Abstract
Prosaccade and antisaccade errors in the context of social and nonsocial stimuli were investigated in youth with autism spectrum disorder (ASD; n = 19) a matched control sample (n = 19), and a small sample of youth with obsessive compulsive disorder (n = 9). Groups did not differ in error rates in the prosaccade condition for any stimulus category. In the antisaccade condition, the ASD group demonstrated more errors than the control group for nonsocial stimuli related to circumscribed interests, but not for other nonsocial stimuli or for social stimuli. Additionally, antisaccade error rates were predictive of core ASD symptom severity. Results indicate that the cognitive control of visual attention in ASD is impaired specifically in the context of nonsocial stimuli related to circumscribed interests.
Keywords: Autism spectrum disorder, Visual attention, Cognitive control, Eyetracking
Introduction
Eyetracking has proven to be a powerful tool to investigate visual attention in autism spectrum disorder (ASD), with critical implications for the development of social communicative impairments in this disorder (Jones and Klin 2013). Most research on visual attention in ASD indicates atypical scanpaths when individuals with ASD view social information (Boraston and Blakemore 2007; Fletcher-Watson et al. 2009; Katarzyna et al. 2010; Klin et al. 2002), though not all studies in this area are consistent (see Jemel et al. 2006 for a review). A smaller body of research has examined visual attention in ASD in the context of both social and nonsocial stimuli, and these studies have typically found both decreased visual attention to social elements of visual scenes and increased visual attention to nonsocial elements of visual scenes (Chawarska et al. 2009; Sasson et al. 2008, 2011). These findings suggest that patterns of social visual attention in ASD are critically dependent on the context within which stimuli are presented.
The studies reviewed above address visual attention in ASD in the context of passive viewing tasks. Relatively few studies have investigated visual attention in ASD in contexts requiring cognitive control of visual attention. This is a significant omission given the evidence indicating impairments in response inhibition and cognitive control in ASD (Mosconi et al. 2009; Mostert-Kerckhoffs et al. 2015). Given that real-world contexts require cognitive control of visual attention, and given that a number of empirically-validated ASD treatments contain elements of training attention towards socially relevant elements of the environment (Turner-Brown et al. 2008), understanding the capacity for individuals with ASD to consciously control visual attention has critical implications for understanding social impairments in ASD as well as for research addressing the development of novel ASD interventions.
The purpose of the present study was to investigate the cognitive control of visual attention in ASD in the context of a prosaccade and antisaccde task that contained both social and nonsocial target stimuli. Prosaccade tasks require participants to look towards a cued peripheral target, whereas antisaccade tasks require participants to look to the opposite direction than the cued peripheral target (Hutton 2008). Thus, antisaccade tasks require relatively greater cognitive control, and there is evidence of relatively greater deficits on anti-relative to prosaccade tasks in ASD (Luna et al. 2004; Manoach et al. 2004). We examined responses to face images as well as to two types of nonsocial stimuli: nonsocial images related to circumscribed interests (CIs) in ASD and nonsocial images unrelated to CIs (Sasson et al. 2012).
Given prior studies indicating impaired control of attention in ASD (Luna et al. 2007; Mosconi et al. 2009), we predicted that the ASD group would make relatively more errors in the antisaccade condition. Additionally, given that ASD is characterized by the canalization of social brain regions in response to nonsocial stimuli related to CIs in ASD (Grelotti et al. 2005) and that recruitment of frontostriatal brain regions during cognitive control is contingent on whether stimuli are related to CIs in ASD (Sabatino et al. 2013), we further predicted that antisaccade errors rates in the ASD group would be larger on trials with nonsocial stimuli related to CIs, suggesting decreased capacity to exert cognitive control over nonsocial stimuli related to CIs relative to social stimuli and nonsocial stimuli unrelated to CIs in the ASD group. We also investigated whether task performance correlated with ASD symptom severity in the ASD group due to previous reports of relations between deficits in response inhibition, cognitive flexibility, and ASD symptom severity (Lopez et al. 2005; Mosconi et al. 2009). Finally, we explored potential differences in saccade latencies to address whether impairments in the cognitive control of visual attention were attributable to deficits in response inhibition and/or differences in visual saccade dynamics.
Given the recent emphasis on dimensional constructs with transdiagnostic relevance (Cuthbert and Insel 2013), we also included a small group of youth with obsessive compulsive disorder (OCD). OCD is characterized by impaired attentional control (Shin et al. 2014), and obsessive–compulsive behaviors are often observed in ASD (see Jacob et al. 2009 for a review). The inclusion of an OCD group allows for an exploratory evaluation of the degree to which impaired cognitive control of visual attention is specifically characteristic of ASD or alternatively extends to another disorder characterized by attentional biases and repetitive behaviors.
Method
Participants
Seventy-two children and adolescents ranging in age from 9 to 18 years old participated; 32 individuals had a diagnosis of ASD; 31 were typically developing controls; and 9 individuals had a diagnosis of OCD. Individuals who (a) were not able to complete both task conditions successfully; (b) had a substantial amount of missing data (i.e., >50 % of trials missing within a condition due to technical problems with eyetracking recording and/or gaze time off screen); and/or (c) had more than 50 % invalid trials due to not beginning a trial with a central fixation were not analyzed, and the remaining 49 individuals were included in the final analysis. Two outliers from the control group were excluded from analysis due to rates of saccade errors that were at least three standard deviations outside the group mean (Howell et al. 1998). Of the remaining participants, 19 had a diagnosis of ASD (mean age (SD) = 13.34 (4.38), 16 male); 19 were typically developing controls (TD; mean age (SD) = 14.00 (2.79), 17 male); and 9 had a diagnosis of OCD (mean age (SD) = 15.43 (2.49). Participant characteristics are presented in Table 1.
