Abstract
Background
Vascular cognitive impairment (VCI) is a spectrum of cognitive impairment caused by various chronic diseases including aging, hypertension, and diabetes mellitus. Oxidative and inflammatory reactions induced by chronic cerebral hypoperfusion (CHP) are believed to cause VCI. Melatonin is reported to possess anti-oxidation and anti-inflammation effects. This study was designed to investigate the effect and mechanisms of melatonin in CHP mice model.
Results
The behavioral function results revealed that CHP mice were significantly impaired when compared with the control. Melatonin improved the cognitive function, but the addition of MT2 receptor antagonist reversed the improvement. The IHC staining showed melatonin significantly improved WM lesions and gliosis in CHP mice. Again, the addition of MT2 receptor antagonist to melatonin worsened the WM lesion and gliosis. Similar results were also found for mRNA and protein expressions of oxidative reaction and inflammatory cytokines.
Materials and Method
Forty C57BL/6 mice were divided into four groups: Group 1: sham control; Group 2: CHP mice; Group 3: CHP with melatonin treatment; Group 4: CHP-melatonin and MT2 receptor antagonist (all groups n = 10). Working memory was assessed with Y–arm test at day-28 post-BCAS (bilateral carotid artery stenosis). All mice were sacrificed at day-30 post-BCAS. The immunohistochemical (IHC) staining was used for white matter (WM) damage and gliosis. The expression of mRNA and proteins about inflammatory and oxidative reaction were measured and compared between groups.
Conclusions
Partially through MT2 receptor, melatonin is effective for CHP-induced brain damage.
Keywords: brain damage, chronic cerebral hypoperfusion, melatonin
INTRODUCTION
Vascular cognitive impairment (VCI) is the secondary cause of dementia in the world [1–3]. VCI increases the morbidity, disability, and health care costs in the growing elderly population, and decreases the quality of life and survival rate of patients despite aggressive therapy [1–8]. Aging, chronic hypertension, and diabetes mellitus have been reported associations with the onset of VCI, which is characterized by white matter (WM) lesions [9–11]. These vascular risk factors result in vascular remodeling, which then lead to chronic cerebral hypoperfusion (CHP). CHP, a consequence of chronic hypertension, diabetes mellitus and aging, is a critical factor that leads to the onset of VCI [9–11]. Characterized by blood-brain barrier (BBB) disruption, WM lesions, damage of oligodendrocytes, activated gliosis and cognitive dysfunction, CHP triggers off oxidative reactions and inflammatory processes, resulting in white matter (WM) damage [12–14].
Melatonin is synthesized primarily in the pineal gland, and is involved with the circadian rhythm [15–17]. Several of its actions are mediated via two G-protein-coupled membrane receptors called MT1 and MT2. [15–17] Additionally, melatonin has been shown to be a powerful antioxidant with multifaceted protective capacities against oxidative stress and the organ dysfunction from the enhancement of reactive oxygen species (ROS) generation [15–19]. Previous studies have shown that melatonin therapy remarkably reduces the generation of ROS as well as oxidative stress in animal models of ischemia-reperfusion injury [18, 19]. Based on the anti-oxidative and inflammatory effects of melatonin, the use of melatonin can be projected to offer benefits for CHP-induced vascular-impaired dementia (VID). However, it is unclear whether these effects are caused solely by melatonin, or also partially by the activation of the melatonin MT2 receptor [20–23]. This study was designed to test whether or not melatonin attenuates CHP induced brain damage and MT2 receptors take part in the aforementioned process.
RESULTS
The effect of melatonin on cerebral blood flow and working memory in CHP mice
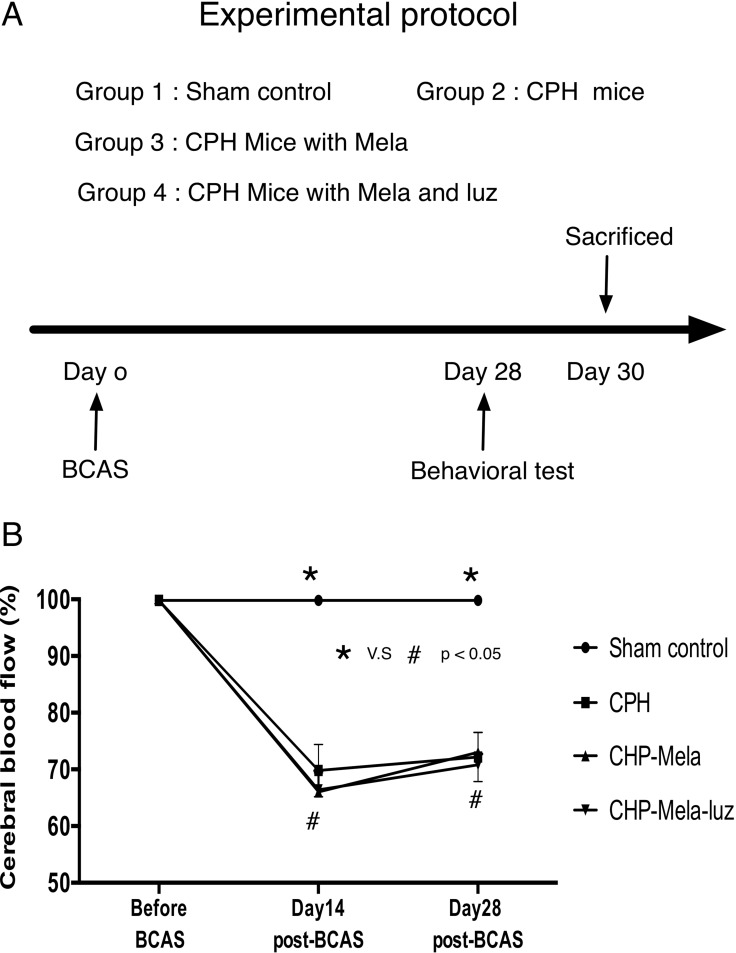
The study protocol is presented in Figure 1A. The cerebral blood flow (CBF) values from the deceased mice were excluded because extremely low CBF was found in these mice, which was resulted from systemic hypotension. In group 1, the mean CBF after the sham operation varied from 97.0% to 102% of the baseline level throughout the entire experiment. In groups 2, 3, and 4, the CBF values showed a significant decrease to around 65% of the baseline level at day-14 post-BCAS. The CBF values remained relatively low, at 75% of the baseline level, at day-28 post-BCAS (Figure 1B).
Figure 1.
