Abstract
Aims
To characterize the histomorphological features of endometrial carcinomas (ECs) harbouring polymerase ε (POLE) mutations.
Methods and results
Forty-three ECs with POLE mutations were compared with a cohort of 202 ECs. Most POLE-mutated ECs were endometrioid [34/43 (79%]; the remaining tumours were mixed [6/43 (14%)], serous [2/43 (5%)], and clear cell [1/43 (2%)]. The endometrioid carcinomas were predominantly International Federation of Gynecology and Obstetrics grade 3 (27/43, 63%). The histotype distribution did not differ from that of control ECs (P = 0.69), but the grade of the EC was higher (P < 0.0005). Both nuclear grade and mitotic index were significantly higher in POLE-mutated ECs than in the comparison cohort. POLE-mutated ECs were associated with peritumoral lymphocytes and numerous tumour-infiltrating lymphocytes. Lymphovascular invasion was present in 20 of 43 tumours. Adjuvant radiotherapy and adjuvant chemotherapy would be offered in up to 80% and 40% of patients, respectively, on the basis of stage, grade, lymphovascular invasion, and histotype.
Conclusions
POLE-mutated ECs are typically of high grade, with prominent lymphocytic infiltration, but they are not sufficiently distinctive to allow accurate diagnosis based on routine haematoxylin and eosin staining. Even though POLE-mutated tumours are associated with an excellent prognosis, current guidelines for giving adjuvant treatment for EC result in most patients receiving adjuvant therapy.
Keywords: endometrial carcinoma, mutations, POLE, TCGA
Introduction
Endometrial carcinoma (EC) is the most common of all gynaecological cancers in the Western world, affecting 52 630 women in the USA in 2014, with 8590 deaths.1 Historically, the pathogenesis of this disease has been divided into types 1 and 2. Type 1 ECs are associated with an earlier age at diagnosis, a higher body mass index (BMI), oestrogen-driven hyperplastic precursor lesions, and an endometrioid histotype. Conversely, type 2 ECs are associated with older age, a lower BMI, atrophic endometrium, and a serous histotype.2,3 This is, however, an oversimplified view of EC. High-grade ECs can arise through progression of typical type 1 carcinomas, e.g. through acquisition of p53 mutations, and such tumours can have a serous histotype. Thus, morphology cannot predict pathogenesis (i.e. type 1 versus type 2) in EC.4 Morphological evaluation of histotype is also subject to very significant interobserver variability.5,6 This problem has recently been overtly acknowledged through the designation of a subset of tumours as ‘EC with ambiguous phenotype’.7
The Cancer Genome Atlas (TCGA) recently provided an integrated molecular analysis of 373 endometrial tumours, consisting almost exclusively of endometrioid and serous histotypes, and was able to define four groups based on genomic characterization of mutation profiles, and to demonstrate that these groups correlate with prognosis.8 One of these groups (ultramutated phenotype) was characterized by very high numbers of mutations throughout the genome, and arises because of mutations in the exonuclease domain of the polymerase ε gene (POLE). This group accounted for 17 cases (4.6%) in the TCGA dataset. Church et al. described 14 POLE-mutated tumours in 173 ECs (7.5%), and the TRANSPORTEC study found POLE mutations in 14 of 116 (12%) ECs.9,10 The inactivating mutations in POLE result in defective proofreading during leading-strand DNA synthesis, which, in turn, leads to significantly higher mutation rates than in other EC subtypes, even the hypermutated tumours with defects in DNA mismatch repair (MMR)/microsatellite instability (MSI) (7.2 × 10−6 versus 1.7 × 10−6 mutations per megabase, respectively).8 Several studies have shown that ECs with POLE exonuclease domain mutations have a very favourable outcome.10–12 Morphological features have been studied for the 17 TCGA POLE-mutated ECs and eight additional POLE-mutated ECs, and it was found that the majority of these tumours: are endometrioid, are of higher grade, show ambiguous morphological features, show intratumoral heterogeneity, and have increased numbers of peritumoral lymphocytes.13 Given that these tumours are frequently of high grade but have a very favourable prognosis, the question is raised of how to integrate the implications of POLE mutational assessment into existing risk assessment strategies for EC.
Currently, decisions regarding post-hysterectomy adjuvant radiation or chemotherapy for patients with EC rely primarily on histopathological assessment of the hysterectomy specimen, with considerable inter-institutional variability in how these decisions are made. International Federation of Gynecology and Obstetrics (FIGO) stage, grade and the presence of lymphovascular invasion (LVI) serve as the basis for risk assessment in the province of British Columbia, determining the need for adjuvant therapy14 (Tables 1 and 2). Tumour histotype is also considered for the purposes of risk assessment in some centres, with serous carcinomas considered to be more aggressive.
