Abstract
Fluoxetine, a selective serotonin reuptake inhibitor (SSRI), has shown favorable effects in some children with autism. There are no previous studies evaluating the connection between clinical outcome and markers of clinical response to fluoxetine treatment. We examined serum brain derived neurotrophic factor (BDNF) concentrations and serotonin transporter (SERT) binding in the medial frontal cortex and midbrain, measured by single photon emission computed tomography (SPECT) scanning, in a group of 13 autistic children and adolescents (12 males, one female; age 5-16 years), who were treated for six months with fluoxetine at a dose range of 10–40 mg/day. Clinical response was evaluated by the Autism Treatment Evaluation Checklist (ATEC). Serum concentrations of BDNF and SERT binding were measured at baseline and two months after termination of fluoxetine treatment.
At baseline, before starting fluoxetine treatment, the serum concentration of BDNF had a bimodal distribution in the autism group with either a low concentration (n = 8, mean 1497 pg/mL) or a high concentration (n = 5, mean 14062 pg/mL) with respect to controls (n = 15, mean 9652 pg/mL), and SERT binding was uniformly low in the autistic subjects in medial frontal cortex and midbrain. Fluoxetine treatment led to positive effects in several aspects of communication, socialization and cognitive awareness, with 6 out 13 subjects being particularly good responders. These six also had a significant decrease in BDNF (p = 0.03) and minimal change in SERT binding after therapy. The other 7 subjects showed a trend towards an increase in BDNF and SERT binding.
Our results indicate that fluoxetine may improve core autistic symptoms, and that this clinical response is linked to a decrease in serum BDNF.
Keywords: Autism, Brain derived neurotrophic factor, Clinical outcome, Fluoxetine, Serotonin transporter, Single photon emission computed tomography
Introduction
Autism is a developmental disorder characterized by: core impairment in social interaction, deviance in communication, and a markedly restricted repertoire of activities and interests [1]. Anatomical and biochemical data suggest that in autism, there is an early disturbance in brain growth (i.e., early acceleration followed by retardation) [2]. This pattern may be the consequence of abnormalities in production and action of neurotrophic factors, which could lead to decreased neuronal apoptosis and/or reduced synaptic pruning. Several studies have demonstrated abnormal serum concentrations of neurotrophic factors, in particular of brain derived neurotrophic factor (BDNF), although direction and timing of the BDNF changes have not been consistent [3, 4]. BDNF is unique because, in addition to its trophic effects, it is involved in synaptic formation and plasticity [5]. Among the neurotransmitters influenced by BDNF is serotonin, which is also implicated in autism and other neurodevelopmental disorders [6].
Evidence linking serotonin and autism includes neuroimaging data suggesting reduced serotonin production [7]. Decreased number of Purkinje cells, a consistent finding in post-mortem studies of autistic subjects, may also be due to serotoninergic abnormalities [8]. Further support for a serotoninergic deficit in autism is provided by our recent study (concerning the same population of autistic children as the present paper) using single photon emission computed tomography (SPECT), which demonstrated reduced serotonin transporter (SERT) binding in the medial frontal cortex and midbrain of autistic children [9]. Fluoxetine and fluvoxamine, selective serotonin reuptake inhibitors (SSRIs), have shown to alleviate some major symptoms in autism [10-12]. However, it is unclear to what extent SSRIs are efficacious in autism, since a recent study demonstrated no effect after twelve weeks treatment with another SSRI, citalopram, on repetitive behaviors [13]. The short term effect of SSRIs is a blockade of SERT, which re-uptakes serotonin from the synaptic cleft back to the afferent neuron, increasing in this way the availability of serotonin at the synapse. Although blocking SERT takes place minutes after SSRI administration, behavioral changes are only seen after several weeks. This temporal profile may reflect SSRIs’ effects on the regulatory function of serotonin, particularly its relationship with brain BDNF concentrations [14]. The precise nature of the abnormalities in the serotonergic system in autism is still unknown, an area that certainly deserves further investigation.
We are the first to study the clinical outcome and the markers of response to fluoxetine treatment, including serum BDNF concentrations and SERT binding in the medial frontal cortex and midbrain, measured by SPECT scanning, in a group of autistic children and adolescents.
Subjects and methods
Subjects
The study was conducted in the Departments of Pediatrics, Unit of Child Neurology, Kuopio University Hospital, Kuopio, Finland. The cohort was composed of 13 autistic children and adolescents, aged 5 to 16 years (mean±SD, 8.7±3.7 years; 12 males, one female). The diagnosis of autism was based on the International Statistical Classification of Diseases and Related Health Problems Tenth Revision (ICD-10). Subjects were also evaluated with the Childhood Autism Rating Scale (CARS) and with the Leiter scale of non-verbal intelligence, by a multiprofessional team including pediatric neurologists and psychologists. At baseline, CARS scores ranged from 28.5 to 40.5 (mean±SD, 33.5±3.0), while non-verbal intelligence quotient (according to the Leiter method) varied from 70 to 109 (mean±SD, 88±12), the latter measure demonstrating that our population was composed of high functioning autistic children. Clinical magnetic resonance brain imaging was normal in all individuals.