Table 1. Means (SDs) of demographic and symptom data.
| ASD (n = 19) | TD (n = 19) | OCD (n = 9) | t(p) | |
|---|---|---|---|---|
| Age | 13.35 (4.38) | 14.00 (2.79) | 15.43 (2.49) | a1.32(p > 0.19) |
| Min: 9.3 | Min: 9.3 | Min: 10.8 | b0.56(p > 0.58) | |
| Max: 18.0 | Max: 18.2 | Max: 16.8 | c−1.30(p > 0.21) | |
| ADOS | ||||
| Calibrated Total Scores | 7.47 (2.46) | 1.67(0.50) | a*6.95(p < 0.001) | |
| Comm Algorithm Score | 4.36 (2.14) | 3.80 (0.33) | a*5.65(p < 0.001) | |
| RSI Algorithm Score | 7.16 (2.11) | 0.55 (0.73) | a*9.04(p < 0.001) | |
| SBRI Algorithm Score | 2.53 (1.93) | 1.22 (0.97) | a1.91(p > 0.06) | |
| K-BIT | ||||
| VIQ | 101.11 (18.78) | 108.31 (11.94) | 111.89 (9.76) | a1.61(p > 0.11) |
| b1.42(p > 0.16) | ||||
| c−0.78(p > 0.44) | ||||
| NVIQ | 103.84 (14.57) | 107.89 (14.01) | 110.89 (10.96) | a1.27(p > 0.21) |
| b0.87(p > 0.38) | ||||
| c−0.55(p > 0.58) | ||||
| IQ Composite | 103.05 (14.29) | 109.68 (13.54) | 113.45 (10.96) | a1.78(p > 0.08) |
| b1.39(p > 0.17) | ||||
| c−0.73(P>0.47) | ||||
| CY-BOCS | ||||
| Obsessions | 5.56 (5.74) | 0.94 (2.32) | 11.22 (5.65) | a*2.43(p < 0.02) |
| b* −3.05(p < 0.004) | ||||
| c* −6.89(p < 0.001) | ||||
| Compulsions | 8.33 (6.09) | 1.21 (3.05) | 12.22 (1.92) | a* −2.02(p < 0.05) |
| b* −4.2(p < 0.001) | ||||
| c* −9.89(p < 0.001) | ||||
| Total | 13.89 (10.07) | 2.16 (5.34) | 23.44 (6.74) | a*2.56(p < 0.01) |
| * −4.46(p < 0.001) | ||||
| c* −9.06(p < 0.001) | ||||
| SRS (raw scores) | 82.74 (17.48) | 51.36 (10.35) | 63.44 (14.94) | a*2.57(p < 0.01) |
| b* −6.96(p < 0.001) | ||||
| c* −3.31(p < 0.001) | ||||
Comm communication, RSI reciprocal social interaction, SBRI stereotyped behaviors and restricted interests, SRS Social Responsiveness Scale (Constantino and Gruber 2002); CY-BOCS the Children's Yale-Brown Obsessive Compulsive Scale (Scahill et al. 1997)
Significant differences between groups (p< 0.05) are indicated (*) in the right column as follows:
HFA versus OCD;
HFA versus TD;
TD versus OCD
Symptom Measures
Parents or legal guardians completed informant-report versions of the Social Responsiveness Scale (SRS) (Constantino and Gruber 2002) and the Children's Yale-Brown Obsessive Compulsive Scale (C-YBOCS) (Scahill et al. 1997). Intelligence was assessed via the Kaufman Brief Intelligence Test (K-BIT) (Kaufman and Kaufman 2004). The OCD and TD groups also were given the Schedule for Affective Disorders and Schizophrenia for School-Age Children-Present and Lifetime Version (K-SADS-PL) (Kaufman et al. 1997). The ASD and OCD groups completed the Autism Diagnostic Observation Schedule (ADOS-G; Lord et al. 2012).
Groups were matched on age, p>0.05, and gender distribution, χ2(2) = 5.26, p> 0.05. The ASD group was recruited through the Autism Research Registry at the Carolina Institute for Developmental Disabilities at the University of North Carolina at Chapel Hill. Autism spectrum diagnoses were based on history of clinical diagnosis and confirmed based on scores on modules 3 or 4 of the ADOS-G administered by a research reliable assessor using standard cutoffs. Calibrated total severity scores were calculated from raw ADOS scores to obtain dimensional measures of ASD symptom severity (Gotham et al. 2008). Children with OCD were recruited from the Program in Child Affective and Anxiety Disorders at Duke University Medical Center via announcements to psychology and psychiatry clinics, pediatricians, schools, and other professional and community settings (e.g., health fairs, parent groups). The TD group was recruited via emails sent through the UNC listserv to university employees and students. Exclusionary criteria for all groups included known genetic/medical conditions; known sensory deficits (e.g., blind or deaf); a diagnosis of a verbal learning disability; and IQ <80. Participants in the ASD group had a primary diagnosis of ASD with no comorbid diagnosis of psychosis, bipolar disorder, or OCD as reported by their primary caretaker. Participants in the OCD group had a primary diagnosis of OCD with no comorbid diagnosis of psychosis, bipolar disorder, ASD, or any other form of PDD confirmed by the K-SADS-PL. Participants with typical development had no significant features of PDD or OCD confirmed by K-SADS-PL, no history of an AXIS-I psychiatric or neurological disorders or use of psychotropic medications, nor family history of psychosis, bipolar disorder, PDD, and OCD. All participants consented to protocols approved by the Human Investigations Committees at UNC-Chapel Hill (ASD and TD groups) and Duke University (OCD group) Medical Centers. All participants had normal or corrected-to-normal vision.