(A) The animal experimental protocol. Time course of individual interventional procedure during the study period. The melatonin and luzindole were treated at 24 hours post-bilateral carotid artery stenosis(BCAS) until 30 days post-BCAS. SC = sham control; CHP = cerebral hypoperfusion mice; CHP-Mela = cerebral hypoperfusion mice with melatonin (10 mg/kg/day) treated; CHP-Mela-Luz = cerebral hypoperfusion mice with melatonin(10 mg/kg/day) and luzindole (30 mg/kg/day, ip). (B) Laser Doppler flow recordings in different groups. The Laser Doppler analysis of cerebral blood flow(CBF) was performed. Results represent the mean values ± SEM in different groups mice. Group 1 = sham control, Group 2 chronic cerebral hypoperfusion(CHP) mice, Group 3: melatonin treatment in CHP mice, Group 4: addition MT2 antagonist with melatonin in CHP mice. The decrease in CBF at day 30 post-BCAS of approximately 70% was similar between CHP, CHP-Mela and CHP-Mela-Luz mice groups. Different symbols (#, *) indicate significance, p < 0.05. All statistical analyses using by repeat two-away ANOVA test, followed by Bonferroni multiple comparison post hoc test.
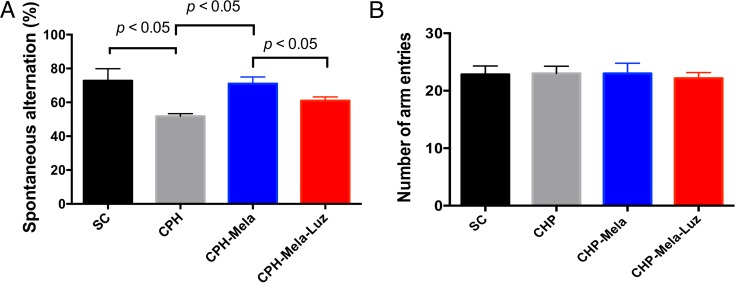
As shown in Figure 2, the Y maze (spontaneous alternation) was performed to discriminate the working memories between the 4 groups. In regards to CHP mice, group 2 showed a significant impairment in comparison with group 1 (P < 0.05). Treatment with melatonin (group 3) significantly improved the spatial working memory when compared with those without additional treatment. However, the positive effect of melatonin was lessened when the MT2 receptor antagonist was added to melatonin (group 4). There was no significant difference between four groups in numbers of arm entries.
Figure 2. Effects of melatonin and melatonin with MT2 receptor antaginist on spatial working memory in mice post-BCAS at day-28.
Spontaneous alternation. Group 1 = sham control, Group 2 chronic cerebral hypoperfusion(CHP) mice, Group 3: melatonin treatment in CHP mice, Group 4: addition MT2 receptor antagonist with melatonin in CHP mice. Values are mean SEM (n =10 in each group). Spontaneous alternation ratio was decreased in group 2 than group 1. The treated melatonin increased the Spontaneous alternation ratios in group 3 than group 2. However, the addition MT2 receptor antagonist with melatonin abolished the protective effect of melatonin in group 4. The p value < 0.05 indicates significance between two groups. All statistical analyses using one-way ANOVA, followed by Bonferroni multiple comparison post hoc test. Number of arm entries. Group 1 = sham control, Group 2 chronic cerebral hypoperfusion(CHP) mice, Group 3 : melatonin treatment in CHP mice, Group 4 addition MT2 receptor antagonist with melatonin in CHP mice. Values are mean SEM (n =10 in each group). There was no significant difference between four groups. All statistical analyses using one-way ANOVA.
Effects of melatonin on WM Lesions, and glial activation in CHP Mice
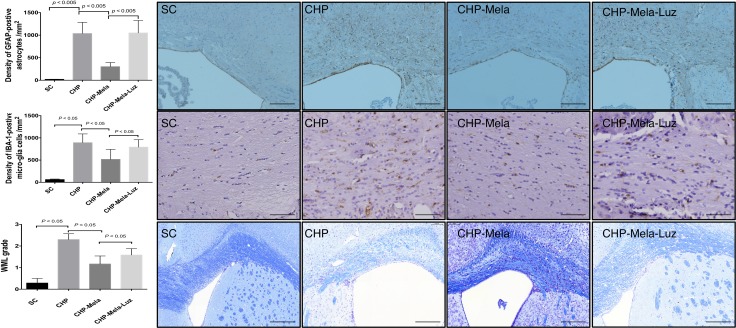
As shown in Figure 3A–3B, the activated microglial cells and astrocytes were significantly increased in CHP mice received BCAS (group 2) as compared with SC (group 1). Besides, the activated microglial cells and astrocytes in CHP mice were significantly attenuated when melatonin was provided (group 3) when compared with mice in group 2. Furthermore, the addition of MT2 antagonist to melatonin treatment raised the number of activated microglial cells in CHP mice (group 4).
Figure 3.
Effects of Melatonin on the activation of astrocytes (A) microglia (B) and white matter lesion (C) in mice with chronic cerebral hypoperfusion. Upper panels: are representative photomicrographs of immunostaining of glial fibrillary acidic protein(GAFA) in the paramedian parts of the corpus callosum. Density of GFAP-positive astrocytes; Scale bar = 200 um. Middle panels: are representative photomicrographs of immunostaining of ionized calcium binding adaptor molecule-1 (IBA-1)-positive microglial cells in the paramedian parts of the corpus callosum. Density of Iba-1-positive microglial cells; Scale bar = 100 um. Lower panel: Immunohistochemical (IHC) images (100×) illustrating white matter lesion (WML) of different severity reflected in the grading; Scale bars in right lower corner represent 200 µm. All statistical analyses using one-way ANOVA, followed by Bonferroni multiple comparison post hoc test. SC = sham control; CHP = cerebral hypoperfusion; CHP-Mela = cerebral hypoperfusion with melatonin (10mg/kg/day,ip); CHP-Mela-Luz = cerebral hypoperfusion with melatonin (10 mg/kg/day,ip) and luzindole (30 mg/kg,ip).
The severity grade of WM lesion of the corpus callosum was significantly higher in group 2 when compared with that in group 1. The WM lesion was found significantly reduced when melatonin was offered (group 3). However, the addition of MT2 receptor antagonist to melatonin worsened the WM lesion in group 4 mice (Figure 3C).