All participants’ families were from middle socio-economic stratum.
Exclusion criteria
Only individuals with autistic disorder were included in the study. In addition to the exclusion of subjects with Asperger syndrome or Pervasive Developmental Disorder - Not Otherwise Specified (PDD-NOS), diagnosis of a genetic disorder (e.g., tuberous sclerosis, fragile X syndrome, or major chromosomal defect) was also an exclusionary criterion. None of the subjects had a history of perinatal asphyxia, severe infection of the central nervous system, or head injury requiring medical care. Clinical symptoms indicating potential attention deficit hyperactivity disorder (ADHD) comorbidity with autism were not separately studied. In autism, an overlap between autistic and ADHD-like behaviors is not uncommon. However, in general terms the participants did not have prominent ADHD symptoms. The autistic subjects did not receive any medication with known effects on serotonin metabolism during the preceding 3 years.
Comparison group
Fifteen neurologically healthy control children (13 males, two females; mean age 8.7 years, SD±3.6 years, range 4–15 y) served as controls for BDNF studies. These children had been admitted for lower body surgery to be performed under spinal anesthesia for the following indications: inguinal herniotomy (n=6), orthopedic procedures (n=6), and operations of urogenital system (n=3). Normative data on SERT binding was available from the recently published study mentioned above [9].
Methods
The SPECT scanning procedure was composed of three serial scans at: 15 min, 6 hr, and 24 hr after an intravenous injection of the tracer [123I] nor-β-CIT. Regions of interest included the medial frontal cortex and the midbrain. For the 15 min and 24 hr SPECT scans subjects were given oral midazolam, while for the 6 hr scan intravenous sedation with: midazolam, propofol, and thiopental were used. The SPECT scanning procedure has been presented in detail in a previous publication [9].
Samples for BDNF studies were collected during the sedation for the 6 hr scan. The samples were frozen immediately and stored at −70°C until analysis. BDNF was assayed by the sandwich-ELISA method, using a commercial kit for human BDNF (Chemicon, Temecula, CA, USA), following the manufacturer's protocol and using a variety of subject sample and technical controls. The sensitivity of the method is 7.8 pg/mL.
Therapy and evaluation of clinical response
Fluoxetine (Seromex® 10 mg soluble tablets, Ratiopharm, Ulm, Germany) was given once daily in the morning. The trial was begun with a dose of 5 mg, which was raised 5 mg (in children) or 10 mg (in adolescents) every two weeks until a highest tolerated dose or a maximum dose of 1 mg/kg or 40 mg total was reached. Children continued receiving special education and other interventions (i.e., speech and language therapy, occupational therapy), without any modification during the treatment trial.
Therapeutic doses ranged from 10–40 mg/day (0.4–0.9 mg/kg/day, mean 0.53 mg/kg/day); they were given for six months, after which fluoxetine was discontinued by decreasing the dose gradually over a period of two to four weeks. Following this, all patients had a two-month wash out period before SPECT scanning, to exclude the acute effect of fluoxetine on serotonin transporters. Clinical response to fluoxetine was evaluated with a follow-up checklist based on the Autism Treatment Evaluation Checklist (ATEC), an instrument that measures treatment response in autistic individuals [15]. The ATEC has four sections with multiple items each; ATEC 1: 14 items on speech, language and communication, ATEC 2: 20 items regarding socialization, ATEC 3: 18 items on sensory or cognitive awareness, and ATEC 4: 25 items dealing with health and physical condition or behavior. In the ATEC, we used a scale from 1 to 5 to express the severity of each item. In addition to these ATEC sections and items, we posed 13 questions regarding possible adverse effects of fluoxetine treatment. The baseline information was gathered from the parents in an interview by the investigators according to the ATEC checklist. During the treatment period, the parents reported the status every week by filling out the checklist by themselves. They were advised to contact the investigators whenever they detected drug intolerability or adverse effects in their child. After the treatment period, in the follow-up visit, the checklist was again filled out by the investigators in an interview with the parents.
Statistical analyses
All data were analyzed with the Statistical Package for Social Sciences (SPSS software version 14.0 for Windows, SPSS Inc., Chicago, USA). Due to data deviations from normal distribution, nonparametric tests were predominantly used: Mann-Whitney U-test in the analyses of BDNF and SERT measurements, and Wilcoxon signed ranks test for the analyses of clinical responses to fluoxetine therapy. Correlations between clinical response and the aforementioned biomarkers were performed by comparison of treatment response categories/groups using the same nonparametric tests. Group differences were considered to be statistically significant at a two-tailed p-value of < 0.05.