Table 1. British Columbia Cancer Agency Chemotherapy Management Guidelines.
| Stage | |||||||||
|---|---|---|---|---|---|---|---|---|---|
| IA (M−) | IA (M+) | IB | II | IIIA | IIIB | IIIC | IVA | IVA | |
| Endometrioid | |||||||||
| Grade 1 | − | − | − | −/+ | −/+ | + | + | + | + |
| Grade 2 | − | − | − | −/+ | + | + | + | + | + |
| Grade 3 | − | − | + | + | + | + | + | + | + |
| Non-endometrioid | |||||||||
| Serous | − | + | + | + | + | + | + | + | + |
| Clear cell | − | − | + | + | + | + | + | + | + |
| M1 carcinosarcoma | − | + | + | + | + | + | + | + | + |
M+, myometrial invasion present; M−, myometrial invasion not present; +, chemotherapy recommended; −, no chemotherapy recommended; +/−, chemotherapy optional.
Table 2. British Columbia Cancer Agency Management Guideline for radiotherapy in endometrioid endometrial carcinomas.
| Stage | ||||
|---|---|---|---|---|
| IA | IB | II | IIIA/B | |
| Grade 1 | O | V | P + V | P |
| Grade 2 | O | V | P + V | P |
| Grade 3 | V | P | P + V | P |
O, observation; P, pelvic radiation; V, vault brachytherapy.
Tumours with lymphovascular invasion (see criteria for diagnosis in Materials and methods) are considered to be high risk, and pelvic radiation is recommended.
This study focuses on 47 ECs identified as having a POLE mutation, out of a series of 483 ECs tested (9.7%). Our aims were to perform a detailed morphological assessment of these POLE-mutated ECs, and to determine the risk group assessment, according to currently used guidelines, on the basis of histopathological and stage considerations. Our hypotheses were that POLE-mutated ECs cannot be recognized by the use of morphological methods alone, and that tumours harbouring POLE mutations may be considered to be at high risk of recurrence according to conventional risk assessment tools, despite accumulating evidence that they are associated with a very favourable prognosis.
Materials and methods
Ethical approval to perform the studies described herein was provided through the oversight of the Clinical Review Ethics Board of the University of British Columbia (ethics certificate H05-60119, issued 25 May 2009). DNA from a series of 483 endometrial tumours was extracted from flash-frozen tissue or formalin-fixed paraffin-embedded (FFPE) tissue, and sequenced by the use of Illumina next-generation sequencing, as described previously.15 Normal DNA was extracted from either buffy coat or FFPE tissue, and sequenced to confirm the somatic status of the POLE mutation.
Forty-seven POLE-mutated tumours were identified from the series. Haematoxylin and eosin-stained slides from hysterectomy specimens could be retrieved for 43 of the 47 specimens. Histotype diagnosis was made by an experienced gynaecological pathologist (C.B.G.). The slides were then independently evaluated by two pathologists (M.K. and S.B.) for a range of morphological criteria. In each case, p53 and MMR status were evaluated by immunohistochemistry.
The following morphological criteria were evaluated:
Nuclear grade
classified as low or high. Tumours with high cytological grade showed variations of greater than three-fold in nuclear size, highly irregular contours, striking hyperchromasia, and/or prominent nucleoli. Nuclear features characteristic of serous or clear cell carcinoma were sufficient to categorize a tumour as cytologically high grade. High-grade nuclear atypia could be appreciated at intermediate magnification (×100).
Peritumoral lymphocytes
defined as present when peritumoral lymphocytes were readily identifiable at scanning power in any area around the tumour, on at least one slide.16
Tumour-infiltrating lymphocytes (TILs)
TIL counts were carried out as previously described.16 Aggregates of lymphocytes within or adjacent to tumour were first detected at scanning magnification, in an effort to obtain the highest possible lymphocyte counts. Ten high-power microscopic fields (total area of 1.96 mm2) were then selected randomly within these areas. Only lymphocytes located within tumour cells, nests or glands were counted.
Mitoses/10 high-power fields (HPFs)
evaluation at scanning magnification was undertaken in an effort to obtain the highest possible mitotic counts. Mitoses were counted when there was clearly condensed chromatin or evidence of telophase, and expressed as mitoses per 10 HPFs (total area of 1.96 mm2). Apoptotic bodies were not counted.
Tumour characteristics
Histotype was defined according to the World Health Organization 2014 criteria, and the endometrioid carcinomas were graded according to the 1988 FIGO grading system.17
Intratumoral heterogeneity
defined as present when a tumour had two or more clearly separate and distinct morphological patterns, juxtaposed, but not intimately admixed, each constituting at least 10% of the tumour.
LVI
defined as clusters of tumour cells within spaces lined by endothelial cells. The clusters are frequently moulded to the shape of the vessel, and lack fibrovascular stroma (unlike tumour fragments that are artefactually displaced into vessels). If one to three vascular spaces contained tumour, LVI was considered to be focal; if four or more vascular spaces contained tumour (in total, based on assessment of all slides), LVI was considered to be extensive.