Task and Procedure
The eyetracking task included a prosaccade condition (“look towards a peripheral target when it appears”) and an antisaccade condition (“look to the opposite side of the screen when a peripheral target appears”). Conditions were blocked and counterbalanced across participants (i.e., some participants completed the prosaccade condition first while others completed the antisaccade condition first). On each trial, a central target was presented for a variable period of 1500-2500 ms, followed by a peripheral stimulus presented for a variable period of 2500-3500 ms at 10° to the left or right of center (Mosconi et al. 2009). Sixty total trials were administered: 20 trials with a social stimulus as the peripheral target, 20 with an object related to CIs as a peripheral target, and 20 with nonsocial images unrelated to CIs as a peripheral target (see below for a description of these stimuli; see Fig. 1 for trial exemplars and sample images). Stimuli across all three categories appeared on the left and right sides an equal number of times and were presented in pseudo-random order within each condition such that a particular category could not appear more than twice in a row. Participants completed 10 practice trials before beginning the task and all participants reached a minimum of eight correct practice trials before proceeding. Stimuli used within the practice trials were simple shapes such as circles, triangles, and squares. During practice trials, participants were asked to verbalize the correct onscreen location (i.e., participants had to correctly answer questions such as “Did you look in the box on the right or the box on the left?”, “Can you point to the box that you looked at?”, “Did you make a mistake?”, “Why do you think you made a mistake?”).
Fig. 1.
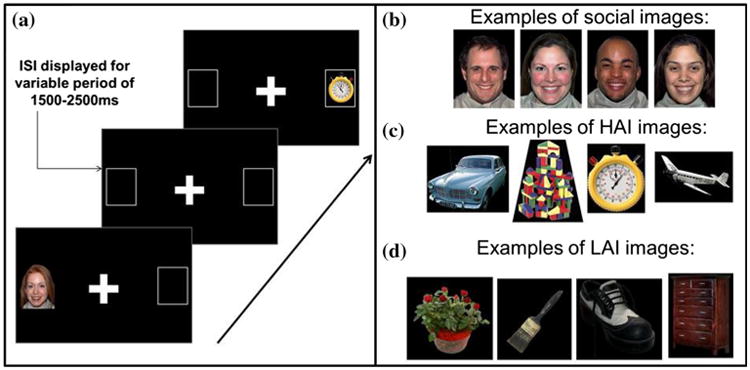
a Two sample trials (one social, one nonsocial) separated by an ISI comprised of a crosshair. Exemplar images from the b social, c HAI, and d LAI stimulus categories
Social stimuli were pictures of open mouth happy faces (with equal numbers of male and female faces) from the NimStim set of facial expressions (Tottenham et al. 2009). The nonsocial images were either related (50 %) or not related (50 %) to CIs in ASD (see Sasson et al. 2012 for a fuller description). Images related to CIs in ASD, referred to as High Autism Interest (HAI) images, were derived as follows: first a large number of potential nonsocial images was selected based on response profiles from semi-structured parent-report interviews about CIs in ASD (e.g., machines, mechanical systems, trains and electronic devices) (South et al. 2005; Turner-Brown et al. 2011). Next, the visual salience of these images was evaluated via passive-viewing visual exploration eyetracking studies of children and adults with and without ASD (Sasson et al. 2011; Sasson et al. 2008). These eyetracking studies identified 40 images without social content that garnered relatively greater visual attention (i.e., greater number of fixations and greater duration of fixations) in ASD samples. A complimentary set of “Low Autism Interest” (LAI) images also contained nonsocial content but were unrelated to CIs in ASD. In a recent study, 56 adults self-identified as having ASD rated these HAI images as significantly more pleasant than these LAI images relative to 213 adults without ASD, while the groups did not differ in their valence ratings of LAI images (Sasson et al. 2012); additionally these HAI images were found to have increased reward value in an econometric choice task (Watson et al. 2015). The HAI and LAI images used in the present study can be found in the Appendix of Dichter et al. (2012).
Eyetracking Data Collection and Analysis
Eye movement data were recorded with a Tobii X120 eye-tracker (Tobii Technology, Stockholm, Sweden). The system is a stand-alone eyetracking unit that monitors pupil movement at 60 Hz by using infrared light to produce reflection patterns on the corneas and then monitors the movements of these reflections relative to eye position. Before the task began, each participant completed a brief procedure to calibrate the eyetracking system that lasted approximately 12–15 s: participants were presented with a five-point calibration display and instructed to follow the moving red dot with their eyes while not moving their head.
Eye movement patterns were analyzed by fixation analyses. A single fixation was defined as gaze that remained within a radius of 30 pixels for a minimum of 100 ms. There were three areas of interest (AOIs) within each stimulus: the correct on-screen location, incorrect on-screen locations and the central fixation cross. Areas of interest were defined as rectangles to left and right of center. Only valid trials were included in analysis, defined as maintaining fixation on the central fixation cross at the start of the trial followed with a saccadic response. If a participant did not start within this central region at the start of the trial, that trial was excluded. As stated previously, if >50 % of trials were characterized as invalid then that participant was excluded from analysis. Groups did not differ in the final number of useable trials within each condition, p's> 0.50.
