Abstract
Heat stress is one of the most detrimental confrontations in tropical and subtropical regions of the world, causing considerable economic losses in poultry production. Propolis, a resinous product of worker honeybees, possesses several biological activities that could be used to alleviate the deleterious effects of high environmental temperature on poultry production. The current study was aimed at evaluating the effects of propolis supplementation to Japanese quail (Coturnix coturnix japonica) diets on the production performance, intestinal histomorphology, relative physiological and immunological parameters, and selected gene expression under heat stress conditions. Three hundred one-day-old Japanese quail chicks were randomly distributed into 20 wired-cages. At 28 d of age, the birds were divided into 2 temperature treatment groups; a normal at 24°C (C group) and a heat stress at 35°C (HS group). The birds in each group were further assigned to 2 subgroups; one of them was fed on a basal diet without propolis supplementation (-Pr subgroup) while the other was supplemented with propolis (+Pr subgroup). Production performance including body weight gain, feed intake and feed conversion ratio were measured. The intestinal histomorphological measurements were also performed for all treatment groups. Relative physiological parameters including body temperature, corticosterone hormone level, malondialdehyde (MDA) and free triiodothyronine hormone (fT3), as well as the relative immunological parameters including the total white blood cells count (TWBC’s), heterophil/lymphocyte (H/L) ratio and lymphocyte proliferation index, were also measured. Furthermore, the mRNA expression for toll like receptor 5 (TLR5), cysteine-aspartic protease-6 (CASP6) and heat shock proteins 70 and 90 (Hsp70 and Hsp90) genes was quantified in this study. The quail production performance was significantly (P<0.05) impaired by HS treatment, while Pr treatment significantly improved the quail production performance. The villus width and area were significantly (P<0.05) lower in the HS compared to the C group, while Pr treatment significantly increased crypts depth of quail. A negative impact of HS treatment was observed on the physiological status of quail; however, propolis significantly alleviated this negative effect. Moreover, quail of the HS group expressed lower immunological parameters than C group, while propolis enhanced the immune status of the quail. The relative mRNA expression of TLR5 gene was down-regulated by HS treatment while it was up-regulated by the Pr treatment. Furthermore, the positive effects of propolis in HS-quail were evidenced by normalizing the high expressions of CASP6 and Hsp70 genes when compared to the C group. Based on these results, the addition of propolis to quail diets as a potential nutritional strategy in order to improve their performance, especially under heat stress conditions, is recommended.
Introduction
Japanese quail (Coturnix coturnix japonica) is considered one of the locally available and a cheap sources of poultry meat [1]. Quail meat is renowned for its dietary properties like high-quality protein and low-caloric content, and its valuable taste to consumers [2,3]. It is also a preferable experimental animal model for its unique characteristics and advantages over other species of poultry, which include early attainment of sexual maturity and short generation interval which normally produce several generations in a year [4].
Undoubtedly, global warming phenomenon is currently a serious challenge facing poultry production in tropical and subtropical regions. Heat stress begins when the ambient temperature raises the thermo-neutral zone which ranges between 16–25°C for poultry species [5]. Recent studies found that poultry flocks subjected to chronic heat stress environment (over 30°C) had poor production performance as well as high morbidity and mortality, causing substantial economic losses in poultry farms [6,7]. In response to the heat stress condition, multiple changes in poultry physiology occur in order to maintain their homeostasis status [8]. Several researchers reported reduced concentrations of thyroid hormones in heat-stressed chickens [9], affecting metabolism and growth processes in target tissues [10]. Other reports concluded that heat stress results in an irregular systemic immune and an imbalance expression of inflammatory molecules throughout the body [11]. In addition, heat stress causes the release of corticosterone [12] and initiates lipid peroxidation in cell membranes as a consequence of increased reactive oxygen species (ROS) and free radicals formation [13]. Moreover, it is indicated that exposure to acute heat stress causes a substantial impairment in the gut formation, intestinal epithelial integrity and villus morphology in laying hens [14]. Furthermore, it has been reported that poultry exposure to stress conditions, including heat stress, could have differential influences on the expression pattern of some genes such as apoptotic and heat shock proteins [15–17].
Recent studies have revealed that supplementation of some antioxidants like vitamin C and E and/or other compounds could counteract the heat stress effects on growth and physiological aspects that are caused by high environmental temperatures in broilers [18,19], in laying hens [20,21], and in quail birds [22,23]. Propolis is an adhesive resinous balsam made by worker honeybees (Apis melifera) [24], and it has been extensively used in poultry feeds to expand their productive performance under high environmental temperature conditions [12,19,25,26]. Studies in the last two decades explored many biological and positive characteristics for propolis such as growth promotion, appetite enhancement, antioxidation, antimicrobial, antitumor and immune modulation [27–29].
Due to this wide range of biological activities of propolis, there is a renewed interest in its use as an alternative and practical way to alleviate the deleterious effects of high environmental temperature on poultry production. However, most investigations on quail were exposed exposed to heat stress have focused on describing the positive effects of propolis on aspects correlated with productive performance while the physiological and biological reasons behind these effects remain poorly understood. Therefore, the present study was conducted to evaluate the effects of propolis supplementation into quail diets on productive performance, intestinal histomorphology, physiological aspects of inflammation, and immunological characteristics, as well as the mRNA expression of some immune-stimulatory, apoptotic and heat-shock-protein genes under heat stress conditions.
Materials and methods
Propolis preparation
Propolis was collected from apiary located at Faculty of Agriculture, Cairo University (Giza province, Egypt). The collected propolis was kept dried in the dark until processing according to methods described by Seven et al. [30]. Briefly, collected propolis was extracted for a week with 100 ml of 70% ethanol at room temperature. After filtration, the extract was evaporated using a vacuum evaporator at 50°C. The extracted propolis was kept in a clean, dark bottle at 4°C until use in the experiment. Propolis was incorporated into the basal diet of quail in a powder form at 1 g of extracted propolis per kg feed.
The free radical scavenging activity of extracted propolis was measured according to methods described by Oktay et al. [31]. In brief, the extracted propolis was added to a solution of 0.1 mM of 1,1-diphenyl-2-picryl-hydrazil (DPPH•) in methanol at different concentrations (25–75 μg/ml). The mixtures were shaken vigorously and were allowed to stand at room temperature for 30 min. Thereafter, the absorbance of reactions was measured using an automatic scanning spectrophotometer at 517 nm. The free radical scavenging activity was calculated for the extracted propolis was 87.3%.
The flavonoids and polyphenols were also measured in the extracted propolis according to methods described by Rolim et al. [32] and Kujala et al. [33], respectively. First, 1 g of extracted propolis was dissolved in 10 ml solution of ethanol 95% and glacial acetic acid 99:1 (v/v). For flavonoids assay, 1 ml of propolis solution was diluted to 100 ml with ethanol 95% and glacial acetic acid solution 99:1 (v/v) and then was measured spectrophotometrically at 363 nm. For polyphenols assay, 5 ml of propolis solution was diluted to 100 ml with ethanol then 1 ml of the diluted solution was added to 4 ml Folin-Ciocalteu reagent/water solution 1:9 (v/v). After vortex, 5 ml of sodium carbonate (7.5%) was added to the solution and left in dark for 30 min. The blue color amount that was developed from the last step was detected using automatic scanning spectrophotometer at 745 nm. As a result, the flavonoids and polyphenols amounts in the propolis samples were 10.2% and 5.6%, respectively.
Birds and management
A total of 300, one-day-old, Japanese quail (Coturnix coturnix japonica) chicks were distributed into 20 wired-cages (15 chicks per cage, measured at 60×50×50 cm) in an environmentally-controlled room. A brooding temperature was set at 37°C on day 1 and then was gradually reduced to 24°C by d 21. Light was provided continuously (24 h) throughout the experiment. The birds were fed according to NRC (1994) guidelines with a starter diet (24% CP and 2900 kcal/kg ME) until 20 d of age followed by a grower diet (20% CP and 3100 kcal/kg ME) from d 21 onwards. The diets and fresh water were offered ad libitum.