Effects of melatonin on the mRNA expression of inflammatory cytokine and oxidative stress in CHP Mice
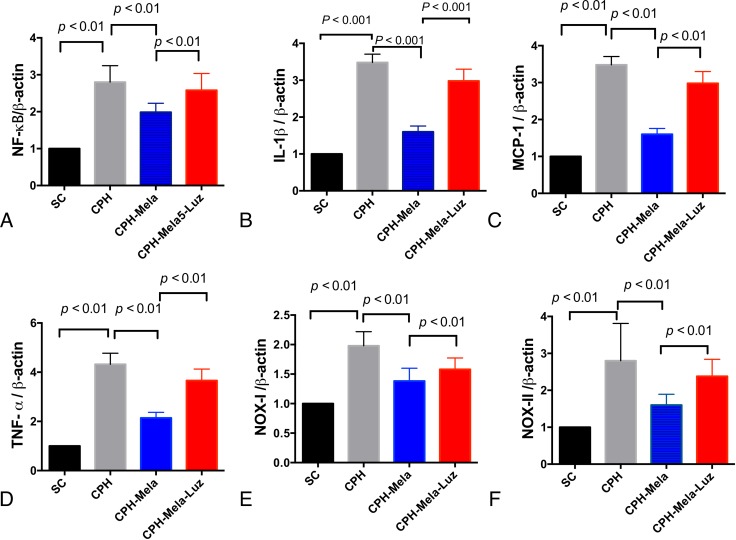
As shown in (Figure 4A–4D), the levels of inflammatory cytokines such as cerebral NF-ƘB, IL-1β, MCP-1, and TNF-α were significantly increased in CHP mice received BCAS (group 2) as compared with SC (group 1). Melatonin treatment significantly attenuated the mRNA expression of these inflammatory cytokines in CHP mice (group 3). However, additions with MT2 antagonist and melatonin comprised the inhibitory effect of melatonin on these mRNA expressions of these inflammatory cytokines in CHP mice (group 4). As shown in Figure 4D–4F), the mRNA expressions of oxidation markers such as NOX-I and NOX-II were significantly increased in CHP mice received BCAS (group 2). The provision of melatonin significantly attenuated the mRNA expressions of these oxidative stress markers in CHP mice, but the reduction of mRNA expressions for these oxidative stress markers were significantly compromised by the addition of MT2 antagonist.
Figure 4. The mRNA expressions of inflammatory and oxidative markers in cerebral cortex by day 30 after chronic cerebral hypoperfusion (CHP) procedure (n = 6).
(A) mRNA expression of IL-1ß; (D, B) mRNA expression of tumor necrotic factor (TNF)-α, (C) The mRNA expression of monocyte chemoattractant protein (MCP)-1; D) The mRNA expression of NF-kB; (E) The mRNA expression of NOX-I; (F) The mRNA expression of NOX-II; The p < 0.05 indicates statistical significance between two groups. All statistical analyses using one-way ANOVA, followed by Bonferroni multiple comparison post hoc test. SC = sham control; CHP = cerebral hypoperfusion; CHP-Mela = cerebral hypoperfusion with melatonin (10 mg/kg/day,ip); CHP-Mela-Luz= cerebral hypoperfusion with melatonin (10 mg/kg/day,ip) and luzindole (30 mg/kg,ip).
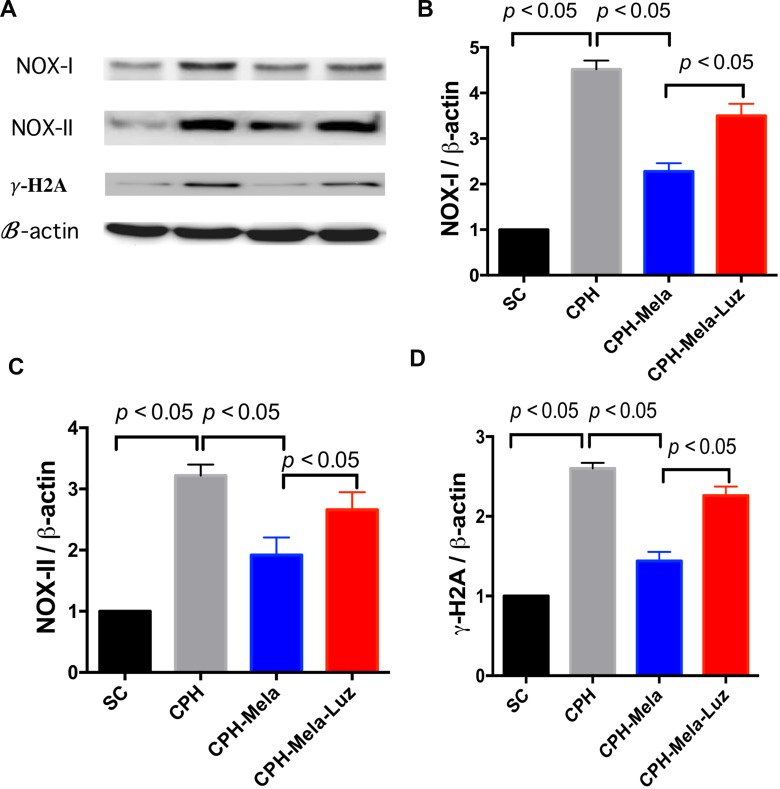
Effects of melatonin on protein expressions of oxidative stress and DNA damage in CHP mice
As shown in (Figure 5A–5D), the protein expressions of oxidation markers such as NOX-I and NOX-II induced by CHP were significantly reduced when melatonin was provided. However, this anti-oxidation protective effect of melatonin was reversed when MT2 receptor antagonist was added. The γ-H2A is a marker of DNA damage, which helps to represent the condition of brain damage. The protein expressions of γ-H2A were significantly increased in CHP mice. The addition of MT2 receptor antagonist to melatonin raised the expression of γ-H2A again.
Figure 5. Changes in protein expressions of oxidative and DNA damage markers at 30 days in mice with chronic cerebral hypoperfusion.
(A) Illustration of Western blot results of ROS (i.e., NOX-I and NOX-II) and r-H2A. (B) Protein expression of NOX-I; (C) Protein expression of NOX-II;. (D) Protein expression of r-H2A; All statistical analyses using one-way ANOVA, followed by Bonferroni multiple comparison post hoc test. The p < 0.05 indicates statistical significance between two groups. SC = sham control; CHP = cerebral hypoperfusion; CHP-Mela = cerebral hypoperfusion with melatonin (10 mg/kg/day,ip); CHP-Mela-Luz = cerebral hypoperfusion with melatonin (10 mg/kg/day, ip) and luzindole (30 mg/kg, ip).
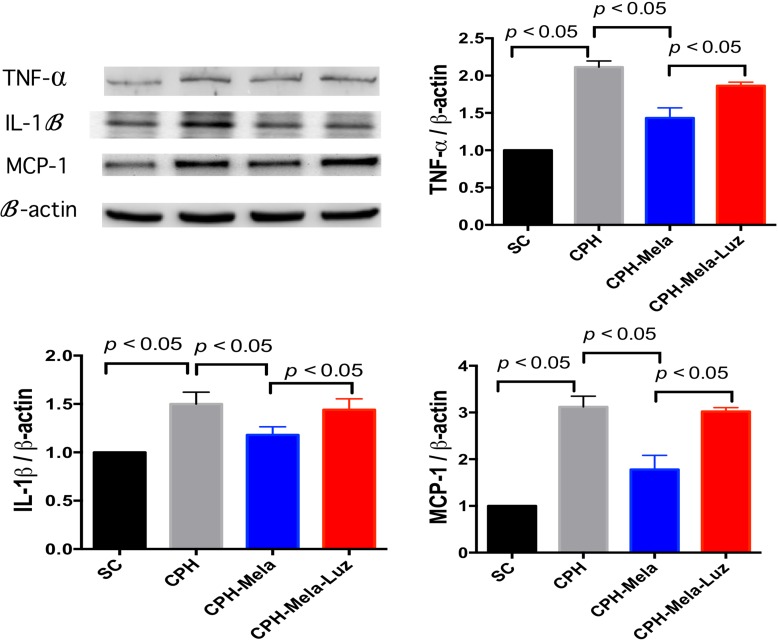
Effects of melatonin on proteins expression of inflammatory markers in CHP mice
As shown in (Figure 6A–6D), the protein expressions of inflammatory markers such as IL-1β, TNF-α and MCP-1 were significantly increased in CHP mice. The protein expressions of IL-1 β, TNF-α and MCP-1 were significantly higher in CHP mice than in SC. Melatonin treatment significantly attenuated these inflammatory protein expressions in CHP mice. However, the addition of MT2 receptor antagonist to melatonin increased these IL-1 β and TNF-α protein expressions again.
