased compared to group A. The expression of P2X2 was also significantly decreased in groups B compared to group A. The expression of TRPV1 transcripts was not different among three groups.
FIGURE 3.
The fold increase of mRNA expression in DRGs of SCI mice compared to normal spinal intact mice. P2X2, P2X3, TRPA1, and TRPV1 were overexpressed in SCI mice compared to spinal intact rats (control). Compared to group A, P2X3 and TRPA1 in groups B and C were decreased, and P2X2 was decreased in groups B with statistical significances. The ratio of each marker to GAPDH mRNA was used for statistical analyses. The fold increase of each marker expression was calculated using the average values of each group. *P < 0.05 versus Group A (once daily bladder squeeze) using one-way ANOVA with Dunnett's post hoc test. †P < 0.05 versus normal spinal intact mice using Student t-test
4 | DISCUSSION
The present study demonstrated that the number of daily bladder squeezes after SCI greatly affected storage and voiding dysfunction as well as the level of NGF in the bladder mucosa and the expressions of TRP and P2X receptor transcripts channels of L6-S1 DRGs, which contain afferent neurons innervating the lower urinary tract. Thus, our results indicate that post-injury bladder management strategy is one of important factors to maintain lower urinary tract function in a better condition for neurogenic LUTD due to SCI. Also, researchers should keep in mind that chronic SCI model animals would have different functional and molecular characteristics depending on the post-injury management methods.
After SCI, dyssynergic urethral activity (ie, DSD) is induced in mice as well as in rats and humans, and voiding efficiency of SCI mice is much lower than that of rats according to our previous research,5 in which we showed that SCI mice exhibit smaller VV, larger PVR, and reduced voiding efficiency compared to spinal intact mice because the urethral pumping activity, which contributes to efficient voiding, does not emerge in SCI mice. DSD and decreased voiding efficiency cause continuous elevation of intravesical pressure and bladder overdistention followed by bladder hypertrophy and bladder ischemia, which could finally impair the bladder contractile force.8,9 In SCI, overdistention of the bladder is considered to stimulate increased levels of bladder NGF that is one of chemical mediators of pathology-induced changes in C-fiber afferent nerve excitability and reflex bladder activity.10 In this study, in SCI mice receiving bladder squeezes twice or three times daily, the emergence of NVCs was less compared to SCI mice with once daily of squeezes. Similarly, PVR was significantly less and the voiding efficiency tended to be improved in twice or three times squeezed SCI mice compared to once daily squeezed SCI mice. These results are consistent with the clinical findings that bladder deterioration is better controlled in patients with neurogenic LUTD who are performing intermittent catheterization more frequently.11 It is probably because frequent bladder squeezes have advantage to prevent bladder overdistention and high bladder pressure during storage. As a result, bladder weight (hypertrophy) of SCI mice receiving three times squeezes was less compared to SCI mice with once daily of squeeze, and the levels of NGF in the bladder mucosa were also lower as the number of bladder squeezes was increased. These findings are similarly observed in animal models of bladder outlet obstruction. Ohnishi et al. demonstrated that the prevention of bladder overdistention with intermittent catheterization for rabbits with bladder outlet obstruction reduced the magnitude of the increased bladder mass and improved the impaired contractile responses.12 Taken together, the frequent bladder emptying after SCI can reduce bladder hypertrophy by avoiding bladder overdistention, and decrease the NGF expression in the bladder, resulting in improvements of storage and voiding dysfunctions.
Because it has been demonstrated that NGF is an important mediator inducing C-fiber bladder afferent hyperexcitability that contribute to neurogenic DO and DSD after SCI,2,13,14 it is possible that the increased number of daily bladder squeezes after SCI that reduces NGF expression in the bladder could reduce bladder afferent hyperexcitability, thereby, improving SCI-induced DO and inefficient voiding. Therefore, we investigated the expression of P2X2, P2X3, TRPA1, and TRPV1 transcripts, which are predominantly expressed in C-fiber afferent pathways,15,16 and showed that they were higher in L6/S1 DRGs of SCI mice compared to spinal intact mice. Furthermore, in SCI mice receiving twice or three times squeezes daily, these increased expressions of P2X3 and TRPA1 transcripts in group B and C, and of P2X2 transcripts in group B were reduced with significant differences when compared to group A, suggesting that more frequent bladder emptying after SCI can reduce the C-fiber afferent hyperexcitability underlying LUTD to improve DO and inefficient voiding induced by DSD after SCI.
As SCI leads initially to areflexic bladder and urinary retention, animals after SCI commonly receive bladder squeezes at different times depending on institutions or researchers.5–7 The present study revealed that the number in daily bladder squeezes could affect the post-injury functional characteristics including urodynamic parameters, NGF expression in the bladder and mRNA levels of TRP and P2X receptor transcripts in lumbosacral DRGs even in the same SCI model mice. Therefore, important aspects derived from this study are that researchers should consider an appropriate post-injury management of animals when creating disease models that fit with their research purposes, and that an appropriate post-injury management would also be useful as a strategy of refinement to improve welfare of animal models used in the basic research of LUTD.