Experimental design and data collection
At 28 d of age, the quail were randomly distributed into two identical environmentally-controlled rooms. The two rooms were fully cleaned before initiation of the experiment and contained the same conditions of size, ventilation, humidity, light intensity, and light schedule. The temperature in the first room was kept at 24°C (C group) and the other room was kept under heat stress at 35°C (HS group). The birds in each group were further assigned to two subgroups according to dietary propolis supplementation (5 cages per subgroup), and one of them was fed on a basal diet without propolis supplementation (-Pr subgroup) while the other was supplemented with propolis (+Pr subgroup). The relative humidity of the two rooms was maintained at 50%. These treatments continued till the birds were 35 d of age.
The production performance was obtained for each treatment group as will be mentioned later. At the end of the experiment (35 d), body temperatures were measured for each treatment group and blood samples were collected in heparinized tubes for physiological and immunological analysis listed later. The intestinal histomorphological measurements were also performed for all treatment groups. In addition, the liver was separated and directly plunged into liquid nitrogen (LN2) for later mRNA quantification of toll-like receptor 5 (TLR5), cysteine-aspartic protease-6 (CASP6), and heat shock proteins 70 and 90 (Hsp70 and Hsp90) genes.
Compliance with ethical standards
Birds were monitored closely twice a day to detect any signs of chronic stress (breathing difficulty, watery discharge of the peak, decreased appetite, ruffled feathers or droopy looking) throughout the experimental period. Accordingly, when one or more of these signs appeared, cervical dislocation was used to end the life of these birds. This process was accomplished to minimize suffering of birds and to allow humane endpoints. All experimental protocols were approved by Cairo University Ethics Committee for the Care and Use of Experimental Animals in Education and Scientific Research (CU-IACUC).
The performance traits
The initial and final body weights (g) were recorded individually at the beginning and at the end of the experiment (28 and 35 d of age). The weight gain (g/bird) was determined for each treatment group. The feed intake (g/bird) was measured for each treatment group. The feed conversion ratio was calculated for each treatment group.
Intestinal histomorphological measurements
Ten birds from each treatment group were sacrificed and the small intestine was separated from the rest of the gastrointestinal tract as a standard procedure. The intestinal length (jejunum and ileum parts) was measured for each treatment group. For histomorphological studies, intestinal samples were cut from the middle part of ileum tissues. Tissue samples were flushed and soaked in 10% neutral buffered formalin for 72 h. Samples were trimmed and processed by dehydration in alcohol, clearing in Xylene, synthetic wax infiltration and blocking out into Paraplast tissue embedding media. At least, 5 cross sections of 3–5 μm per sample with 50 μm in-between were cut by a rotatory microtome. The sections were stained with Harris Hematoxylin and Eosin as a general staining method as outlined by Suvarna et al. [34]. The villus length, villus width, villus area and crypts depth of tissue sections were examined for each treatment group at 40X magnification under light microscope equipped with HD microscopic camera and Image analysis software (Leica Microsystems, Germany).
Physiological parameters
At the end of the experiment (35 d), the body temperatures were measured for 10 birds of each treatment group (2 birds x 5 cages) using thermocouple rectal thermometer with a 3-cm insertion probe. Blood samples were collected in heparinized tubes and centrifuged at 2000 xg for 10 min at 4°C. The plasma was separated and stored at -20°C until analysed. The plasma corticosterone concentration (5 samples per treatment group) was measured by ELISA reader (BIOTEKELX808) using corticosterone ELISA kits (MBS701668, MyBioSource Inc., San Diego, CA, USA). The free triiodothyronine concentration in plasma (fT3; 5 samples per treatment group) was also measured by the automated ELISA Microplate reader using enzyme immunoassay ELISA kits (EIA-10301, Chemux BioScience Inc., South San Francisco, CA, USA). The plasma malondialdehyde (MDA; 5 samples per treatment group) concentration was determined using colorimetric assay kit (AB118970, Abcam, UK), according to the manufacturer's directions.
Immunological parameters
Five blood samples from each treatment group were collected into heparinized tubes and assigned to measure the total white blood cells (TWBC’s) and the heterophil/lymphocyte (H/L) ratio. The TWBC’s were manually determined by mixing 490 μl of brilliant cresyl blue dye with 10 μl of the whole blood sample, and then the total leukocytes were counted under a microscope at a magnification of 200X using a hemocytometer slide [35]. The H/L ratio was determined using Hema-3 stain (cat# 22–122911, Fisher scientific, USA), according to Zhang et al. [36].
The lymphocyte proliferation assay was measured in 5 blood samples in each treatment group as described in a previous work [37]. Briefly, the peripheral blood mononuclear cells (PBMCs) were isolated from blood using histopaque-1077 (Sigma Chemical Co., St. Louis, MO) and the viable lymphocytes were detected using Trypan Blue dye. Cells were plated at 5x105 cells/well and incubated with Concanavalin-A (Con-A, Sigma Chemical Co., St. Louis, MO) at 41°C in a humidified atmosphere of 5% CO2 for 68 h. Thereafter, cells were incubated with 3-[4,5-dimethylthiazol]-2,5-diphenyltetrazolium bromide (MTT) for further 4 h, followed by addition of 10% sodium dodecyl sulfates dissolved in 0.04 M HCl solution to each single well. Subsequently, the optical density at 570 nm was obtained using an automated ELISA reader (model 550 Microplate Reader, Bio-Rad) and the stimulation index (SI) of T-lymphocyte cells was calculated for each treatment group as follows: SI = OD570 (stimulated cells) / OD570 (unstimulated cells).
mRNA quantification
Total RNA was extracted from liver tissues (10 samples for each treatment group) using the standard TRIzol® Reagent extraction method. Total RNA was treated with 1 U of RQ1 RNase-free DNase (Invitrogen, Germany) to digest DNA residues, and then re-suspended in diethylpyrocarbonate (DEPC)-treated water. The purity of total RNA was assessed spectrophotometrically at 260/280 nm, and the integrity of extracted RNA was determined by using 1.5% agarose gel electrophoresis. Then, total RNA was reverse-transcribed into cDNA by using RevertAidTM First Strand cDNA Synthesis Kit (MBI Fermentas, Germany) according to the manufacturer's directions. The cDNA templates were stored at -20°C for future quantitative real time-polymerase chain reaction (qRT-PCR).
The qRT-PCR of TLR5, CASP6, Hsp70 and Hsp90 genes were normalized to the main expression of β-actin gene, and transformed using the comparative cycle threshold (CT) method [38]. Sequence-specific primers for the qRT-PCR of the selected genes were designed using the primer-blast web interface (http://www.ncbi.nlm.nih.gov/tools/primer-blast/index.cgi) as shown in Table 1. To perform the qRT-PCR reactions, 5 μl of cDNA template was mixed with 12.5 μl of 1× SYBR® Premix Ex TaqTM (TaKaRa, Biotech. Co. Ltd., Germany), 0.5 μl sense primer (0.2 μM), 0.5 μl antisense primer (0.2 μM), 6.5 μl distilled water. The reaction program consisted of three thermal cycling phases. The first phase was set to 95.0°C for 3 min. The second phase consisted of 40 cycles in which each cycle was divided to 3 steps (95°C for 15 sec, 55°C for 30 sec, and 72°C for 30 sec). The last phase consisted of 71 cycles, started at 60°C and then increased by 0.5°C every 10 sec up to 95°C. Each experiment included a distilled water control. At the end of each qRT-PCR, a melting curve analysis was performed at 95°C to check the quality of the used primers.