Figure 6. Changes in protein expressions of inflammatory markers at 30 days in mice with chronic cerebral hypoperfusion.
(A) Illustration of Western blot results of inflammatory markers: IL-1ß, T TNF-α and MCP-1. (B) Protein expression of IL-1ß; (C) Protein expression of TNF-α; (D) Protein expression of MCP-1; All statistical analyses using one-way ANOVA, followed by Bonferroni multiple comparison post hoc test. The p < 0.05 indicates statistical significance between two groups. SC = sham control; CHP = cerebral hypoperfusion; CHP-Mela = cerebral hypoperfusion with melatonin (10mg/kg/day, ip); CHP-Mela-Luz = cerebral hypoperfusion with melatonin (10 mg/kg/day, ip) and luzindole (30 mg/kg, ip)
DISCUSSION
This was an investigational study aimed to reveal the therapeutic effects and mechanisms of action for melatonin for the cognitive impairment in CHP mice. The study results demonstrated interesting findings. First, melatonin attenuates both the activation of microglial cells and astrocytes and WM damage were attenuated by melatonin in CHP mice. Second, the cognitive function was improved by melatonin in CHP mice. Third, the action mechanisms of melatonin to improve cognitive function, WM lesions and gliosis were partially mediated through the MT2 receptor pathway which showed anti-oxidative and anti-inflammatory effects. Brain damage and cognitive dysfunction in CHP mice were improved after melatonin treatment. Melatonin might thus be considered as a putative treatment to protect the cognitive function in CHP mice.
Oxidative stress and inflammation process are major factors in pathogenesis of CHP-induced brain damage
Oxidative stress and inflammation process are reported to be major factors for CHP-induced brain damage and the progression of cognitive dysfunction in the setting of chronic CHP. Bio-markers of oxidative stress such us 8-isoprostaglandin F(2a) (8-isoPGF(2a)) and nitrotyrosine (NT) are obviously elevated in patients with VCI and dementia [24–30]. Inflammatory cytokines such us interleukin (IL)-6, and hs-CRP (high sensitivity- chronic reactive protein) are also elevated in patients with dementia [31–35]. Interestingly, (IL)-6 and hs-CRP are predictors used for cognitive decline. In additional, the decrease of glutathione (GSH) and the elevation of oxidative stress and inflammatory cytokines have been linked to the WM damage and gliosis in animal CHP model [36–41]. The expressions of oxidative stress and inflammation reactions were obviously elevated and correlated with the severity of WM lesion and gliosis in CHP mice in the study. Besides, the brain damage was attenuated and cognitive function improved after oxidative stress and inflammation were inhibited.
Benefits of melatonin therapy in attenuating CHP brain damage
The study aimed to explore the therapeutic effect and action mechanisms of melatonin to improve cognitive impairment in CHP mice models. The WM lesion and glial cells activation were exhibited in the CHP mice model just as they demonstrated in previous studies [13, 14]. Melatonin was showed to both attenuate the WM lesions and glial cells activation and improve cognitive function in our study. Melatonin is found to possess strong anti-oxidative effect by inhibiting the expression of NADPH oxidases (NOX) in many studies [15, 16, 18, 19, 42]. Among the NOXs, NOX-I and NOX-II play major regulating roles in oxidative stress reactions [15, 16, 18, 19, 42]. Brain damage in CHP mice was effectively attenuated by reducing the oxidative stress in previously published studies [43, 44]. Our study demonstrated that melatonin attenuated the expression of oxidative stress in CHP mice, indicating that melatonin possessed with anti-oxidative effect.
Cerebral hypoxia induced by CHP leads to the increase of inflammation reaction, which plays an important role in the pathogenesis of WM lesion, gliosis and cognitive dysfunction [13, 14]. The expression of inflammatory cytokines (i.e. MCP-1, TNF-α, and IL-1β) was found increased in the CHP mice in our study and this was consistent with the findings in previous reports [13, 14] Melatonin was then found to attenuate the expression of inflammatory cytokines in mRNA and protein levels and improved the WM lesion, gliosis and cognitive function in our study. These results were also consistent with the results in previous reports [12–14]. We therefore conclude that melatonin is effective to attenuate the brain damage induced by CHP through inhibiting oxidative stress and inflammation.
Protection against brain damage of melatonin may through MT2 receptor pathway
Brain damage induced by CHP would also lead to ischemia which could later result in DNA damage and lipid per-oxidation [43]. The G-protein–coupled melatonin MT1 and MT2 receptors are all expressed in the brain [45]. In addition, melatonin activates the Nrf2-antioxidant responsive element (Nrf2-ARE) pathway in experimental diabetic neuropathy, a subarachnoid hemorrhage model, and ischemic stroke through melatonin receptor [46–48]. It is unclear whether melatonin attenuates the oxidative stress through spontaneous antioxidant effect or the activation of MT2 receptor in CHP model. Melatonin demonstrates cellular protective effect through both anti-oxidative stress and MT2 receptor [49–52]. The reduction of oxidative stress is revealed to alleviate the ischemia-related damage such as stroke and CHP in previous studies [12, 23, 53]. This study was designed to reveal the therapeutic mechanisms of melatonin in CHP mice. Interestingly, we found that the addition of MT2 receptor antagonist to melatonin for CHP mice worsened the WM lesion, glial cell activation and cognitive function. Besides, the comeback of oxidative stress and inflammation in CHP mice was obviously observed after MT2 receptor antagonist was added to melatonin treatment. The protective effect of melatonin in the CHP mice was also reversed by the addition of MT2 receptor antagonist. These findings indicated that MT2 receptor is involved in the anti-oxidative and anti-inflammation effects of melatonin in this CHP mice model. Nevertheless, luzindole (MT2 receptor antagonist) is found to antagonize the protective effect of melatonin [20]. Luzindole is found to possess anti-depression effect as well [22]. Melatonin has also been reported to attenuate ischemic neuronal damage with up-regulation of the MT2 melatonin receptor [21]. Luzindole (MT2 receptor antagonist) significantly reversed the anti-oxidative and anti-inflammation effects of melatonin in CHP mice. These findings suggested that melatonin attenuate the brain damage induced by CHP partially through MT2 receptor to inhibiting oxidative stress and inflammation.