Dependent measures were the percentage of saccade errors made in the pro- and antisaccade conditions and the latency to saccade to the correct onscreen location during the pro- and antisaccade condition. A saccadic error was defined as a saccade within 3 degrees of the incorrect onscreen location (Manoach et al. 2004). Analyses with and without age, IQ, and the order of condition presentation as covariates yielded nearly identical results, and results without covariates are reported.
Results
Saccade Errors Rates
A Group (ASD, TD, OCD) × Stimulus Category (Social, HAI, LAI) × Condition (Prosaccade, Antisaccade) repeated measures ANOVA was conducted on error rates (calculated as a proportion of errors across useable trials for each individual). The Group × Stimulus Category × Condition interaction was not significant, F(4,88) = 0.43, p >0.78. A main effect of Condition, F(1,44) = 67.71, p <0.0001, was found that reflected that more errors were made in the antisaccade condition relative to the prosac-cade condition across Groups and Stimulus Categories, and follow-up t-tests revealed that this effect was significant in all three groups, p's < 0.02. The main effect of Stimulus Category was not significant, F(2,43) = 0.13, p > 0.88. There was a significant Group x Condition interaction, F(2,44) = 3.45, p < 0.04 but the interactions of Stimulus Category × Group, F(4,88) = 1.42, p>0.23, and Stimulus Category × Condition, F(2,43) = 1.26, p > 0.30 were not significant.
Due to specific a priori hypotheses regarding saccade performance in response to each stimulus category, follow-up analyses were conducted within the prosaccade and antisaccade conditions separately, as described in the next two sections.
Prosaccade Error Rates
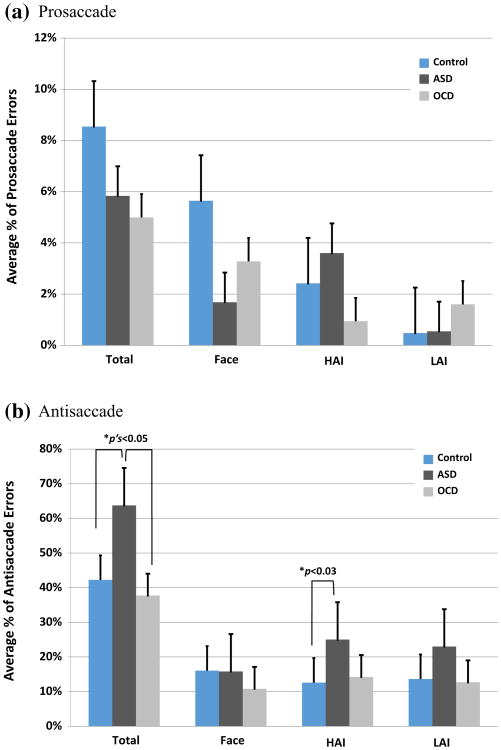
Prosaccade error rates across all stimulus categories did not differ between the TD and ASD groups, t(36) = 0.60, p>0.55. There were no differences between the TD and ASD groups in prosaccade error rate within each stimulus category, p's > 0.21. There were no differences between the ASD and OCD groups in the rate of prosaccade errors across all stimulus categories, t(26) = 0.15, p> 0.88, nor the rate of prosaccade errors within each stimulus category, p's > 0.16. Similarly, there were no differences between the TD group and the OCD group in the rate of prosaccade errors across categories, t(26) = 0.63, p > 0.53, nor the rate of errors within each stimulus category, p's > 0.21. Figure 2a illustrates average error rates for the prosaccade condition.
Fig. 2.
Group-averaged error rates for the a prosaccade condition and b the antisaccade condition across all trials (“Total”), Face, HAI, and LAI trials. Error bars reflect SE of the mean
Antisaccade Error Rates
Relative to TD controls, the ASD group showed an increased overall rate of antisaccade errors across all stimulus categories, t(36) = 2.32, p < 0.03. Within each stimulus category, the ASD group made more antisaccade errors in response to HAI stimuli, t(36) = 2.65, p < 0.01, than did TD controls, but did not differ in antisaccade errors in response to social stimuli, t(36) = 0.02, p > 0.98, or the LAI stimuli, t(38) = 1.84, p > 0.08. Relative to the OCD group, the ASD group made more antisaccade errors across all stimulus categories, t(26) = 2.06, p < 0.05. There were no differences between the ASD and OCD groups in the number of antisaccade errors within each stimulus category, Social: t(26) = 0.95, p > 0.35; HAI: t(26) = 1.66, p > 0.11; LAI: t(26) = 1.70, p > 0.10. There were no differences between the TD controls and OCD group in the total rate of antisaccade errors, t(26) = 0.63, p > 0.53, nor the number of antisaccade errors within each stimulus category, Social: t(26) = 1.10, p > 0.28; HAI: t(26) = 0.32, p > 0.75; LAI: t(26) = 0.14, p > 0.89. Figure 2b illustrates average error rates for the antisaccade condition.