Table 1. Details of primers used for real-time PCR quantitative analysis.
| Gene symbol |
Forward primer sequences | Reverse primer sequences | Product size (bp) |
|---|---|---|---|
| TLR5 | TCACACTCAACTGTCCGAGC | CGGCAGATCGATGCACTTTG | 176 |
| CASP6 | CAGGGACCGAAACGAGGAAA | ACAGCTTCACTGCCCTTCTC | 271 |
| Hsp70 | ATGAAACTGAGTCGCTCGCA | CAGTCTGTTGCACCTTTGGC | 166 |
| Hsp90 | GAAACACTCTGGGACGTGGT | TTCGACAGTCTCCGTCTTGC | 158 |
| β-actin | GGATGCAGAAGGAGATCACTG | CAAGTACTCCGTGTGGATCG | 90 |
Statistical analysis
All statistical analyses were performed using IBM SPSS 22.0 Software Package (IBM corp., NY, USA, 2013). Two-way ANOVA was used to to analyze the main effects of the heat stress (HS), dietary propolis supplementation (Pr) and their interactions (HSxPr) on the production performance traits, the intestinal histomorphological measurements, the relative physiological and immunological parameters, and the mRNA expression data for selected genes. When significant differences due to the HS, Pr or interaction effects were detected, a multiple pairwise comparison among treatment groups was performed using Tukey’s HSD test. The experimental unit for each test done is the number of observations taken from each treatment group. The level of statistical significance was set at P<0.05.
Results
The performance traits
Results of the quail production performance under heat stress with or without dietary propolis supplementation are shown in Table 2. The average body weight gain from 28–35 d of age was significantly (P<0.05) less in the HS group compared to the C group. However, dietary propolis supplementation significantly increased the body weight gain in both HS and C groups. Quail in the HS group had significantly less feed intake and worse feed conversion ratio compared to the C group. However, dietary propolis supplementation ameliorates the negative effects of HS on quail and significantly increased the feed intake and feed efficiency.
Table 2. Least square means for the production performance traits as affected by heat stress and dietary propolis supplementation in Japanese quail.
| Items | n | Treatment groups 1 | SEM | Main effects 2 | |||||
|---|---|---|---|---|---|---|---|---|---|
| C | HS | ||||||||
| - Pr | + Pr | - Pr | + Pr | HS | Pr | HSxPr | |||
| Initial body weight (g) | 25 | 192.0 | 193.7 | 190.1 | 189.5 | 2.58 | NS | NS | NS |
| Body weight gain (g/bird) | 25 | 29.0 b | 37.9 a | 16.0 d | 26.0 c | 0.33 | S | S | NS |
| Feed Intake (g/bird) | 5 | 101.0 b | 108.7 a | 65.0 d | 83.2 c | 1.32 | S | S | S |
| Feed conversion ratio | 5 | 3.5 b | 2.9 d | 4.1 a | 3.2 c | 0.04 | S | S | S |
a-d Means with different superscripts, within the same row, are significantly different (P<0.05).
n: number of observations per treatment group. SEM: standard error of the mean.
1 C: control groups that were exposed to 24°C; HS: heat stress groups that were exposed to 35°C; -Pr: subgroups without dietary propolis supplementation; +Pr: subgroups with dietary propolis supplementation.
2 HS: heat stress effect; Pr: propolis effect; HSxPr: interaction between HS and Pr effect. S: significant; NS: non-significant.
Intestinal histomorphological measurements
The effects of heat stress and dietary propolis supplementation on the histomorphological measurements of quail intestines are presented in Table 3. No significant interactions between HS and Pr treatments were obtained for the histomorphological measurements of quail intestine. However, a significant decrease in the villus width and area was observed due to heat stress. In addition, it was found that dietary propolis supplementation significantly increased the crypts depth of quail intestines.
Table 3. Least square means for the histomorphological measurements of small intestines as affected by heat stress and dietary propolis supplementation in Japanese quail.
| Items | n | Treatment groups 1 | SEM | Main effects 2 | |||||
|---|---|---|---|---|---|---|---|---|---|
| C | HS | ||||||||
| - Pr | + Pr | - Pr | + Pr | HS | Pr | HSxPr | |||
| Intestinal length (cm) | 10 | 60.0 | 66.3 | 63.3 | 61.7 | 4.19 | NS | NS | NS |
| Villus length (μm) | 10 | 915.4 | 940.3 | 751.9 | 919.4 | 72.57 | NS | NS | NS |
| Villus width (μm) | 10 | 192.4 a | 155.4 bc | 128.0 c | 149.7 bc | 15.32 | S | NS | NS |
| Villus area (x103 μm2) | 10 | 166.1 a | 126.3 b | 84.5 c | 82.7 c | 19.41 | S | NS | NS |
| Crypts depth (μm) | 10 | 80.3 b | 171.7 a | 109.4 ab | 131.3 ab | 19.10 | NS | S | NS |
a-c Means with different superscripts, within the same row, are significantly different (P<0.05).
n: number of observations per treatment group. SEM: standard error of the mean.
1 C: control groups that were exposed to 24°C; HS: heat stress groups that were exposed to 35°C; -Pr: subgroups without dietary propolis supplementation; +Pr: subgroups with dietary propolis supplementation.
2 HS: heat stress effect; Pr: propolis effect; HSxPr: interaction between HS and Pr effect. S: significant; NS: non-significant.
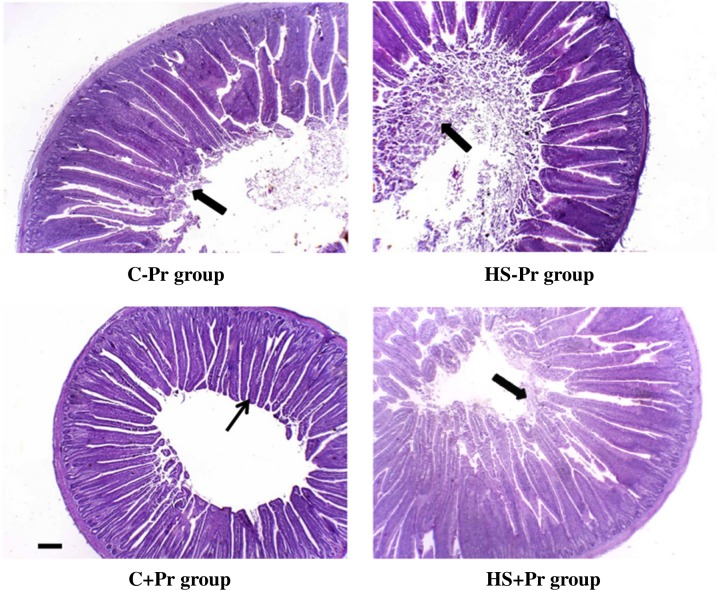
On the other hand, the histology of quail intestines shows desquamation of the mucosal epithelium and serious disruption of villi due to heat stress treatment. In contrast, dietary propolis supplementation maintains the villus structure relatively normal and alleviates the severe lesions by heat stress (Fig 1).
Fig 1. Visual examples for the histomorphological alteration in the intestinal villi of Japanese quail subjected to heat stress and supplemented with propolis in the basal diet (Scale bars 100 μm).
C: control groups that were exposed to 24°C; HS: heat stress groups that were exposed to 35°C; -Pr: subgroups without dietary propolis supplementation; +Pr: subgroups with dietary propolis supplementation. The thick arrow indicates the damage and desquamation observed at the tips of the intestinal villi, while the thin arrow indicates the normal structure of intestinal villi.
Physiological parameters
The effects of heat stress and dietary propolis supplementation on some physiological parameters in Japanese quail are summarized in Table 4. A significant (P<0.05) increase in the body temperature, the plasma corticosterone hormone and the plasma MDA levels was observed in the HS quail group when compared to the C group. While the plasma fT3 hormone was significantly lower in the HS group compared to the C group. On the other hand, the body temperature and corticosterone level were significantly decreased while the fT3 level was increased due to Pr treatment. Moreover, the high corticosterone level of HS-quail was significantly (P<0.05) reduced by the Pr treatment.