This study has several limitations. First, this study did not investigate the optimal dosage of melatonin for maximizing the brain-protecting effects in this CHP mice model. However, previous studies have demonstrated that the dose of melatonin (10 mg/kg/day) offer adequate protective effects in mice with ischemic organ dysfunction [23, 54]. We therefore followed the dosage they used in previous studies [23, 54]. It proved in the end to be suitable for our study. Second, we didn’t provide the CCH mice with Luzindole only treatment to investigate whether MT2 antagonist were associated with the effects of endogenous melatonin mediated brain damage. However, recent paper found chronic use of MT2 antagonist were involved in the effects of endogenous melatonin to mediate the hippocampal neurogenic process in C57BL/6 mice [55]. This finding provided that MT2 antagonist has an effect on its own, which was contrasts the improvement due to endogenous melatonin. Third, the age of mice is twelve-week-old with a duration of 30 days for cerebral hypoperfusion in the study. Clinical VCI is a long-term progressive disease found in elderly patients. This study did not evaluate the long-term impact of melatonin treatment; it would not be suitable to directly apply these results to a clinical setting. Forth, although the exploration of the therapeutic effects of melatonin was attempted, the precise signaling pathways through melatonin in protecting brain damage in CHP mice model were not completely elucidated.
In conclusion, melatonin offers a substantial therapeutic advantage of protection against CHP-induced brain damage and cognitive dysfunction in a murine model. The results of this study encourage a prospective clinical trial in order to evaluate the therapeutic potential of melatonin in the clinical setting of VCI.
MATERIALS AND METHODS
Experimental protocol and bilateral carotid artery surgery
The study protocol is presented in Figure 1A. Twelve-week-old male C57Bl/6J mice (n = 40) weighing 25–30 g (Charles River Technology, BioLASCO Taiwan Co. Ltd., Taiwan) were randomized and equally divided into group 1 (sham controls, n = 10), group 2 [chronic cerebral hypoperfusion (CHP) with bilateral carotid artery stenosis (BCAS) only, n = 10], group 3 [CHP with intraperitoneal (i.p) 10 mg/kg melatonin treatment, beginning 24 hours post BCAS until 30 days post BCAS, n = 10], group 4 [CHP with intraperitoneal 10 mg/kg melatonin and 5 mg/kg MT2 receptor antagonist (luzozide), beginning 24 hours post BCAS until 30 days post BCAS, n = 10] to determine the therapeutic effect of melatonin on mice with BCAS. Immediately after their working memories were assessed by the Y maze at day-28 post BCAS, mice were sacrificed for a histological and real-time quantitative reverse transcriptase–polymerase chain reaction (RTqPCR) examination at day-30 post-BCAS. All animals were anesthetized by inhalational 3.0% isoflurane and placed supine on a warming pad at 37°C during the BCAS procedure. The chronic cerebral hypoperfusion mice model was induced by BCAS which introducing wire micro coils (Sawane Spring Co, Shizuoka, Japan) with an internal diameter of 0.16 mm at left common carotid artery and 0.18mm around right common carotid artery to result in luminal narrowing (n = 30) [12, 13]. Sham-control mice received identical surgical interventions without the coil placement (n = 10). All animal experimental procedures were approved by the Institute of Animal Care and Use Committee at Kaohsiung Chang Gung Memorial Hospital and performed in accordance with the Guide for the Care and Use of Laboratory Animals (NIH publication No. 85–23, National Academy Press, Washington, DC, USA, revised 1996).
Cerebral blood flow measurement
Under deep anesthesia by inhalational 3.0% isoflurane in a 50:50 mixture of oxygen to room air, mice were placed in a prone position on a warming pad at 37°C. The skin overlying the right skull was reflected. Cerebral blood flow was measured using laser Doppler imaging (MoorLD1- 2l; Moor Instruments, Devon, UK). After equilibration to the experimental setup, laser point was fixed perpendicular to the skull at 1 mm posterior and 2.5 mm lateral to the bregma and local cerebral blood flow was obtained. The baseline CBF recordings were obtained before and on day-14 and day-28 after the BCAS. The CBF values were expressed as a percentage of the baseline value.
The behavioral test: Y arm maze test
Y-maze is designed with 3 arms with 40 cm long, 13.5 cm high and 4 cm wide and locates in a dim room. Each mouse will be placed at the end of an arm and will move through the maze for 10 minutes per session. Spontaneous alternation can be defined as the consecutive entry of a mouse into all three different arms to form a triplet of non-repeated components. The percentage of spontaneous alternation can be calculated as the ratio of actual to possible alterations automatically by the Y-maze system [defined as the number of spontaneous alternation behavior / (the total number of arm entries - 2) × 100] [14].
Histopathology scoring staining
After BCAS, the mice were anesthetized by inhalational 3.0% isoflurane and perfused transcardially with 0.01 mol/L PBS (pH 7.4), then with a fixative containing 4% paraformaldehyde and 0.2% picric acid in 0.1mol/L phosphate buffer (pH7.4), and stored in 20% sucrose in 0.1 mol/L PBS (pH 7.4). The brains were embedded in paraffin and sliced into 5 μm-thick coronal sections. Kluver-Barrera (KB) staining was used to determine the demyelination changes in different groups. The severity of the WM lesions was graded as normal (grade 0), disarrangement of the nerve fibers (grade 1), the formation of marked vacuoles (grade 2), and the disappearance of myelinated fibers (grade 3) as described elsewhere. [12] For immunohistochemistry, the rest of the coronal blocks were cut into serial sections (10μm thick) in a cryostat and incubated overnight with a rabbit anti–glial fibrillary acidic protein (GFAP) antibody (diluted 1: 1000; DAKO, Denmark) and goat anti-ionized calcium binding adaptor molecule-1 (Iba-1) antibody (1:200, Abcam). Subsequently, these sections were treated with the appropriate biotinylated secondary antibodies (Santa Cruz Biotechnology) and were visualized with 0.01% diaminobenzidine tetrahydrochloride and 0.005% H2O2 in 50 mmol/L Tris HCl (pH 7.6). The WM lesions were evaluated in corpus callosum. We counted the numerical density of the glial cell nuclei with immunopositive perikarya in a 0.125 mm2 area of corpus callosum in 4 animals from each group. We counted the numerical density of the glial cell nuclei with immunopositive perikarya in the white matter, as described [12].
Real-time quantitative PCR analysis
The mRNA expressions of MCP-1, IL-1β, TNF-α, NF-ϰB, NOX-I and NOX-II in each of the three groups of animals were analyzed with RT-qPCR and compared. RNA was extracted from brain using EuroGold Trifast (EuroClone). Quantitec Reverse Transcription Kit (Qiagen), according to manufacturer’s protocol. Quantitative analysis was performed using SYBR Green 2X PCR Master Mix (Applied Biosystem). Each sample was run in triplicate and normalized to the expression of housekeeping B-actin gene as previously described [12].