Saccade Latency
A Group (ASD, TD, OCD) × Stimulus Category (Social, HAI, LAI) × Condition (Prosaccade, Antisaccade) repeated measures ANOVA on saccade latency revealed a main effect of Condition, F(1,44) = 7.78, p <0.03, reflecting that latencies were significantly longer in the antisaccade relative to the prosaccade conditions, across stimulus categories for all three groups, p’s's < 0.001. The three-way interaction and all two-way interactions were not significant, p's > 0.50. Relative to the OCD group, the ASD group was significantly faster to prosaccade in the HAI condition, t(26) = 2.92, p < 0.007. All other group differences in saccade latencies across Stimulus Categories and Conditions were not significant, p's> 0.15. Figure 3 illustrates average latencies for the pro- and antisaccade conditions.
Fig. 3.
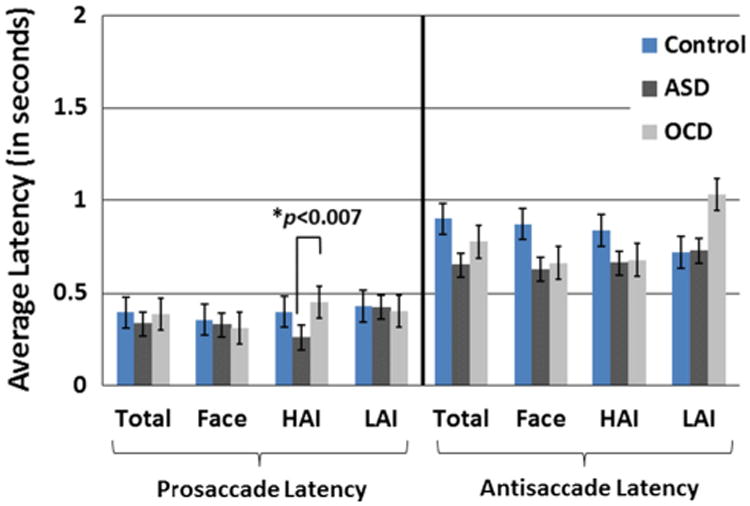
Group-averaged latencies for the prosaccade and antisaccade condition across all trials (“Total”), Face, HAI. And LAI trials. Error bars reflect SE of the mean
Correlations Between Saccade Performance and Symptom Severities
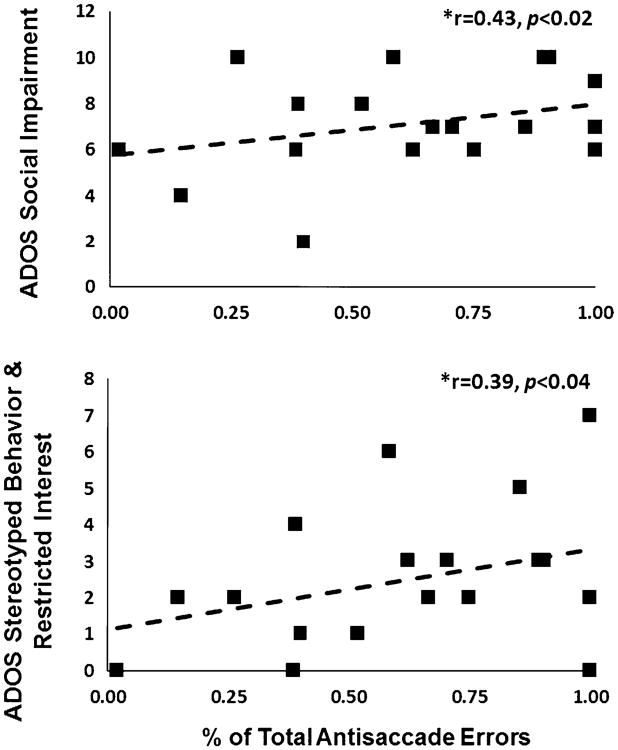
Within the ASD group, antisaccade error rates across all three stimulus categories were significantly related to reciprocal social interaction algorithm scores, r = 0.43, p <0.02, and stereotyped behaviors and restricted interests algorithm scores, r = 0.39, p< 0.04, from the ADOS (see Fig. 4). In the ASD group, IQ was indirectly related to total antisaccade error rates, r = −0.54, p < 0.02. Within the ASD group, correlations between age and antisaccade error rates to social images, r = −0.58, p <0.01, and HAI images, r = −0.49, p < 0.03, were also significant.
Fig. 4.
Top Scatterplot depicting the relation between ADOS reciprocal social interaction algorithm scores and total antisaccade errors (across all image categories) in the ASD group. Bottom Scatterplot depicting the relation between ADOS stereotyped behavior and restricted interests algorithm scores and total antisaccade errors in the ASD group
Within the OCD group, the correlations between total antisaccade error rates across all three stimulus categories and the severity of compulsive behaviors from the C-YBOCS approached significance, r = 0.60, p>0.08. In the ASD and OCD groups, error rates within stimulus categories were not significantly related to ADOS or C-YBOCS scores, p's > 0.14. Correlations between saccade latencies within the ASD and OCD groups and symptom severity were not significant, p's > 0.13. Correlations between age, IQ and antisaccade error rates were not significant within the OCD group.