Table 4. Least square means for some physiological parameters as affected by heat stress and dietary propolis supplementation in Japanese quail.
| Items | n | Treatment groups 1 | SEM | Main effects 2 | |||||
|---|---|---|---|---|---|---|---|---|---|
| C | HS | ||||||||
| - Pr | + Pr | - Pr | + Pr | HS | Pr | HSxPr | |||
| Body temperature (°C) | 10 | 40.5 bc | 40.2 c | 41.4 a | 40.7 b | 0.15 | S | S | NS |
| Corticosterone (ng/ml) | 5 | 2.8 c | 2.6 c | 8.0 a | 5.0 b | 0.56 | S | S | S |
| MDA (nmol/ml) | 5 | 1.8 c | 1.8 c | 3.4 a | 2.4 b | 0.24 | S | NS | NS |
| Free T3 (pg/ml) | 5 | 4.0 a | 5.1 a | 2.3 b | 4.0 a | 0.52 | S | S | NS |
a-c Means with different superscripts, within the same row, are significantly different (P<0.05).
n: number of observations per treatment group. SEM: standard error of the mean.
1 C: control groups that were exposed to 24°C; HS: heat stress groups that were exposed to 35°C; -Pr: subgroups without dietary propolis supplementation; +Pr: subgroups with dietary propolis supplementation.
2 HS: heat stress effect; Pr: propolis effect; HSxPr: interaction between HS and Pr effect. S: significant; NS: non-significant.
Immunological parameters
The results shown in Table 5 indicate that heat stress negatively affected the studied immune response parameters while dietary propolis supplementation enhanced these parameters, in Japanese quail. However, no significant interactions between HS and Pr treatments were obtained for the immunological parameters in the current study.
Table 5. Least square means for some immunological parameters as affected by heat stress and dietary propolis supplementation in Japanese quail.
| Items | n | Treatment groups 1 | SEM | Main effects 2 | |||||
|---|---|---|---|---|---|---|---|---|---|
| C | HS | ||||||||
| - Pr | + Pr | - Pr | + Pr | HS | Pr | HSxPr | |||
| TWBC’s (x103/mm3) | 5 | 81.8 c | 131.2 a | 60.4 d | 108.8 b | 8.24 | S | S | NS |
| H/L ratio | 5 | 0.4 c | 0.3 d | 0.8 a | 0.6 b | 0.04 | S | S | NS |
| T-lymphocyte proliferation (SI) | 5 | 4.2 b | 6.3 a | 1.6 d | 3.0 c | 0.40 | S | S | NS |
a-d Means with different superscripts, within the same row, are significantly different (P<0.05).
n: number of observations per treatment group. SEM: standard error of the mean.
1 C: control groups that were exposed to 24°C; HS: heat stress groups that were exposed to 35°C; -Pr: subgroups without dietary propolis supplementation; +Pr: subgroups with dietary propolis supplementation.
2 HS: heat stress effect; Pr: propolis effect; HSxPr: interaction between HS and Pr effect. S: significant; NS: non-significant.
mRNA quantification
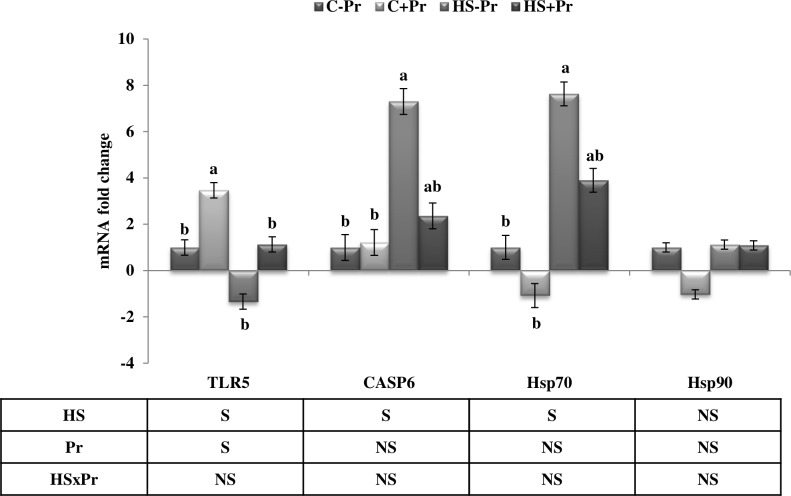
The effects of heat stress and dietary propolis supplementation on the relative expression of examined genes in the liver of Japanese quail are illustrated in Fig 2. There were no significant interactions between HS and Pr treatments for the mRNA expression of selected genes in the present study. However, a significant (P<0.05) augmentation in the expression of TLR5 gene was occurred due to Pr treatment in the C group. In addition, the exposure of quail to heat stress significantly (P<0.05) increased the expression of CASP6 and Hsp70 genes by 7.3 and 7.6 fold, respectively, in the HS-Pr group as compared to the C-Pr group. While propolis decreased the expression of these genes to intermediate levels (2.4 and 3.9 fold for CASP6 and Hsp70, respectively) in the HS+Pr group. No significant differences (P>0.05) were observed in Hsp90 gene expression among the treatment groups.
Fig 2. The hepatic relative expression of TLR5, CASP6, Hsp70 and Hsp90 genes as affected by heat stress and dietary propolis supplementation in Japanese quail.
Bars express the means ± standard error of means (n = 10). C: control groups that were exposed to 24°C; HS: heat stress groups that were exposed to 35°C; -Pr: subgroups without dietary propolis supplementation; +Pr: subgroups with dietary propolis supplementation. a-b Means within the same gene with different superscripts are significantly different (P<0.05). The main effects of heat stress (HS), propolis (Pr) and their interaction (HSxPr) are provided for each gene in the table (S: significant; NS: non-significant).
Discussion
Global warming became one of the main meteorology factors that may change both the production and the consumption of livestock products in the future. Heat stress has many substantial effects on the biological functions of animals that in turn contribute significantly to the decrease in their productive performance. Exposure of poultry species to heat stress substantially decreases their products which are important and invaluable source of nutrition and livelihood for millions of low income people [39]. It is, therefore, essential to find means to minimize the negative effects of heat stress. Recently, propolis has been used as an alternative and practical way to alleviate the deterioration effects of high environmental temperature in poultry production [12,19]. In the current research, the Japanese quail was used as an experimental animal model to study the capability of dietary propolis supplementation to ameliorate the deleterious effects of heat stress on the production performance, intestinal histomorphology, physiological aspects, immunological responses and the mRNA expression of some immune-stimulatory, apoptotic and heat-shock-protein genes.
In the present study, all production performance traits of the quail were negatively affected by heat stress compared to the control. The low weight gain and feed efficiency were previously reported in broilers [25] and laying hens [20] when they were exposed to heat stress. As indicated by Vona et al. [40], birds decrease their feed intake under heat stress to lower the heat production in their body caused by increased respiration. In addition, the activities of trypsin, chymotrypsin, and amylase become low at a temperature of 32°C [41]. Therefore, high ambient temperatures are associated with lower nutrient digestibility in animals and poultry [42]. On the other hand, the addition of propolis in quail diets significantly increased the weight gain and feed intake, and improved the feed conversion ratio. Similar results were obtained when propolis was added to the quail diets [43] or drinking water [44]. These positive effects of dietary propolis on quail performance could be linked to the palatable substances included in propolis diets like resin, wax, honey and vanillin [45], and these could give beneficial results when birds are not kept at optimal conditions [26].