Western blot analysis of brain specimens
Equal amounts (30 μg) of protein extracts from brain of the animals (n = 6, for each group) were loaded and separated by SDS-PAGE using 7% or 12% acrylamide gradients. The membranes were incubated with monoclonal antibodies against NAPH oxidase (NOX)-I (1:1500, Sigma), NOX-II (1:500, Sigma), IL-1β (1:1000, proteintech), r-H2A (1:1000 Abcam), MCP-1 (1:1000, Millipore), TNF-⍺ (1: 1000, Cell Signaling) were used. Signals were detected with horseradish peroxidase (HRP)-conjugated goat anti-mouse, goat anti-rat or goat anti-rabbit IgG. Proteins were transferred to nitrocellulose membranes which were then incubated in the primary antibody solution overnight, followed by a washing procedure carried out three times within 15 minutes. The nitrocellulose membranes were then incubated with the second antibody solution for one hour at room temperature. The washing procedure was repeated three times within 15 minutes. Immunoreactive bands were visualized by enhanced chemiluminescence (ECL; Amersham Biosciences), which was then exposed to Biomax L film (Kodak). For quantification, ECL signals were digitized using Labwork software (UVP).
Statistical analysis
All data were presented as mean ± SD. Statistical analyses were performed using SPSS 18.0 (SPSS Inc, Chicago, IL) to conduct one-way and two-way analysis of variance (ANOVA), followed by a Bonferroni multiple-comparison post hoc test to compare the physiological parameters. A Mann–Whitney U test was used to compare the severity of WM lesions between the groups. P < 0.05 was considered to be statistically significant.
ACKNOWLEDGMENTS AND FUNDING
This study was supported by a program grant from Chang Gung Memorial Hospital, Chang Gung University (Grant number: CMRPG8D0591) and NMRPG8E0301.
Footnotes
CONFLICTS OF INTEREST
The authors declare that they have no competing interests of any sort, including commercial association, such as consultancies, stock ownership or other equity interests or patent-licensing arrangements.
REFERENCES
- 1.Iadecola C, Gorelick PB. Converging pathogenic mechanisms in vascular and neurodegenerative dementia. Stroke. 2003;34:335–7. doi: 10.1161/01.str.0000054050.51530.76. [DOI] [PubMed] [Google Scholar]
- 2.Mauri M, Corbetta S, Pianezzola C, Ambrosoni E, Riboldazzi G, Bono G. Progression to vascular dementia of patients with mild cognitive impairment: relevance of mild parkinsonian signs. Neuropsychiatr Dis Treat. 2008;4:1267–71. doi: 10.2147/ndt.s4288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Wentzel C, Rockwood K, MacKnight C, Hachinski V, Hogan DB, Feldman H, Ostbye T, Wolfson C, Gauthier S, Verreault R, McDowell I. Progression of impairment in patients with vascular cognitive impairment without dementia. Neurology. 2001;57:714–6. doi: 10.1212/wnl.57.4.714. [DOI] [PubMed] [Google Scholar]
- 4.Genao L, White H, Twersky J. The clinical course of advanced dementia. N Engl J Med. 2010;362:363–4. author reply 4-5. [PubMed] [Google Scholar]
- 5.Savva GM, Wharton SB, Ince PG, Forster G, Matthews FE, Brayne C. Age, neuropathology, and dementia. N Engl J Med. 2009;360:2302–9. doi: 10.1056/NEJMoa0806142. [DOI] [PubMed] [Google Scholar]
- 6.Irish M, Addis DR, Hodges JR, Piguet O. Exploring the content and quality of episodic future simulations in semantic dementia. Neuropsychologia. 2012;50:3488–95. doi: 10.1016/j.neuropsychologia.2012.09.012. [DOI] [PubMed] [Google Scholar]
- 7.Novella JL, Dhaussy G, Wolak A, Morrone I, Drame M, Blanchard F, Jolly D. Quality of life in dementia: state of the knowledge. Geriatr Psychol Neuropsychiatr Vieil. 2012;10:365–72. doi: 10.1684/pnv.2012.0375. [DOI] [PubMed] [Google Scholar]
- 8.Gitlin LN, Kales HC, Lyketsos CG. Nonpharmacologic management of behavioral symptoms in dementia. JAMA. 2012;308:2020–9. doi: 10.1001/jama.2012.36918. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Wiesmann M, Kiliaan AJ, Claassen JA. Vascular aspects of cognitive impairment and dementia. J Cereb Blood Flow Metab. 2013 doi: 10.1038/jcbfm.2013.159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Gorelick PB, Pantoni L. Advances in vascular cognitive impairment. Stroke. 2013;44:307–8. doi: 10.1161/STROKEAHA.111.000219. [DOI] [PubMed] [Google Scholar]
- 11.Rincon F, Wright CB. Vascular cognitive impairment. Curr Opin Neurol. 2013;26:29–36. doi: 10.1097/WCO.0b013e32835c4f04. [DOI] [PubMed] [Google Scholar]
- 12.Tsai TH, Sun CK, Su CH, Sung PH, Chua S, Zhen YY, Leu S, Chang HW, Yang JL, Yip HK. Sitagliptin attenuated brain damage and cognitive impairment in mice with chronic cerebral hypo-perfusion through suppressing oxidative stress and inflammatory reaction. J Hypertens. 2015;33:1001–13. doi: 10.1097/HJH.0000000000000529. [DOI] [PubMed] [Google Scholar]
- 13.Shibata M, Ohtani R, Ihara M, Tomimoto H. White matter lesions and glial activation in a novel mouse model of chronic cerebral hypoperfusion. Stroke. 2004;35:2598–603. doi: 10.1161/01.STR.0000143725.19053.60. [DOI] [PubMed] [Google Scholar]
- 14.Miyamoto N, Maki T, Pham LD, Hayakawa K, Seo JH, Mandeville ET, Mandeville JB, Kim KW, Lo EH, Arai K. Oxidative stress interferes with white matter renewal after prolonged cerebral hypoperfusion in mice. Stroke. 2013;44:3516–21. doi: 10.1161/STROKEAHA.113.002813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Poeggeler B, Saarela S, Reiter RJ, Tan DX, Chen LD, Manchester LC, Barlow-Walden LR. Melatonin--a highly potent endogenous radical scavenger and electron donor: new aspects of the oxidation chemistry of this indole accessed in vitro. Ann N Y Acad Sci. 1994;738:419–20. doi: 10.1111/j.1749-6632.1994.tb21831.x. [DOI] [PubMed] [Google Scholar]