Discussion
The current study investigated the cognitive control of visual attention in ASD in the context of a prosaccade and antisaccade paradigm that included social stimuli, nonsocial stimuli related to CIs in ASD, and nonsocial stimuli unrelated to CIs in ASD. The hypothesis was that the ASD group would make more antisaccade errors specifically in the context of nonsocial stimuli related to CIs was confirmed. The finding that the ASD and TD control groups did not differ in antisaccade rates in the context of social stimuli suggests that, in the domain of cognitive control of visual attention, impairments in ASD appear to be constrained to nonsocial stimuli related to CIs, though visual inspection of Fig. 2b suggests a somewhat similar pattern of responses to nonsocial stimuli not related to CIs, suggesting that ASD may be characterized by impaired cognitive control of visual attention in responses to nonsocial stimuli more generally. Additionally, the ASD group showed no differences in prosaccade error rates for any stimulus category, indicating that saccade errors in ASD are most prominent in contexts requiring response inhibition, a finding that is consistent with prior domain-general evidence of relatively greater deficits on anti-relative to prosaccade tasks in ASD (Luna et al. 2004; Manoach et al. 2004). There were also no differences between the ASD and TD control group in saccade latencies for either the prosaccade or antisaccade conditions. This suggests that deficits in the cognitive control of visual attention in ASD were not attributed to impaired saccade dynamics, but rather were a result of the demands placed on mechanisms subserving behavioral inhibition and the cognitive control of visual attention. Finally, the OCD group demonstrated overall fewer antisaccade errors than the ASD group, though this effect was not significant for any specific condition category, highlighting the specificity of antisaccade impairments to the ASD group, though the small OCD sample size in this study clearly dictates the need for further research in this area with larger samples.
Results compliment previous ASD findings that have incorporated passive viewing paradigms. Sasson and Touchstone (2014), using a passive preferential looking paradigm, found that toddlers with ASD presented similarly to typically developing peers in visual attention to faces presented concurrently with LAI stimuli; however, attention to faces was significantly diminished when displayed concurrently with HAI images. Additionally, Sasson et al. (2011) reported increased visual attention for HAI stimuli in the context of passively viewing complex visual arrays in ASD. It would thus appear that visual attention in ASD is impacted specifically by nonsocial stimuli related to CIs in ASD in passive viewing contexts as well as tasks requiring cognitive control of visual attention.
Antisaccade errors predicted the severity of social impairments and repetitive behaviors and restricted interests in the ASD group. This aligns with previous research highlighting relations between impairments in cognitive flexibility and response inhibition with restricted and repetitive behaviors in ASD (Lopez et al. 2005). Deficits in behavior monitoring and response inhibition may be tightly coupled with other complex executive function capacities (i.e. generativity, meta-representation) utilized in the processing of multifaceted or abstract information (Turner 1999).
Further studies will be needed to better characterize mechanisms subserving cognitive control of visual attention and the differential impact of social and nonsocial information on the ability to adaptively engage and disengage visual attention in ASD. Previous studies of executive function and higher cognitive processes mediated by social and nonsocial information in ASD via the use of functional neuroimaging and electrophysiological methods have illustrated differential patterns of neural activation and evoked responses to social versus nonsocial stimuli (Agam et al. 2010; Dichter et al. 2009; Manoach et al. 2004; McMahon and Henderson 2014; Sabatino et al. 2013). It thus may be the case that social and nonsocial information compete for processing resources that are limited in capacity in ASD. Inefficiencies in the neural substrates of cognitive control may therefore contribute to visual attentional impairments and the greater number of saccade errors made by the ASD group observed in the current context.
Limitations of this study should be addressed in future research. All participants viewed the same images; despite the internal validity this approach, CIs in ASD are typically person-specific. In the present context, HAI stimuli were used as a proxy for common CIs in ASD, and the use of standardized nonsocial image sets likely resulted in a conservative estimate of volitional control over visual attention in response to idiosyncratic images. Future research utilizing person-specific CI images will be needed to address whether our findings generalize to idiosyncratic CIs. We also note that findings from within condition t-tests would not survive correction for multiple comparisons and thus should be interpreted with caution pending replication. Additional limitations include the sample size of the OCD group and wide age ranges of all participants. Furthermore, the large proportion of males in this study suggests that findings may be specific to males.
Despite these limitations, the present study extends the literature on cognitive control impairments in ASD to the domain of cognitive control of nonsocial visual attention. Results are informative for future clinical research and the development of interventions focused on embedding nonsocial interests into structured activities to help engage individuals with ASD in adaptive behaviors (Boyd et al. 2007). Finally, the assessment of oculomotor behavior may be used to quantify impairments across clinical contexts as well as discrete aspects of symptom presentations in ASD and related neurodevelopmental disorders with overlapping phenotypes. A comprehensive understanding of cognitive control deficits and impairments in social and nonsocial visual attention and their roles across atypical development is a valuable first step in the use of these metrics as outcome measures in future clinical trials.
Acknowledgments
We would like to extend our sincere gratitude to the families who participated in this study. This research was supported by MH081285, MH073402, HD079124, by a UNC Jessie Ball DuPont Dissertation Fellowship to MK and by a UNC Graduate School Summer Research Fellowship to ASD.
Footnotes
Author Contributions The first author, ASD, worked closely with GD regarding the conception, design, analysis, and interpretation of the data. SJM, EH, and MK made contributed substantially with data acquistion. Authors made significant contributions to drafting or revising the article or contributed to the intellectual content. This study was a portion of the dissertation of ASD under the supervision of GD.