The changes in intestinal morphology that were observed in the current study could explain the quail production performance under heat stress and effects of dietary propolis supplementation. The villus width and area were significantly lower in the HS group than the C group. Similar results were reported in a previous study on Japanese quail exposed to heat stress [22]. These results could be an outcome of the decreased feed intake in the HS quail, as previously reported in chickens [46]. Consequently, exposure of quail to heat stress may reduce the intact spaces of intestinal epithelia [47], affecting the intestinal digestion and absorption functions [48]; and cause changes that may be sufficient to impair the quail performance as was observed in the current study. No clear improvement was found in the intestinal length and villus measurements after dietary propolis supplementation. Similarly, Denli et al. [43] did not observe changes in the intestinal length of Japanese quail fed with propolis. Such changes may require a longer time of exposure [49]. However, some morphological alterations among the treatment groups were noticed by the visual microscopic examination of villi histology slides. The damage observed in the intestinal villi of the HS-quail groups vs. the integrity of villi structure in the Pr-quail groups indicates that propolis may provide better protection of villi, which is likely to enhance its ability for nutrient absorption [21]. Moreover, crypts depth was significantly deeper in the Pr group. These results are consistent with another study [50], and confirm the positive effect of dietary propolis supplementation on the intestinal morphology and mucosal development. Therefore, the overall histomorphometric changes induced by propolis could be remarkably useful, particularly during heat stress, when the intestinal mucosa is damaged and requires a rapid renewal of villus cells [51].
The data on body temperature from the current research agree with that obtained by Bobek et al. [52] and show that the quail’s body temperature was significantly elevated in the HS group compared to the C group. The failure of thermoregulation process in the HS group may be due to the exergonic reactions of free radicals that are generated by heat stress [53]. In addition, the effects of heat stress on circulating corticosterone and MDA levels in the present study are similar to that obtained in previous works on both broilers and quail [16,54]. These results demonstrate that heat stress induced inflammation and lipid peroxidation of the quail as previously reported [12,55,56]. The excessive amounts of corticosterone may induce the fever that observed in the HS group [16]. Furthermore, the decline in fT3 concentrations upon heat exposure is consistent with the reduction in feed consumption of the same quail group in the present study. This might have happened in order to decrease heat accumulation and control body temperature which favors survivability of birds under heat stress condition [57].
It was found that some components extracted from propolis, like flavonoids and caffeic acid phenethyl ester (CAPE), have anti-inflammation capacity [58], anti-oxidant potency [30] and anti-thyroid effects [59], especially under the heat stress environment [54]. In the present study, Pr treatment decreased the plasma corticosterone of the HS quail. The positive effects of propolis partially interfere with oxidative protein denaturation and decrease the protein breakdown [56]. Therefore, the presence of propolis in the quail diets would likely improve the feed conversion ratio and the digestibility of nutrients, reflecting in a high production performance of quail, especially under heat stress conditions.
In line with other works [11,60,61], the current research indicates that heat stress significantly impaired the immunological parameters of Japanese quail. The high H/L ratio and low lymphocyte proliferation observed in the heat-stressed quail may be due to the high plasma corticosterone concentration that observed in the same treatment group [55]. The present study demonstrates that the dietary propolis supplementation significantly improved the immunity parameters in the quail. Other reports suggest that some propolis constituents could augment immune-modulation in the heat-stressed quail via influencing the lymphocyte proliferation and antibody production [62–65]. These compounds also inhibit prostaglandin synthesis as anti-immune substances, thus contribute in getting a better humoral response [66].
To the best of our knowledge, there is no information on the direct effect of propolis at molecular levels in poultry either under thermoneuteral or heat stress conditions. The present research aimed to gain a better understanding of propolis effects following heat stress using qRT-PCR analysis of many genes expressed in the liver of Japanese quail and involved in immunity (TLR5), apoptosis (CASP6) and heat shock proteins (Hsp70 and Hsp90); and at the same time, their expressions have been widely altered in response to stress conditions. The TLR5 gene is a member of the toll-like receptor family which plays a fundamental role in pathogen recognition and activation of innate immunity [67]. While the TLR5 mRNA expression was negatively affected by HS treatment in the current study, the up-regulation effect of propolis on the TLR5 expression leads to the conclusion that supplementing propolis to quail diets could maintain the quail in adequate immune responses, probably by keeping these birds in a well-nourished status [68].
On the other hand, it was observed that the exposure of quail to heat stress significantly increased the expression of CASP6 gene while it was normalized again after propolis supplementation. The CASP6 gene plays a central role in the execution-phase of cell apoptosis [69], and could be up-regulated as a result of the increased fever, corticosterone and MDA levels in the heat-stressed quail [16]. The protective effects of propolis and its components against the activation of the caspase cascade that leads to apoptosis and subsequent cell death were also reported in previous works on human cells [29,70]. Furthermore, two classes of heat shock proteins; Hsp70 and Hsp90, which are mainly synthesized to protect cells when stressed by elevated temperatures [71]; were also analyzed in the present study. The up-regulation of hepatic Hsp70 expression in the heat-stressed quail may be promoted in a consequence of the elevated plasma levels of MDA in these quail in order to protect cells against peroxidation damage [72]. The protective effect of Hsp70 does not correlate with the intestinal morphology under heat stress, but it may be strongly correlated with the increased activity of the digestive enzymes [49]. Moreover, many signaling pathways correlated with apoptosis and protein transcription are modulated by Hsp90 to improve the tolerance to stress [73]. The study of Xie et al. [15] indicated that mRNA expression of Hsp90 in the liver of broiler breeder hens increased after a long-term heat treatment. However, the current study does not show any differences in the hepatic Hsp90 gene expression in the quail groups exposed to heat stress when compared to the control group.
Conclusion
The results of the present study indicate that dietary propolis supplementation can alleviate the negative effects of heat stress on the growth performance, intestinal histomorphometry, physiology and immunity of Japanese quail. Propolis maintained the normal levels of plasma free T3 hormone, preserved the integrity of intestinal villi and increased the crypts depth in the heat-stressed quail, which consequently enhanced the feed efficiency and growth performance of the quail. The plasma corticosterone and body temperature were decreased in the quail that were fed with propolis, indicating that propolis decreases the stress indicators in quail exposed to heat stress conditions. Moreover, the quail that were fed with propolis expressed good immunity status as presented by a lower H/L ratio and higher lymphocyte proliferation together with the high expression of TLR5 gene. Furthermore, the positive effects of propolis were evidenced by normalizing the high expressions of CASP6-apoptotic gene and Hsp70-stress gene to lower levels in the heat-stressed quail. Therefore, the addition of propolis at a rate of 1 g/kg to the diet of quail could be recommended as a potential nutritional strategy in order to improve their performance, especially under heat stress conditions.
Supporting information
(PDF)
Acknowledgments
This study was funded by the General Scientific Research Department at Cairo University (GSRD-CU) under activities carried out by the Project of Rapid Climate Change in Poultry Cellular and Molecular Physiology (RCC-PCMP). Dr. Ahmed O. Abass was the principal investigator and research team leader of the project. The authors thank Prof. Dr. Farid Stino (Professor of Poultry Breeding, Cairo University), Prof. Dr. Magdi Mashaly (Professor of Poultry Immunology, Cairo University), and Prof. Dr. Abdel-Rahman M. M. Atta (Professor of Poultry Immunology, Cairo University), for their technical support during this study. We are very grateful to all the personnel from the Poultry Biotechnology Lab and members of Poultry Services Center at Faculty of Agriculture, Cairo University, for their assistance in sample preparation and monitoring of birds throughout the experimental period.
Data Availability
All relevant data are within the paper.
Funding Statement
This work was supported by the project “Rapid Climate Change in Poultry Cellular and Molecular Physiology (RCC-PCMP)”. It was funded by the General Scientific Research Department at Cairo University (GSRD-CU); http://www.gsrd.cu.edu.eg/. Dr. Ahmed O. Abass was the principal investigator of the project and the fund was awarded to him during the implementation of the project. The funders approved the study design, data collection and analysis, the decision to publish, and preparation of the manuscript.