- 16.Galano A, Tan DX, Reiter RJ. Melatonin as a natural ally against oxidative stress: a physicochemical examination. J Pineal Res. 2011;51:1–16. doi: 10.1111/j.1600-079X.2011.00916.x. [DOI] [PubMed] [Google Scholar]
- 17.Rodriguez C, Mayo JC, Sainz RM, Antolin I, Herrera F, Martin V, Reiter RJ. Regulation of antioxidant enzymes: a significant role for melatonin. J Pineal Res. 2004;36:1–9. doi: 10.1046/j.1600-079x.2003.00092.x. [DOI] [PubMed] [Google Scholar]
- 18.Lin HW, Lee EJ. Effects of melatonin in experimental stroke models in acute, sub-acute, and chronic stages. Neuropsychiatr Dis Treat. 2009;5:157–62. doi: 10.2147/ndt.s4815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Reiter RJ, Paredes SD, Manchester LC, Tan DX. Reducing oxidative/nitrosative stress: a newly-discovered genre for melatonin. Crit Rev Biochem Mol Biol. 2009;44:175–200. doi: 10.1080/10409230903044914. [DOI] [PubMed] [Google Scholar]
- 20.Browning C, Beresford I, Fraser N, Giles H. Pharmacological characterization of human recombinant melatonin mt(1) and MT(2) receptors. Br J Pharmacol. 2000;129:877–86. doi: 10.1038/sj.bjp.0703130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Dubocovich ML, Yun K, Al-Ghoul WM, Benloucif S, Masana MI. Selective MT2 melatonin receptor antagonists block melatonin-mediated phase advances of circadian rhythms. FASEB J. 1998;12:1211–20. doi: 10.1096/fasebj.12.12.1211. [DOI] [PubMed] [Google Scholar]
- 22.Sumaya IC, Masana MI, Dubocovich ML. The antidepressant-like effect of the melatonin receptor ligand luzindole in mice during forced swimming requires expression of MT2 but not MT1 melatonin receptors. J Pineal Res. 2005;39:170–7. doi: 10.1111/j.1600-079X.2005.00233.x. [DOI] [PubMed] [Google Scholar]
- 23.Chern CM, Liao JF, Wang YH, Shen YC. Melatonin ameliorates neural function by promoting endogenous neurogenesis through the MT2 melatonin receptor in ischemic-stroke mice. Free Radic Biol Med. 2012;52:1634–47. doi: 10.1016/j.freeradbiomed.2012.01.030. [DOI] [PubMed] [Google Scholar]
- 24.Bennett S, Grant MM, Aldred S. Oxidative stress in vascular dementia and Alzheimer's disease: a common pathology. J Alzheimers Dis. 2009;17:245–57. doi: 10.3233/JAD-2009-1041. [DOI] [PubMed] [Google Scholar]
- 25.Mao P. Oxidative Stress and Its Clinical Applications in Dementia. J Neurodegener Dis. 2013;2013:319898. doi: 10.1155/2013/319898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Mecocci P, Cherubini A, Polidori MC, Cecchetti R, Chionne F, Senin U. Oxidative stress and dementia: new perspectives in AD pathogenesis. Aging (Milano) 1997;9:51–2. doi: 10.1007/BF03339704. [DOI] [PubMed] [Google Scholar]
- 27.Butterfield DA, Reed T, Newman SF, Sultana R. Roles of amyloid beta-peptide-associated oxidative stress and brain protein modifications in the pathogenesis of Alzheimer's disease and mild cognitive impairment. Free Radic Biol Med. 2007;43:658–77. doi: 10.1016/j.freeradbiomed.2007.05.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Kolosova NG, Shcheglova TV, Sergeeva SV, Loskutova LV. Long-term antioxidant supplementation attenuates oxidative stress markers and cognitive deficits in senescent-accelerated OXYS rats. Neurobiol Aging. 2006;27:1289–97. doi: 10.1016/j.neurobiolaging.2005.07.022. [DOI] [PubMed] [Google Scholar]
- 29.Pratico D, Clark CM, Liun F, Rokach J, Lee VY, Trojanowski JQ. Increase of brain oxidative stress in mild cognitive impairment: a possible predictor of Alzheimer disease. Arch Neurol. 2002;59:972–6. doi: 10.1001/archneur.59.6.972. [DOI] [PubMed] [Google Scholar]
- 30.Sanchez-Rodriguez MA, Santiago E, Arronte-Rosales A, Vargas-Guadarrama LA, Mendoza-Nunez VM. Relationship between oxidative stress and cognitive impairment in the elderly of rural vs. urban communities. Life Sci. 2006;78:1682–7. doi: 10.1016/j.lfs.2005.08.007. [DOI] [PubMed] [Google Scholar]
- 31.Singh-Manoux A, Dugravot A, Brunner E, Kumari M, Shipley M, Elbaz A, Kivimaki M. Interleukin-6 and C-reactive protein as predictors of cognitive decline in late midlife. Neurology. 2014;83:486–93. doi: 10.1212/WNL.0000000000000665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Grammas P, Ovase R. Inflammatory factors are elevated in brain microvessels in Alzheimer's disease. Neurobiol Aging. 2001;22:837–42. doi: 10.1016/s0197-4580(01)00276-7. [DOI] [PubMed] [Google Scholar]
- 33.Chen W, Liu B, Zhang F, Xue P, Cui R, Lei W. The effects of dexmedetomidine on post-operative cognitive dysfunction and inflammatory factors in senile patients. Int J Clin Exp Med. 2015;8:4601–5. [PMC free article] [PubMed] [Google Scholar]
- 34.Harrison SL, de Craen AJ, Kerse N, Teh R, Granic A, Davies K, Wesnes KA, den Elzen WP, Gussekloo J, Kirkwood TB, Robinson L, Jagger C, Siervo M, et al. Predicting Risk of Cognitive Decline in Very Old Adults Using Three Models: The Framingham Stroke Risk Profile; the Cardiovascular Risk Factors, Aging, and Dementia Model; and Oxi-Inflammatory Biomarkers. J Am Geriatr Soc. 2017;65:381–9. doi: 10.1111/jgs.14532. [DOI] [PubMed] [Google Scholar]
- 35.Huckans M, Fuller BE, Chalker AL, Adams M, Loftis JM. Plasma Inflammatory Factors Are Associated with Anxiety, Depression, and Cognitive Problems in Adults with and without Methamphetamine Dependence: An Exploratory Protein Array Study. Front Psychiatry. 2015;6:178. doi: 10.3389/fpsyt.2015.00178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Saxena AK, Abdul-Majeed SS, Gurtu S, Mohamed WM. Investigation of redox status in chronic cerebral hypoperfusion-induced neurodegeneration in rats. Appl Transl Genom. 2015;5:30–2. doi: 10.1016/j.atg.2015.05.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Tomimoto H, Ihara M, Wakita H, Ohtani R, Lin JX, Akiguchi I, Kinoshita M, Shibasaki H. Chronic cerebral hypoperfusion induces white matter lesions and loss of oligodendroglia with DNA fragmentation in the rat. Acta Neuropathol. 2003;106:527–34. doi: 10.1007/s00401-003-0749-3. [DOI] [PubMed] [Google Scholar]