Comparison Group of ECs
The study cases were compared with a previously described series of 202 ECs, with respect to histotype, FIGO grade, nuclear atypia, and mitotic activity;18 this series consisted of all ECs from Vancouver General Hospital (VGH) for the period 1983–1998. Carcinosarcomas were excluded. MMR status and POLE status were not evaluated for this case series.
Immunostaining for p53 and MMR Proteins
Tissue microarrays were constructed as described previously, with duplicate 0.6-mm tissue cores.15 Detection of DNA MMR deficiencies by immunohistochemistry can effectively enable the diagnosis of the MSI phenotype in ECs.18 The details of immunostaining assessment of the MMR proteins MLH1, MSH2, MSH6 and PMS2 have also been previously reported, and the immunoassessment of MMR has been validated against molecular testing for MSI.18 Anti-hMLH1 (1:150, ES05; Novocastra, Vector Laboratories, Burlington, Ontario, Canada), anti-hMSH2 (1:100, 25D12; Novocastra, Vector Laboratories), anti-hMSH6 (1:600, PU29; Novocastra, Vector Laboratories), anti-hPMS2 (1:150, MOR4G; Novocastra, Vector Laboratories) and anti-p53 (1:400, DO-7; Dako, Carpenteria, CA, USA) primary antibodies were used, on a semi-automated Benchmark XT instrument (Ventana Medical Systems, Tucson, AZ, USA). Tissue sections were incubated with the primary antibody for 32 min at 37°C, followed by the prediluted Ventana UltraMap detection reagent, used according to the manufacturer's protocol. Antigen retrieval was performed with the ‘Mild CC1’ on-machine protocol. Appropriate on-slide positive and negative controls were used. MMR protein expression was scored as present or absent, with an internal control of nuclear staining of benign nuclei being required for interpretation. p53 expression was scored in three tiers: complete loss of expression, focal expression, or overexpression, defined as strong staining in >80% of positive tumour cell nuclei. Complete loss or overexpression was considered to indicate abnormal p53 expression.19 Abnormal results were confirmed by staining of whole sections.
Risk Assessment
The demographic and clinical data of the patients were collected, and an assignment of risk as low, intermediate or high was made for each case, based on the following risk factors: FIGO grade 3 endometrioid or serous histotype, outer-half myoinvasion, and lymphovascular invasion, as described in Tables 1 and 2. This assignment is for stage I tumours, because all tumours of higher stage would receive adjuvant therapy. For adjuvant radiotherapy, low risk (i.e. observation only; no radiotherapy) is defined as zero risk factors, intermediate risk as one risk factor, and high risk as two or more risk factors. For chemotherapy, low risk is defined as zero or one risk factor, and high risk as two risk factors.
Results
A complete dataset, with data for all features assessed at the time of morphological review, is shown in Table S1.
The median age of the patients with POLE-mutated carcinomas was 58 years (range 38–92 years) (Table 3). The overwhelming majority (42/43, 98%) of tumours presented at an early stage (FIGO stage I), with only one patient (2%) presenting with a FIGO stage III tumour, with uterine serosal involvement and involvement of one pelvic lymph node.
Table 3. Patient demographics.
| Variable | Value |
|---|---|
| Age (years), median (range) | 58 (38–92) |
| Histotype, n (%) | |
| Endometrioid | 34 (79) |
| Serous | 2 (5) |
| Clear cell | 1 (2) |
| Mixed | 6 (14) |
| FIGO grade, n (%) | |
| 1 | 8 (24) |
| 2 | 7 (21) |
| 3 | 19 (56) |
| Stage, n (%) | |
| IA | 22 (51) |
| IB | 20 (47) |
| II | 0 |
| III–IV | 1 (2) |
| LVI, n (%) | |
| Present | 21 (55) |
| Not identified | 17 (45) |
FIGO, International Federation of Gynecology and Obstetrics; LVI, lymphovascular invasion.
Endometrioid carcinomas accounted for 34 of the 43 tumours (79%); six were mixed (14%), two were serous (5%), and one was clear cell (2%) (Figure 1). The mixed carcinomas were endometrioid/serous in five tumours, and one was an admixture of serous and undifferentiated. On comparison of the histotype distribution in this series of 43 POLE-mutated ECs with that of a consecutive series of ECs from VGH [202 cases, of which 158 (78%) were endometrioid, 22 (11%) were mixed, 13 (6%) were serous, five were (2%) clear cell, and four (2%) were of other histotypes], there was not a significant difference (P = 0.69).
Figure 1.