References
- 1.Runjaic-Antic D, Pavkov S, Levic J. Herbs in a sustainable animal nutrition. Biotechnol Anim Husb. 2010;26: 203–214. doi: 10.2298/BAH1004203R [Google Scholar]
- 2.Genchev A, Mihaylova G, Ribarski S, Pavlov A, Kabakchiev M. Meat quality and composition in Japanese quails. Trakia J Sci. 2008;66: 72–82. Available: http://www.uni-sz.bg [Google Scholar]
- 3.Agiang EA, Oko OOK, Essien GE. Quails response to aqueous extract of bush marigold (Aspilia africana) leaf. Am J Anim Vet Sci. 2011;6: 130–134. [Google Scholar]
- 4.Ruskin FR. Microlivestock [Internet]. BOSTID, editor. Micro-livestock: Little known small animals with promising economic future. Washington, D.C.: National Academies Press; 1991. doi: 10.17226/1831 [Google Scholar]
- 5.Cerci IH, Tath P, Azman MA, Birben N. The effect of restricted feed on feed intake, egg production and feed conversion in pullets. Indian Vet J. 2003;80: 1153–1157. Available: http://agris.fao.org/agris-search/search.do?recordID=IN2004000602 [Google Scholar]
- 6.Niu ZY, Liu FZ, Yan QL, Li WC. Effects of different levels of vitamin E on growth performance and immune responses of broilers under heat stress. Poult Sci. 2009;88: 2101–2107. doi: 10.3382/ps.2009-00220 [DOI] [PubMed] [Google Scholar]
- 7.Quinteiro-Filho WM, Gomes a. VS, Pinheiro ML, Ribeiro A, Ferraz-de-Paula V, Astolfi-Ferreira CS, et al. Heat stress impairs performance and induces intestinal inflammation in broiler chickens infected with Salmonella Enteritidis. Avian Pathol. 2012;41: 421–427. doi: 10.1080/03079457.2012.709315 [DOI] [PubMed] [Google Scholar]
- 8.Miller DB, O’Callaghan JP. Neuroendocrine aspects of the response to stress. Metabolism. 2002;51: 5–10. Available: http://www.ncbi.nlm.nih.gov/pubmed/12040534 [DOI] [PubMed] [Google Scholar]
- 9.Bowen SJ, Washburn KW, Huston TM. Involvement of the thyroid gland in the response of young chickens to heat stress. Poult Sci. 1984;63: 66–69. Available: http://www.ncbi.nlm.nih.gov/pubmed/6701144 [DOI] [PubMed] [Google Scholar]
- 10.Yen PM. Physiological and molecular basis of thyroid hormone action. Physiol Rev. 2001;81: 1097–1142. Available: http://www.ncbi.nlm.nih.gov/pubmed/11427693 [DOI] [PubMed] [Google Scholar]
- 11.Mashaly MM, Hendricks GL, Kalama MA, Gehad AE, Abbas AO, Patterson PH. Effect of Heat Stress on Production Parameters and Immune Responses of Commercial Laying Hens. Poult Sci. 2004;83: 889–894. doi: 10.1093/ps/83.6.889 [DOI] [PubMed] [Google Scholar]
- 12.Seven PT, Yılmaz S, Seven I, Cercı IH, Azman MA, Yılmaz M. Effects of Propolis on Selected Blood Indicators and Antioxidant Enzyme Activities in Broilers under Heat Stress. Acta Vet Brno. 2009;78: 75–83. doi: 10.2754/avb200978010075 [Google Scholar]
- 13.Sahin K, Sahin N, Onderci M. Vitamin E supplementation can alleviate negative effects of heat stress on egg production, egg quality, digestibility of nutrients and egg yolk mineral concentrations of Japanese quails. Res Vet Sci. 2002;73: 307–312. doi: 10.1016/S0034-5288(02)00126-1 [DOI] [PubMed] [Google Scholar]
- 14.Burkholder KM, Thompson KL, Einstein ME, Applegate TJ, Patterson JA. Influence of stressors on normal intestinal microbiota, intestinal morphology, and susceptibility to salmonella enteritidis colonization in broilers. Poult Sci. 2008;87: 1734–1741. doi: 10.3382/ps.2008-00107 [DOI] [PubMed] [Google Scholar]
- 15.Xie J, Tang L, Lu L, Zhang L, Xi L, Liu H, et al. Differential expression of heat shock transcription factors and heat shock proteins after acute and chronic heat stress in laying chickens (Gallus gallus). PLoS One. 2014;9: e102204 doi: 10.1371/journal.pone.0102204 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Mehaisen GMK, Eshak MG, El Sabry MI, Abass AO. Expression of inflammatory and cell death program genes and comet DNA damage assay induced by Escherichia coli in layer hens. PLoS One. 2016;11: e0158314 doi: 10.1371/journal.pone.0158314 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Mehaisen GMK, Eshak MG, Elkaiaty AM, Atta AMM, Mashaly MM, Abass AO. Comprehensive growth performance, immune function, plasma biochemistry, gene expressions and cell death morphology responses to a daily corticosterone injection course in broiler chickens. van den Bos R, editor. PLoS One. CABI; 2017;12: e0172684 doi: 10.1371/journal.pone.0172684 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Patra T, Pati PK, Mohapatra AK. Study on carcass quality of coloured broiler chicks supplemented with vitamin E and C during summer stress. SAARC J Agric. 2011;9: 123–132. Available: https://www.cabdirect.org/cabdirect/abstract/20123050083 [Google Scholar]
- 19.Mahmoud UT, Abdel-Rahman MAM, Darwish MHA. Effects of propolis, ascorbic acid and vitamin E on thyroid and corticosterone hormones in heat stressed broilers. J Adv Vet Res. 2014;4: 18–27. Available: http://www.advetresearch.com/index.php/avr/article/viewFile/227/168 [Google Scholar]
- 20.Seven PT. The effects of dietary Turkish propolis and vitamin C on performance, digestibility, egg production and egg quality in laying hens under different environmental temperatures. Asian-Australasian J Anim Sci. 2008;21: 1164–1170. Available: http://ajas.info/journal/view.php?number=21899 [Google Scholar]
- 21.Deng W, Dong XF, Tong JM, Zhang Q. The probiotic Bacillus licheniformis ameliorates heat stress-induced impairment of egg production, gut morphology, and intestinal mucosal immunity in laying hens. Poult Sci. 2012;91: 575–582. doi: 10.3382/ps.2010-01293 [DOI] [PubMed] [Google Scholar]
- 22.Sandikci M, Eren U, Onol AG, Kum S. The effect of heat stress and the use of Saccharomyces cerevisiae or (and) bacitracin zinc against heat stress on the intestinal mucosa in quails. Rev Med Vet (Toulouse). 2004;11: 552–556. Available: http://www.revmedvet.com/artdes-us.php?id=1273 [Google Scholar]
- 23.Sahin N, Orhan C, Tuzcu M, Sahin K, Kucuk O. The Effects of Tomato Powder Supplementation on Performance and Lipid Peroxidation in Quail. Poult Sci. 2008;87: 276–283. doi: 10.3382/ps.2007-00207 [DOI] [PubMed] [Google Scholar]
- 24.Bankova VS, de Castro SL, Marcucci MC. Propolis: recent advances in chemistry and plant origin. Apidologie. EDP Sciences; 2000;31: 3–15. doi: 10.1051/apido:2000102 [Google Scholar]