Results
At baseline, there was no group difference in BDNF serum concentrations between, autistic subjects (range < 7.8 – 17149 pg/mL, [mean 6330 pg/mL, SD ± 6591]) and the control group (n=15) (range 4575 – 14927 pg/mL, [mean 9652 pg/mL, SD ± 3223]) (p = 0.08). However, there was a bimodal distribution within the autistic group; subgroups had either remarkably low BDNF concentrations (n= 8, mean 1497 pg/mL, SD ± 1651) or higher than the mean concentration of the controls (n=5, mean 14062 pg/mL, SD ± 2026) (Table 1).
Table 1.
Brain derived neurotrophic factor (BDNF) concentrations and serotonin transporter (SERT) binding and clinical response after fluoxetine treatment in autistic children
| Age (years) | BDNF (pg/mL) | SERT (mL/mL) | ||||
|---|---|---|---|---|---|---|
| medial frontal cortex | midbrain | |||||
| at baseline | after treatment | at baseline | after treatment | at baseline | after treatment | |
| Good clinical responders* | ||||||
| 6 | 2954 | 711 | 0.05 | 0.12 | 1.30 | 1.11 |
| 6 | 12837 | 1290 | 0.28 | 0.15 | 1.34 | 1.25 |
| 8 | 17148 | 5557 | 0.08 | 0.33 | 1.07 | 1.25 |
| 11** | 11760 | 1728 | 0.11 | 0.06 | 1.00 | 1.02 |
| 13 | 1375 | 1229 | 0.23 | 0.29 | 1.07 | 1.10 |
| 16 | 14320 | 1821 | 0.16 | 0.20 | 1.09 | 1.17 |
| Poor clinical responders* | ||||||
| 5 | 56 | 10390 | 0.20 | 0.19 | 1.18 | 1.49 |
| 5 | 14247 | 13652 | 0.06 | 0.15 | 1.09 | 1.15 |
| 5 | 556 | 5283 | 0.32 | 0.34 | 1.11 | 1.32 |
| 5 | < 8*** | 2909 | 0.24 | 0.19 | 1.24 | 1.01 |
| 8 | 1941 | 1229 | 0.08 | 0.14 | 1.02 | 1.11 |
| 12 | 379 | 10035 | 0.08 | 0.22 | 1.06 | 1.14 |
| 13 | 4709 | 5374 | 0.07 | 0.29 | 1.07 | 1.35 |
according to the Autism Treatment Evaluation Checklist, section 2 (socialization)
female autistic patient, all other are males
below the detection range (7.8 pg/mL) of the ELISA assay
In autistic individuals, after fluoxetine treatment, BDNF concentration was lower (range 711–13652 pg/mL, [mean 4891 pg/mL, SD ± 4194]) than at baseline, but the change was not significant statistically (p = 0.44). BDNF concentration decreased in eight patients and increased in five, eliminating the bimodality observed at baseline.
At baseline, SERT binding capacity was significantly lower in both the midbrain and the medial frontal cortex of these autistic patients than in their age-matched controls, as it has been reported in a previous study [9]. After the fluoxetine treatment, there was an increased SERT binding capacity in both the midbrain and in the medial frontal cortex, but the change was not statistically significant (p= 0.09 in both areas) (Table 1). There were no significant correlations between SERT binding capacity in the abovementioned areas and BDNF concentrations, either at baseline or after treatment.
In terms of clinical response, there was a statistically significant favorable effect, after fluoxetine, on 23 items out of the 77 in ATEC; 7 items on speech, language, and communication (ATEC 1), 8 items concerning socialization (ATEC 2), and 8 items on sensory/cognitive awareness (ATEC 3) (Table 2). There were no significant changes in ATEC items dealing with health and physical conditions or behavior (ATEC 4). The positive effects found in the autistic cohort were mainly driven by a subgroup of six subjects who had a particularly high frequency of favorable scores on the ATEC 2 scale (i.e., socialization). Therefore, subsequent analyses were carried out by dividing the autistic cohort into good (above the ATEC 2 median) and poor (at or below the ATEC 2 median) clinical responders (Table 1), as this was a distinctive division in the autistic cohort.
Table 2.