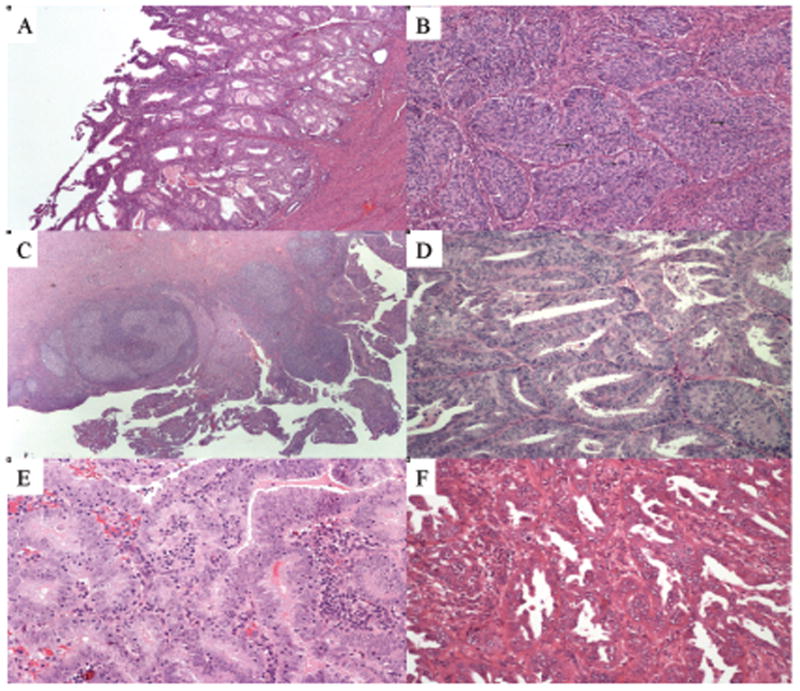
Examples of endometrial carcinomas with POLE mutations. A, Low-grade endometrioid carcinoma without peritumoral lymphocytes. B, High-grade endometrioid carcinoma with nuclear pleomorphism. C, Peritumoral lymphocytes with germinal centres. D,E, Endometrial carcinomas with ambiguous morphology. F, Serous carcinoma.
The FIGO grades of the endometrioid carcinomas were as follows: grade 1, eight of 43 (19%); grade 2, eight of 43 (19%); and grade 3, 27 of 43 (63%) On comparison of the FIGO grade distribution in the endometrioid ECs in this series with that of the comparison group of consecutive ECs from VGH20 [158 cases, of which 101 (64%) were grade 1, 27 (17%) were grade 2, and 30 (19%) were grade 3], the POLE-mutated cases were significantly more likely to be of higher grade (P < 0.0005). There was an atypical hyperplasia precursor lesion in six cases.
Peritumoral lymphocytes were identified in 34 of 43 (79%) tumours (Figure 1). TILs ranged from 19 per 10 HPFs to 1398 per 10 HPFs (mean 294 per 10 HPFs). The subjective assessment of intratumoral heterogeneity, as defined in Materials and methods, was made independently by two reviewers, who noted its presence in 18 and 19 of 43 cases, respectively.
LVI was present in 20 of 43 (47%) tumours, and was extensive in eight. In 17 cases, lymph node dissection was performed as part of staging surgery, with only one having lymph node metastasis. Nine of 17 cases with lymph node sampling showed LVI in the myometrium, and this was extensive in five of these nine tumours.
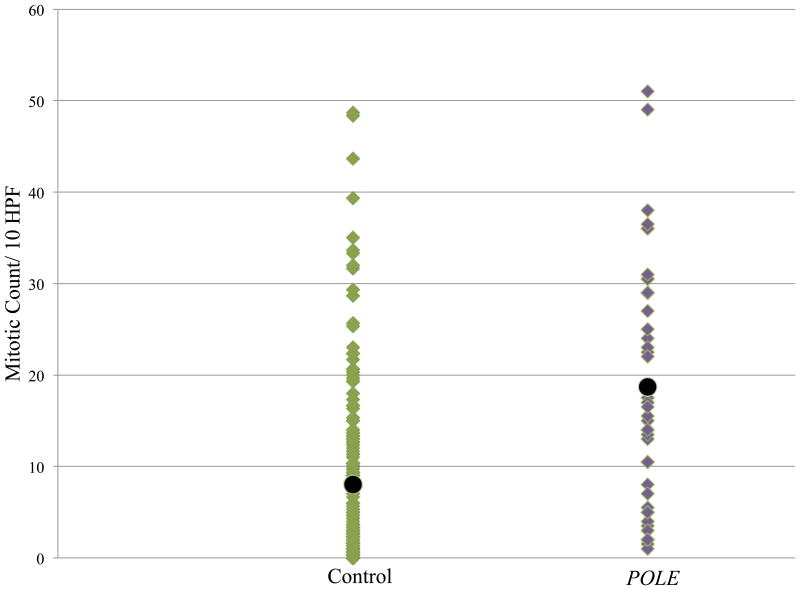
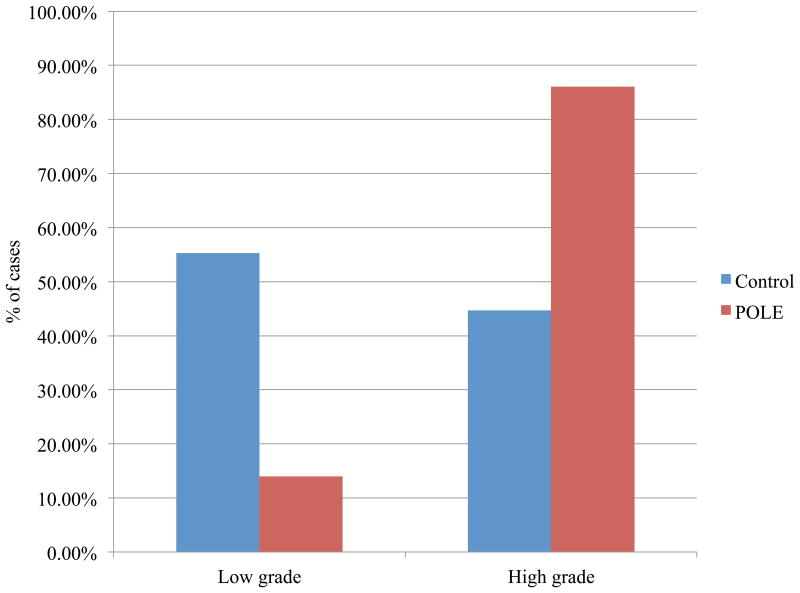