- 25.Seven PT, Seven İ. Effect of dietary Turkish propolis as alternative to antibiotic on performance and digestibility in broilers exposed to heat stress. J Appl Anim Res. 2008;34: 193–196. doi: 10.1080/09712119.2008.9706970 [Google Scholar]
- 26.Seven PT, Seven I, Yilmaz M, Şimşek ÜG. The effects of Turkish propolis on growth and carcass characteristics in broilers under heat stress. Anim Feed Sci Technol. 2008;146: 137–148. doi: 10.1016/j.anifeedsci.2007.11.003 [Google Scholar]
- 27.Prytzyk E, Dantas AP, Salomão K, Pereira AS, Bankova VS, De Castro SL, et al. Flavonoids and trypanocidal activity of Bulgarian propolis. J Ethnopharmacol. 2003;88: 189–193. Available: http://www.ncbi.nlm.nih.gov/pubmed/12963141 [DOI] [PubMed] [Google Scholar]
- 28.Wang B-J, Lien Y-H, Yu Z-R. Supercritical fluid extractive fractionation–study of the antioxidant activities of propolis. Food Chem. 2004;86: 237–243. doi: 10.1016/j.foodchem.2003.09.031 [Google Scholar]
- 29.Chen YJ, Huang AC, Chang HH, Liao HF, Jiang CM, Lai LY, et al. Caffeic acid phenethyl ester, an antioxidant from propolis, protects peripheral blood mononuclear cells of competitive cyclists against hyperthermal stress. J Food Sci. 2009;74: 162–167. doi: 10.1111/j.1750-3841.2009.01199.x [DOI] [PubMed] [Google Scholar]
- 30.Seven I, Aksu T, Seven PT. The Effects of Propolis on Biochemical Parameters and Activity of Antioxidant Enzymes in Broilers Exposed to Lead-Induced Oxidative Stress. Asian-Australasian J Anim Sci. 2010;23: 1482–1489. doi: 10.5713/ajas.2010.10009 [Google Scholar]
- 31.Oktay M, Gülçin İ, Küfrevioğlu Öİ. Determination of in vitro antioxidant activity of fennel (Foeniculum vulgare) seed extracts. LWT—Food Sci Technol. 2003;36: 263–271. doi: 10.1016/S0023-6438(02)00226-8 [Google Scholar]
- 32.Rolim A, Maciel CPM, Kaneko TM, Consiglieri VO, Salgado-Santos IMN, Velasco MVR. Validation assay for total flavonoids, as rutin equivalents, from Trichilia catigua Adr. Juss (Meliaceae) and Ptychopetalum olacoides Bentham (Olacaceae) commercial extract. J AOAC Int. 2005;88: 1015–1019. Available: http://www.ncbi.nlm.nih.gov/pubmed/16152916 [PubMed] [Google Scholar]
- 33.Kujala TS, Loponen JM, Klika KD, Pihlaja K. Phenolics and betacyanins in red beetroot (Beta vulgaris) root: distribution and effect of cold storage on the content of total phenolics and three individual compounds. J Agric Food Chem. 2000;48: 5338–5342. Available: http://www.ncbi.nlm.nih.gov/pubmed/11087483 [DOI] [PubMed] [Google Scholar]
- 34.Suvarna KS, Layton C, Bancroft JD. Bancroft’s theory and practice of histological techniques. 7th ed. 2013.
- 35.Gehad A E., Mehaisen G M., Abbas A O., Mashaly M M. The Role of Light Program and Melatonin on Alleviation of Inflammation Induced by Lipopolysaccharide Injection in Broiler Chickens. Int J Poult Sci. 2008;7: 193–201. doi: 10.3923/ijps.2008.193.201 [Google Scholar]
- 36.Zhang L, Yue HY, Zhang HJ, Xu L, Wu SG, Yan HJ, et al. Transport stress in broilers: I. Blood metabolism, glycolytic potential, and meat quality. Poult Sci. 2009;88: 2033–2041. doi: 10.3382/ps.2009-00128 [DOI] [PubMed] [Google Scholar]
- 37.Kamel NN, Ahmed AMH, Mehaisen GMK, Mashaly MM, Abass AO. Depression of leukocyte protein synthesis, immune function and growth performance induced by high environmental temperature in broiler chickens. Int J Biometeorol. Springer Berlin Heidelberg; 2017; 1–9. doi: 10.1007/s00484-017-1342-0 [DOI] [PubMed] [Google Scholar]
- 38.Ellestad LE, Malkiewicz SA, Guthrie HD, Welch GR, Porter TE. Expression and regulation of glucocorticoid-induced leucine zipper in the developing anterior pituitary gland. J Mol Endocrinol. 2009;42: 171–183. doi: 10.1677/JME-08-0066 [DOI] [PubMed] [Google Scholar]
- 39.Havlík P, Valin H, Herrero M, Obersteiner M, Schmid E, Rufino MC, et al. Climate change mitigation through livestock system transitions. Proc Natl Acad Sci U S A. 2014;111: 3709–3714. doi: 10.1073/pnas.1308044111 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Vona LC, Jung GA, Reid RL, Sharp WC. Nutritive value of warm-season grass hays for beef cattle and sheep; digestibility, intake and mineral utilization. J Anim Sci. 1984;59: 1582–1593. doi: 10.2527/jas1984.5961582x [Google Scholar]
- 41.Hai L, Rong D, Zhang Z-Y. The effect of thermal environment on the digestion of broilers. J Anim Physiol Anim Nutr (Berl). 2000;83: 57–64. doi: 10.1046/j.1439-0396.2000.00223.x [Google Scholar]
- 42.Habibian M, Sadeghi G, Ghazi S, Moeini MM. Selenium as a Feed Supplement for Heat-Stressed Poultry: a Review. Biol Trace Elem Res. 2015;165: 183–193. doi: 10.1007/s12011-015-0275-x [DOI] [PubMed] [Google Scholar]
- 43.Denli M, Cankaya S, Silici S, Okan F, Uluocak AN. Effect of Dietary Addition of Turkish Propolis on the Growth Performance, Carcass Characteristics and Serum Variables of Quail (Coturnix coturnix japonica). Asian-Australasian J Anim Sci. 2005;18: 848–854. doi: 10.5713/ajas.2005.848 [Google Scholar]
- 44.Tayeb IT, Sulaiman BF. Effect of Propolis Supplementation on Productive Performance in Local Quail. Iran J Appl Anim Sci. 2014;4: 621–627. Available: http://www.scopemed.org/?mno=169533 [Google Scholar]
- 45.Shalmany SK, Shivazad M. The effect of diet propolis supplementation on Ross broiler chicks performance. Int J Poult Sci. 2006; 84–88. [Google Scholar]
- 46.Shamoto K, Yamauchi K. Recovery responses of chick intestinal villus morphology to different refeeding procedures. Poult Sci. 2000;79: 718–23. Available: http://www.ncbi.nlm.nih.gov/pubmed/10824961 [DOI] [PubMed] [Google Scholar]
- 47.Yu J, Yin P, Liu F, Cheng G, Guo K, Lu A, et al. Effect of heat stress on the porcine small intestine: A morphological and gene expression study. Comp Biochem Physiol Part A Mol Integr Physiol. 2010;156: 119–128. doi: 10.1016/j.cbpa.2010.01.008 [DOI] [PubMed] [Google Scholar]
- 48.Ryder AA, Feddes JJR, Zuidhof MJ. Field study to relate heat stress index to broiler performance. J Appl Poult Res. 2004;13: 493–499. doi: 10.1093/japr/13.3.493 [Google Scholar]
- 49.Hao Y, Gu XH, Wang XL. Overexpression of heat shock protein 70 and its relationship to intestine under acute heat stress in broilers: 1. Intestinal structure and digestive function. Poult Sci. 2012;91: 781–789. doi: 10.3382/ps.2011-01627 [DOI] [PubMed] [Google Scholar]
- 50.Toman R, Hajkova Z, Hluchy S. Changes in intestinal morphology of rats fed with different levels of bee pollen. Pharmacogn Commun. 2015;5: 261–264. doi: 10.5530/pc.2015.4.8 [Google Scholar]