References
- Agam Y, Joseph RM, Barton JJS, Manoach DS. Reduced cognitive control of response inhibition by the anterior cingulate cortex in autism spectrum disorders. NeuroImage. 2010;52(1):336–347. doi: 10.1016/j.neuroimage.2010.04.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boraston Z, Blakemore SJ. The application of eye-tracking technology in the study of autism. The Journal of Physiology. 2007;581(3):893–898. doi: 10.1113/jphysiol.2007.133587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boyd BA, Conroy MA, Mancil GR, Nakao T, Alter PJ. Effects of circumscribed interests on the social behaviors of children with autism spectrum disorders. Journal of Autism and Developmental Disorders. 2007;37(8):1550–1561. doi: 10.1007/s10803-006-0286-8. [DOI] [PubMed] [Google Scholar]
- Chawarska K, Klin A, Paul R, Macari S, Volkmar F. A prospective study of toddlers with ASD: Short-term diagnostic and cognitive outcomes. Journal of Child Psychology and Psychiatry. 2009;50(10):1235–1245. doi: 10.1111/j.1469-7610.2009.02101.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Constantino JN, Gruber CP. The Social Responsiveness Scale. Los Angeles: Western Psychological Services; 2002. [Google Scholar]
- Cuthbert BN, Insel TR. Toward the future of psychiatric diagnosis: The seven pillars of RDoC. BMC Medicine. 2013;11:126. doi: 10.1186/1741-7015-11-126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dichter GS, Felder JN, Bodfish JW. Autism is characterized by dorsal anterior cingulate hyperactivation during social target detection. Social Cognitive and Affective Neuroscience. 2009 doi: 10.1093/scan/nsp017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dichter GS, Felder JN, Green SR, Rittenberg AM, Sasson NJ, Bodfish JW. Reward circuitry function in autism spectrum disorders. Social Cognitive and Affective Neuroscience. 2012;7:160–172. doi: 10.1093/scan/nsq095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fletcher-Watson S, Leekam SR, Benson V, Frank MC, Findlay JM. Eye-movements reveal attention to social information in autism spectrum disorder. Neuropsychologia. 2009;47(1):248–257. doi: 10.1016/j.neuropsychologia.2008.07.016. [DOI] [PubMed] [Google Scholar]
- Gotham K, Risi S, Dawson G, Tager-Flusberg H, Joseph R, Carter A, et al. A replication of the Autism Diagnostic Observation Schedule (ADOS) revised algorithms. Journal of the American Academy of Child & Adolescent Psychiatry. 2008;47(6):642–651. doi: 10.1097/CHI.0b013e31816bffb7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grelotti DJ, Klin AJ, Gauthier I, Skudlarski P, Cohen DJ, Gore JC, et al. fMRI activation of the fusiform gyrus and amygdala to cartoon characters but not to faces in a boy with autism. Neuropsychologia. 2005;43(3):373–385. doi: 10.1016/j.neuropsychologia.2004.06.015. [DOI] [PubMed] [Google Scholar]
- Howell DC, Rogier M, Yzerbyt V, Bestgen Y. Statistical methods in human sciences. New York: Wadsworth; 1998. [Google Scholar]
- Hutton SB. Cognitive control of saccadic eye movements. Brain and Cognition. 2008;68(3):327–340. doi: 10.1016/j.bandc.2008.08.021. [DOI] [PubMed] [Google Scholar]
- Jacob S, Landeros-Weisenberger A, Leckman JF. Autism spectrum and obsessive-compulsive disorders: OC behaviors, phenotypes and genetics. Autism Research. 2009;2(6):293–311. doi: 10.1002/aur.108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jemel B, Mottron L, Dawson M. Impaired face processing in autism: Fact or artifact? Journal of Autism and Developmental Disorders. 2006;36(1):91–106. doi: 10.1007/s10803-005-0050-5. [DOI] [PubMed] [Google Scholar]
- Jones W, Klin A. Attention to eyes is present but in decline in 2–6-month-old infants later diagnosed with autism. Nature. 2013;504(7480):427–431. doi: 10.1038/nature12715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Katarzyna C, Fred V, Ami K. Limited attentional bias for faces in toddlers with autism spectrum disorders. Archives of General Psychiatry. 2010;67(2):178. doi: 10.1001/archgenpsychiatry.2009.194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaufman J, Birmaher B, Brent D, Rao UMA, Flynn C, Moreci P, et al. Schedule for affective disorders and schizophrenia for school-age children-present and lifetime version (K-SADS-PL): Initial reliability and validity data. Journal of the American Academy of Child & Adolescent Psychiatry. 1997;36(7):980–988. doi: 10.1097/00004583-199707000-00021. [DOI] [PubMed] [Google Scholar]
- Kaufman A, Kaufman N. Kaufman Brief Intellifence Test. 2nd. Bloomington, MN: Pearson; 2004. [Google Scholar]
- Klin A, Jones W, Schultz R, Volkmar F, Cohen D. Visual fixation patterns during viewing of naturalistic social situations as predictors of social competence in individuals with autism. Archives of General Psychiatry. 2002;59(9):809. doi: 10.1001/archpsyc.59.9.809. [DOI] [PubMed] [Google Scholar]
- Lopez BR, Lincoln AJ, Ozonoff S, Lai Z. Examining the relationship between executive functions and restricted, repetitive symptoms of autistic disorder. Journal of Autism and Developmental Disorders. 2005;35(4):445–460. doi: 10.1007/s10803-005-5035-x. [DOI] [PubMed] [Google Scholar]
- Lord C, Rutter M, DiLavore P, Risi S, Gotham K, Bishop S. Autism Diagnostic Observation Schedule (ADOS-2) 2nd. Torrance, CA: Western Psychological Services; 2012. [Google Scholar]