Mitotic index and nuclear grade were compared with previously reported data from a cohort of 202 ECs from the archives of VGH (1983–1998).20 The POLE-mutated carcinomas were more mitotically active than the control group (19 per 10 HPFs versus eight per 10 HPFs, P < 0.005) (Figure 2), and were also more likely to be of high nuclear grade (P < 0.05), even though the tumours in this group were predominantly of low architectural grade (Figures 3 and 4).
Figure 2.
Comparison of mitotic index of endometrial carcinomas (ECs) with POLE mutations and a comparison series of ECs. The average mitotic count in the POLE-mutated cases [20 per 10 high-power fields (HPFs)] was significantly higher than in the comparison series of unselected ECs (eight per 10 HPFs) (P < 0.005).
Figure 3.
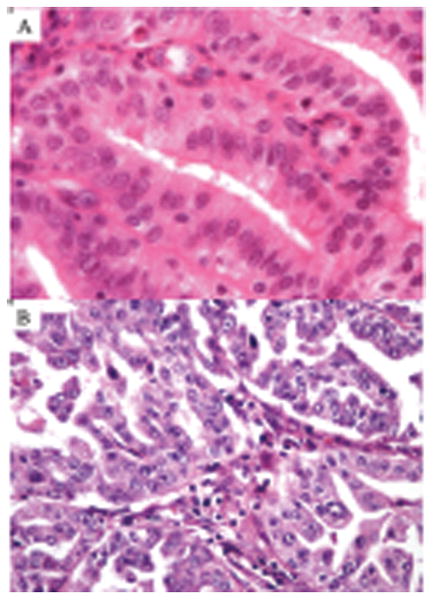
Endometrioid carcinomas with POLE mutations showing low-grade nuclear features (A) and high-grade nuclear features (B).
Figure 4.
Percentage of cases showing low-grade and high-grade nuclear features in endometrial carcinomas (ECs) with POLE mutations and in a comparison series of unselected ECs; high-grade nuclear features are more common in the POLE-mutated ECs (P < 0.05).
In five of the 43 tumours, there was abnormal p53 expression. On whole slide staining, two of these five tumours showed only focal abnormal p53 staining, with an abrupt transition between overexpressed and wild-type staining patterns (Figure 5). The three remaining cases showed uniform p53 mutant overexpression. Three of 43 showed abnormal MMR protein immunostaining (one MLH-1/PMS-2 loss of expression, one MSH-2/MSH-6 loss of expression, and one MSH-6 loss of expression). MMR protein loss was uniformly present in the tumour cells on whole section staining.
Figure 5.
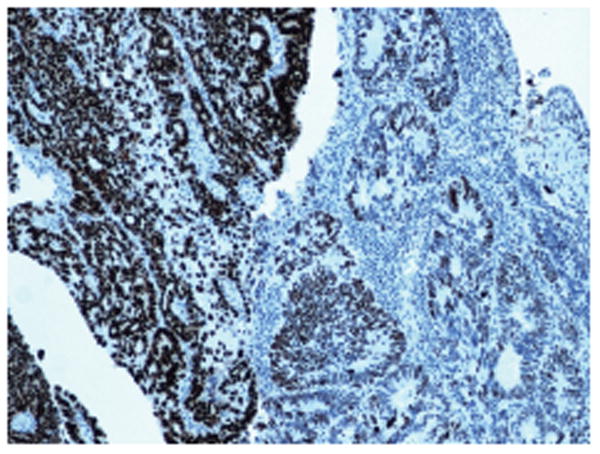
p53 immunostaining showing focal abnormal (strong diffuse) p53 staining with an abrupt transition to wild-type p53 expression.