Clinical response to fluoxetine treatment in 13 autistic children measured by the Autism treatment evaluation checklist (ATEC)
| Baseline* | After treatment* | p-value** | ||||
|---|---|---|---|---|---|---|
| mean | SD*** | mean | SD | |||
|
Section One (ATEC 1): Speech/Language/Communication
|
||||||
| 3. | Can follow some commands | 3.3 | 0.8 | 3.8 | 0.7 | 0.006 |
| 7. | Knows 10 or more words | 3.8 | 1.2 | 4.4 | 0.9 | 0.027 |
| 9. | Explains what he/she wants | 2.4 | 1.1 | 3.2 | 1.1 | 0.007 |
| 10. | Asks meaningful questions | 1.6 | 1.0 | 2.3 | 1.6 | 0.027 |
| 11. | Speech tends to be meaningful/relevant | 2.0 | 0.8 | 2.9 | 1.2 | 0.007 |
| 12. | Often uses several successive sentences | 1.7 | 1.1 | 2.1 | 1.3 | 0.041 |
| 13. | Carries on fairly good conversation | 1.3 | 0.4 | 1.6 | 0.7 | 0.038 |
| Mean | 2.3 | 2.9 | ||||
|
Section Two (ATEC 2): Socialization
|
||||||
| 1. | Seems to be in a shell – cannot be reached | 2.5 | 1.1 | 2.0 | 1.0 | 0.026 |
| 2. | Ignores other people | 3.0 | 0.7 | 2.5 | 0.9 | 0.020 |
| 3. | Pays little or no attention when addressed | 2.5 | 0.9 | 2.0 | 0.8 | 0.007 |
| 5. | No eye contact | 2.5 | 1.2 | 2.1 | 0.9 | 0.028 |
| 9. | Avoids contact with others | 2.8 | 0.9 | 2.2 | 0.7 | 0.010 |
| 10. | Does not imitate | 2.8 | 0.7 | 2.4 | 0.7 | 0.050 |
| 12. | Does not share or show | 3.6 | 1.0 | 3.2 | 1.2 | 0.037 |
| 18. | Insensitive to other's feelings | 3.2 | 1.0 | 2.8 | 1.1 | 0.041 |
| Mean | 2.9 | 2.4 | ||||
|
Section Three (ATEC 3): Sensory/Cognitive Awareness
|
||||||
| 2. | Responds to praise | 3.8 | 0.7 | 4.2 | 0.5 | 0.026 |
| 10. | Aware of environment | 3.1 | 0.7 | 3.4 | 0.7 | 0.041 |
| 12. | Shows imagination | 2.1 | 1.1 | 2.5 | 1.3 | 0.004 |
| 13. | Initiates activities | 2.7 | 0.9 | 3.2 | 0.9 | 0.006 |
| 14. | Dresses self | 3.8 | 0.6 | 4.2 | 0.4 | 0.011 |
| 15. | Curious, interested | 2.9 | 0.8 | 3.4 | 0.8 | 0.006 |
| 16. | Venturesome - explores | 2.7 | 0.9 | 3.1 | 1.0 | 0.010 |
| 17. | “Tuned in” - Not spacey | 1.9 | 0.8 | 2.4 | 1.0 | 0.016 |
| Mean | 2.6 | 3.3 | ||||
ATEC scale ranged from 1 to 5; in ATEC 1 and ATEC 3 increasing values mean more favorable situation (or treatment response), in ATEC 2 decreasing values, respectively.
Wilcoxon signed ranks test.
SD= standard deviation
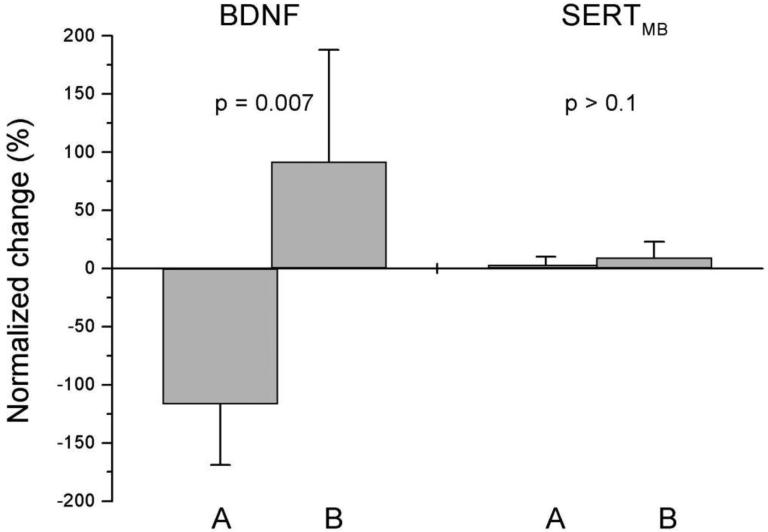
Post hoc analyses revealed that, the six good responders had a significant decrease in BDNF serum concentrations compared to baseline (p= 0.03), in contrast with the seven poor responders, who showed an increase in BDNF or no change. Actually, as a group, there was a trend towards increased BDNF concentrations among the poor clinical responders (p= 0.09) (Table 1). SERT binding changes in the midbrain were less pronounced; good clinical responders showed minimal or no differences and most responders in the poor response group showed an increase. Figure 1 illustrates these BDNF and SERT binding profiles in terms of scores on the ATEC 2.
Figure 1.
Change in serum brain derived neurotrophic factor concentrations (BDNF) and in serotonin transporter binding in midbrain (SERTMB), in two groups with different clinical responses to fluoxetine, according to the Autism Treatment Evaluation Checklist Section Two, Socialization. Group A (good responders, n = 6) had the best clinical response, while group B (poor responders, n = 7) had less beneficial clinical effect. Error bars indicate the standard deviations (SD). The difference of BDNF between the groups was significant. Normalized change was calculated as follows: 100 % (treatment – baseline) / 0.5·(treatment + baseline).