- 51.Sayrafi R, Shahrooz R, Soltanalineja F, Rahimi S. Histomorphometrical Study of the Prebiotic Effects on Intestine Morphology and Growth Performance of Broiler Chickens. Vet Res Forum. Urmia University; 2011;2: 45–51. [Google Scholar]
- 52.Bobek S, Niezgoda J, Pietras M, Kacińska M, Ewy Z. The effect of acute cold and warm ambient temperatures on the thyroid hormone concentration in blood plasma, blood supply, and oxygen consumption in Japanese quail. Gen Comp Endocrinol. 1980;40: 201–210. Available: http://www.ncbi.nlm.nih.gov/pubmed/7364210 [DOI] [PubMed] [Google Scholar]
- 53.Ciftçi M, Ertas ON, Güler T. Effects of vitamin E and vitamin C dietary sup- plementation on egg production and egg quality. Rev Med Vet (Toulouse). 2005;156: 107–111. [Google Scholar]
- 54.Akdemir F, Sahin N, Orhan C, Tuzcu M, Sahin K, Hayirli A. Chromium-histidinate ameliorates productivity in heat-stressed Japanese quails through reducing oxidative stress and inhibiting heat-shock protein expression. Br Poult Sci. 2015;56: 247–254. doi: 10.1080/00071668.2015.1008992 [DOI] [PubMed] [Google Scholar]
- 55.Shini S, Huff GR, Shini A, Kaiser P. Understanding stress-induced immunosuppression: exploration of cytokine and chemokine gene profiles in chicken peripheral leukocytes. Poult Sci. Oxford University Press; 2010;89: 841–851. doi: 10.3382/ps.2009-00483 [DOI] [PubMed] [Google Scholar]
- 56.Sahin K, Onderci M, Sahin N, Gursu MF, Khachik F, Kucuk O. Effects of lycopene supplementation on antioxidant status, oxidative stress, performance and carcass characteristics in heat-stressed Japanese quail. J Therm Biol. 2006;31: 307–312. doi: 10.1016/j.jtherbio.2005.12.006 [Google Scholar]
- 57.Bogin E, Avidar Y, Pech-Waffenschmidt V, Doron Y, Israeli BA, Kevkhayev E. The relationship between heat stress, survivability and blood composition of the domestic chicken. Eur J Clin Chem Clin Biochem. 1996;34: 463–469. Available: http://www.ncbi.nlm.nih.gov/pubmed/8831047 [DOI] [PubMed] [Google Scholar]
- 58.Hori JI, Zamboni DS, Carrão DB, Goldman GH, Berretta AA. The Inhibition of Inflammasome by Brazilian Propolis (EPP-AF). Evidence-Based Complement Altern Med. 2013;2013: 418508 doi: 10.1155/2013/418508 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Lueprasitsakul W, Alex S, Fang SL, Pino S, Irmscher K, Kohrle J, et al. Flavonoid administration immediately displaces thyroxine (T 4) from serum transthyretin, increases serum free T 4, and decreases serum thyrotropin in the rat. Endocrinology. 1990;126: 2890–2895. doi: 10.1210/endo-126-6-2890 [DOI] [PubMed] [Google Scholar]
- 60.Prieto MT, Campo JL. Effect of heat and several additives related to stress levels on fluctuating asymmetry, heterophil:lymphocyte ratio, and tonic immobility duration in White Leghorn chicks. Poult Sci. 2010;89: 2071–2077. doi: 10.3382/ps.2010-00716 [DOI] [PubMed] [Google Scholar]
- 61.Felver-Gant JN, Mack LA, Dennis RL, Eicher SD, Cheng HW. Genetic variations alter physiological responses following heat stress in 2 strains of laying hens. Poult Sci. 2012;91: 1542–1551. doi: 10.3382/ps.2011-01988 [DOI] [PubMed] [Google Scholar]
- 62.Sforcin JM, Orsi RO, Bankova V. Effect of propolis, some isolated compounds and its source plant on antibody production. J Ethnopharmacol. 2005;98: 301–305. doi: 10.1016/j.jep.2005.01.042 [DOI] [PubMed] [Google Scholar]
- 63.Fan Y, Lu Y, Wang D, Liu J, Song X, Zhang W, et al. Effect of epimedium polysaccharide-propolis flavone immunopotentiator on immunosuppression induced by cyclophosphamide in chickens. Cell Immunol. 2013;281: 37–43. doi: 10.1016/j.cellimm.2013.01.008 [DOI] [PubMed] [Google Scholar]
- 64.Yuan J, Liu J, Hu Y, Fan Y, Wang D, Guo L, et al. The immunological activity of propolis flavonoids liposome on the immune response against ND vaccine. Int J Biol Macromol. 2012;51: 400–405. doi: 10.1016/j.ijbiomac.2012.06.002 [DOI] [PubMed] [Google Scholar]
- 65.Borrelli F, Maffia P, Pinto L, Ianaro A, Russo A, Capasso F, et al. Phytochemical compounds involved in the anti-inflammatory effect of propolis extract. Fitoterapia. 2002;73: S53–S63. doi: 10.1016/S0367-326X(02)00191-0 [DOI] [PubMed] [Google Scholar]
- 66.Namgoong SY, Son KH, Chang HW, Kang SS, Kim HP. Effects of naturally occurring flavonoids on mitogen-induced lymphocyte proliferation and mixed lymphocyte culture. Life Sci. 1994;54: 313–320. Available: http://www.ncbi.nlm.nih.gov/pubmed/8289592 [DOI] [PubMed] [Google Scholar]
- 67.Miao EA, Andersen-Nissen E, Warren SE, Aderem A. TLR5 and Ipaf: dual sensors of bacterial flagellin in the innate immune system. Semin Immunopathol. 2007;29: 275–288. doi: 10.1007/s00281-007-0078-z [DOI] [PubMed] [Google Scholar]
- 68.Sophia I, Sejian V, Bagath M, Bhatta R. Quantitative expression of hepatic toll-like receptors 1–10 mRNA in Osmanabadi goats during different climatic stresses. Small Rumin Res. 2016;141: 11–16. doi: 10.1016/j.smallrumres.2016.06.005 [Google Scholar]
- 69.Kumar S, Colussi PA. Prodomains–adaptors–oligomerization: the pursuit of caspase activation in apoptosis. Trends Biochem Sci. 1999;24: 1–4. doi: 10.1016/S0968-0004(98)01332-2 [DOI] [PubMed] [Google Scholar]
- 70.Izuta H, Shimazawa M, Tazawa S, Araki Y, Mishima S, Hara H. Protective effects of Chinese propolis and its component, chrysin, against neuronal cell death via inhibition of mitochondrial apoptosis pathway in SH-SY5Y cells. J Agric Food Chem. 2008;56: 8944–8953. doi: 10.1021/jf8014206 [DOI] [PubMed] [Google Scholar]
- 71.Al-Aqil A, Zulkifli I. Changes in heat shock protein 70 expression and blood characteristics in transported broiler chickens as affected by housing and early age feed restriction. Poult Sci. 2009;88: 1358–1364. doi: 10.3382/ps.2008-00554 [DOI] [PubMed] [Google Scholar]
- 72.Kregel KC. Heat shock proteins: modifying factors in physiological stress responses and acquired thermotolerance. J Appl Physiol. 2002;92: 2177–2186. doi: 10.1152/japplphysiol.01267.2001 [DOI] [PubMed] [Google Scholar]
- 73.Padmini E, Usha Rani M. Heat-shock protein 90 alpha (HSP90α) modulates signaling pathways towards tolerance of oxidative stress and enhanced survival of hepatocytes of Mugil cephalus. Cell Stress Chaperones. Springer; 2011;16: 411–425. doi: 10.1007/s12192-011-0255-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
(PDF)
Data Availability Statement
All relevant data are within the paper.