Current guidelines for giving adjuvant chemotherapy and radiotherapy rely on FIGO grade, FIGO stage, histotype, and LVI. Of the 42 stage I tumours in this study, 83% (35/42) fell into the intermediate-risk or high-risk groups with respect to radiotherapy (Table 4), and the patients would therefore potentially have been offered adjuvant radiotherapy. In addition, 36% (15/42) patients would potentially have been offered some form of chemotherapy. Only 17% (7/42) were of sufficiently low risk that adjuvant therapy would not have been considered, and the patients would have been followed with observation only (Table 4).
Table 4. Adjuvant therapy risk assessment.
| Risk level | Radiotherapy | Chemotherapy |
|---|---|---|
| Low risk, n (%) | 7 (17) | 27 (64) |
| Intermediate risk, n (%) | 11 (26) | NA |
| High risk, n (%) | 24 (57) | 15 (36) |
NA.
For all tumours that are stage I, n = 42.
Radiotherapy risk assessment was assessed on the basis of zero risk factors (low), one risk factor (intermediate), or at least two (high) of the following risk factors: grade 3 endometrioid or non-endometrioid histotype; at least outer-half myometrial invasion; lymphovascular invasion.
Chemotherapy risk assessment was assessed on the basis of one (low) or two (high) of the following risk factors: grade 3 endometrioid or non-endometrioid histotype; at least deep myometrial invasion.
Discussion
We have morphologically characterized the largest cohort of POLE-mutated ECs to date. The histotypes of the 43 POLE-mutated ECs included 34 endometrioid (79%), six mixed (14%), two serous (5%), and one clear cell (2%), a distribution that does not differ significantly from the histotype of ECs seen at VGH. Of the 34 endometrioid tumours, eight were FIGO grade 1, eight were grade 2, and 27 were grade 3, a distribution that is significantly different from that of the comparison group of unselected endometrioid ECs, with a pronounced tendency for there to be higher grade in POLE-mutated tumours (P < 0.0005). Forty-two tumours were stage I (98%), and one patient presented with a stage III tumour (2%). Peritumoral lymphocytic infiltrate and TILs were prominent in most cases, and LVI was identified in 20 tumours (47%). The percentage of cases with high-grade nuclear features and the mitotic index were both significantly higher in POLE-mutated ECs than in the comparison group of unselected ECs. It is unclear why these tumours show a higher frequency of FIGO grade 3 and high-grade nuclear features. One explanation could be that the very high mutational burden is reflected in changes to the nuclear chromatin configuration.
We and others have shown that ECs can be grouped according to the four TCGA subtypes by the use of immunohistochemistry for p53 (a marker of the high copy number abnormality group, which consists predominantly of serous carcinomas), MMR protein immunostaining (to identify the MSI group), and POLE exonuclease sequencing (to identify the ultramutator group); the remaining tumours are designated copy number low, and consist predominantly of low-grade endometrioid carcinomas.10,15 The markers of the different subgroups are not mutually exclusive, however, and occasional ECs with POLE mutation show abnormal p53 or MMR protein expression, as was seen in this series (five and three cases, respectively). This is not surprising, given that a POLE mutation results in massive numbers of mutations throughout the genome, and the occasional involvement of p53 or MMR genes is expected. What is not clear is the consequence of acquiring these additional mutations. It could be postulated that, if POLE-mutated tumours acquired p53 or MMR mutations during tumour progression, these would be heterogeneously expressed within the tumour and would not be driver mutations, and would therefore not be associated with the worse outcome associated with p53 or MMR abnormalities than in POLE-mutated ECs. We did observe heterogeneous staining for p53 in whole sections in two of five tumours but, in the remaining three cases, expression was uniformly abnormal throughout, suggesting that p53 abnormalities in these POLE-mutated tumours would be present in all cells. The correct classification of ECs with both POLE and either p53 or MMR abnormalities is uncertain at this time, and investigation of more examples of these uncommon tumours is needed to determine whether the prognosis is determined by the POLE mutation or by the p53 or MMR abnormalities.