There was no correlation detected between: changes in BDNF concentrations or SERT binding and the baseline cognitive function (i.e., Leiter scale of non-verbal intelligence), or severity of autistic behavior (i.e., CARS scores). Neither, did these baseline measurements correlate with the clinical response during the study. There was also no correlation between fluoxetine dosage and any of the above mentioned variables.
During the trial, symptoms representing possible drug intolerability or adverse events were detected temporarily, including: hyperactivity (n= 5), increase in aggressive behavior (n=5), and sleep disturbances (n=4). Reducing or maintaining doses (i.e., not continuing increases) relieved these symptoms. None of the patients needed to discontinue medication because of adverse events during the 6 month treatment period. Parents of nine autistic subjects requested beginning fluoxetine treatment again, after the wash-out period and the follow-up investigations were completed, because the positive clinical effects clearly outweighed the side effects.
Discussion
At baseline, we detected a bimodal distribution of BDNF concentrations in the autistic cohort. Although the basis for this finding remains unknown, it may be additional evidence of the heterogeneous etiology and pathogenesis of otherwise homogenous autistic behavioral phenotypes. Our study indicates that, children with relatively higher baseline BDNF concentrations, followed by a decrease in this biomarker after fluoxetine treatment, had a more beneficial clinical outcome. Consistent with this, behavioral response to antidepressant treatment, is lost in depressed individuals with either reduced brain BDNF concentration or inhibited signaling of its receptor tyrosine kinase B (TrkB) [16]. A specific level of BDNF might be required for a positive response to SSRIs and this could, at least in part, explain the less favorable response in autistic children with low baseline BDNF. On the other hand, the decrease in serum BDNF concentrations after fluoxetine treatment, in the good responder group, may reflect correction of BDNF hyperactivity. Indeed, excessive BDNF can be deleterious for neuronal development, leading to premature ending of cortical plasticity [17]. Thus, in autism, BDNF may be a factor leading to precocious maturation, limiting the brain's ability to refine synaptic processes. Therefore, a hypothetical target for early intervention in autism is a decrease in BDNF signaling via blockade of TrkB receptors or reduction in BDNF synthesis, which could extend periods of synaptic plasticity and make it possible to overcome the atypical development seen in this disorder [18, 19].
As other biological parameters in autism, serum BDNF concentrations have been quite inconsistent in the literature. In a recent report, in serum samples collected at birth, BDNF concentrations were similar for infants who were later diagnosed as autistic, intellectual disabled, or typically developing [20]. Connolly and colleagues found elevated serum BDNF concentrations in pre-school age children with autism or childhood disintegrative disorder, when compared with children with normal development or other neurological disorders [3]. They also reported an association between immunological abnormalities, namely autoantibodies against BDNF and other antigens (e.g., myelin basic protein), and BDNF concentrations. These findings are consistent with Miyazaki et al. [21], who found elevated BDNF concentrations in children and young adults with autism (and others with intellectual disability) when compared with adult control subjects. However, a previous study demonstrated low serum BDNF levels in adult males with autism [4]. This discrepancy suggests that BDNF concentrations may be variable in autistic individuals and that they may also depend on their age. To our knowledge, no longitudinal studies of BDNF concentrations, in autistic subjects, have been reported, nor evaluations of BDNF changes after treatment of the disorder. For these reasons, our findings represent a contribution to the increasing body of literature on BDNF concentrations and genotype in autism [3, 20-22].
Additional support for the link between BDNF and autism has been provided by the study of specific genetic disorders associated with autism. In Rett syndrome, (a neurodevelopmental disorder with a regressive phase characterized by autistic features) although serum BDNF concentrations are not different from controls [23], the mutated protein in most cases (MeCP2) regulates BDNF transcription and cortical BDNF levels are decreased in mouse models [24]. Furthermore, BDNF polymorphisms appear to modify key phenotypical features of the disorder such as seizures [25]. Similarly, in fragile X syndrome, BDNF seems to regulate the expression of the deficient protein (FMRP) [26] and, as in Rett syndrome, BDNF polymorphisms influence the severity of seizures [27].
We have earlier shown that in autism, serotonergic activity is reduced in: the medial frontal cortex, the midbrain, and the temporal lobe areas [9]. Likewise, in a recent study of high functioning autistic subjects, SERT binding was reduced in several cortical areas [28]. In addition, the authors found correlations between decreased SERT binding and impaired social cognition in anterior and posterior cingulate cortices, and obsessive behavior in the thalamus. Our data suggests that fluoxetine has an effect on serotonergic metabolism via changes in SERT binding in the medial frontal cortex and the midbrain [9]. Nevertheless, connections between BDNF and serotonin signaling pathways, appear to be interesting targets for medical interventions in pathogenesis of neurological disorders [29]. On the other hand, several studies indicate that a subgroup of autistic patients (up to 36%) have elevated blood serotonin concentrations [30].