5 | CONCLUSION
The post-injury bladder management with increased number of daily bladder emptying improves the storage and voiding LUTD after SCI, and the improvement of SCI-induced LUTD is associated with the decrease of bladder NGF and reductions of C-fiber afferent marker receptors in bladder afferent pathways. Thus, it is assumed that the well-planned initial management strategy would be important for the control of LUTD that develops after the initial areflexic phase of SCI. Also, in the basic research of LUTD, researchers should consider the tailored plan of animal care of chronic disease models after surgical manipulation depending on their intended purposes.
Supplementary Material
Footnotes
POTENTIAL CONFLICTS OF INTEREST
Nothing to disclose.
SUPPORTING INFORMATION
Additional Supporting Information may be found online in the supporting information tab for this article.
References
- 1.Vizzard MA. Neurochemical plasticity and the role of neurotrophic factors in bladder reflex pathways after spinal cord injury. Prog Brain Res. 2006;152:97–115. doi: 10.1016/S0079-6123(05)52007-7. [DOI] [PubMed] [Google Scholar]
- 2.Yoshimura N, Bennett NE, Hayashi Y, et al. Bladder overactivity and hyperexcitability of bladder afferent neurons following intrathecal delivery of nerve growth factor in rats. J Neurosci. 2006;26:10847–10855. doi: 10.1523/JNEUROSCI.3023-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.de Groat WC, Yoshimura N. Plasticity in reflex pathways to the lower urinary tract following spinal cord injury. Exp Neurol. 2012;235:123–132. doi: 10.1016/j.expneurol.2011.05.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Takahashi R, Yoshizawa T, Yunoki T, et al. Hyperexcitability of bladder afferent neurons associated with reduction of Kv 1.4α-subunit in rats with spinal cord injury. J Urol. 2013;190:2296–2304. doi: 10.1016/j.juro.2013.07.058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kadekawa K, Yoshimura N, Majima T, et al. Characterization of bladder and external urethral activity in mice with or without spinal cord injury—a comparison study with rats. Am J Physiol Regul Integr Comp Physiol. 2016;310:R752–R758. doi: 10.1152/ajpregu.00450.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Wilson TS, Aziz KA, Vazques D, et al. Changes in detrusor smooth muscle myosin heavy chain mRNA expression following spinal cord injury in the mouse. Neurourol Urodyn. 2005;24:89–95. doi: 10.1002/nau.20077. [DOI] [PubMed] [Google Scholar]
- 7.Yoshiyama M, deGroat WC, Fraser MO. Influences of external urethral sphincter relaxation induced by alpha-bungarotoxin, a neuromuscular junction blocking agent, on voiding dysfunction in the rat with spinal cord injury. Urology. 2000;55:956–960. doi: 10.1016/s0090-4295(00)00474-x. [DOI] [PubMed] [Google Scholar]
- 8.Azadzoi KM, Pontari M, Vlachiotis J, et al. Canine bladder blood flow and oxygenation: changes induced by filling, contraction and outlet obstruction. J Urol. 1996;155:1459–1465. doi: 10.1016/s0022-5347(01)66307-9. [DOI] [PubMed] [Google Scholar]
- 9.Lin AT, Chen MT, Yang CH, et al. Blood flow of the urinary bladder: effects of outlet obstruction and correlation with bioenergetic metabolism. Neurourol Urodyn. 1995;14:285–292. doi: 10.1002/nau.1930140309. [DOI] [PubMed] [Google Scholar]
- 10.Vizzard MA. Changes in urinary bladder neurotrophic factor mRNA and NGF protein following urinary bladder dysfunction. Exp Neurol. 2000;161:273–284. doi: 10.1006/exnr.1999.7254. [DOI] [PubMed] [Google Scholar]
- 11.Yokoyama O, Hasegawa T, Ishiura Y, et al. Morphological and functional factors predicting bladder deterioration after spinal cord injury. J Urol. 1996;155:271–274. [PubMed] [Google Scholar]
- 12.Ohnishi N, Horan P, Levin SS, et al. Intermittent catheterization limits rabbit bladder dysfunction in response to partial outlet obstruction. J Urol. 2000;163:292–295. [PubMed] [Google Scholar]
- 13.Seki S, Sasaki K, Fraser MO, et al. Immunoneutralization of nerve growth factor in lumbosacral spinal cord reduces detrusor hyperreflexia in spinal cord injured rats. J Urol. 2002;168:2269–2274. doi: 10.1016/S0022-5347(05)64369-8. [DOI] [PubMed] [Google Scholar]
- 14.Seki S, Sasaki K, Igawa Y, et al. Suppression of detrusor-sphincter dyssynergia by immunoneutralization of nerve growth factor in lumbosacral spinal cord in spinal cord injured rats. J Urol. 2004;171:478–482. doi: 10.1097/01.ju.0000088340.26588.74. [DOI] [PubMed] [Google Scholar]
- 15.Zhong Y, Banning AS, Cockayne DA, et al. Bladder and cutaneous sensory neurons of the rat express different functional P2X receptors. Neuroscience. 2003;120:667–675. doi: 10.1016/s0306-4522(03)00243-4. [DOI] [PubMed] [Google Scholar]
- 16.Julius D. TRP channels and pain. Annu Rev Cell Dev Biol. 2013;29:355–384. doi: 10.1146/annurev-cellbio-101011-155833. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.