The POLE exonuclease domain-mutated ECs frequently showed intratumoral heterogeneity, as has been noted previously.13 This earlier study also noted the frequent presence of ambiguous morphology, a finding associated with difficulties in classification as endometrioid versus serous histotype. Reproducible histotype diagnosis remains a challenge in EC;5–7 a TCGA-based classification system for EC, with recognition of the POLE-mutated and MMR groups, may dramatically improve the reproducibility of EC classification. Approximately one-third of ECs can be identified on the basis of sequencing of POLE exonuclease domains and MMR immunostaining, respectively, and it is possible that these tumours, showing frequent intratumoral heterogeneity and ambiguous morphology, may account for a large majority of the cases in which histotype diagnosis is problematic, and that the remaining ECs may prove to be relatively easily subclassified as copy number high/type 2/serous versus copy number low/type 1/endometrioid. It is conceivable that subclassification could then approach the very high degree of reproducibility seen for histotype assignment with ovarian carcinomas.21
The finding of this POLE-ultramutated phenotype within EC was an exciting discovery by TCGA. The excellent prognosis associated with this tumour group raises the possibility that these patients may need less aggressive treatment than those with other EC subtypes. One theory is these tumours possess an enhanced antitumour immune response to the mutational burden. We observed frequent LVI in POLE-mutated ECs, but infrequent nodal metastasis, suggesting that the cells may lack full metastatic potential, with a reduced capacity to move out of lymphatics and establish themselves at distant sites. Another explanation for prominent LVI with infrequent metastasis would be the intense immune response characteristic of POLE-mutated ECs22 preventing the survival of tumour cells in the lymphatic system. It is important to acknowledge, however, that it is not currently clear whether the favourable prognosis is a result of a good response to current therapy, or whether POLE-mutated ECs are inherently less aggressive. If the latter turns out to be the case, it may become important to recognize POLE-mutant cases on biopsy specimens, as the patients may not benefit from more extensive surgical treatment, such as lymph node dissection. Le et al. recently demonstrated the ability to use the host immune response to tumours with MMR abnormalities and neoantigen formation, a phenomenon also seen in POLE-mutated ECs, to identify those patients who respond to blockade of the PD-1 programmed death receptor by pembrolizumab; this class of cancer therapeutic agent may also be active in POLE-mutated ECs, but this remains to be tested.23 As routine histomorphological assessment does not allow recognition of POLE-mutated ECs, exonuclease domain sequencing of POLE will be required, at least until a surrogate assay, such as an immunostain, can be developed.
The routine identification of POLE exonuclease domain mutation status will also allow us to further address the need for adjuvant therapy in this subset of ECs. In VGH, any one of the following features will place a stage I tumour within the intermediate-risk or high-risk category, and result in possible recommendation of adjuvant therapy: outer-half myometrial invasion, LVI, grade 3, or non-endometrioid histotype. In other words, only grade 1 or 2 endometrioid carcinomas, stage IA, without LVI, are considered to be of sufficiently low risk that adjuvant therapy would not be considered. The implication of using current morphological criteria to risk-stratify patients with POLE-mutated tumours, however, is that a significant number of patients with POLE-mutated ECs (83% in this series) will potentially be offered adjuvant therapy on the basis of current risk assessment algorithms. As noted previously, further research is required to determine whether patients with POLE-mutated ECs benefit from adjuvant therapy or would do well regardless, relieving patients from receiving adjuvant therapy when it is not required.11,15,24
Supplementary Material
Table S1: Complete Dataset for Clinical and Histopathological Variables Assessed.
Acknowledgments
Grant support: P30 CA008748
Footnotes
Conflicts of interest: The authors declare no conflicts of interest.
Author contributions: J. N. McAlpine, M. K. McConechy and C. Blake Gilks conceived the study. S. Bakhsh and M.Kinloch. performed independent detailed morphological review of all cases. L. N. Hoang and C. Blake Gilks performed histopathological review of selected cases. R. Soslow, M.Köbel and C.-H. Lee provided input on study design and histopathological review parameters. M. K. McConechy performed POLE mutational analysis. J. N. McAlpine provided interpretation of application of guidelines for adjuvant therapy. M.Kinloch. and C. Blake Gilks wrote the initial draft manuscript. All authors provided input on subsequent draft revisions, including approval of the final manuscript.
References
- 1.Howlader N, Noone A, Krapcho M. SEER cancer statistics review, 1975–2011. Bethesda, MD: National Cancer Institute; Available at: http://Seer.cancer.gov/csr/1975_2011/ [Google Scholar]