We hypothesized that children with autistic disorder would respond favorably to a six-month fluoxetine treatment trial, and that the clinical outcome would correlate with specific profiles of BDNF concentrations and SERT binding. In this way, the latter biomarkers would serve to identify autistic patients, who would benefit from SSRI treatment. We indeed found, positive clinical effects on several aspects, including: communication skills, socialization, and sensory and cognitive awareness, according to the ATEC scales. At least half of the patients had a favorable clinical response on 10 of the 23 ATEC items.
Some reports indicate that SSRI drugs may be effective for some autistic symptoms, including core social interaction and communication impairments [10-12]. DeLong and colleagues [10, 31] reported positive effects of fluoxetine treatment in autistic children, mainly on language function. Descriptions of individual cases, in these studies, indicate that marked responses were detected during the first weeks of treatment. In our study, we did not find such immediate or dramatic changes. Most of the favorable responses were seen at 12 weeks after the onset of the trial, but became more evident at the end of the six-month treatment period. Moreover, we found more prominent progress in social skills, sensory awareness, and cognitive functions, rather than in verbal abilities. Symptoms dealing with obsessive-compulsive-like behavior or stereotypes did not differ between the good and poor responders, neither during the baseline nor at the end of the treatment period.
In a recent placebo-controlled study, no efficacy of shorter-term (i.e., 3 months) citalopram therapy was detected on repetitive behavior symptoms in children with autistic spectrum disorders; including Asperger disorder and PDD-NOS [13]. The discrepancy in outcomes, between this study and ours, may be influenced by the differences in cohort composition (i.e., we only included children with autistic disorder) and length of treatment (i.e., 6 months in our study).
This study has some limitations. Firstly, we had a small sample size, which may have affected the ability to detect clinically important relationships. The open label design and the use of a parent-completed diary to collect outcome data, could have also led to subjective and non-consistent information. Moreover, during the six-month fluoxetine treatment and the subsequent two-month wash out period, there were unforeseeable factors (e.g., changes in family relationships) that may have affected the autistic subjects. The effect of concurrent school attendance and/or individual therapies (including speech and occupational therapy) is certainly impossible to be ruled out during such a long period of time. However, as stated in Methods, no changes in these non-pharmacological treatments occurred during the study.
Conclusions
The present study indicates that fluoxetine may have an effect on salient autistic manifestations in a subgroup of patients (46% in our study) and that this favorable clinical response to fluoxetine is possibly linked to a decrease in serum BDNF, further supporting the notion that BDNF is involved in the neurobiology of autism.
Acknowledgements
The authors would like to thank Mrs. Hilkka Kinnunen for caring assistance with the subjects and their families, and Vesa Kiviniemi PhL. for valuable guidance on statistical analyses, and Justin Martello for technical assistance with the BDNF assays.
Role of the Funding Source
This study was supported by the EVO Fund of the Hospital District of Northern Savo, Kuopio, Finland; the Arvo and Lea Ylppö Foundation, Helsinki, Finland; and NIH grant P01 HD24448.
Footnotes
Statement of Informed Consent
The study protocol was approved by the Research Ethics Committee of the Hospital District of Northern Savo, Kuopio, Finland. Written informed consent was obtained from the parents of the autistic subjects. For the BDNF components of the study, the protocol was explained to the control children and their parents and written informed consent was obtained. Children able to understand the protocol gave assent. The protocol for serum and CSF sample analysis was also approved by the Johns Hopkins Medical Institutions’ IRB.
Conflict of Interest
The authors have no conflicts of interest to disclose with regard to the content of this article.
Contributor Information
Ismo Makkonen, Department of Pediatrics, Unit of Child Neurology, Kuopio University Hospital, Kuopio, Finland; ismo.makkonen@kuh.fi.
Raili Riikonen, Department of Pediatrics, Unit of Child Neurology, Kuopio University Hospital, Kuopio, Finland; raili.riikonen@kolumbus.fi.
Jyrki T. Kuikka, Imaging Center, Kuopio University Hospital, and Niuvanniemi Hospital, Kuopio, Finland; jyrki.kuikka@kuh.fi.
Hannu Kokki, Department of Anesthesiology and Intensive Care, Kuopio University Hospital, Kuopio, Finland; hannu.kokki@kuh.fi.
Joseph P. Bressler, Center for Genetic Disorders of Cognition & Behavior, Kennedy Krieger Institute and the Johns Hopkins Bloomberg School of Public Health, Baltimore, Maryland, United States of America; bressler@kennedykrieger.org.
Cathleen Marshall, Center for Genetic Disorders of Cognition & Behavior, Kennedy Krieger Institute and the Johns Hopkins University School of Medicine, Baltimore, Maryland, United States of America; cathleen.marshall@gmail.com.