- 38.Masumura M, Hata R, Nagai Y, Sawada T. Oligodendroglial cell death with DNA fragmentation in the white matter under chronic cerebral hypoperfusion: comparison between normotensive and spontaneously hypertensive rats. Neurosci Res. 2001;39:401–12. doi: 10.1016/s0168-0102(01)00195-x. [DOI] [PubMed] [Google Scholar]
- 39.Nishio K, Ihara M, Yamasaki N, Kalaria RN, Maki T, Fujita Y, Ito H, Oishi N, Fukuyama H, Miyakawa T, Takahashi R, Tomimoto H. A mouse model characterizing features of vascular dementia with hippocampal atrophy. Stroke. 2010;41:1278–84. doi: 10.1161/STROKEAHA.110.581686. [DOI] [PubMed] [Google Scholar]
- 40.Love S. Oxidative stress in brain ischemia. Brain Pathol. 1999;9:119–31. doi: 10.1111/j.1750-3639.1999.tb00214.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Rizk NN, Rafols JA, Dunbar JC. Cerebral ischemia-induced apoptosis and necrosis in normal and diabetic rats: effects of insulin and C-peptide. Brain Res. 2006;1096:204–12. doi: 10.1016/j.brainres.2006.04.060. [DOI] [PubMed] [Google Scholar]
- 42.Okutan H, Savas C, Delibas N. The antioxidant effect of melatonin in lung injury after aortic occlusion-reperfusion. Interact Cardiovasc Thorac Surg. 2004;3:519–22. doi: 10.1016/j.icvts.2004.05.005. [DOI] [PubMed] [Google Scholar]
- 43.Ueno Y, Koike M, Shimada Y, Shimura H, Hira K, Tanaka R, Uchiyama Y, Hattori N, Urabe T. L-carnitine enhances axonal plasticity and improves white-matter lesions after chronic hypoperfusion in rat brain. J Cereb Blood Flow Metab. 2015;35:382–91. doi: 10.1038/jcbfm.2014.210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Ueno Y, Zhang N, Miyamoto N, Tanaka R, Hattori N, Urabe T. Edaravone attenuates white matter lesions through endothelial protection in a rat chronic hypoperfusion model. Neuroscience. 2009;162:317–27. doi: 10.1016/j.neuroscience.2009.04.065. [DOI] [PubMed] [Google Scholar]
- 45.Imbesi M, Uz T, Dzitoyeva S, Giusti P, Manev H. Melatonin signaling in mouse cerebellar granule cells with variable native MT1 and MT2 melatonin receptors. Brain Res. 2008;1227:19–25. doi: 10.1016/j.brainres.2008.06.082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Negi G, Kumar A, Sharma SS. Melatonin modulates neuroinflammation and oxidative stress in experimental diabetic neuropathy: effects on NF-kappaB and Nrf2 cascades. J Pineal Res. 2011;50:124–31. doi: 10.1111/j.1600-079X.2010.00821.x. [DOI] [PubMed] [Google Scholar]
- 47.Wang Z, Ma C, Meng CJ, Zhu GQ, Sun XB, Huo L, Zhang J, Liu HX, He WC, Shen XM, Shu Z, Chen G. Melatonin activates the Nrf2-ARE pathway when it protects against early brain injury in a subarachnoid hemorrhage model. J Pineal Res. 2012;53:129–37. doi: 10.1111/j.1600-079X.2012.00978.x. [DOI] [PubMed] [Google Scholar]
- 48.Parada E, Buendia I, Leon R, Negredo P, Romero A, Cuadrado A, Lopez MG, Egea J. Neuroprotective effect of melatonin against ischemia is partially mediated by alpha-7 nicotinic receptor modulation and HO-1 overexpression. J Pineal Res. 2014;56:204–12. doi: 10.1111/jpi.12113. [DOI] [PubMed] [Google Scholar]
- 49.Jang H, Lee OH, Lee Y, Yoon H, Chang EM, Park M, Lee JW, Hong K, Kim JO, Kim NK, Ko JJ, Lee DR, Yoon TK, et al. Melatonin prevents cisplatin-induced primordial follicle loss via suppression of PTEN/AKT/FOXO3a pathway activation in the mouse ovary. J Pineal Res. 2016;60:336–47. doi: 10.1111/jpi.12316. [DOI] [PubMed] [Google Scholar]
- 50.Deng W, Zhang Y, Gu L, Cui J, Duan B, Wang Y, Du J. Heat shock protein 27 downstream of P38-PI3K/Akt signaling antagonizes melatonin-induced apoptosis of SGC-7901 gastric cancer cells. Cancer Cell Int. 2016;16:5. doi: 10.1186/s12935-016-0283-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Ferreira GM, Martinez M, Camargo IC, Domeniconi RF, Martinez FE, Chuffa LG. Melatonin Attenuates Her-2, p38 MAPK, p-AKT, and mTOR Levels in Ovarian Carcinoma of Ethanol-Preferring Rats. J Cancer. 2014;5:728–35. doi: 10.7150/jca.10196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Faria JA, Kinote A, Ignacio-Souza LM, de Araujo TM, Razolli DS, Doneda DL, Paschoal LB, Lellis-Santos C, Bertolini GL, Velloso LA, Bordin S, Anhe GF. Melatonin acts through MT1/MT2 receptors to activate hypothalamic Akt and suppress hepatic gluconeogenesis in rats. Am J Physiol Endocrinol Metab. 2013;305:E230–42. doi: 10.1152/ajpendo.00094.2013. [DOI] [PubMed] [Google Scholar]
- 53.Shin EJ, Chung YH, Le HL, Jeong JH, Dang DK, Nam Y, Wie MB, Nah SY, Nabeshima Y, Nabeshima T, Kim HC. Melatonin attenuates memory impairment induced by Klotho gene deficiency via interactive signaling between MT2 receptor, ERK, and Nrf2-related antioxidant potential. Int J Neuropsychopharmacol. 2014;18 doi: 10.1093/ijnp/pyu105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Yang Y, Jiang S, Dong Y, Fan C, Zhao L, Yang X, Li J, Di S, Yue L, Liang G, Reiter RJ, Qu Y. Melatonin prevents cell death and mitochondrial dysfunction via a SIRT1-dependent mechanism during ischemic-stroke in mice. J Pineal Res. 2015;58:61–70. doi: 10.1111/jpi.12193. [DOI] [PubMed] [Google Scholar]
- 55.Ortiz-Lopez L, Perez-Beltran C, Ramirez-Rodriguez G. Chronic administration of a melatonin membrane receptor antagonist, luzindole, affects hippocampal neurogenesis without changes in hopelessness-like behavior in adult mice. Neuropharmacology. 2016;103:211–21. doi: 10.1016/j.neuropharm.2015.11.030. [DOI] [PubMed] [Google Scholar]