- 2.Bansal N, Yendluri V, Wenham RM. The molecular biology of endometrial cancers and the implications for pathogenesis, classification, and targeted therapies. Cancer Control. 2009;16:8–13. doi: 10.1177/107327480901600102. [DOI] [PubMed] [Google Scholar]
- 3.Bokhman JV. Two pathogenetic types of endometrial carcinoma. Gynecol Oncol. 1983;15:10–17. doi: 10.1016/0090-8258(83)90111-7. [DOI] [PubMed] [Google Scholar]
- 4.McConechy MK, Ding J, Cheang MC, et al. Use of mutation profiles to refine the classification of endometrial carcinomas. J Pathol. 2012;228:20–30. doi: 10.1002/path.4056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Hoang LN, McConechy MK, Kobel M, et al. Histotype–genotype correlation in 36 high-grade endometrial carcinomas. Am J Surg Pathol. 2013;37:1421–1432. doi: 10.1097/PAS.0b013e31828c63ed. [DOI] [PubMed] [Google Scholar]
- 6.Han G, Sidhu D, Duggan MA, et al. Reproducibility of histological cell type in high-grade endometrial carcinoma. Mod Pathol. 2013;26:1594–1604. doi: 10.1038/modpathol.2013.102. [DOI] [PubMed] [Google Scholar]
- 7.Soslow RA. Endometrial carcinomas with ambiguous features. Semin Diagn Pathol. 2010;27:261–273. doi: 10.1053/j.semdp.2010.09.003. [DOI] [PubMed] [Google Scholar]
- 8.Cancer Genome Atlas Research Network. Kandoth C, Schultz N, et al. Integrated genomic characterization of endometrial carcinoma. Nature. 2013;497:67–73. doi: 10.1038/nature12113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Church DN, Briggs SE, Palles C, et al. DNA polymerase epsilon and delta exonuclease domain mutations in endometrial cancer. Hum Mol Genet. 2013;22:2820–2828. doi: 10.1093/hmg/ddt131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Stelloo E, Bosse T, Nout RA, et al. Refining prognosis and identifying targetable pathways for high-risk endometrial cancer; a TransPORTEC initiative. Mod Pathol. 2015;28:836–844. doi: 10.1038/modpathol.2015.43. [DOI] [PubMed] [Google Scholar]
- 11.Church DN, Stelloo E, Nout RA, et al. Prognostic significance of POLE proofreading mutations in endometrial cancer. J Natl Cancer Inst. 2014;107:402. doi: 10.1093/jnci/dju402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Meng B, Hoang LN, McIntyre JB, et al. POLE exonuclease domain mutation predicts long progression-free survival in grade 3 endometrioid carcinoma of the endometrium. Gynecol Oncol. 2014;134:15–19. doi: 10.1016/j.ygyno.2014.05.006. [DOI] [PubMed] [Google Scholar]
- 13.Hussein YR, Weigelt B, Levine DA, et al. Clinicopathological analysis of endometrial carcinomas harboring somatic POLE exonuclease domain mutations. Mod Pathol. 2015;28:505–514. doi: 10.1038/modpathol.2014.143. [DOI] [PubMed] [Google Scholar]
- 14.BC Cancer Agency. Available at: http://Www.bccancer.bc.ca/HPI/CancerManagementGuidelines/gynecology/endometrium/mngmt.htm. (updated 2012)
- 15.Talhouk A, McConechy M, Leung S, et al. A clinically applicable molecular-based classification for endometrial cancers. Br J Cancer. 2015;113:299–310. doi: 10.1038/bjc.2015.190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Shia J, Black D, Hummer AJ, Boyd J, Soslow RA. Routinely assessed morphological features correlate with microsatellite instability status in endometrial cancer. Hum Pathol. 2008;39:116–125. doi: 10.1016/j.humpath.2007.05.022. [DOI] [PubMed] [Google Scholar]
- 17.Kurman R, Carcangiu M, Herrington S, Young R, editors. World Health Organization classification of tumors of female reproductive organs. 4th edn. Lyon: IARC Press; 2014. [Google Scholar]
- 18.McConechy MK, Talhouk A, Li-Chang HH, et al. Detection of DNA mismatch repair (MMR) deficiencies by immunohistochemistry can effectively diagnose the microsatellite instability (MSI) phenotype in endometrial carcinomas. Gynecol Oncol. 2015;137:306–310. doi: 10.1016/j.ygyno.2015.01.541. [DOI] [PubMed] [Google Scholar]
- 19.Kobel M, Reuss A, Bois A, et al. The biological and clinical value of p53 expression in pelvic high-grade serous carcinomas. J Pathol. 2010;222:191–198. doi: 10.1002/path.2744. [DOI] [PubMed] [Google Scholar]
- 20.Alkushi A, Abdul-Rahman ZH, Lim P, et al. Description of a novel system for grading of endometrial carcinoma and comparison with existing grading systems. Am J Surg Pathol. 2005;29:295–304. doi: 10.1097/01.pas.0000152129.81363.d2. [DOI] [PubMed] [Google Scholar]
- 21.Kobel M, Bak J, Bertelsen BI, et al. Ovarian carcinoma histotype determination is highly reproducible, and is improved through the use of immunohistochemistry. Histopathology. 2014;64:1004–1013. doi: 10.1111/his.12349. [DOI] [PubMed] [Google Scholar]
- 22.van Gool IC, Eggink FA, Freeman-Mills L, et al. POLE Proofreading mutations elicit an antitumor immune response in endometrial cancer. Clin Cancer Res. 2015;21:3347–3355. doi: 10.1158/1078-0432.CCR-15-0057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Le DT, Uram JN, Wang H, et al. PD-1 blockade in tumors with mismatch-repair deficiency. N Engl J Med. 2015;372:2509–2520. doi: 10.1056/NEJMoa1500596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Billingsley CC, Cohn DE, Mutch DG, Stephens JA, Suarez AA, Goodfellow PJ. Polymerase varepsilon (POLE) mutations in endometrial cancer: clinical outcomes and implications for Lynch syndrome testing. Cancer. 2015;121:386–394. doi: 10.1002/cncr.29046. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Table S1: Complete Dataset for Clinical and Histopathological Variables Assessed.