Walter E. Kaufmann, Center for Genetic Disorders of Cognition & Behavior, Kennedy Krieger Institute and the Johns Hopkins University School of Medicine, Baltimore, Maryland, United States of America; kaufmann@kennedykrieger.org.
References
- 1.American Psychiatric Association . Diagnostic and Statistical Manual of Mental Disorders, Fourth Edition, Text Revision (DSM-IV-TR) American Psychiatric Association; Washington, DC: 2000. [Google Scholar]
- 2.Riikonen R. Insulin-like growth factors. Neurobiological regulators of brain growth in autism? In: Zimmerman A, editor. Autism: Current theories and evidence. Humana Press; Totowa, NJ: 2008. pp. 233–44. [Google Scholar]
- 3.Connolly AM, Chez M, Streif EM, Keeling RM, Golumbek PT, Kwon JM, et al. Brain-derived neurotrophic factor and autoantibodies to neural antigens in sera of children with autistic spectrum disorders, Landau-Kleffner syndrome, and epilepsy. Biol Psychiatry. 2006;59(4):354–63. doi: 10.1016/j.biopsych.2005.07.004. [DOI] [PubMed] [Google Scholar]
- 4.Hashimoto K, Iwata Y, Nakamura K, Tsujii M, Tsuchiya KJ, Sekine Y, et al. Reduced serum levels of brain-derived neurotrophic factor in adult male patients with autism. Prog Neuropsychopharmacol Biol Psychiatry. 2006;30(8):1529–31. doi: 10.1016/j.pnpbp.2006.06.018. [DOI] [PubMed] [Google Scholar]
- 5.Binder DK, Scharfman HE. Brain-derived neurotrophic factor. Growth Factors. 2004;22(3):123–31. doi: 10.1080/08977190410001723308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Pardo CA, Eberhart CG. The neurobiology of autism. Brain Pathol. 2007;17(4):434–47. doi: 10.1111/j.1750-3639.2007.00102.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Chugani DC, Muzik O, Rothermel R, Behen M, Chakraborty P, Mangner T, et al. Altered serotonin synthesis in the dentatothalamocortical pathway in autistic boys. Ann Neurol. 1997;42(4):666–9. doi: 10.1002/ana.410420420. [DOI] [PubMed] [Google Scholar]
- 8.Bauman ML, Kemper TL. Neuroanatomic observations of the brain in autism: a review and future directions. Int J Dev Neurosci. 2005;23(2-3):183–7. doi: 10.1016/j.ijdevneu.2004.09.006. [DOI] [PubMed] [Google Scholar]
- 9.Makkonen I, Riikonen R, Kokki H, Airaksinen MM, Kuikka JT. Serotonin and dopamine transporter binding in children with autism determined by SPECT. Dev Med Child Neurol. 2008;50(8):593–7. doi: 10.1111/j.1469-8749.2008.03027.x. [DOI] [PubMed] [Google Scholar]
- 10.DeLong GR, Ritch CR, Burch S. Fluoxetine response in children with autistic spectrum disorders: correlation with familial major affective disorder and intellectual achievement. Dev Med Child Neurol. 2002;44(10):652–9. doi: 10.1017/s0012162201002717. [DOI] [PubMed] [Google Scholar]
- 11.Hollander E, Phillips A, Chaplin W, Zagursky K, Novotny S, Wasserman S, et al. A placebo controlled crossover trial of liquid fluoxetine on repetitive behaviors in childhood and adolescent autism. Neuropsychopharmacology. 2005;30(3):582–9. doi: 10.1038/sj.npp.1300627. [DOI] [PubMed] [Google Scholar]
- 12.McDougle CJ, Naylor ST, Cohen DJ, Volkmar FR, Heninger GR, Price LH. A double-blind, placebo-controlled study of fluvoxamine in adults with autistic disorder. Arch Gen Psychiatry. 1996;53(11):1001–8. doi: 10.1001/archpsyc.1996.01830110037005. [DOI] [PubMed] [Google Scholar]
- 13.King BH, Hollander E, Sikich L, McCracken JT, Scahill L, Bregman JD, et al. Lack of efficacy of citalopram in children with autism spectrum disorders and high levels of repetitive behavior: citalopram ineffective in children with autism. Arch Gen Psychiatry. 2009;66(6):583–90. doi: 10.1001/archgenpsychiatry.2009.30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Castren E. Neurotrophic effects of antidepressant drugs. Curr Opin Pharmacol. 2004;4(1):58–64. doi: 10.1016/j.coph.2003.10.004. [DOI] [PubMed] [Google Scholar]
- 15.Rimland B, Edelson SM. Autism Treatment Evaluation Checklist (ATEC) Autism Research Institute; San Diego, CA: 2000. [April 19, 2010]. Available at: http://www.autism.com/ari/atec/atec-online.htm. [Google Scholar]