- Luna B, Doll SK, Hegedus SJ, Minshew NJ, Sweeney JA. Maturation of executive function in autism. Biological Psychiatry. 2007;61(4):474–481. doi: 10.1016/j.biopsych.2006.02.030. [DOI] [PubMed] [Google Scholar]
- Luna B, Garver KE, Urban TA, Lazar NA, Sweeney JA. Maturation of cognitive processes from late childhood to adulthood. Child Development. 2004;75(5):1357–1372. doi: 10.1111/j.1467-8624.2004.00745.x. [DOI] [PubMed] [Google Scholar]
- Manoach DS, Lindgren KA, Barton JJS. Deficient saccadic inhibition in Asperger's disorder and the social-emotional processing disorder. Journal of Neurology, Neurosurgery and Psychiatry. 2004;75(12):1719–1726. doi: 10.1136/jnnp.2003.025981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McMahon CM, Henderson HA. Error-monitoring in response to social stimuli in individuals with higher-functioning autism spectrum disorder. Developmental Science. 2014 doi: 10.1111/desc.12220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mosconi MW, Kay M, D'Cruz AM, Seidenfeld A, Guter S, Stanford LD, Sweeney JA. Impaired inhibitory control is associated with higher-order repetitive behaviors in autism spectrum disorders. Psychological Medicine. 2009;39(9):1559–1566. doi: 10.1017/S0033291708004984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mostert-Kerckhoffs MAL, Staal WG, Houben RH, de Jonge MV. Stop and change: Inhibition and flexibility skills are related to repetitive behavior in children and young adults with autism spectrum disorders. Journal of Autism and Developmental Disorders. 2015;45(10):3148–3158. doi: 10.1007/s10803-015-2473-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sabatino A, Rittenberg A, Sasson NJ, Turner-Brown L, Bodfish JW, Dichter GS. Functional neuroimaging of social and nonsocial cognitive control in autism. Journal of Autism and Developmental Disorders. 2013;43(12):2903–2913. doi: 10.1007/s10803-013-1837-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sasson NJ, Dichter GS, Bodfish JW. Affective responses by adults with autism are reduced to social images but elevated to images related to circumscribed interests. PLoS ONE. 2012;7(8):e42457. doi: 10.1371/journal.pone.0042457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sasson NJ, Elison JT, Turner-Brown LM, Dichter GS, Bodfish JW. Brief report: Circumscribed attention in young children with autism. Journal of Autism and Developmental Disorders. 2011;41(2):242–247. doi: 10.1007/s10803-010-1038-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sasson NJ, Touchstone EW. Visual attention to competing social and object images by preschool children with autism spectrum disorder. Journal of Autism and Developmental Disorders. 2014;44(3):584–592. doi: 10.1007/s10803-013-1910-z. [DOI] [PubMed] [Google Scholar]
- Sasson NJ, Turner-Brown LM, Holtzclaw TN, Lam KSL, Bodfish JW. Children with autism demonstrate circumscribed attention during passive viewing of complex social and nonsocial picture arrays. Autism Research. 2008 doi: 10.1002/aur.4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scahill L, Riddle MA, McSwiggin-Hardin M, Ort SI, King RA, Goodman WK, et al. Children's Yale-Brown obsessive compulsive scale: reliability and validity. Journal of the American Academy of Child and Adolescent Psychiatry. 1997;36(6):844–852. doi: 10.1097/00004583-199706000-00023. [DOI] [PubMed] [Google Scholar]
- Shin NY, Lee TY, Kim E, Kwon JS. Cognitive functioning in obsessive-compulsive disorder: A meta-analysis. Psychological medicine. 2014;44(6):1121–1130. doi: 10.1017/S0033291713001803. [DOI] [PubMed] [Google Scholar]
- South M, Ozonoff S, McMahon WM. Repetitive behavior profiles in Asperger syndrome and high-functioning autism. Journal of Autism and Developmental Disorders. 2005;35(2):145–158. doi: 10.1007/s10803-004-1992-8. [DOI] [PubMed] [Google Scholar]
- Tottenham N, Tanaka JW, Leon AC, McCarry T, Nurse M, Hare TA, et al. The NimStim set of facial expressions: Judgments from untrained research participants. Psychiatry Research. 2009;168(3):242–249. doi: 10.1016/j.psychres.2008.05.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Turner MA. Generating novel ideas: Fluency performance in high-functioning and learning disabled individuals with autism. Journal of Child Psychology and Psychiatry. 1999;40(2):189–201. doi: 10.1111/1469-7610.00432. [DOI] [PubMed] [Google Scholar]
- Turner-Brown LM, Lam KSL, Holtzclaw TN, Dichter GS, Bodfish JW. Phenomenology and measurement of circumscribed interests in autism spectrum disorders. Autism. 2011;15(4):437–456. doi: 10.1177/1362361310386507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Turner-Brown LM, Perry TD, Dichter GS, Bodfish JW, Penn DL. Brief report: Feasibility of social cognition and interaction training for adults with high functioning autism. Journal of Autism and Developmental Disorders. 2008;38(9):1777–1784. doi: 10.1007/s10803-008-0545-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watson KK, Miller S, Hannah E, Kovac M, Damiano CR, Sabatino-DiCrisco A, et al. Increased reward value of non-social stimuli in children and adolescents with autism. Frontiers in Psychology. 2015 doi: 10.3389/fpsyg.2015.01026. [DOI] [PMC free article] [PubMed] [Google Scholar]