- 16.Castren E, Voikar V, Rantamaki T. Role of neurotrophic factors in depression. Curr Opin Pharmacol. 2007;7(1):18–21. doi: 10.1016/j.coph.2006.08.009. [DOI] [PubMed] [Google Scholar]
- 17.Huang ZJ, Kirkwood A, Pizzorusso T, Porciatti V, Morales B, Bear MF, et al. BDNF regulates the maturation of inhibition and the critical period of plasticity in mouse visual cortex. Cell. 1999;98(6):739–55. doi: 10.1016/s0092-8674(00)81509-3. [DOI] [PubMed] [Google Scholar]
- 18.Bethea TC, Sikich L. Early pharmacological treatment of autism: a rationale for developmental treatment. Biol Psychiatry. 2007;61(4):521–37. doi: 10.1016/j.biopsych.2006.09.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Tsai SJ. TrkB partial agonists: potential treatment strategy for epilepsy, mania, and autism. Med Hypotheses. 2006;66(1):173–5. doi: 10.1016/j.mehy.2005.05.033. [DOI] [PubMed] [Google Scholar]
- 20.Croen LA, Goines P, Braunschweig D, Yolken R, Yoshida CK, Grether JK, et al. Brain-derived neurotrophic factor and autism: maternal and infant peripheral blood levels in the Early Markers for Autism (EMA) Study. Autism Res. 2008;1(2):130–7. doi: 10.1002/aur.14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Miyazaki K, Narita N, Sakuta R, Miyahara T, Naruse H, Okado N, et al. Serum neurotrophin concentrations in autism and mental retardation: a pilot study. Brain Dev. 2004;26(5):292–5. doi: 10.1016/S0387-7604(03)00168-2. [DOI] [PubMed] [Google Scholar]
- 22.Nishimura K, Nakamura K, Anitha A, Yamada K, Tsujii M, Iwayama Y, et al. Genetic analyses of the brain-derived neurotrophic factor (BDNF) gene in autism. Biochem Biophys Res Commun. 2007;356(1):200–6. doi: 10.1016/j.bbrc.2007.02.135. [DOI] [PubMed] [Google Scholar]
- 23.Vanhala R, Korhonen L, Mikelsaar M, Lindholm D, Riikonen R. Neurotrophic factors in cerebrospinal fluid and serum of patients with Rett syndrome. J Child Neurol. 1998;13(9):429–33. doi: 10.1177/088307389801300903. [DOI] [PubMed] [Google Scholar]
- 24.Kaufmann WE, Johnston MV, Blue ME. MeCP2 expression and function during brain development: implications for Rett syndrome's pathogenesis and clinical evolution. Brain Dev. 2005;27(Suppl 1):S77–S87. doi: 10.1016/j.braindev.2004.10.008. [DOI] [PubMed] [Google Scholar]
- 25.Zeev BB, Bebbington A, Ho G, Leonard H, de Klerk N, Gak E, et al. The common BDNF polymorphism may be a modifier of disease severity in Rett syndrome. Neurology. 2009;72(14):1242–7. doi: 10.1212/01.wnl.0000345664.72220.6a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Castren M, Lampinen KE, Miettinen R, Koponen E, Sipola I, Bakker CE, et al. BDNF regulates the expression of fragile X mental retardation protein mRNA in the hippocampus. Neurobiol Dis. 2002;11(1):221–9. doi: 10.1006/nbdi.2002.0544. [DOI] [PubMed] [Google Scholar]
- 27.Louhivuori V, Arvio M, Soronen P, Oksanen V, Paunio T, Castren ML. The Val66Met polymorphism in the BDNF gene is associated with epilepsy in fragile X syndrome. Epilepsy Res. 2009;85(1):114–7. doi: 10.1016/j.eplepsyres.2009.01.005. [DOI] [PubMed] [Google Scholar]
- 28.Nakamura K, Sekine Y, Ouchi Y, Tsujii M, Yoshikawa E, Futatsubashi M, et al. Brain serotonin and dopamine transporter bindings in adults with high-functioning autism. Arch Gen Psychiatry. 2010;67(1):59–68. doi: 10.1001/archgenpsychiatry.2009.137. [DOI] [PubMed] [Google Scholar]
- 29.Mattson MP, Maudsley S, Martin B. BDNF and 5-HT: a dynamic duo in age-related neuronal plasticity and neurodegenerative disorders. Trends Neurosci. 2004;27(10):589–94. doi: 10.1016/j.tins.2004.08.001. [DOI] [PubMed] [Google Scholar]
- 30.Cook EH, Leventhal BL. The serotonin system in autism. Curr Opin Pediatr. 1996;8(4):348–54. doi: 10.1097/00008480-199608000-00008. [DOI] [PubMed] [Google Scholar]
- 31.DeLong GR, Teague LA, McSwain Kamran M. Effects of fluoxetine treatment in young children with idiopathic autism. Dev Med Child Neurol. 1998;40(8):551–62. doi: 10.1111/j.1469-8749.1998.tb15414.x. [DOI] [PubMed] [Google Scholar]